Abstract
The mechanism of the non-catalysed and the MgBr2-catalysed [3+2] cycloaddition (32CA) reactions between C-methoxycarbonyl nitrone and 2-propen-1-ol has been theoretically investigated within the molecular electron density theory using DFT methods at the B3LYP/6-31G(d) computational level. Analysis of DFT reactivity indices allows explaining the role of the MgBr2 Lewis acid in the catalysed 32CA reaction. The 32CA reaction between C-methoxycarbonyl nitrone and 2-propen-1-ol takes place with a relative high activation enthalpy, 13.5 kcal mol−1, as a consequence of the non-polar character of this zw-type 32CA reaction. Coordination of the MgBr2 LA to C-methoxycarbonyl nitrone accelerates the corresponding zw-type 32CA reaction by taking place through a polar mechanism and with lower activation enthalpy, 8.5 kcal mol−1. Both 32CA reactions, which take place through a one-step mechanism, are completely meta regioselective and present low exo stereoselectivity, which increases in the catalysed process. Energy and non-covalent interaction analyses at the transition-state structures indicate that the formation of an intramolecular H–Br hydrogen bond in the catalysed process could be responsible for the exo selectivity experimentally observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
[3+2] Cycloaddition (32CA) reactions are among the most powerful methods for the synthesis of five-membered heterocyclic compounds since 1961, when Huisgen experimentally developed their mechanistic study [1]. Starting from relatively simple and easily accessible molecules, 32CA reactions can offer a highly yielding and selective method for the synthesis of a wide variety of simple as well as complex heterocycles of great biological importance [2].
Nitrones 1 are stable and easily prepared compounds widely used as efficient three-atom components (TACs) in 32CA reactions with alkenes. In particular, the 32CA reaction of nitrones 1 with electron-deficient (ED) ethylenes 2 is one of the most attractive methods for the synthesis of isoxazolidines [3], which are important precursors for the synthesis of a large number of biologically active molecules with a diversity of applications, mainly as amino alcohols and alkaloids antiviral agents [4,5,6,7]. In addition, the use of non-symmetric alkenes opens up the possibility of obtaining two isomeric isoxazolidines (see Scheme 1).
Unlike 1,3-dienes participating in Diels–Alder reactions [8], the electronic structure of TACs participating in 32CA reactions strongly depends on the type and hybridisation of the atoms present in the TAC. Thus, depending on their electronic structure, TACs have recently been classified as pseudodiradical, carbenoid and zwitterionic TACs (see Scheme 2) [9, 10]. Note that only 1,2-zwitterionic TACs such as nitrones 1 have the electronic structure of a 1,3-dipole as Huisgen proposed [1].
Very recently, Domingo has proposed a new reactivity model in organic chemistry named the molecular electron density theory (MEDT) [11], in which changes in the electron density along an organic reaction, and not MO interactions, are responsible for the feasibility of an organic reaction. Within MEDT, besides a deep exploration and characterisation of the potential energy surfaces (PESs) associated with the studied reaction, quantum chemical tools based on the analysis of the electron density, such as the analysis of the conceptual density functional theory (CDFT) reactivity indices at the ground state of the molecules [12, 13], the topological analysis of the electron localisation function (ELF) [14] focused on the progress of the bonding changes along the reaction coordinate and non-covalent interaction [15] (NCI) analysis at the TSs in order to characterise weak interactions determining the selectivity in organic reactions, are used to study the molecular reactivity in organic reactions rigorously.
MEDT studies [10, 16] devoted to the understanding of the mechanisms of 32CA reactions have allowed establishing a useful classification of these reactions into pseudodiradical-type [9] (pr-type), carbenoid-type (cb-type) [10] and zwitterionic-type [9] (zw-type) reactions, in such a manner that TACs with a pseudodiradical character participate in pr-type 32CA reactions taking place easily through earlier transition-state structures (TSs) with a very low polar character [9, 17]; TACs with a carbenoid character participate in cb-type 32CA reactions whose feasibility depends on the polar character of the reaction, i.e. the nucleophilic character of the carbenoid TAC and the electrophilic character of the ethylene derivative [10]; and finally, TACs with a zwitterionic character participate in zw-type 32CA reactions controlled by nucleophilic/electrophilic interactions taking place at the TSs, similarly to cb-type reactions [9, 18].
Nitrones 1, which present a high nucleophilic character, are able to participate in zw-type 32CA reactions with ED ethylenes 2, usually via a polar one-step mechanism with low activation energy [18]. However, nitrones generally have no tendency to react with electron-rich (ER) ethylenes. In these cases, the presence of a Lewis acid (LA) catalyst coordinated to the nitrone oxygen atom is required in order to increase its electrophilic character [18].
