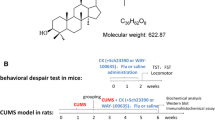
Abstract
Background
Depression causes significant debilitating symptoms and economic burden. Current management is challenged by slow onset of action and modest efficacies of antidepressants; thus, the search for newer antidepressants remains relevant. We evaluated the antidepressant effects of a kaurene diterpene, xylopic acid (XA), in zebrafish and mouse models.
Methods
The chronic unpredictable stress (CUS) protocol in zebrafish and the tail suspension test (TST), forced swim test (FST), lipopolysaccharide-induced depression-like behaviour test (LID) and repeated open space swimming test (OSST) in mice were used. We further examined the impact of depleting monoamines on XA’s antidepressant effects. The contribution of glutamatergic and nitrergic pathways on the antidepressant effect of XA in mice and XA’s effects on 5-HT receptors and monoamine oxidase (MAO) enzymes were also evaluated. Finally, XA’s influence on neuroprotection was evaluated by measuring BDNF and oxidative stress enzymes in whole brain. XA doses (1–10 μM) in zebrafish and (10, 30, 100 mg kg−1) in mice exerted potent antidepressant-like potential in FST, TST, LID and showed fast-onset antidepressant-like property in the OSST.
Results
The antidepressant-like properties in mice were reversed by blocking synthesis/release of serotonin but not noradrenaline using p-chlorophenylalanine and α-methyl-p-tyrosine, respectively. This antidepressant-like effect was potentiated by d-cycloserine and Nω-Nitro-l-arginine methyl ester (l-NAME) but not by d-serine and L-arginine. XA also evoked partial agonist-like effects on 5-hydroxytrptamine receptors on the rat fundus but it did not have MAO inhibition effect. It also increased BDNF, glutathione and antioxidant enzymes.
Conclusion
Therefore, xylopic acid possesses antidepressant-like effects largely mediated by serotonergic and neuroprotective mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is the most predominant chronic mental health disorder, and causes significant burden on quality of life. It is a leading cause of disability worldwide and poses huge economic burden (James et al. 2018). It is characterised by debilitating symptoms including low self-esteem, changes in mood, reduced interest in pleasure and an overall negative affect (APA 2015). One out of sixteen people worldwide experiences depression yearly (Otte et al. 2016). Furthermore, major depressive disorder comes with an elevated risk of other burdensome diseases such as stroke, cardiovascular disorders and diabetes mellitus (Whooley and Wong 2013).
Pharmacological management of MDD is mainly with antidepressants. Although significant strides have been made in the clinical management of depression, there is still an unmet need for new antidepressants. This has been due to reduced efficacies of current antidepressants which in some cases had only modest effects compared to placebos (Cipriani et al. 2018). Also limiting current therapy is the slow onset of the first-line drugs in addition to clusters of patients who are completely unresponsive to the current antidepressants (Cipriani et al. 2018; Bear et al. 2019). These gaps in the management of depression make the search from novel therapeutics still relevant.
Xylopic acid (15-(acetyloxy) kaur-16-en-18-oic acid) is a kaurene diterpene previously isolated from plants such as Xylopia frutescens, Xylopia sericeae and Xylopia aethiopica, where it has been shown to be one of the major secondary metabolites (Takahashi et al. 1995; Cavalcanti et al. 2010). Some of the plants from which it has been isolated have traditionally been used in the management of brain disorders or have compounds isolated from them with CNS effects (Biney et al. 2016). We have previously shown the anxiolytic-like effects of xylopic acid in mouse and zebrafish protocols (Biney et al. 2018) as well as its effects in the CNS core battery test (Biney et al. 2014). Anxiety and depression are usually co-morbid conditions with some antidepressants being used in managing anxiety and vice versa (Strawn et al. 2018). Thus, we hypothesise that xylopic acid may possess antidepressant effects. With a significant role of neuroinflammation in several CNS disorders including MDD (Rossi et al. 2017; Woelfer et al. 2019) and the reported anti-inflammatory property of XA (Osafo et al. 2016; Ekuadzi et al. 2018), it is possible that xylopic acid will reduce neuroinflammation and thus ameliorate depressive symptoms.
Materials and methods
Animals
Mice (ICR, male, 20–25 g) were obtained from Noguchi Memorial Institute for Medical Research (NMIMR) (Accra, Ghana), housed at the vivarium (25 °C, 12/12-h light cycle, 75% humidity) of the School of Biological Sciences, University of Cape Coast, and were used for the study after an 8-day acclimatisation period. They were grouped 10 mice in a cage and allowed ad libitum access to mice chow (Agricare, Kumasi, Ghana) and tap water. Four to six (4–6)-month-old zebrafish (Danio rerio) (adult short fin wild-type) supplied by Aqua Marshall (Accra, Ghana) and kept in 30-L glass tanks (5 fishes/L) were used. They were maintained at 25 °C, 12/12-h light cycle and 30% constantly aerated water replaced daily (pH 7–8). They were fed 2 times a day with commercial flakes (Aquafin Professional, Guangzhou, China). Experimental procedures were subject to NIH guidelines for the Care and Use of Laboratory Animals, EU Directive 2010/63, for zebrafish with ethics approval from the Institutional Review Board of UCC (UCCIRB/CoHAS/17/073). All behavioural experiments were observed by experienced experimenters blinded to the various treatment groups.
Drugs and chemicals
Xylopic acid (structure, Fig. 1a) was isolated and purified by reflux recrystallisation from unripe fruits of Xylopia aethiopica as reported earlier (Biney et al. 2018). Its purity was confirmed by LC-MS to be 99.8% w/w. It was formulated as an emulsion by emulsifying using 1% Cremophor EL before administration to mice. Fluoxetine and methysergide (Eli Lilly and Co., England), l-NAME, p-chlorophenylalanine and l-arginine (Sigma, Switzerland), Selegiline, serotonin, d-serine, tryptamine, desipramine, α-Methyl-p-tyrosine, d-cycloserine and Cremophor EL were purchased from Sigma, St. Louis, MO.
Drugs were administered per os via oral gavage and did not exceed 10 ml kg−1 (final volume 0.3–0.5 ml). Doses of drugs including XA, fluoxetine and desipramine were selected based on work published earlier.
