Abstract
The problem of heavy metal pollution in nature has increased rapidly in recent years. Hexavalent chromium (Cr+6) is one of the most toxic heavy metals that cause environmental pollution. Although many studies in the literature that illuminate the stress response mechanisms of biological organisms such as bacteria, algae, and plants against heavy metals, there is limited information about revealing the protein level changes of lichen species in response to heavy metal stress. Here, we used a MALDI-TOF-based proteomic assay to determine protein level changes in Pseudevernia furfuracea after exposure to Cr+6 heavy metal stress at 6, 18 and 24 h. It was determined that expression levels of 26, 149 and 66 proteins changed in P. furfuracea. 6, 18 and 24 h after Cr+6 application compared to the control sample, respectively. We identified 9 common proteins expressed at three different time levels (6, 18, 24 h) and evaluated their protein–protein interaction profiles with the STRING tool. According to the results of the study, it was determined that the expression level of six proteins was up-regulated (OP4, KIP3, BNI5, VSP64, HSP 60, BCK1) and three proteins were down-regulated (MNS1, ABZ2, ATG4) from the expression level of nine proteins in total with Cr+6 exposure. It was determined that nine proteins were also found to be effective in biological processes such as stress signaling, transcription regulation and cellular detoxification metabolisms. To confirm the protein expression level, we analyzed the HSP60 protein by western blot assay. It has been shown that exposure to Cr+6 exposure in P. furfuracea caused an increase in HSP60 protein level compared to the control sample (non-exposed Cr+6). In this study, new knowledge are presented for the use of P. furfuracea as a biosorption agent in the removal of industrial wastes in biotechnological applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are caused important environmental pollutions in recent years. Chromium (Cr) is a potent toxic heavy metal to all biological organisms and highly common environmental pollutants in industrial site (Dhal et al. 2013; Viti et al. 2014). Cr occurs as a result of many different industrial activities such as discharge of heavy metal contaminated waste and mining (Manikandan et al. 2016). Oxidation level is a marker in Cr toxicity, and hexavalent chromium (Cr+6) is much more toxic than other chromium metals (Cr+3) (Garcίa-Hernández et al. 2017; Tchounwou et al. 2014). Hexavalent chromium produces reactive oxygen species, which causes toxic effects on the cell as it causes oxidative damage (Wu et al. 2010; Li et al. 2017). The carcinogenicity of hexavalent chromium can induce many effects on biological organisms such as mutation, gene expression, and DNA damage (Das et al. 2014; Shi et al. 2018). Especially, the rapid increase of cancer disease makes us think that it can be caused by the effects of these metals (Torun et al. 2018). Therefore, it is very important to find low-cost and effective remediation methods for Cr+6 polluted soil or water treatment.
Instead of traditional technologies, bacteria and fungi are used for chromium removal, moreover, the use of these organisms enables cheaper and environmentally friendly methods (Coreño-Alonso et al. 2014; Murugavelh and Mohanty 2013; Dhal et al. 2013). Lichens have also been used as biomonitors and bioaccumulators for the assessment of heavy metal pollution. Due to the fact that lichens have some advantages, they are preferred primarily in the removal of heavy metals. Thanks to the high sensitivity of lichens to pollutants and their tolerance to difficult weather conditions, they are abundant and widely available in nature (Bargagli 2016). Lichens have a simple thallus structure with a large surface area but have no root systems, thus lichen species easily accumulate heavy metals directly from the air (Boamponsem et al. 2017; Zhou et al. 2017). In addition, lichens have the potential to retain metals from the air thanks to their cuticle structure (Salo et al. 2012). Our previous studies demonstrated that Pseudevernia furfuracea was a suitable species for monitoring heavy metals accumulation in Yenice, Karabük, Turkey (Cansaran-Duman et al. 2009). As a result of this study, P. furfuracea has been shown to be abundant and easily found in nature, low cost, and very resistant to the adverse effects of heavy metal accumulation. For these reasons, we preferred to use P. furfuracea lichen species in this study for determining effect of Cr+6 heavy metal.
Studies based on transcriptional analyzes cannot provide efective results due to changes in mRNA expression and slight correlation at the protein level (Rose et al. 2004; Gygi et al. 1999). However, research based on protein analysis enables us to obtain information about the protein response and function to the stress source by providing information at both the transcription and translation level (Ahsan et al. 2009). Determining the function of genes and proteins that respond to heavy metal stress makes it possible to understand the molecular mechanism against stress response in detail. The obtained results from proteomics data provide information about the actively translated part of the genome and complements DNA and mRNA analyzes. It is very important to determine the function of proteins in resistance to the source of stress, pathway analysis, transcription regulation and other processes (Ahsan et al. 2009). Therefore, proteomic analysis is a powerful and effective tool for studying the stress responses of biological organisms.