LA-catalysed 32CA reactions constitute a new research field and have attracted a continuous growing interest in some unexplored areas, which need to be investigated in both experimental and theoretical ways [19]. A considerable amount of experimental and theoretical studies of the mechanisms and selectivities of LA-catalysed 32CA reactions of nitrones with ethylene derivatives can be found in the literature. The 32CA reaction of nitrone 5 with the ER ethylene 6 giving exclusively the 5-substituted isoxazolidine 7 was experimentally studied by Jorgensen et al. [20] (see Scheme 3). The reaction rate was enhanced by the presence of a LA. The 32CA reaction presented a total regioselectivity, while the exo stereoselectivity depended on the bulky chiral LA used as catalyst [20]. A further DFT study permitted establishing that inclusion of the BH3 LA and solvent effects causes the corresponding nitrone/BH3 complex and ER vinyl ether to behave as an electrophile and a nucleophile, respectively, in a polar reaction [21]; thus, these reactions should be considered as a nucleophilic attack of the α-carbon of the vinyl ether on the carbon atom of the LA-coordinated nitrone.
LA-catalysed 32CA reactions of nitrone 5 with ER vinyl ether 6 [20]
Sousa et al. [22] experimentally studied the 32CA reaction of C-methoxycarbonyl nitrone 8 with ER cyclopentene 9, finding that this reaction gives exclusively the trans-isoxazolidine 10 proceeding from the exo approach (see Scheme 4). A recent DFT study [23] of the role of the BF3 LA in this zw-type 32CA reaction showed that in the absence of the LA catalyst, the reaction presents a high activation energy, 11.2 kcal mol−1, as a consequence of the low electrophilic character of C-methoxycarbonyl nitrone 8, ω = 1.46 eV [23]. Coordination of the BF3 LA to the nitrone oxygen atom accelerated the 32CA reaction by increasing markedly the polar character of the reaction, ΔE ≠ = 2.9 kcal mol−1. Both non-catalysed and catalysed 32CA reactions showed to be completely exo selective, in total agreement with the experimental outcomes [23].
32CA reaction of C-methoxycarbonyl nitrone 8 with ER cyclopentene 9 yielding trans-isoxazolidine 10 [22]
A recent DFT study[23] devoted to the participation of nitrones in zw-type reactions showed that the C-phenyl nitrone 11a presents a low electrophilicity ω index [24], ω = 1.57 eV, and a high nucleophilicity N index [25, 26], N = 3.51 eV, being classified as a moderate electrophile and a strong nucleophile (see Scheme 5). Inclusion of a strong electron-withdrawing group (EWG) such as NO2 in the phenyl substituent notably increases the electrophilicity of nitrone 11b, ω = 3.13 eV. However, in spite of this strong electrophilic activation, the 32CA reaction of nitrone 11b with an ER ethylene presents a high activation energy, ΔE ≠ = 13.2 kcal mol−1 [13, 18]. This study revealed that coordination of the BF3 LA to the nitrone oxygen atom effectively activates the participation of complex 11c, ω = 4.20 eV, in zw-type 32CA reactions with ER ethylenes. In this case, the most favourable regioisomeric TS was energetically found below the separated reagents, ΔE = −3.7 kcal mol−1. The LA-catalysed 32CA reaction showed to be completely regioselective, giving the corresponding 1,2-isoxazolidine as single product [23].
The 32CA reaction of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13, yielding a 44:56 mixture of 3,5-trans- and 3,5-cis-isoxazolidines 14n and 14x, was experimentally studied by Kanemasa (see Scheme 6) [27]. Interestingly, a high rate acceleration was observed in the presence of an equimolar amount of MgBr2·OEt2 in dichloromethane (DCM), being the 3,5-cis-isomer 14x obtained as single stereoisomer in 71% yield.
32CA reactions between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13 studied by Kanemasa [27]
Herein, in order to understand the effects of the MgBr2 LA catalyst on the molecular mechanism and the reaction rate of the 32CA reaction between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13, as well as the origin of the selectivities experimentally found by Kanemasa et al. [27], a theoretical characterisation of the molecular mechanism of these zw-type 32CA reactions is carried out within the MEDT using DFT methods at the B3LYP/6-31G(d) computational level. To this end, besides the exploration and characterisation of the potential energy surfaces (PESs) associated with the studied reactions, quantum chemical tools based on the analysis of the electron density, such as CDFT reactivity indices [13, 28] and NCI [15] analysis, will be employed.
2 Computational methods
All stationary points involved in these 32CA reactions were optimised using the B3LYP functional [29, 30] together with the standard 6-31G(d) basis set [31] The optimisations were carried out using the Berny analytical gradient optimisation method [32, 33] The stationary points were characterised by frequency computations in order to verify that TSs have one and only one imaginary frequency. The intrinsic reaction coordinate (IRC) paths [34] were traced in order to check the energy profiles connecting each TS to the two associated minima of the proposed mechanism using the second-order González–Schlegel integration method [35, 36]. Solvent effects of DCM were taken into account by full optimisation of gas-phase stationary points using the polarisable continuum model (PCM) developed by Tomasi’s group[37, 38] in the framework of the self-consistent reaction field (SCRF) [39,40,41]. Values of enthalpies, entropies and Gibbs free energies in DCM were calculated with standard statistical thermodynamics at 298.15 K and 1 atm [31]. Natural atomic charges were obtained through a natural population analysis (NPA) within the natural bond orbital (NBO) method [42, 43]. NCIs were computed by evaluating the promolecular density and using the methodology previously described [15, 44, 45]. All computations were carried out with the Gaussian 09 suite of programs [46].