Preliminary screening for antidepressant-like effect in zebrafish
Chronic unpredictable mild stress
We first used a zebrafish model for chronic unpredictable mild stress (CUS) to rapidly screen for potential antidepressant effect of XA using methods described by Piato et al. (2011) and Chakravarty et al. (2013) (Fig. 1b). Wild-type adult zebrafish were put through two stressors twice a day consecutively for 14 days. The stressors applied in a random manner included the following: congestion—10 fishes in 250 ml beaker containing 150 ml of water for 1 h; confinement—restraint for 15 min in narrow 5-ml tubes; dorsal body exposure—2 min exposure of the dorsal side of fishes by reducing tank water to a shallow level; social isolation—single housing for 60 min in 250-ml beakers; cold stress—reducing water temperature to 23 °C for 30 min; chasing—8 min pursuit with a harvesting net; heat stress—increasing tank water temperature to 33 °C for 30 min; repeated tank change—rapid movement of fishes from one tank to another repeatedly in 6 cycles. Twenty-four hours after the last stressor, they were placed in 8 groups (n = 9) and exposed to XA 1, 3, 10 μM or FLX 0.3, 3, 30 μM or tank water daily for 3 days by immersing them in respective drug solutions for 30 min. A naïve group did not undergo any of the stressors nor treatments. The shoaling, novel tank diving and light-dark tests were conducted 24 h after the final drug treatment as detailed below.
Novel tank test
In the novel tank test, done on day 18 of CUS paradigm, zebrafish were gently placed a new arena (15 (l) × 10 (w) × 25 (h) cm3) containing water (10 cm deep), segmented horizontally to form two 5-cm halves. The total duration in the upper half was videotaped and later computed with JWatcher™.
Scototaxis test
In this test, conducted after the NTT test, zebrafish were gentle placed water (25 °C) in a thin arena (9 (l) × 55 (w) × 10 (h) cm3), segmented vertically to form 2 zones of either white or black backgrounds. Time spent in the light region was videotaped for 5 min and later computed with JWatcher™.
Test for shoaling
In the shoaling behaviour analyses performed after the ST, three fishes were transferred into a rectangular tank (20 ×10 ×15 cm3) containing water (25–26 °C, depth = 10 cm) and allowing them to swim freely. The average area occupied by the 3 zebrafishes every 10 s was computed with ImageJ® for a total duration of 10 min.
Antidepressant effect in mice models
Forced swim test
The forced swim test by Porsolt et al. (1977) was used (Fig. 1c). Mice (n = 8) were treated p.o. with xylopic acid (XA) 10, 30 and 100 mg kg−1, fluoxetine (FLX) 3, 10 and 30 mg kg−1, desipramine (DES) 3, 10 and 30 mg kg−1 or vehicle 10 ml kg−1. Six-minute swim sessions were conducted in plastic cylinders (height = 25 cm radius = 6 cm) filled with water (23 ± 1 °C, depth = 15 cm), 2 h after XA or 1 h after FLX, DES and vehicle treatment. The total time of behaviours directed at escaping (climbing—vertically moving with forepaws on the wall of the cylinders and swimming—moving horizontally in the water) and immobility were recorded and quantified for the last 4 min of the test using JWatcher™ 1.0 and compared to the common control group (vehicle 10 ml kg−1).
Tail suspension test
Mice (n = 8) received p.o. same XA, FLX, DES and vehicle dosage regimen as in the FST. This was followed by the tail suspension test described by Steru et al. (1985). They were individually held by the tail onto a horizontal bar 52 cm high from the top of a laboratory bench with the aid of adhesive tape. Duration of immobility (no movements save those required for breathing) was videotaped for 6 min and computed with JWatcher™ and compared to the vehicle (10 ml kg−1) group.
Lipopolysaccharide-induced depression-like behaviour
The method described by Sulakhiya et al. (2016) was used to induce depressive-like behaviour using lipopolysaccharide (LPS 0111: B4, (Sigma, St. Louis, MO)) (Fig. 1d). Seven groups of mice (n = 10 per group), housed one mouse/plastic cage, were treated for 14 days with XA 10, 30, 100 mg kg−1, FLX 3, 10, 30 mg kg−1 or vehicle 10 ml kg−1 p.o. once daily. Twenty-four (24) hours after the last treatment, all mice were injected with 830 μg kg−1 LPS i.p. Subsequently, social interaction and sucrose preference tests as well as neurochemical assays of brain samples were assessed as described below.
Social interaction test
After 4 h following LPS injection, the social interaction test (Crestani et al. 1991) with minor modifications was conducted by introducing into the cages of the LPS-treated mice, a younger mouse of the same strain but which has had no prior contact with it. The total time for social exploration (pursuits, sniffing and social grooming) was videotaped for 5 min.
Sucrose preference test
A 2-bottle-model sucrose preference test described in Jangra et al. (2014) was used to evaluate anhedonia. The test includes a 7-day adaptation time in which mice have unlimited access to bottles: one containing 50-g tap water and the other 50 g of 2% sucrose solution to determine baseline sucrose consumption. The amount of each fluid drunk was computed as the change in bottles’ weight 24 h prior to and 24 h post-LPS injection on day 15. The preference for consuming sucrose was computed as percentage using the formula:
Neurochemical assays
On day 16, 24 h post-LPS injection, mice were sacrificed by cervical dislocation. Whole brain samples were isolated and quickly kept at −80 °C for further assays. 10% w/v extracts were prepared by sonicating isolated brain samples in TNGT buffer (1% Triton X 100 (Sigma, St. Louis, MO), 150 mM Tris HCl (Sigma, St. Louis, MO), 10% glycerol, 150 mM NaCl, protease inhibitor cocktail (Sigma, St. Louis, MO), pH 7.4) and centrifuging the homogenate for 20 min at 6000g. Total protein of the resultant homogenate was assayed (Bradford 1976) and the levels of oxidative stress enzymes and BDNF determined in triplicates and expressed per mg protein.
Effect on reduced glutathione, catalase and superoxide dismutase
The concentration of reduced glutathione (GSH) was assayed by the method of Ellman (1959) using 100-μl supernatant of tissue extract. Absorbance of coloured product was read at 412 nm. Catalase activity was determined with a method described by Sinha (1972) in 100 μl of tissue extract. Absorbance of the final coloured product was read at read 620 nm. Superoxide dismutase (SOD) was assessed with procedures described by Sun et al. (1988) using 500 μl of tissue extract. The absorbance of final product was read at 560 nm and enzymatic activity quantified in unit of activity per weight of protein according to the formula:
Lipid peroxidation assessment
Malondialdehyde levels were assayed with a method described by Draper and Hadley (1990) using 1-ml tissue extract. Absorbance was read at 532 nm and 600 nm (nonspecific absorbance) and concentration of malondialdehyde then computed according to the formula:
Effect on BDNF
Whole brain samples were sonicated in cold extraction buffer after thawing to extract bound BDNF according to procedures by Kolbeck et al. (1999). The extraction buffer contained 3 portions of neutralising buffer (0.1 M Na2HPO4, 0.1 M KH2PO4 (Sigma, St. Louis, MO), final pH 7.6) and 1 portion acid-extraction buffer (1 M NaCl (BDH Poole, England), 0.1% Triton X 100, 50 mM sodium acetate (BDH Poole, England), protease inhibitor cocktail, final pH 4). The resultant mixture was centrifuged for 20 min (4 °C) at 6000g and the supernatant quantified for BDNF by ELISA as per manufacturer-prescribed protocols (Boster Biological Technology, Pleasanton, CA; Catalog # EK0309).