The scope of this study is to determine the response of Cr+6 stress with P. furfuracea lichen species at the proteome level. This is the first study to determine the protein profile of the Cr+6 effect on P. furfuracea as one of the most important bioaccumulators. Results obtained from proteome analysis were also confirmed by western blot analysis.
Materials and method
Lichen species and hexavalent chromium treatment
The thalli of P. furfuracea were collected from Yenice Forest/Karabük, Turkey. 25 mg to 1 g of fresh lichen samples were soaked on shaker in 5 mM HEPES buffer containing Cr+6, pH 6.8 at 0 (control) and 36 µM solution for 6, 18 and 24 h. Lichen samples were analyzed after exposure to Cr+6 stress.
Determination of hexavalent chromium content of Pseudevernia furfuracea thalli
Heavy metal contents were made according to the method determined in Cansaran-Duman (2011). The amount of Cr+6 in lichen samples was analyzed by spectrophometer (540 nm) using 1.5-diphenylcarbazide.
Protein extraction
Lichen sample (0.5 g) was pulverized in liquid nitrogen and the powder was re-suspended 1 ml of urea-lysis buffer (0.15 M NaCl, 50 mM Tris–HCl, 5 mM EDTA, Urea and protease inhibitor mixture). The solution was done sonication 10 times every 5 s at 40 amplude with sonicator for better homogenized of sample. Lichen samples were centrifuged at 17,000g for 45 min and then the supernatant was collected and protein concentrations were quantified by Bradford method (Bradford 1976) with BSA standard as triplicate.
Two-dimensional gel electrophoresis
In our study, 7 cm strips (Bio-Rad) with pH 3–10 gradient were used for protein samples that were applied control and Cr+6 stress. The strips were exposed to the rehydration phase at 50 V for 16 h. After this step, IEFs were treated at room temperature with the conditions of 250 V for 15 min, 10 kV for 3 h, and 10 kV until reaching 60 kV/h using Protean IEF Cell (Bio-Rad). IPG strips were balanced with buffers at room temperature. After this stage, separation of IPGs with 4% stacking and 12.5% running gels was performed at 100 V for 15 min. After the samples were stained, they were imaged using the VersaDoc Imaging System (Bio-Rad). Each sample was studied in four replicates.
Gel analysis
The 2D gels of the control (0 h) and Cr+6 stressed samples at different times (6, 18 and 24 h) were analyzed by PDQuest Version 8 software (Bio-Rad). One master gel was created from four replicates of the control group and the chromium stressed samples were compared with this master gel. Protein spots with different expressions between control and stressed samples were determined and the significance rate (p < 0.05) was determined by statistical analysis (Student’s t-test).
Enzymatic digestion
Enzymatic digestion method was applied according to the protocol determined by İgci et al. (2012). After the enzymatic digestion of the control and Cr+6 stressed samples, the samples were evaporated at room temperature about 5 h.
Matrix-assisted laser desorption ionization/time of flight (MALDI-TOF) mass spectrometry
We performed four biological replications at 0, 6, 18 and 24 h time points for proteomics assays.
Samples digested with trypsin were resolved using 0.1% trifluoroacetic acid (TFA) in 50% acetonitrile solvent. The samples were mixed with CHCA in the matrix solution. 1.5 µl of each solution was added to the MALDI plate and allowed to dry. Samples were analyzed with MALDI-TOF mass spectrometer (Micromass, Waters, UK) between 500–1000 Da. The spectra obtained were calibrated with five peptide mixes (angiotensin 1, substance P, Glu-fibrinopeptide, renin-14, ACTH-18–19).
Validation of differentially abundant protein by western blot analysis
Total soluble protein from each Cr+6 treated sample was separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane (Thermo Fischer Scientific, USA), and then blocked for 1 h with the usage of 5% nonfat milk. The membranes were incubated at 4 °C including diluted HSP60 primary antibodies (Santa Cruz Biotechnology, USA) and β-Actin primary antibodies (Cell Signaling, USA). Membranes were incubated again at room temperature for 1.5 h with the application of horseradish peroxide (HRP) conjugated secondary antibodies (1:20.000 dilutions) (Abcam, USA). Bands were visualized by chemiluminescence system ((Licor Imaging System, LI-COR, Odyssey Fc 2800, USA). The protein expression of samples evaluated ImageJ program.