The global electrophilicity index [24], ω, is given by the following expression, \( \omega = (\mu^{2} /2\eta ) \), in terms of the electronic chemical potential, μ, and the chemical hardness, η. Both quantities may be approached in terms of the one-electron energies of the frontier molecular orbitals HOMO and LUMO, \( \varepsilon_{H} \) and \( \varepsilon_{L} \), as \( \mu \approx (\varepsilon_{H} + \varepsilon_{L} )/2 \) and \( \eta = (\varepsilon_{L} - \varepsilon_{H} ) \), respectively [47, 48]. The empirical (relative) nucleophilicity index [25, 26] N, based on the HOMO energies obtained within the Kohn–Sham scheme [49], is defined as N = E HOMO(Nu) − E HOMO(TCE), where tetracyanoethylene (TCE) is the reference, because it presents the lowest HOMO energy in a long series of molecules already investigated in the context of polar organic reactions. This choice allows conveniently to hand a nucleophilicity scale of positive values. Nucleophilic \( P_{k}^{ - } \) and electrophilic \( P_{k}^{ + } \) Parr functions [50] were obtained through the analysis of the Mulliken atomic spin density (ASD) of the corresponding radical cations or anions, respectively.
3 Results and discussion
The present theoretical study has been divided into four sections: (1) first, an analysis of DFT reactivity indices of the reagents involved in the non-catalysed and in the LA-catalysed 32CA reactions is carried out; (2) then, the PESs associated with the 32CA reaction between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13 are explored and characterised; (3) third, the 32CA reaction between complex 12:Mg and 2-propen-1-ol 13 is studied; and (4) finally, the origin of the selectivity experimentally found in the LA-catalysed process is analysed.
3.1 Analysis of CDFT reactivity indices
Global reactivity indices, as defined in the context of CDFT [16, 28], are useful tools to understand the reactivity of molecules in their ground state as shown in recent studies devoted to DA [8] and 32CA reactions [51]. Table 1 shows the static global properties: electronic chemical potential, μ, chemical hardness, η, and global electrophilicity, ω, and nucleophilicity, N, of C-methoxycarbonyl nitrone 12, complex 12:Mg, 2-propen-1-ol 13 and ethylene 16.
The electronic chemical potential of 2-propen-1-ol 13, μ = −3.27 eV, is higher than that of C-methoxycarbonyl nitrone 12, μ = −4.17 eV, and complex 12:Mg, μ = −5.00 eV, suggesting that along a polar reaction, the global electron density transfer (GEDT) [52] will take place from 2-propen-1-ol 13 to C-methoxycarbonyl nitrone 12 and complex 12:Mg.
Along a polar reaction, there is an electron density transfer from the nucleophile to the electrophile, which is measured by the GEDT [52] value at the TS of the reaction; the larger GEDT at the TS is the more polar the reaction. Cycloadditions with GEDT values near 0.0e correspond to non-polar processes, whereas values higher than 0.2e correspond to polar processes. Interestingly, thorough studies have permitted to establish good correlations between the polar character of the reactions and their feasibility [8,9,10]. It is noteworthy that the GEDT concept comes from the observation that the electron density transfer taking place from a nucleophile to an electrophile along a polar reaction is not a local process, but a global one involving the two interacting frameworks [52] and depending on the electrophilic/nucleophilic interactions taking place between them.
The electrophilicity and nucleophilicity indices of C-methoxycarbonyl nitrone 12, ω = 1.81 and N = 2.54 eV, allow its classification on the borderline of strong electrophiles and as a moderate nucleophile based on the electrophilicity [53] and nucleophilicity [54] scales. Coordination of MgBr2 LA to nitrone 12 considerably increases the electrophilicity of complex 12:Mg, ω = 4.72 eV, and slightly increases the nucleophilicity, N = 2.84 eV, being now classified as a strong electrophile and remaining as moderate nucleophile.
On the other hand, the electrophilicity and nucleophilicity indices of 2-propen-1-ol 13 are ω = 0.74 and N = 2.20 eV, being classified on the borderline of marginal electrophiles and as a moderate nucleophile. Note that 2-propen-1-ol 13 is only slightly more nucleophilic than ethylene 16, which has no tendency to participate in polar cycloaddition reactions.
Analysis of these global reactivity indices indicates that the low electrophilic character of C-methoxycarbonyl nitrone 12 and the low nucleophilic character of 2-propen-1-ol 13 make that the corresponding zw-type 32CA reaction will have a very low polar character. Coordination of MgBr2 to nitrone 12 considerably increases the electrophilicity of complex 12:Mg, allowing the corresponding 32CA reaction to have somewhat polar character, thus having a lower activation energy that the non-catalysed process.