Open space swimming test
The repeated open space swimming test described by Stone and Lin (2011) was adapted and use to assess time-course of antidepressant effect (Fig. 1e). Each mouse (20–22 g) swam for 15 min each day for 4 consecutive days. This was carried out in rectangular plastic containers (25 × 35 × 50 cm3) filled with water (33 ± 1 °C) to a depth of 15 cm. On the 5th day, they were placed in 7 groups (n = 8) where they received p.o. daily for 14 days, either XA 3, 10, 30 mg kg−1 or FLX 3, 10, 30 mg kg−1 or vehicle 10 ml kg−1. Individual weights of mice were measured daily before to drug dosing. Swimming was repeated on the 5th, 7th, 10th, 14th and 18th days while being videotaped. Total time spent being immobile and the distance travelled were then computed with JWatcher™. A tail suspension test was repeated on day 19 to rule out if immobility had become a learned behaviour.
Effect on coat state
On the 17th day of the open space swimming test (OSST), a coat state assessment was performed as described by Yalcin et al. (2007). Each mouse was tenderly taken out of its cage for a visual examination of the dorsal and ventral coats, head, neck, genital area, front and hind paws, and tail. A clean well laid coat in each region was assigned 0 while an obviously altered, piloerected, unclean, messy or marred coat was assigned 1 by an experienced experimenter who was blinded to the various treatment groups. The cumulative score of the 8 body areas is the coat index.
Spontaneous novelty-induced grooming behaviour
Grooming behaviour was also measured on day 17 of the OSST. The mice were tenderly removed from their cages and individually put in Perspex observation arenas before grooming behaviour videotaped for 5 min. The mice were observed for the type of grooming they performed. This included passive grooming of the dorsal, ventral and genital regions as well as active grooming of the fore paws, head and neck (rostral grooming). The total time spent in fore paw grooming and head and neck washing (rostral grooming) was quantified by a blinded experienced experimenter.
Evaluation of possible mechanism(s) of action
Effects of depleting monoamines on antidepressant-like property of XA
The impact of serotonin and noradrenaline on the established antidepressant-like property of XA was evaluated by selectively inhibiting either their storage and/or synthesis (O’Leary et al. 2007). To exclusively deplete 5-HT, mice received 3 daily intraperitoneal pre-treatments of p-chlorophenylalanine (pCPA) 300 mg kg−1. Twenty-four hours post-pCPA injection, both naïve and treated mice, they were further treated with either vehicle or equipotent doses of XA (50 mg kg−1), FLX (10 mg kg−1) or DES (10 mg kg−1). One hour later, the FST was repeated.
Using a similar approach, noradrenaline and dopamine were selectively depleted with α-methyl-p-tyrosine (AMPT) 100 mg kg−1 i. p. and subsequently orally treated with equipotent doses of XA (50 mg kg−1), FLX (10 mg kg−1) or DES (10 mg kg−1), 4 h after which the FST was repeated.
Furthermore, 5–HT, NA and DA pools in both the cytoplasm and in vesicles were depleted after mice received intraperitoneal injection of 1 mg kg−1 reserpine. Eighteen hours later, animals received equipotent doses as described above, and subsequently, the FST was repeated.
Effect of XA on 5-HT receptors on a rat fundus strip
Male Sprague-Dawley rats were sacrificed to transversely cut out the stomach and isolate the fundus. From the fundus, longitudinal strips (20 × 2 mm) were removed and bathed in Krebs-Henseleit buffer: KCl (4.7), NaCl (118), MgSO4 .7H2O (1.2), CaCl2 (2.5), glucose (11.1) NaHCO3 (1.2) (mmol/L) (BDH Poole, England). The strips were isotonically mounted in 10-ml tissue baths (tension = 1 g, temperature = 37 °C, aeration = carbogen (95%)). Mounted tissues equilibrated for 1 h with regular tissue washing every 10 min. A total of 67-mM KCl was first used to generate the highest contractile response against which subsequent contractions were normalised. Cumulative concentration response tracings (CRTs) were recorded and measured with a Harvard kymograph after cumulative addition of either tryptamine, XA or 5-HT in the presence or absence of the 5HT1 and 5-HT2 non-selective antagonist methysergide (10 nM).
Interaction of XA with MAO enzyme
Rat fundus strips were isolated and mounted as described above. Initial tryptamine-provoked CRTs was recorded after cumulatively adding tryptamine (Sigma, St. Louis, MO) in ½ log increases. To examine the inhibitory actions of XA on monoamine oxidase enzyme, the CRTs of tryptamine were regenerated in the presence or absence of XA (1 μM) or selegiline (10 mM).
Effects of pre-treatment with d-cycloserine, d-serine, L-NAME and L-arginine
The role of NMDA glutamatergic modulation on XA’s effect was evaluated. Mice received either equipotent doses of XA, FLX or DES alone or in a combination with d-cycloserine (2.5 mg kg−1 i. p.) a glycineB partial agonist, or in a combination with d-serine (320 mg kg−1 i. p) a glycineB agonist. One hour later, the FST was repeated.
To evaluate the influence of nitric oxide pathways in XA’s antidepressant-like effects, mice received either an equipotent dose of XA, FLX or DES alone or in combination l-NAME 20 mg kg−1 i. p. administered 15 min earlier or in combination with l-arginine 750 mg kg−1 administered 30 min earlier. FST was repeated 1 h post-XA, FLX or DES treatment.
Data analyses
Data are reported as means ± SEM using GraphPad Prism version 6.0. Unless specified, data were compared using one-way analysis of variance with significance level of p < 0.05. F-statistic was computed using the Brown-Forsythe test. Data in the OSST and effects of pre-treatment with D-cycloserine, D-serine, L-NAME and L-arginine were compared by two-way analysis of variance with repeated measures (treatment × time). Data on swimming and climbing time in the FST was also analysed by 2-way ANOVA. When ANOVA was significant, Holm-Sidak test for significant difference was performed except in the LPS-induced depression behaviour tests where Kruskal-Wallis post hoc was used. Dose-response curves were fitted using iterative nonlinear regression (3-parameter logistic) equation:
where the logarithm of dose and response are represented by X and Y, respectively, with Y assuming a sigmoid shape from a (bottom) to b (top). The fitted midpoints ED50s) were computed from the fitted midpoints of the curves and compared with the F test.