Protein interaction network analysis
Gene Ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathways were used to evaluate the functions of proteins. Protein–protein interaction networks were analyzed STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) program.
Statistical analysis
The data obtained as a result of the study were analyzed by applying Student’s t-test analysis and the protein expression change rate barrier 2 folds and above compared with control (p < 0.05).
Results
Lichen species bioaccumulate from the environment contaminated with heavy metals. While lichens accumulate toxic metals, lichen samples develop a variety of defense strategies to regulate the uptake, distribution, and intracellular concentration of toxic heavy metal ions to decrease stress damage and survive. In this study, we determined the networks behind toxicity response to Cr+6 heavy metal stress in P. furfuraceae lichen species by MALDI-TOF-based proteomics approach.
Determination of hexavalent chromium content of P. furfuracea thalli
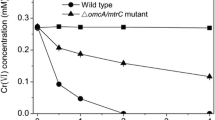
After applying 36 µM Cr+6 stress to P. furfuraceae lichen sample at different time intervals, the biosorption capacity of lichen sample for Cr+6 was measured by spectrophometer. According to the results, P. furfuraceae lichen sample showed important biosorption capacity from 0 to 6 h against Cr+6 stress (Fig. 1).
The proteome analysis of P. furfuracea after exposed of hexavalent chromium
After applying Cr+6 stress to the lichen sample, protein isolation was performed for MALDI-TOF analysis. A large number of protein isolation methods have been applied in this study. In order to obtain the optimal protein concentration of the P. furfuracea lichen sample, the protein isolation method was tried using different buffers. As a result of optimization experiments, the most appropriate protein concentration from lichen samples was obtained with urea-lysis buffer. Thus, it was possible to obtain a sufficient amount of protein until MALDI-TOF assay. The results of 2D gel electrophoresis and MALDI-TOF analysis were evaluated, it was determined that the spot similarity rate in the gels formed as a result of applying Cr+6 heavy metal stress at different time intervals (6, 18 and 24 h) was about 90%. The 90% similarity rate in the spot rates showed that it was interpreted as accurate and reliable from the gel spots obtained as a result of the repetitions in our study.
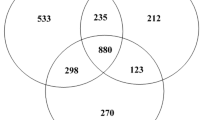
To obtain detailed information on the proteomic responses of P. furfuracea to Cr+6 stress, we determined the changes in time-dependent profile of the P. furfuracea at 0, 6, 18 and 24 h after 36 µM Cr+6 treatment and were analyzed by 2-DE (Fig. 2). Four independent biological replicates were performed in 2-DE assay. A total protein obtained from P. furfuracea were detected into more than 700 spots in each reproducible SDS-PAGE gels. Protein spots were visualized by bromophenol blue and spots compare to 2-DE gels from the control and 36 µM Cr+6 treated samples for 6, 18 and 24 h showed many differences in protein profile (Fig. 2). Comparison between the four biological replicates, there were 26, 149 and 66 differentially expressed proteins (DEPs) at 6, 18 and 24 h of Cr+6 treatment, respectively (Fig. 3). The densitometric analysis of 37 proteins showed common changes in response to Cr+6 stress, these proteins showed at least two-fold increase or decrease in expression in Cr+6 stress. From the examined protein spots, a total of 9 proteins with statistically significant increase or decrease expression levels in response to Cr+6 treatment at 6, 18 and 24 h were analyzed by MALDI-TOF (Table 1) (p < 0.05). The protein levels of 57, 65, 89 spots were found to be altered by Cr+6 stress on P. furfuracea at 6, 18 and 24 h, respectively (Fig. 2). Of these altered proteins, 6 proteins upregulated by Cr+6, while 3 proteins were down-regulated (Table 2). Our results indicate that proteomic assay can be successfully applied to lichen species.
The 9 common proteins can be classified into several groups according to their physiological functions. According to bioinformatics analysis, 9 proteins that respond to Cr+6 stress applied at different times mostly function in stress signaling (53%), transcription regulation (29%), cellular detoxification (18%) and other processes. Stress (Cr+6) metabolism may have damaged these pathways, in which case protein and histone modification may play a role in Cr+6 tolerance.