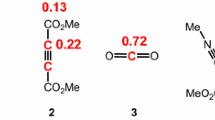
In polar cycloaddition reactions, the most favourable reactive channel is that involving the initial two-centre interaction between the most electrophilic and nucleophilic centres of both reagents. Recently, Domingo et al. [50] proposed the electrophilic \( P_{k}^{ + } \) and nucleophilic \( P_{k}^{ - } \) Parr functions derived from the changes of spin electron density reached via the GEDT process from the nucleophile to the electrophile as powerful tools in the study of the local reactivity in polar processes. Accordingly, the electrophilic \( P_{k}^{ + } \) Parr functions for complex 12:Mg and the nucleophilic \( P_{k}^{ - } \) Parr functions for 2-propen-1-ol 13 were computed (see Fig. 1).
Analysis of the electrophilic \( P_{k}^{ + } \) Parr functions for complex 12:Mg indicates that the nitrone nitrogen atom is the most electrophilic centre, \( P_{k}^{ + } = 0.36 \), being twice as electrophilically activated than the O1 and C3 atoms, which have the same electrophilic activation, \( P_{k}^{ + } = 0.14 \) (see Scheme 7 for atom numbering). The carbonyl carbon atom has a moderate electrophilic activation, \( P_{k}^{ + } = 0.20 \). On the other hand, analysis of the nucleophilic \( P_{k}^{ - } \) Parr functions at 2-propen-1-ol 13 indicates that the C4 carbon, \( P_{k}^{ - } = 0.40 \), is twice as nucleophilically activated as the C4 carbon, \( P_{k}^{ - } = 0.24 \).
Although the O1 and C3 atoms of nitrone have the same electrophilic activation, the high electrophilic activation of the carbonyl carbon atom adjacent to the nitrone C3 carbon suggests that the most favourable regioisomeric reactive channel will correspond to that associated with the C3–C4 bond formation. Note the larger and overlapped ASD surfaces at the C3 and carbonyl carbon atoms at the radical anion of complex 12:Mg in Fig. 1.
3.2 Study of the 32CA reaction between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13
Due to the non-symmetry of both reagents, four reactive channels for the 32CA reaction between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13 are possible. They are related to the two regioisomeric approach modes of 2-propen-1-ol 13 to nitrone 12, namely meta and ortho, and the two possible stereoisomeric approach modes, namely endo and exo. The meta and ortho regioisomeric channels are associated with the formation of the 3,5- and 3,4-isoxazolidines, respectively, while the endo and exo stereoisomeric channels are related to the relative position of the hydroxyl group of 2-propen-1-ol 13 with respect to the N2 nitrogen atom of C-methoxycarbonyl nitrone 12 (see Scheme 7). Analysis of the PESs associated with this 32CA reaction allows locating and characterising four TSs, TS-mn, TS-mx, TS-on and TS-ox, and the corresponding CAs, 14-n 14-x, 15-n and 15-x, thus indicating that it takes place through a one-step mechanism. Relative energies, in the gas phase and in the presence of DCM, for the species involved in the 32CA reaction between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13 are given in Table 2.
The gas-phase activation energies associated with the four competitive channels are 9.3 (TS-mn), 8.5 (TS-mx), 14.7 (TS-on) and 12.7 (TS-ox) kcal mol−1. Thus, in gas phase, this 32CA reaction is completely regioselective as TS-mx is 4.2 kcal mol−1 lower in energy than TS-ox and slightly exo stereoselective as TS-mx is 0.8 kcal mol−1 lower in energy than TS-mn. This 32CA reaction is strongly exothermic, between 27 and 31 kcal mol−1; consequently, it can be considered irreversible. These energy results indicate that the major product isoxazolidine 14x is formed by kinetic control in good agreement with the experimental outcomes [27].
With the inclusion of solvent effects of DCM, the activation energies increase to 12.0 (TS-mn), 11.8 (TS-mx), 17.5 (TS-on) and 16.3 (TS-ox) kcal mol−1, as a consequence of a larger solvation of the reagents than the TSs and CAs [55]. In DCM, while the total regioselectivity found in gas phase remains unchanged, as the most favourable TS-mx is 4.5 kcal mol−1 lower in energy than TS-mx, the exo stereoselectivity decreases, as now TS-mx is only 0.2 kcal mol−1 more stable than TS-mn.
Values of relative enthalpies, entropies and Gibbs free energies of the stationary points involved in the 32CA reaction of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13 are summarised in Table 3. Analysis of the relative activation enthalpies indicates that the most favourable approach mode remains associated with TS-mx, ΔH = 13.3 kcal mol−1. Addition of the entropic contribution to the enthalpy increases the activation Gibbs free energy of this reactive channel to 26.5 kcal mol−1 as the consequence of the unfavourable negative activation entropy associated with this bimolecular process, ΔS = −44.1 cal mol−1 K−1. Formation of the more favourable meta CAs is exergonic by 9.3 (14n) and 7.6 (14x) kcal mol−1. Inclusion of the thermodynamic parameters to the electronic energies does not modify the regio- and stereoselectivity previously found, in agreement with the experimental outcomes in which a 44:56 mixture of isoxazolidines 14n and 14x is obtained [27].