Results
Effects of XA on CUS in zebrafish
Antidepressant-like property was first screened in a CUS model in zebrafish to rapidly determine a potential antidepressant effect. The stress paradigm employed evoked depression-like behaviours after 14 days. This manifested as decreased time in the top half of the novel tank diving test, increased scototaxis and reduced shoaling in stressed zebrafish. Both XA and FLX reversed these behaviours. XA increased frequency and duration in the upper half (F 4, 25 = 35.4, p < 0.001) (Fig. 2a, b). Likewise, both XA and FLX treatments enhanced shoaling in zebrafishes (Fig. 2c). Also, the XA 10 μM increased the frequency and duration in the light region during the scototaxis test (p = 0.041) but this behaviour was not modified by FLX treatment (F 4, 20 = 1.683, p = 0.193) (Fig. 2d, e).
Effects of XA (1, 3, 10 μM) and FLX (0.3, 3, 30 μM) pre-treatments on duration and frequency in the upper segment of novel tank (a and b) shoaling behaviour (c) and scototaxis test (d and e) after 14-day CUS. Results show mean ± SEM *p < 0.05, **p < 0.01, ***p < 0.001, comparing to CUS control. 1-way ANOVA
Forced swim test
Having exhibited an antidepressant-like potential in zebrafish, XA was further assessed in mice models. XA at 10, 30 and 100 mg kg−1 decreased immobility in a dose-dependent fashion in the FST (F3, 55 = 35.17, p < 0.001) similar to fluoxetine and desipramine (p < 0.001) (Fig. 3a). Comparing dose-response curves (DRCs), XA had similar efficacies in reducing immobility (ED50 = 8.40 ± 0.91; Emax = 76.43 ± 1.29) compared to FLX (ED50 = 2.37 ± 0.06; Emax = 78.65 ± 3.88) and DES (ED50 = 3.75 ± 0.52; Emax = 66.04 ± 2.28) (Fig. 3b). In Fig. 3c, XA and FLX increased total time spent swimming but not climbing whereas DES increased total time spent climbing.
Antidepressant-like effect of XA in the forced swim test in mice. a Effect of acute treatment of XA (10–100 mg kg−1), FLX (3–30 mg kg−1) and DES (3–30 mg kg−1) on duration of immobility during the forced swim test. Results indicate group means ±SEM (n = 8). One-way ANOVA and Holm-Sidak post hoc test. *p < 0.05, **p < 0.01 compared to ctrl. b DRCs showing % reduction of immobility by XA, FLX and DES in the FST. c Effect of XA, FLX and DES on swimming and climbing time in FST. Data represents group mean ± SEM. Two-way ANOVA, comparing within treatment groups: **p < 0.01
Tail suspension test
In the TST, XA decreased immobility significantly at 100 mg kg−1 (p < 0.01) while DES and FLX reduced immobility at all doses tested (F3, 53 = 13.23, p < 0.001) (Fig. 4a). From the DRCs, the rank order of efficacy in deceasing immobility during the tail suspension test was FLX > XA > DES with ED50 and Emax values as follows: XA (ED50 = 19.25 ± 1.07; Emax = 71.57 ± 4.48), FLX (ED50 = 2.03 ± 0.14; Emax = 73.09 ± 1.16) and DES (ED50 = 4.64 ± 0.8; Emax = 68.04 ± 4.46) Fig. 4b.
Antidepressant-like property of XA in the TST in mice. a Effect of acute treatment of XA (10–100 mg kg−1), FLX (3–30 mg kg−1) and DES (3–30 mg kg−1) on duration of immobility during the tail suspension. Results indicate group means ±SEM (n = 8). One-way ANOVA *p < 0.05, **p < 0.01 comparing to ctrl. b DRCs showing % reduction of immobility by XA, FLX and DES in the TST
LPS-induced depression-like behaviours
The influence of XA on the neuroinflammation hypothesis of depression was assessed using the LPS-induced depression-like behaviours model in mice. Administering 830 μg kg−1 lipopolysaccharide produced depression-like behaviours including decreased social interaction and % sucrose preference. XA 3, 10 and 30 mg kg−1 -treated animals exhibited dose-dependent sucrose preference (p < 0.01) (Fig. 4a) coupled with enhanced social interaction (p < 0.001) (Fig. 5c) as was also observed in fluoxetine-treated mice (p < 0.001). DRCs show that XA has higher efficacy than FLX in ameliorating anhedonia induced by lipopolysaccharide at the tested dose levels (Emax: XA: 78.23 ± 11.9; FLX, 61.71 ± 7.21) (Fig. 5b). A similar trend in efficacy is seen in the social interaction test (Emax: XA: 85.79 ± 6.8; FLX, 81.98 ± 4.05) (Fig. 5d). Overall, total fluid intake did not vary significantly across groups (Table 1).
Effect of XA on LPS-induced depression-like behaviours. Top panel a Impact of 14 days of treatment with XA (3, 10, 30 mg kg−1) or FLX (3, 10, 30 mg kg−1) in the anhedonia test. Data show group means ±SEM (n = 10). Kruskal-Wallis test * p < 0.05, ** p < 0.01 comparing to ctrl. b DRCs showing % decrease in anhedonia by XA and FLX. Bottom panel c Influence of XA or FLX in social interaction test. Data show group means ±SEM (n = 10). One-way ANOVA, ** p < 0.01, *** p < 0.001 comparing to ctrl. d DRCs of % increase in social interaction by XA and FLX
Neurochemical assays
Reduced glutathione, superoxide dismutase and lipid peroxidation assays
Glutathione, SOD and malondialdehyde were assayed during the LPS-induced depression test to assess influence of XA on these markers of oxidative stress. Administration of LPS-reduced significantly brain glutathione levels of untreated mice. This reduction was overcame in a dose-dependent manner by both XA and FLX treatments (F7,32 = 0.75, p < 0.01) (Fig. 6a). Activity of SOD increased with XA and FLX treatments (F7,32 = 1.00, p < 0.01). Similar observations were made with respect to the effect of XA and FLX on catalase (F7,32 = 1.23, p < 0.01) (Fig. 6b, c). Lipid peroxidation levels in XA- and FLX-treated mice were reduced as indicated by reduction in brain concentrations of malondialdehyde (F7,32 = 1.01, p < 0.01) (Fig. 6d).