We also evaluated nine common proteins for determining protein–protein interaction by STRING bioinformatic tool (Fig. 4). In addition, the interaction of 6 proteins up-regulated from common proteins against Cr+6 stress in Fig. 5 and the interaction of 3 down-regulated proteins were evaluated in Fig. 6 were evaluated. In our study, the expression of Opaque-phase-specific protein OP4, Kinesin-like protein KIP3 OS, Bud neck protein 5, HSP60, and Vacuolar protein sorting/targeting protein 10 increased protein expression level without at 6 h and 18 h. In contrast, Mannosyl-oligosaccharide alpha-1,2-mannosidase, Aminodeoxychorismate lyase and cysteine protease ATG4 also decreased in all expression rates except at 6 h and 18 h. The Cytochrome P450 CYP2 subfamily, on the other hand, showed only a four-fold increase in the 6 h and not observe in 18 h and 24 h. The data obtained in our study were found to be compatible with the results in the literature (Table 2). Opaque-phase-specific protein OP4 is formed within the cell against the occurrence of environmental stress in three different time intervals against chromium stress and this protein related the cell grow and function in stress conditions. It is possible to increase the stress tolerance levels of P. furfuracea lichen with genetic engineering methods.
Heat shock protein 60 (HSP60) functions as a mitochondrial chaperonin, usually responsible for the transport and refolding of proteins from the cytoplasm to the mitochondrial matrix. HSP60 is essential for the synthesis and transport of significant mitochondrial proteins from the cell’s cytoplasm to the mitochondrial matrix. As a result of studies carried out to date, HSP60 is determined as a marker against diabetes, stress response, cancer and some immunological disorders. Likewise, as a result of our study with P. furfuracea, which is one of the lichen samples known to have a very high tolerance to stress, the response of HSP60 against stress has made it possible to use as a marker of the lichen sample. HSPs may play role in protecting the cells from damage following Cr+6 toxicity and we evaluated protein–protein interaction of HSP60 protein by STRING tool (Fig. 7). For further validation of the changes in proteomic profiling, HSP60 was analyzed by western blot assay. β-Actin was used as an internal protein reference. Results showed that significantly increase HSP60 densities in lichen species compared with control (p < 005) (Fig. 8). As shown in Fig. 8, treatment of Cr+6 on P. furfuracea revealed to 4.3-fold increased HSP60 level.
As a result of our study, protein cysteine protease ATG4 showed the most fold change in 6, 18 and 24 h. Cysteine protease ATG4, mainly related with the apoptotic pathway. Cysteine protease ATG4 plays the role of an autophagy regulator that provides the link between mitochondrial dysfunction and apoptosis. Mitochondrial transfer of cysteine protease ATG4 during cellular stress and differentiation plays an important role in regulating mitochondrial physiology, ROS, mitophagia and cell viability. For this reason, it shows that the response mechanism of the cysteine protease ATG4 protein formed against stress by P. furfuracea lichen sample might be related to apoptosis.
In the light of the data obtained, the capacity of P. furfuracea lichen to accumulate Cr+6 in high amounts has made it possible to use it as a biomonitor against metal pollutants in polluted areas. Along with the results of our protein-based study, we performed this study to determine the effect of Cr+6 on lichens at the proteome level. Thus, as a result of the identification of some of the proteins with this method, the products of specific genes that show different expression changes during metal exposure are also defined. The results obtained from the study is the first report in literature and will illuminate the future studies that will be performed in this important field about determination of environmental pollutants in lichen by using proteomic analysis.
Discussion
Environmental pollution has emerged as a result of the start of urban life, and it has increased to parallel with industrial development. The epiphytic lichens have been used extensively to monitor air quality around urban areas, industrial sites and to document spatial distribution and accumulation of heavy metals. Excessive use of chromium, which is very toxic especially in many industries, also causes serious danger to the environment and human health. Chromium has effects on biological organisms such as suppression of enzyme activity (Farid et al. 2017), inhibition of pigment biosynthesis, negatively affecting the photosynthesis process through lower CO2 exchange, decrease in cell division rate, over-production and accumulation of reactive oxygen species (ROS). In addition, the effect of chromium on lipids, proteins, enzymes and DNA has been determined by studies (Farid et al. 2017; Panda 2007; Christoua et al. 2020). This study determined the potential toxic effects of chromium (VI) on oxidative stress using Venus verrucosa (Chaabane et al. 2020). A significant increase in malondialdehyde levels, lipid hydroperoxides and hydrogen peroxide were detected in V. verrucosa exposed to chromium (VI). In the study, chromium (VI)-induced toxicity was determined in V. verrucosa (Chaabane et al. 2020). In our study, the effect of chromium (VI) was determined at the proteome level by using P. furfuracea lichen species. There is no studies have yet been studied on the protein response in P. furfuracea exposed to Cr+6 stress, and the detailed mechanism of the response of Cr+6 on the P. furfuracea lichen species was clearly explained in this study at protein level.