Figure 2 shows the geometries of the TSs involved in the 32CA reaction between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13. At the meta TSs, the lengths of the O1–C5 and C3–C4 forming bonds are 2.152 and 2.157 Å at TS-mn and 2.136 and 2.175 Å at TS-mx, while at the ortho TSs, the lengths of the O1–C4 and C3–C5 forming bonds are 2.023 and 2.205 Å at TS-on, and 2.291 and 1.994 Å at TS-ox, respectively. In addition, all TSs present a hydrogen bond (HB) between the nitrone O1 oxygen and the hydroxyl hydrogen of the propenol framework. Note that this 32CA reaction implies the formation of two single bonds C–C and C–O of different nature, and consequently, neither analysis of geometries nor of bond order values can be used as a measure of the extent of the bond formation, but an ELF topological characterisation of the bonding changes along the reaction path [56]. Inclusion of solvent effects of DCM in the geometry optimisation does not produce appreciable changes in gas-phase geometries (see Fig. 2).
In order to evaluate the electronic nature of this 32CA reaction, the GEDT in the four competitive TSs was analysed. The GEDT at the TSs computed as the sum of the natural atomic charges, obtained through an NPA, of all the atoms belonging to the propenol framework is 0.06e at TS-mn, 0.06e at TS-mx, 0.03e at TS-on and 0.08e at TS-ox. These negligible GEDT values point out the low polar character of the non-catalysed process and account for the computed high activation energy of this 32CA reaction [18]. This behaviour is in agreement with the relative low electrophilicity of C-methoxycarbonyl nitrone 12 and the relative low nucleophilicity of 2-propen-1-ol 13 (see Sect. 3.1).
3.3 Study of the MgBr2 LA-catalysed 32CA reaction of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13
Coordination of the MgBr2 LA to the carbonyl and nitrone oxygen atoms of C-methoxycarbonyl nitrone 12 allows the formation of complex 12:Mg, thus increasing strongly the electrophilic character of C-methoxycarbonyl nitrone 12. As the non-catalysed process, due to the non-symmetry of the reagents, the MgBr 2 LA-catalysed 32CA reaction of C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13 can take place along four competitive channels (see Scheme 8). Analysis of the stationary points found along the PESs associated with this LA-catalysed 32CA reaction indicates that it takes place also through a one-step mechanism. Thus, four TSs, TS-Mg-mn, TS-Mg-mx, TS-Mg-on and TS-Mg-ox, and the corresponding CAs, 14-Mg-n, 14-Mg-x, 15-Mg-n and 15-Mg-x, were located and characterised. Relative energies, in the gas phase and in the presence of DCM, for the species involved in the 32CA reaction between complex 12:Mg and 2-propen-1-ol 13 are given in Table 4.
The gas-phase activation energies associated with the four reaction channels are: 8.3 (TS-Mg-mn), 5.7 (TS-Mg-mx), 11.0 (TS-Mg-on) and 11.9 (TS-Mg-ox) kcal mol−1 (see Table 4). In gas phase, this LA-catalysed 32CA reaction is completely regioselective as TS-Mg-mx is 5.3 kcal mol−1 lower in energy than TS-Mg-on, and highly exo selective as TS-Mg-mx is 2.6 kcal mol−1 lower in energy than TS-Mg-mn. This 32CA reaction is strongly exothermic, between 24 and 30 kcal mol−1; consequently, this LA-catalysed 32CA reaction can also be considered irreversible, the major product isoxazolidine 14-Mg-x being formed by kinetic control. Coordination of the MgBr2 LA to the two oxygen atoms of C-methoxycarbonyl nitrone 12 not only accelerates the zw-type 32CA reaction of complex 12:Mg with 2-propen-1-ol 13, but also notably increases the exo stereoselectivity, in complete agreement with the experimental outcomes [27].
Solvent effects of DCM increase the activation energies to 10.2 (TS-Mg-mn), 9.7 (TS-Mg-mx), 14.3 (TS-Mg-on) and 15.8 (TS-Mg-ox) kcal mol−1 as a consequence of a larger solvation of the reagents than TSs and CAs [55]. In DCM, the total regioselectivity found in gas phase remains unchanged as the most favourable TS-Mg-mx is 4.6 kcal mol−1 lower in energy than TS-Mg-mn, while the exo selectivity decreases, being TS-Mg-ox 0.5 kcal mol−1 lower in energy than TS-Mg-mn.
Values of relative enthalpies, entropies and Gibbs free energies of the stationary points involved in the 32CA reaction of complex 12:Mg with 2-propen-1-ol 13 are given in Table 5. Analysis of the relative activation enthalpies of the four reactive channels associated with the 32CA reaction of complex 12:Mg with 2-propen-1-ol 13 shows that the most favourable approach mode remains associated with TS-Mg-mx, ΔH = 10.3 kcal mol−1. Coordination of the MgBr2 LA to C-methoxycarbonyl nitrone 12 decreases the activation enthalpy by 3.0 kcal/mol with respect to that associated with the non-catalysed process. Addition of the entropic contribution to the enthalpy increases the activation Gibbs free energy of the most favourable meta/exo reactive channel to 26.5 kcal mol−1 as the consequence of the unfavourable negative activation entropy associated with this bimolecular 32CA reaction, ΔS = −52.7 cal mol−1 K−1. Inclusion of the thermodynamic parameters to the electronic energies does not modify the regio- and stereoselectivity found by analysis of the electronic energies in DCM. At this computational level, formation of the most favourable isoxazolidine 14-Mg-x is slightly endergonic.