Influence of XA (3, 10, 30 mg kg−1) and FLX (3, 10, 30 mg kg−1) on (a) reduced glutathione (GSH), (b) catalase, (c) superoxide dismutase, (d) lipid peroxidation and (e) brain-derived neurotropic factor in LPS-challenged mice. f Dose-response curves indicating % rise in BDNF concentrations. Results mean ± SEM (n = 3). 1-way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, comparing to LPS control and †† p < 0.01, ††† p < 0.001, comparing to LPS-naïve mice
Brain-derived neurotropic factor assay
Whole brain levels of BDNF measured after the LPS challenge indicated that XA 3, 10 and 30 mg kg−1 increased brain-derived neurotropic factor levels in a dose-dependent manner (F7,32 = 21.57, p < 0.001) (Fig. 6e) and was more potent and efficacious than fluoxetine at tested doses (XA ED50 = 1.72 mg kg−1, Emax = 93.92%) (FLX ED50 = 4.25 mg kg−1, Emax = 77.10%) (Fig. 6f).
Repeated open space swimming test
The OSST was conducted to establish the time-course of antidepressant effect. Repeated swim sessions caused increased total immobility time, a behaviour which remained elevated for the rest the test (Fig. 7a, c). Treatment with XA (3, 10, 30 mg kg−1) significantly (F3, 28 = 42.85, p < 0.001) reduced immobility on day 1 of treatment (i.e. day 5 of OSST) and persistently reduced to reach the pre-induction immobility time at the end of the OSST (Fig. 7a). Significant reduction in immobility was only observed from day 10 in the fluoxetine-treated mice (F 3, 28 = 14.19, p < 0.001) (Fig. 7c). Both XA and FLX produced significant reduction in overall immobility (Fig. 7b, d). The antidepressant-like behaviour exhibited in the repeated swimming test remained even 24 h post-drug treatment when measured in the TST (Fig. 7e). The various drug treatment however did not have any significant effect (p = 0.1278) on weight of mice at the end of the experiment.
Effects of xylopic acid in the open space swimming test for antidepressants. a–d Effect of XA (3–30 mg kg−1) and FLX (3–30 mg kg−1) on immobility time in the OSST. Results indicate time-course curves (TCCs) (a, c) and area under the TCCs as whisker plots (5–95th percentile) (b, d). ***p < 0.001, **p < 0.01 and *p < 0.05 comparing to saline (2-way ANOVA for TCCs and 1-way ANOVA for AUC’s). e Effect of XA (3–30 mg kg−1) and FLX (3–30 mg kg−1) in the TST 24 h post-OSST. ***p < 0.001, compared to saline. f Effect of XA (3–30 mg kg−1) and FLX (3–30 mg kg−1) on rostral grooming in the novelty-induced grooming behaviour test conducted on day 17 of OSST. Data presented as mean ± SEM. ***p < 0.001 comparing to saline. g Effects of XA (3–30 mg kg−1), and FLX (3–30 mg kg−1) on coat index on day 17 of the OSST. Data show mean scores ± SEM of eight body segments (well-mannered coat = 0, unkempt coat = 1). **p < 0.01, ***p < 0.001 comparing to saline
Effect on novelty-induced grooming and coat index
In comparison to untreated mice, XA- and FLX-treated mice showed significant (F3, 27 = 7.125 p < 0.001) drop in total time spent in rostral grooming (grooming of head and fore paws) when in a new environment (Fig. 7f). Furthermore, the rigorous OSST protocol reduced overall physical appearance of mice. However, both XA and FLX treatments reversed the decline in physical outlook indicated by reduced coat index at 10 and 30 mg kg−1 (F3, 28 = 12.9, p < 0.001) for XA and at all doses for FLX (Fig. 7g).
Evaluation of possible mechanism(s) of action
Effects of depleting monoamines on antidepressant-like property of XA
Reversal of antidepressant-like behaviour of XA, FLX and DES was observed in reserpine pre-treated mice in the forced swim test (Fig. 8a). Selective depletion of catecholamines with AMPT did not reverse the ability of XA and FLX to reduce immobility (F4, 60 = 2.731, p = 0.072) (Fig. 8b) but was rather reversed in pCPA pre-treated mice (F1, 60 = 32.65, p < 0.001) (Fig. 8c). The opposite scenario was observed in mice that received DES.
Contractile properties of XA on rat fundus strip preparation
The pharmacological properties of XA on 5-HT receptors were evaluated using the rat fundus tissue. XA evoked a partial agonist response on an isolated rat stomach fundus preparation in a concentration-dependent manner (EC50 = 45.88 ± 0.35 μM; Emax = 46.53) (Fig. 9a). Cumulative responses to XA were antagonised by 10 mM methysergide with a shift in the concentration response curve (CRC) to the right without depressing the Emax (p = 0.016) (Fig. 9b). Similar responses were observed with 5-HT (EC50 = 4.04 ± 0.16 nM) which was also abolished by methysergide (p < 0.001) (Fig. 9c). In the presence of a fixed sub-threshold concentration of XA (1 μM), the responses of 5-HT were potentiated (Fig. 9d).
Effects of XA on contractile properties of XA on rat fundus strip preparation. a XA and 5-HT evoked concentration-dependent contractions of the fundus (normalised response to 67 mM KCl). b–c Effect of methysergide (10 nM) on concentration response curves of xylopic acid and 5-HT. d Xylopic acid potentiated CRC of 5-HT. e–f Potentiation of tryptamine-induced response by selegiline (e) and not XA (f). Data points indicate mean ± SEM (n = 4–6)
Involvement of monoamine oxidase enzyme
In the presence of a sub-threshold concentration of XA (1 μM), the contractile response of tryptamine was not potentiated (F1, 89 = 0.755, p = 0.382) (Fig. 9f). In contrast, selegiline (10 nM) significantly (F1, 91 = 13.95, p = 0.0023) potentiated responses produced by tryptamine causing a leftward shift with no significant change in Emax (Fig. 9e).
Effects of pre-treatment with d-cycloserine, d-serine, L-NAME and L-arginine
Mice that received a combination of d-cycloserine and XA or FLX exhibited potentiated antidepressant-like effects when compared to mice that received only XA or FLX. d-cycloserine pre-treatment did not affect DES-treated animals (Fig. 10a). The established antidepressant-like property of XA, FLX and DES was absent in d-serine-pre-treated animals (Fig. 10b).