After many optimization studies, the most optimal result was obtained at IEF stage with 75 ng protein amount of P. furfuracea exposed to Cr+6 stress. However, Toorchi et al. (2009) performed protein isolation after PEG application to soybean roots. They have completed the IEF stage with 50 ng protein and 37 different proteins have been successfully identified (Toorchi et al. 2009). In our study, sufficient protein separation was not observed in gels loaded with less than 75 ng protein. The reason for this is interpreted that the amount and purity of the protein obtained from different biological organisms can differentiate the optimization stage.
The response of lichen samples to Cr+6 stress, which has been increasing due to the rapidly increasing industrialization process in recent years, has been determined as an increase and decrease in the level of protein expression. In this resistance pathway, stress conditions in answer of ascending or descending of some proteins play a key role. This type of proteins can be defined in stress conditions with can be possible to proteome study. In this study, P. furfuracea was exposed to control (non-treated Cr+6) and 36 µM Cr+6 for 6, 18 and 24 h of Cr+6 exposure. As a result of the MALDI-TOF MS analysis, we determined Opaque-phase-specific protein OP4, Kinesin-like protein KIP3 OS = , Mannosyl-oligosaccharide alpha-1,2-mannosidase, Aminodeoxychorismate lyase, Probable cysteine protease atg4, Bud neck protein 5, Vacuolar protein sorting/targeting protein 10, HSP 60, Cytochrome P450 CYP2 subfamily proteins for 6, 18 and 24 h of Cr+6 exposure. Rustichelli et al. investigated the mechanisms occurring in response to cadmium (Cd) stress to investigate the proteomic potential of the Physica adscendens lichen species (Rustichelli et al. 2008). P. furfuracea was exposed to a concentration of 36 µM Cd for 6, 18, 24 and 48 h. Two-dimensional electrophoresis and mass spectrometry analysis show a spot identity of 80–85% between 6 and 18, 24 and 48 h Cd stress. The assumed heat-shock proteins and glutathione S-transferase generally increased their expression in all applications of Cd stress. ABC transport proteins decreased their expression under 6 and 18 h Cd stress conditions. All these data led to the understanding of the importance of the protein level response of the P. adscendens thallus against Cd stress by the different expression of various protein families (Rustichelli et al. 2008). In the study carried out by Rustichelli et al. (2008), despite the 16 protein definitions after the Cd heavy metal application to P. adscendens lichen species, 9 proteins of the P. furfuracea lichen species were identified within our study. Two proteins (HSP60 and Cytochrome P450 CYP2 subfamily) were identified as common against different heavy metal stress sources applied in both studies in different lichen species. Nicolardia et al. (2012) identified changes in the Evernia prunastri lichen proteome exposed to constant mercury concentrations. Changes in proteome level showed that the chloroplastic photosystem I reaction center subunit II contains proteins belonging to the photosynthetic pathway, the oxygen-evolving protein and the chloroplastic ATP synthase b-subunit. The lichen response to mercury also included stress-related proteins such as HSP70 (Nicolardia et al. 2012). Nicolardi et al. and our study, protein expression differentiation in HSP70 and HSP60 belonging to the HSP family during metal removal was highly detected. To the best of our knowledge, Rustichelli et al., Nicolardia et al. and our study are three studies that show the response of lichens to heavy metal stress at the level of proteome and there are no other studies in this subject in the literature (Rustichelli et al. 2008; Nicolardia et al. 2012). This study will constitute an important step for the studies that will enable the use of lichens as a biosorption agent for the detoxification of industrial wastes with their protein response to Cr+ 6 stress.
Proteome analysis based on MALDI-TOF is a widely used method in the field of environmental biotechnology. Shashirekha et al. investigated the response of six cyanobacteria species to Cr+3 stress in biochemical and protein level (Shashirekha et al. 2015). The change in the protein profile was confirmed by SDS-PAGE assay. Among the studied species, the response of S. platensis to Cr+3 stress was higher than the other studied species. According to the data obtained, it shows the ability of cyanobacteria to resist Cr+3 stress and their possible use in wastewater treatment (Shashirekha et al. 2015). In our study, P. furfuracea lichen type Cr+6 optimal intake concentration was stabled after 35 µM concentration. In the current study, Cr+6 stress at 36 µM concentration was applied to be able to fully compare with Rustichelli et al. study (Rustichelli et al. 2008). In the light of the data we obtained as a result of the research, similar to S. platensis, P. furfuracea is a candidate biological organism that can be used for Cr+6 removal.