Figure 3 shows the geometries of the TSs involved in the LA-catalysed zw-type 32CA reaction between complex 12:Mg and 2-propen-1-ol 13. At the meta TSs, the lengths of the O1–C5 and C3–C4 forming bonds are 2.250 and 2.123 Å at TS-Mg-mn and 2.285 and 2.124 Å at TS-Mg-mx, while at the ortho TSs, the lengths of the O1–C4 and C3–C5 forming bonds are 2.128 and 2.161 Å at TS-Mg-on and 2.069 and 2.254 Å at TS-Mg-ox, respectively. Interestingly, while at the more favourable exo TSs the hydroxyl hydrogen atom of the propenol moiety is disposed towards one of the bromide anions of the MgBr2 salt at Br–H distances of 2.478 Å (TS-Mg-mx) and 2.388 Å (TS-Mg-ox), at TS-Mg-mn this hydrogen is oriented towards the nitrone oxygen atom at a O–H distance of 2.046 Å. These geometrical arrangements suggest that while TS-Mg-mn presents an intramolecular HB between the hydroxyl hydrogen and the nitrone oxygen (O–H), the former forms a HB with the bromide anion of the LA catalyst (Br–H) in TS-Mg-mx. Note that due to the formation of these HBs, the C–C–O–H dihedral angles decrease from 180 to 42.9 (TS-Mg-mn) and −103.1 (TS-Mg-mx) degrees. Thus, the stronger interaction with the bromide anion could explain the exo selectivity experimentally found in the LA-catalysed process. Inclusion of solvent effects of DCM in the geometry optimisation turns the TSs slightly more delayed (see Fig. 2). In DCM, the H–Br HB is 0.1 Å shorter than in gas phase.
The computed GEDT at the TSs associated with this LA-catalysed 32CA reaction, which fluxes from the propenol framework towards the complex one, is 0.19e at TS-Mg-mn, 0.16e at TS-Mg-mx, 0.15e at TS-Mg-on and 0.18e at TS-Mg-ox. These GEDT values account for the polar character of this LA-catalysed zw-type 32CA reaction, justifying the acceleration experimentally found as a consequence of the high electrophilic character of complex 12:Mg [18]. Consequently, the role of the LA catalysis is to increase the electrophilicity of C-methoxycarbonyl nitrone 12, favouring the corresponding zw-type 32CA reaction to take place via more polar TS with lower activation energy.
3.4 NCI analysis of the origin of the meta/exo selectivity in the LA-catalysed 32CA reaction of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13
As commented in previous sections, the LA-catalysed 32CA reaction of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13 yields the 3,5-cis-isomer 14x, resulting from the meta/exo approach mode of alkene 13, as the single stereoisomer in 71% yield. Analysis of the B3LYP/6-31G(d) gas-phase relative energies of the TSs associated with the two meta endo/exo competitive reactive channels gives a ΔE ≠ = 2.6 kcal mol−1 between TS-Mg-mn and TS-Mg-mx, in reasonable agreement with the experimental outcomes.
As previously commented, analysis of the geometries of both TSs suggests the presence of a HB between the hydroxyl hydrogen and the nitrone oxygen (O–H) or bromide anion (Br–H), respectively (see Fig. 3); thus, the different nature of these non-covalent interactions could justify the preference for the meta/exo reactive pathway in the LA-catalysed 32CA reaction of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13.
In order to characterise the O–H and Br–H HBs, an NCI analysis [15] of the electron density of TS-Mg-mn and TS-Mg-mx was performed. NCI low-gradient isosurfaces for these meta TSs are displayed in Fig. 4.
Figure 4 reveals the presence of a small circular turquoise surface between the hydroxyl hydrogen of the propenol framework and the nitrone oxygen atom in TS-Mg-mn and between the former and the bromide anion of the MgBr2 LA catalyst in TS-Mg-mx, confirming the existence of favourable O–H and Br–H HBs.
NCI gradient isosurfaces are coloured according to a colour scale ranging from blue, which is indicative of strong attractive interactions, to red, which is characteristic of strong repulsive interactions, in such a manner that this colour code can be used as a qualitative measure of the strength of NCI interactions. As shown in Fig. 4, the colour of the NCI gradient isosurfaces associated with the two HBs is indistinguishable, indicating the similar strength of both HBs. Consequently, in order to quantify the stabilisation of both meta TSs by the formation of these HBs, single-point energy calculations over the structures in which the C–C–O–H dihedral angles are set to 180 degrees were performed.