Antidepressant-like property of XA is enhanced by d-cycloserine and L-NAME but not d-serine and L-arginine. XA, FLX and DES administered alone or together with (a) d-cycloserine, (b) d-serine, (c) l-NAME or (d) l-arginine on immobility time. Results show group means ±SEM. Two-way ANOVA *p < 0.05, ***p < 0.01, **p < 0.001 comparing within treatment groups
Also, mice that received a combination of XA and l-NAME showed significant (F1,70 = 47.6, p < 0.001) potentiation in the antidepressant-like property in the FST (Fig. 10c). Similar observation was made for FLX but not in animals that received DES which rather seem to have been reversed. The antidepressant-like effects of XA and FLX and DES were all abolished in l-arginine-treated mice (Fig. 10d).
Discussion
We isolated xylopic acid, a kaurene diterpene, from Xylopia aethiopica: a plant that has antidepressant effect in rodent models (Biney et al. 2016) in addition to significant CNS depressant, analgesic and anti-inflammatory effects (Ameyaw et al. 2014; Biney et al. 2014).
A potential antidepressant effect was first explored in zebrafish using a CUS model where XA demonstrated effects akin to antidepressant agents. The zebrafish has become a model organism for neurobehavioural assays due to its high homology to the human CNS as well as ease of use in experimentation (Kalueff et al. 2014; Stewart et al. 2014; Fontana et al. 2018). It has been used to screen anxiolytics (Maximino et al. 2010; Benneh et al. 2017), anticonvulsants (Mussulini et al. 2013), antipsychotics (Kokel and Peterson 2011) and antidepressants (Meshalkina et al. 2018; Nowakowska et al. 2020) in recent times. XA-treated zebrafish, which have undergone significant stressors, showed reduced duration of bottom dwelling, a feature characteristic of depression and anxiety in this species (Maximino et al. 2010). This effect was similarly observed in fluoxetine-treated zebrafishes.
The initial antidepressant-like effect in zebrafish was also confirmed in mice by the significant reduction in immobility duration in both FST and TST even though XA does not have stimulatory effects in the spontaneous activity and open field tests (Biney et al. 2018). Generally, there were minor differences in the antidepressant-like properties of XA between FST and TST. XA was more potent and efficacious in FST than in TST. Antidepressants have been reported to produce different magnitudes of responses in both tests (Bourin et al. 2005). For example, dopaminergic antidepressants do not reduce immobility in FST in most species while noradrenaline reuptake inhibitors were devoid of effect in the tail suspension test in NMRI mice (Ripoll et al. 2003; Bourin 2019). Thus, these differences in response in the two most popular tests provide a first shot at the potential mechanism of action of xylopic acid.
Having shown an antidepressant potential, XA was further evaluated in the neuroinflammation hypothesis of depression. Undoubtedly, a significant connection exists between inflammation and major depressive disorder with depressed patients exhibiting hyper functioning of the HPA axis and elevated pro-inflammatory cytokines which has been associated with interference in glutamate and monoamine neurotransmission and neurogenesis in the hippocampus (Allison and Ditor 2014; Amodeo et al. 2017). With the knowledge that XA has anti-inflammatory effects (Osafo et al. 2018; Osafo et al. 2019), we evaluated its ability to mitigate LPS-induced depressive behaviour. Lipopolysaccharide induces depression-like behaviours through induction of pro-inflammatory cytokines that permeate the CNS and cause neuroinflammation (Bian et al. 2020). The inflammatory response evoked leads to increased catabolism of serotonin, production of free radicals and increased NMDA activity (Zhang et al. 2020; Dantzer et al. 2008). This ultimately leads to reduced extracellular serotonin and increased hippocampal atrophy all of which are associated with depression (Wang et al. 2020). XA treatment attenuated the depression-like behaviours of reduced social interaction and anhedonia, a core symptom of MDD and a very important measure in antidepressant screening of novel compounds (APA 2015). The ability of XA to reduce these behaviours adds another layer of evidence to support its antidepressant-like effect and gives credence to a potential for further drug development as an antidepressant.
To delineate the time-course of antidepressant action of XA, we evaluated XA in a chronic model for testing antidepressants: the repeated open space swimming test (OSST). Acute test such as the TST and FST may lack face and construct validity. Though they are of good predictive value, they may pick false positives such as atypical antipsychotics and calcium channel antagonists that have central stimulatory effects (Harro 2019). Chronic models for evaluating antidepressants such as the chronic unpredictable stress (CUS) test and repeated open space swimming test (OSST) have more construct validity, and hence, such false positives fail to exhibit antidepressant-like effects.
XA showed a comparatively fast-onset antidepressant effect in the OSST compared to fluoxetine. This effect was still present 24 h after halting treatment as was observed for fluoxetine. The search for fast onset of action antidepressants is very active in current antidepressant drug development (Ramaker and Dulawa 2017) which makes this finding exciting. In recent times, modulation of some serotonin receptors and glutamatergic neurotransmission have been linked to fast unremitting antidepressant actions (Wang et al. 2015; Zanos et al. 2018). Although we have not examined the direct effect of XA on 5-HT4 5-HT7 and 5-HT2c receptors, it is possible that XA may be eliciting the fast-onset antidepressant-like action by enhancing serotonergic transmission pathways involving these receptors. This is because agonists of 5-HT4 (Vidal et al. 2014) and antagonist of 5-HT7 (Mnie-Filali et al. 2011) and 5-HT2c (Opal et al. 2014) receptors have been shown to possess rapid-onset antidepressant-like activities. Indeed, our results showed a partial agonist effect on 5-HT2 receptors in the rat fundus strip, and therefore, XA can have antagonistic effect on the 5-HT2, a mechanism implicated in fast-onset antidepressant-like effects. Additionally, XA reduced other signature symptoms of MDD during open space swim test. Classical antidepressants like the SSRI exhibit a lag in antidepressant effect in the OSSTt while agents with rapid-onset antidepressant show almost immediate response (Ramaker and Dulawa 2017). XA-treated mice had well-kept hair and reduced rostral grooming in comparison to untreated animals. Analyses of grooming behaviour in depressed mice indicates high level of rostral grooming compared to naïve animals (Smolinsky et al. 2009; Bergner et al. 2016) but this was also reversed by XA treatment. Our findings indicate that XA shows fast-onset effect in the OSST, a model that has sensitivity, reliability and specificity in identifying rapid-onset antidepressant effect (Ramaker and Dulawa 2017) and has been used to identify 5-HT2c antagonists possessing fast-onset effects (Opal et al. 2014).