Yıldız and Terzi examined the response of Cr+6 stress in canola using a proteomic approach (Yıldız and Terzi, 2016). Samples exposed at 100 µM Cr+6 were analyzed by two-dimensional electrophoresis (2-DE) and 58 protein differentiation was determined. Of these, 39 proteins were identified by MALDI-TOF/TOF mass spectrometry. It has been determined that proteins exhibiting differentiation function in photosynthesis, energy metabolism, stress defense, protein folding and stabilization, signal transmission, redox regulation and sulfur metabolism. Stress-related proteins have been shown to play an important role in the detoxification of Cr+6 (Yıldız and Terzi 2016). As a result of proteome assay, proteins associated with stress defense were determined, but it was revealed that there was not common stress-related protein in P. furfuracea and canola.
In the study of Fang et al. (2019), 2-DE and MALDI-TOF-TOF MS/MS analysis were performed after Cd application and in this study was selected Cd-sensitive D69 and Cd tolerant D28 line in brass. A total of 53 differently expressed phosphoproteins have been identified, mostly related to metabolism, signal transduction, gene expression regulation, material transport and membrane fusion. These results clarified phosphorylated pathways in response and resistance to Cd stress in rice. Although cadmium is a toxic metal like Cr+6, P. furfuracea protein profile with a common response could not be determined (Fang et al. 2019).
There are few studies on determining the heavy metal stress response of lichens with proteomic methods. However, there are proteome studies in the literature investigating the reactions of plant species in exposed to heavy metal stress and proteins used in defense mechanisms. Ahsan et al. investigated the physiological and proteomic differentiation of germinated rice seeds depending on copper (Cu) stress (Ahsan et al., 2007). As a result of protein profiles analyzed by two-dimensional gel electrophoresis, the result that 25 protein points were expressed differently in samples treated with Cu. Among them, an expression increase in 18 protein points and decrease in expression in 7 protein points were detected. An increase in the expression of some antioxidants has been determined in studies that some regulator proteins such as DNAK-type molecular chaperones, U1P1 protease and receptor-like kinases, and stress-related proteins such as glycose I, peroxyledoxin and aldose reductase, can increase oxidative stress (Ahsan et al. 2007). To investigate the effect of cadmium (Cd) heavy metal in rice, Lee et al. (2010) showed the effect of Cd on the leaf and root on the proteome size, after applying Cd in different concentrations, the root and leaf tissues were collected separately and performed protein isolation from the leaf. Change (increase/decrease) in the expression of 36 proteins after Cd heavy metal application was determined. With these results, it was concluded that the antioxidative effect in the roots may be necessary for the reduction of oxidative stress. In addition, RNA gel blot analysis and proteins determined in proteome analysis were regulated differently at the transcriptional level (Lee et al. 2010). Zhao et al. examined changes in the level of the proteome due to cadmium (Cd) stress in the leaves of the Phytolacca americana hyperacumulator plant (Zhao et al. 2011). In this study, the change in protein expression level in P. americana due to Cd stress was investigated by two-dimensional gel electrophoresis. In total, 32 differently expressed protein points corresponding to 25 gene products were detected using MALDI-TOF/TOF mass spectrometry. The expression of 14 of the 25 gene products increased while level of 11 gene decreased in Cd stress (Zhao et al. 2011). In this study, we evaluated the effect of a different metal on a different biological sample. A total of 9 proteins were identified that differentially expressed in Cr+6 treated P. furfuracea. Of the 9 proteins identified by the Cr+6 effect, the HSP60 protein was showed the highest difference in protein expression. HSP proteins play a role in repairing the damage caused by the cellular response to stress conditions. Changes in temperature or other physical factors can induce the synthesis of HSPs in biological organisms in the environment (Rios-Arana et al. 2005). Rios-Arana et al. (2005) evaluated physiological responses of P. patulus through HSP60 induction to As and other heavy metals. In their study was validated stress protein 60 (HSP60) using western blotting. HSP60 expression was twofold increase in rotifers exposed to elements at both low and high concentrations as compared to control rotifers (Rios-Arana et al. 2005). As our result of western blot analysis, HSP60 increased 4.3-fold after Cr+6 application in our study. Cr+6 heavy metals stimulate HSP60 production and HSP60 might be used to as biomarkers for determine to effect of Cr+6 on P. furfuracea.