The energies of the corresponding structures in which the hydroxyl hydrogen is situated away from the oxygen atom or bromide anion, thus preventing the HB interaction, were found 6.7 (TS-Mg-mn-rot) and 8.3 (TS-Mg-mx) kcal mol−1 higher than those of the corresponding TSs. These energy results suggest that the Br–H HB is ca. 1.6 kcal mol−1 stronger than the O–H one, allowing establishing that the Br–H HB has an important role in the exo stereoselectivity experimentally found in the LA-catalysed 32CA reaction.
The present energy and NCI topological analyses allow concluding that although favourable HB interactions are distinguished in the two TSs associated with the more favourable meta regioisomeric channels, the stronger Br–H HB formed along the meta/exo approach could be responsible for the exo stereoselectivity experimentally found in the LA-catalysed 32CA reactions of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13 [27].
4 Conclusions
The mechanisms of the non-catalysed and MgBr2 LA-catalysed 32CA reactions between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13 have been theoretically investigated within MEDT by using DFT methods at the B3LYP/6-31G(d) computational level.
Analysis of CDFT reactivity indices indicated that while the 32CA reaction of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13 will have a non-polar character, coordination of MgBr2 to nitrone 12 favours the corresponding zw-type 32CA reaction by taking place through a polar process as a consequence of the high electrophilic character of complex 12:Mg.
Due the non-symmetry of both reagents, these 32CA reactions can take place through four competitive reactive channels, the ortho and meta regioisomeric channels and the endo and exo stereoisomeric ones, following a one-step mechanism.
The 32CA reaction between C-methoxycarbonyl nitrone 12 and 2-propen-1-ol 13 takes place with a high activation enthalpy, 13.5 kcal mol−1, as a consequence of the non-polar character of this zw-type 32CA reaction. This 32CA reaction is completely meta regioselective and very low exo stereoselective, in clear agreement with the experimental outcomes in which a 44:56 mixture of isoxazolidines 14n and 14x is obtained [27].
Coordination of the MgBr2 LA to C-methoxycarbonyl nitrone 12 accelerates the corresponding zw-type 32CA reaction by taking place through a more polar mechanism. According to the zw-type mechanism, coordination of the MgBr2 LA to C-methoxycarbonyl nitrone 12 increases the electrophilicity of the corresponding complex 12:Mg, thus increasing the polarity of the reaction. The activation enthalpy associated with the catalysed process is 8.5 kcal mol−1. The catalysed 32CA reaction is completely meta regioselective and presents a low exo stereoselectivity, but higher than in the non-catalysed process, in reasonable agreement with the experimental outcomes in which the isoxazolidine proceeding from the meta/exo approach is obtained as single product [27].
Analysis of the TS geometries involved in the catalysed 32CA reaction suggests the formation of an intramolecular HB between the hydroxyl hydrogen of 2-propen-1-ol 13 and one of the two bromide anions of the MgBr2 salt. Energy and NCI topological analyses allow concluding that although favourable intramolecular HB interactions are distinguished in the two TSs associated with the more favourable meta regioisomeric channels, the stronger Br–H HB formed along the meta/exo approach could be responsible for the exo stereoselectivity experimentally found in the LA-catalysed 32CA reactions of C-methoxycarbonyl nitrone 12 with 2-propen-1-ol 13.
4.1 Theoretical background of the NCI analysis
NCIs have a unique fingerprint, and their presence can be revealed solely by means of electron density analysis. They are highly non-local and manifest in real space as low-gradient isosurfaces with low densities. The sign of the Laplacian of the density, \( \overline{{\nabla^{2} \rho }} \), is a widely used tool to distinguish between different types of strong interactions [57] To analyse bonding in more detail, the Laplacian is often decomposed into a sum of contributions. These components are the three eigenvalues λ i of the electron density Hessian matrix, such that \( \overline{{\nabla^{2} \rho }} = \lambda_{1} + \lambda_{2} + \lambda_{3} \). Analysis of these components has been widely applied to chemical bonding. The sign of λ 2 can be used to distinguish bonded (λ 2 < 0) from non-bonded (λ 2 > 0) interactions. Analysis of the sign of λ 2 thus helps to differentiate between different types of NCIs, whereas the density itself provides information about their strength.
The gradient isosurfaces provide a useful visualisation of NCIs as broad regions of real space, rather than simple pairwise contacts between atoms, and are coloured according to the corresponding values of sign(λ2)ρ. Surfaces with very low density values (ρ < |0.005| a.u.) generally represent weaker dispersion interactions, while surfaces with slightly higher density values (|0.005| < ρ < |0.05| a.u.) represent stronger NCIs, including both attractive HBs and steric clashes. Thus, large negative values of sign(λ 2)ρ are indicative of attractive interactions (such as dipole–dipole or hydrogen bonding) and are coloured in blue, while if the sign(λ 2)ρ is large and positive, the interactions are non-bonding and are coloured in red; values near zero indicate very weak van der Waals interactions and are coloured in green.