Having established an antidepressant-like property, the potential mechanisms of action of XA were investigated. We first assessed its effects on monoamines considering their role in depression. It is known that inhibiting the synthesis and or storage of specific a neurotransmitter will abolish the action of an antidepressant if that specific neurotransmitter contributes to antidepressant effects. For example, by selectively reducing serotonin levels, the antidepressant action of fluoxetine, a SSRI, is reversed (O’Leary et al. 2007; Ruhé et al. 2007; Adeoluwa et al. 2019). In our study, pre-treatment with the tryptophan hydroxylase inhibitor, p-chlorophenylalanine (pCPA), specifically inhibited 5-HT synthesis and reversed the established antidepressant-like effect of XA. The inhibition of synthesis or storage of catecholamines with α-methyl-p-tyrosine (AMPT) could not offset the effects of XA nor fluoxetine. According to work done by O’Leary et al. (2007), by pre-treating mice with the vesicular monoamine transporter (VMAT) reserpine, all monoamines including 5-HT are negatively affected and consequently both noradrenaline-based and 5-HT-based antidepressant will not work. Thus, not surprisingly, the antidepressant effects of XA, FLX and DES were all abolished in the presence or reserpine. These findings suggest a dominant role of 5-hydroxytryptamine in the observed antidepressant-like action of XA.
To further assess the serotonergic mechanism, we evaluated the effects of XA on serotonin receptors on the rat stomach fundus preparation. This tissue preparation has 5-HT1 and 5-HT2 receptors that evoke contractile responses when stimulated (Vane 1959). XA evoked partial agonist-like responses. 5-HT partial agonists like vortioxetine and buspirone have successfully been used to manage MDD thus making this finding another exciting one. The XA-induced responses were antagonised by the 5-HT receptor antagonist methysergide. The same preparation was also used to assess the effect of XA on monoamine oxidase (MAO) enzymes. MAO inhibitors are known to exert a potentiating effect on contractile responses produced by tryptamine an isolated rat fundus strip (Barlow 1961). However, unlike the monoamine oxidase inhibitor selegiline, XA could not significantly enhance tryptamine-evoked responses signifying a lack of monoamine oxidase inhibiting effect by XA.
Having identified a role of 5-HT in the observed antidepressant-like effects of XA, we sought to further confirm this role using the regulatory role of 5-HT on glutamate and nitric oxide pathways. The regulatory role of nitric oxide on glutamate neurotransmission in depression has been established. Subsequently, the efficacy of nitric oxide (NO) synthase inhibitors in depression is known with some antidepressants found to reduce expression and activation of NMDA receptors (Réus et al. 2016; Zhou et al. 2018). Stimulating NMDA glutamate receptors causes downstream activation of nNOS and the production of NO which is believed to be significant in the neurotoxic effects of glutamate (Zhou et al. 2018). However, 5-HT has negative modulatory effect on glutamate neurotransmission via activation of 5-HT7 and 5-HT2A receptors (Pehrson and Sanchez 2014; Li 2020). Therefore, agents that promote 5-HT transmission have synergism with negative modulators of this pathway. For example, decreasing the activity of nNOS enzyme with L-NAME will produce synergism with 5-HT-based antidepressants without affecting antidepressants whose actions are exclusively based on noradrenaline (Ostadhadi et al. 2016). We observed a similar effect in mice treated with XA or fluoxetine but not in desipramine-treated mice. L-NAME potentiated XA and fluoxetine’s effects but L-arginine reversed it. Same pattern of results was seen when the NMDA glutamate receptor was negatively modulated allosterically with d-cycloserine, an antagonist of the gylcineB co-bind site of the NMDA receptor. We observed an increase in antidepressant-like action of XA and FLX but not desipramine in mice pre-treated d-cycloserine whereas in mice pre-treated with glycineB agonist d-serine, the already established antidepressant effects were abolished (Poleszak et al. 2011; Poleszak et al. 2016). Again, this synergistic effects of XA with glutamate/nitric oxide pathway inhibitors confirm an association of serotonin neurotransmission in the observed antidepressant-like effects of XA.
Finally, we explored the effect of XA on oxidative stress and neuroprotection, both of which are implicated in depression (Czarny et al. 2018; Gross and Seroogy 2020). LPS stimulates oxidative stress in several brain regions causing dysregulation in oxidative stress makers like glutathione, malondialdehyde, CAT and SOD in the brain (Tomaz et al. 2014; Jangra et al. 2016). XA increased glutathione, SOD and CAT which constitute important antioxidant mechanisms in the first-line of attacking reactive oxygen species. Thus, XA reduced oxidative stress and protected the brain. The neuroprotective effect was further advanced by xylopic acid’s ability to increase brain level BDNF which is known to play a key role preventing depression and is suggested as a biomarker of efficacy for antidepressants (Autry and Monteggia 2012; Schröter et al. 2020). Patients diagnosed of major depression disorder are reported to present with lower hippocampal and prefrontal cortex levels of BDNF. By promoting branch formation and reducing neurodegeneration, BDNF promotes repair and survival of monoaminergic neurons (Mizui et al. 2016). The ability of XA to increase BDNF and exert this protective role is not surprising as some diterpenes have been shown to have neuroprotective effects (Adaramoye et al. 2010; Xu et al. 2011). This appreciable neuroprotective role of XA seen here supports the established antidepressant-like effects.
In conclusion, we have demonstrated that the kaurene diterpene, xylopic acid, has appreciable antidepressant-like effects that are dependent on serotonergic and neuroprotection mechanisms. It thus has a potential for further drug development as a therapeutic agent for managing MDD.