Conclusion
To the best of our knowledge, this is the first study for determining Cr+6 stress in P. furfuracea by proteomic assay. The identification of Cr+6 responsive proteins might provide new insights into the heavy metal effect at the proteome level. Based on these results, more studies are needed to determine the potential of lichen species to be used as a biomarker by identifying proteins against heavy metal stress.
References
Ahsan N, Lee DG, Lee SH, Kang KY, Lee JJ, Kim PJ, Yoon HS, Kim JS, Lee BH (2007) Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 67:1182–1193
Ahsan N, Renaut J, Komatsu S (2009) Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteomics 9(10):2602–2621
Bargagli R (2016) Moss and lichen biomonitoring of atmospheric mercury: a review. Sci Total Environ 572:216–231
Boamponsem LK, de Freitas CR, Williams D (2017) Source apportionment of air pollutants in the Greater Auckland Region of New Zealand using receptor models and elemental levels in the lichen, Parmotrema reticulatum. Atmos Pollut Res 8:101–113
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cansaran-Duman D (2011) Study on accumulation ability of two lichen species (Hypogymnia physodes and Usnea hirta at iron-steel factory site, Turkey. J Environ Biol 32:839–844
Cansaran-Duman D, Atakol O, Atasoy İ, Kahya D, Aras S, Beyaztaş T (2009) Heavy metal accumulation in Pseudevernia furfuracea (L.) Zopf from the Karabük iron-steel factory in Karabük, Turkey. Z Naturforsch 64c:717–723
Chaabanea M, Bejaouib S, Trabelsib W, Telahigueb K, Chetouib I, Chalghafc M, Zeghala N, Cafsib ME, Soudania N (2020) The potential toxic effects of hexavalent chromium on oxidative stress biomarkers and fatty acids profile in soft tissues of Venus verrucosa. Ecotoxicol Environ Saf 196:110562
Christoua A, Georgiadoua EC, Zissimosb AM, Christoforoub IC, Christofib C, Neocleousa D, Daliasa P, Torradoc S, Argyrakid A, Fotopoulos V (2020) Hexavalent chromium leads to differential hormetic or damaging effects in alfalfa (Medicago sativa L.) plants in a concentration-dependent manner by regulating nitro-oxidative and proline metabolism. Environ Pollut 267:115379
Coreño-Alonso A, Solé A, Diestra E, Esteve I, Gutiérrez-Corona JF, López GR (2014) Mechanisms of interaction of chromium with Aspergillus niger var tubingensis strain Ed8. Bioresour Technol 158:188–192
Das S, Mishra J, Das SK, Pandey S, Rao DS, Chakraborty A, Sudarshan M, Das N, Thatoi H (2014) Investigation on mechanism of Cr(VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere 96:112–121
Dhal B, Thatoi H, Das N, Pandey B (2013) Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J Hazard Mater 250:272–291
Fang Y, Xiangxiong D, Xueli L, Junjun Z, Hua J, Yuchun R, Dali Z, Jiang H, Xiaoqin Z, Dawei X (2019) Differential phosphoproteome study of the response to cadmium stress in rice. Ecotoxicol Environ Saf 180:780–788
Farid M, Ali S, Akram NA, Rizwan M, Abbas F, Bukhari SAH, Saeed R (2017) Phyto-management of Cr-contaminated soils by sunflower hybrids: physiological and biochemical response and metal extractability under Cr stress. Environ Sci Pollut Res 24(20):16845–16859
Garcίa-Hernández MA, Villarreal-Chiu JF, Garza-González MT (2017) Metallophilic fungi research: an alternative for its use in the bioremediation of hexavalent chromium. Int J Environ Sci Technol 14:2023–2038
Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19(3):1720–1730
Igci N, Özel-Demiralp D (2012) A preliminary investigation into the venom proteome of Macrovipera lebetina obtusa (Dwigubsky, 1832) from Southeastern Anatolia by MALDI-TOF mass spectrometry and comparison of venom protein profiles with Macrovipera lebetina lebetina (Linnaeus, 1758) from Cyprus by 2D-PAGE. Arch Toxicol 86(3):441–451
Lee K, Bae DW, Kim SH, Han HJ, Liu X, Park HJ, OhLim C, Lee SY, Chung WS (2010) Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. J Plant Physiol 167:161–168
Li X, Wang Y, Pan Y, Yu H, Zhang X, Shen Y, Jiao S, Wu K, La G, Yuan Y, Zhang S (2017) Mechanisms of Cd and Cr removal and tolerance by macrofungus Pleurotus ostreatus HAU-2. J Hazard Mater 15(330):1–18