References
Huisgen R (1963) Angew Chem Int Ed 2:565–598
Padwa A (1984) 1, 3-Dipolar cycloaddition chemistry, vol 1-2. Wiley Interscience, New York
Padwa A (2002) Synthetic applications of 1,3-dipolar cycloaddition chemistry toward heterocycles and natural products, vol 59. Wiley, New York
Gothelf KV, Jorgensen KA (1998) Chem Rev 98:863–909
Tufariello JJ (1984) 1,3-Dipolar cycloaddition chemistry. A. Padwa, Wiley, New York
Torssell KBG (1988) Nitrile oxides, nitrones and nitronates in organic synthesis. VCH, New York
Tufariello JJ (1979) Acc Chem Res 12:396–403
Domingo LR, Sáez JA (2009) Org Biomol Chem 7:3576–3583
Domingo LR, Emamian SR (2014) Tetrahedron 70:1267–1273
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Tetrahedron 72:1524–1532
Domingo LR (2016) Molecules 21:1319
De Proft F, Geerlings P (2001) Chem Rev 101:1451–1464
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Molecules 21:748
Becke AD, Edgecombe KE (1990) J Chem Phys 92:5397–5403
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen J, Yang AW (2010) J Am Chem Soc 132:6498–6506
Ríos-Gutiérrez M, Domingo LR, Pérez P (2015) RSC Adv 5:84797–84809
Domingo LR, Aurell MJ, Pérez P (2015) Tetrahedron 71:1050–1057
Domingo LR, Aurell MJ, Pérez P (2014) Tetrahedron 70:4519–4525
Kanemasa S (2010) Heterocycles 82:87–200
Simonsen KB, Bayón P, Hazell RG, Gothelf KV, Jorgensen KA (1999) J Am Chem Soc 121:3845–3853
Domingo LR (2000) Eur J Org Chem 2265–2272
Sousa CAD, Vale MLC, Garcia-Mera X, Rodriguez-Borges JE (2012) Tetrahedron 68:1682–1687
Nacereddinea AK, Layeb H, Chafaa F, Yahia W, Djerourou A, Domingo LR (2015) RSC Adv 5:64098–64105
Parr RG, von Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
Domingo LR, Chamorro E, Pérez P (2008) J Org Chem 73:4615–4624
Domingo LR, Pérez P (2011) Org Biomol Chem 9:7168–7175
S. Kanemasa S, Tsuruoka T (1995) Chem Lett 49–50
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1874
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hehre WJ, Radom L, Schleyer PVR, Pople J (1986) Ab initio Mol Orbital Theory. Wiley, New York
Schlegel HB (1982) J Comput Chem 2:214–218
Schlegel HB (1994) In modern electronic structure theory. In: Yarkony DR (ed) World Scientific Publishing, Singapore
Fukui K (1970) J Phys Chem 74:4161–4163
González C, Schlegel HB (1990) J Phys Chem 94:5523–5527
González C, Schlegel HB (1991) J Chem Phys 95:5853–5860
Tomasi J, Persico M (1994) Chem Rev 94:2027–2094
Simkin BY, Sheikhet I (1995) Quantum chemical and statistical theory of solutions—computational approach. Ellis Horwood, London
Cances E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255:327–335
Barone V, Cossi M, Tomasi J (1998) J Comput Chem 19:404–417
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Lane JR, Contreras-Garcia J, Piquemal J-P, Miller BJ, Kjaergaard HG (2013) J Chem Theor Comput 9:3263–3266
Contreras-Garcia J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) J Chem Theor Comput 7:625–632
Frisch MJ et al (2009) Gaussian 09, revision A.02. Gaussian Inc, Wallingford
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7514
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Kohn W, Sham L (1965) J Phys Rev 140:1133–1138
Domingo LR, Pérez P, Sáez JA (2013) RSC Adv 3:1486–1494
Domingo LR, Chamorro E,Pérez P (2009) Eur J Org Chem 3036–3044
Domingo LR (2014) RSC Adv 4:32415–32428
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Tetrahedron 58:4417–4423
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) J Mol Struct (Theochem) 865:68–72
Benchouk W, Mekelleche SM, Silvi B, Aurell MJ, Domingo LR (2011) J Phys Org Chem 24:611–618
Ríos-Gutiérrez M, Pérez P, Domingo LR (2015) RSC Adv 5:58464–58477
Bader RFW, Essén HJ (1984) Chem Phys 80:1943–1960
Acknowledgements
This work has been supported by the Ministry of Economy and Competitiveness of the Spanish Government; Project CTQ2013-45646-P. M. Ríos-Gutiérrez also thanks the Ministry of Economy and Competitiveness for a pre-doctoral contract co-financed by the European Social Fund (BES-2014-068258). A.I. Adjieufack, I. M. Ndassa and J. K. Mbadcam are grateful to the Ministry of Higher Education of the Republic of Cameroon to finance the project with modernisation research allowance. The authors also thank the University of Yaoundé I and High Teacher Training College (Cameroon) for infrastructural facilities for generous allocation of computer time.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Adjieufack, A.I., Ndassa, I.M., Mbadcam, J.K. et al. Understanding the reaction mechanism of the Lewis acid (MgBr2)-catalysed [3+2] cycloaddition reaction between C-methoxycarbonyl nitrone and 2-propen-1-ol: a DFT study. Theor Chem Acc 136, 5 (2017). https://doi.org/10.1007/s00214-016-2028-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-2028-0