Manikandan NA, Alemu AK, Goswami L, Pakshirajan K, Pugazhenthi G (2016) Waste litchi peels for Cr(VI) removal from synthetic wastewater in batch and continuous systems: sorbent characterization, regeneration and reuse study. J Environ Eng 142:4016001
Murugavelh S, Mohanty K (2013) Bioreduction of Cr (VI) using live and immobilized Phanerochaete chrysosporium. Water Treat 51(16–18):3482–3488
Nicolardia V, Giampiero C, Luigi P, Michele P, Laura B, Luca B, Carlo G (2012) The adaptive response of lichens to mercury exposure involves changes in the photosynthetic machinery. Environ Pollut 160:1–10
Panda SK (2007) Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. J Plant Physiol 164(11):1419–1428
Rios-Arana JV, Gardea-Torresdey JL, Webb R, Walsh EJ (2005) Heat shock protein 60 (HSP60) response of Plationus patulus (Rotifera: Monogononta) to combined exposures of arsenic and heavy metals. Hydrobiologia 546:577–585
Rose JK, Bashir S, Giovannoni JJ, Jahn MM, Saravanan RS (2004) Tackling the plant proteome: practical approaches, hurdles and experimental tools. Plant J 39(5):715–733
Rustichelli C, Visioli G, Kostecka D, Vurro E, Sanita di Toppi L, Marmıroli N (2008) Proteomic analysis in the lichen Psysica adscendens expose to cadmium stress. Environ Pollut 156(3):1121–1127
Salo H, Buko MS, Vaahtovuo E, Limo J, Mkinen J, Pesonen LJ (2012) Biomonitoring of air pollution in SW Finland by magnetic and chemical measurements of moss bags and lichens. J Geochem Explor 115:69–81
Shashirekha V, Sridharan MR, Swamy M (2015) Biochemical response of cyanobacterial species to trivalent chromium stress. Algal Res 12:421–430
Shi L, Xue J, Liu B, Dong P, Wen Z, Shen Z, Chen Y (2018) Hydrogen ions and organic acids secreted by ectomycorrhizal fungi, Pisolithus sp., are involved in the efficient removal of hexavalent chromium from waste water. Ecotoxicol Environ Saf 161:430–436
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2014) Heavy metals toxicity and the environment. EXS 101:133–164
Toorchi M, Yukawa K, NouriKomatsu MZS (2009) Proteomics approach for identifying osmotic-stress-related proteins in soybean roots. Peptides 12:2108–2117
Torun V, Değerli E, Cansaran-Duman D (2018) A promising role of lichens for early detection and their secondary metabolites on treatment of cancer disease after exposure to carcinogenic heavy metals. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (eds) Phytoremediation, vol 6. Springer, Switzerland, pp 203–214
Viti C, Marchi E, Decorosi F, Giovannetti L (2014) Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiol Rev 38:633–659
Wu GL, Cui J, Tao L, Yang H (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 19(1):124
Yıldız M, Terzi H (2016) Proteomic analysis of chromium stress and sülfür deficiency responses in leave softwo canola (Brassicanapus L.) cultivars differing in Cr(VI) tolerance. Ecotoxicol Environ Saf 124:255–266
Zhao L, Sun YL, Cui SX, Chen M, Yang HM, Liu HM, Chai TY, Huang F (2011) Cd-induced changes in leaf proteome of the hyperaccumilator plant Phytolacca americana. Chemosphere 1:56–66
Zhou R, Yang R, Jing C (2017) Polycyclic aromatic hydrocarbons in soils and lichen from the western Tibetan Plateau: concentration profiles, distribution and its influencing factors. Ecotoxicol Environ Saf 152:151–158
Acknowledgements
We thank TUBITAK (The Scientific and Technical Research Council of Turkey), Project no. 115Z041, for the financial support. We would like to thank Prof. Dr. Duygu Özel-Demiralp and Dr. Beycan Ayhan for their valuable contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, and that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Özenoğlu-Aydınoğlu, S., Yıldızhan, H. & Cansaran-Duman, D. A proteomic analysis of Pseudevernia furfuracea after exposure to Cr+6 by MALDI-TOF mass spectrometry. 3 Biotech 11, 444 (2021). https://doi.org/10.1007/s13205-021-02986-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02986-3