References
Adaramoye OA, Adedara IA, Popoola B, Farombi EO (2010) Extract of Xylopia aethiopica (Annonaceae) protects against gamma-radiation-induced testicular damage in Wistar rats. J Basic Clin Physiol Pharmacol 21(4):295–314
Adeoluwa AO, Aderibigbe OA, Agboola IO, Olonode TE, Ben-Azu B (2019) Butanol fraction of Olax subscorpioidea produces antidepressant effect: evidence for the involvement of monoaminergic neurotransmission. Drug Res 69(01):53–60
Allison DJ, Ditor DS (2014) The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation 11(1):151
Ameyaw EO, Woode E, Boakye-Gyasi E, Abotsi WK, Kyekyeku JO, Adosraku RK (2014) Anti-allodynic and anti-hyperalgesic effects of an ethanolic extract and xylopic acid from the fruits of Xylopia aethiopica in murine models of neuropathic pain. Pharm Res 6(2):172–179
Amodeo G, Trusso MA, Fagiolini A (2017) Depression and inflammation: disentangling a clear yet complex and multifaceted link. Neuropsychiatry 7(4):448–457
Association AP (2015) Depressive disorders: DSM-5® Selections. edn. American Psychiatric Pub
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64(2):238–258
Barlow R (1961) Effects on amine oxidase of substances which antagonize 5-hydroxytryptamine more than tryptamine on the rat fundus strip. Br J Pharmacol Chemother 16(2):153–162
Bear HA, Edbrooke-Childs J, Norton S, Krause KR, Wolpert M (2019) Systematic review and meta-analysis: outcomes of routine specialist mental health care for young people with depression and/or anxiety. J Am Acad Child Adolesc Psychiatry
Benneh CK, Biney RP, Mante PK, Tandoh A, Adongo DW, Woode E (2017) Maerua angolensis stem bark extract reverses anxiety and related behaviours in zebrafish—involvement of GABAergic and 5-HT systems. J Ethnopharmacol 207:129–145
Bergner CL, Smolinsky AN, Hart PC, Dufour BD, Egan RJ, LaPorte JL et al (2016) Mouse models for studying depression-like states and antidepressant drugs. In: Mouse Models for Drug Discovery. Springer, pp 255–269
Bian HT, Wang GH, Huang JJ, Liang L, Xiao L, Wang HL (2020) Scutellarin protects against lipopolysaccharide-induced behavioral deficits by inhibiting neuroinflammation and microglia activation in rats. Int Immunopharmacol 88:106943
Biney RP, Mante PK, Boakye-Gyasi E, Kukuia KE, Woode E (2014) Neuropharmacological effects of an ethanolic fruit extract of Xylopia aethiopica and xylopic acid, a kaurene diterpene isolate, in mice. West Afr J Pharm 25(1):106–117
Biney RP, Benneh CK, Ameyaw EO, Boakye-Gyasi E, Woode E (2016) Xylopia aethiopica fruit extract exhibits antidepressant-like effect via interaction with serotonergic neurotransmission in mice. J Ethnopharmacol 184:49–57
Biney RP, Benneh CK, Kyekyeku JO, Ameyaw EO, Boakye-Gyasi E, Woode E (2018) Attenuation of anxiety behaviours by xylopic acid in mice and zebrafish models of anxiety disorder. UKJPB 6(3):7–16
Bourin M (2019) Animal research in psychiatry. In: Frontiers in Psychiatry. Springer, pp 283–296
Bourin M, Chenu F, Ripoll N, David DJP (2005) A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension tests. Behav Brain Res 164(2):266–269
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cavalcanti B, Ferreira J, Moura D, Rosa R, Furtado G, Burbano R et al (2010) Structure–mutagenicity relationship of kaurenoic acid from Xylopia sericeae (Annonaceae). Mutat Res Genet Toxicol Environ Mutagen 701(2):153–163
Chakravarty S, Reddy BR, Sudhakar SR, Saxena S, Das T, Meghah V et al (2013) Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: altered brain proteome profile implicates mitochondrial dysfunction. PLoS One 8(5):e63302
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y et al (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391(10128):1357–1366
Crestani F, Seguy F, Dantzer R (1991) Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res 542(2):330–335
Czarny P, Wigner P, Galecki P, Sliwinski T (2018) The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuro-Psychopharmacol Biol Psychiatry 80:309–321
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9(1):46–56
Draper H, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. In: Methods in enzymology, vol 186. Elsevier, pp 421–431
Ekuadzi E, Biney RP, Benneh CK, Osei Amankwaa B, Jato J (2018) Antiinflammatory properties of betulinic acid and xylopic acid in the carrageenan-induced pleurisy model of lung inflammation in mice. Phytother Res 32(3):480–487
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Fontana BD, Mezzomo NJ, Kalueff AV, Rosemberg DB (2018) The developing utility of zebrafish models of neurological and neuropsychiatric disorders: a critical review. Exp Neurol 299:157–171
Gross C, Seroogy KB (2020) Neuroprotective roles of neurotrophic factors in depression. In: Neuroprotection in Autism, Schizophrenia and Alzheimer’s Disease. Elsevier, pp 125–144
Harro J (2019) Animal models of depression: pros and cons. Cell Tissue Res 1–16
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N et al (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858
Jangra A, Lukhi MM, Sulakhiya K, Baruah CC, Lahkar M (2014) Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur J Pharmacol 740:337–345
Jangra A, Kwatra M, Singh T, Pant R, Kushwah P, Sharma Y et al (2016) Piperine augments the protective effect of curcumin against lipopolysaccharide-induced neurobehavioral and neurochemical deficits in mice. Inflammation 39(3):1025–1038
Kalueff AV, Stewart AM, Gerlai R (2014) Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35(2):63–75
Kokel D, Peterson RT (2011) Using the zebrafish photomotor response for psychotropic drug screening. Methods Cell Biol 105:517–524
Kolbeck R, Bartke I, Eberle W, Barde YA (1999) Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem 72(5):1930–1938
Li Y-F (2020) A hypothesis of monoamine (5-HT) – glutamate/GABA long neural circuit: aiming for fast-onset antidepressant discovery. Pharmacol Ther 208:107494
Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A Jr (2010) Measuring anxiety in zebrafish: a critical review. Behav Brain Res 214(2):157–171
Meshalkina DA, Kysil EV, Antonova KA, Demin KA, Kolesnikova TO, Khatsko SL et al (2018) The effects of chronic amitriptyline on zebrafish behavior and monoamine neurochemistry. Neurochem Res 43(6):1191–1199
Mizui T, Ishikawa Y, Kumanogoh H, Kojima M (2016) Neurobiological actions by three distinct subtypes of brain-derived neurotrophic factor: multi-ligand model of growth factor signaling. Pharmacol Res 105:93–98
Mnie-Filali O, Faure C, Lambás-Señas L, El Mansari M, Belblidia H, Gondard E et al (2011) Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 36(6):1275–1288
Mussulini BHM, Leite CE, Zenki KC, Moro L, Baggio S, Rico EP et al (2013) Seizures induced by pentylenetetrazole in the adult zebrafish: a detailed behavioral characterization. PLoS One 8(1):e54515
Nowakowska K, Giebułtowicz J, Kamaszewski M, Adamski A, Szudrowicz H, Ostaszewska T et al (2020) Acute exposure of zebrafish (Danio rerio) larvae to environmental concentrations of selected antidepressants: bioaccumulation, physiological and histological changes. Comp Biochem Physiol, Part C: Toxicol Pharmacol 229:108670
O’Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I (2007) Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology 192(3):357–371
Opal MD, Klenotich SC, Morais M, Bessa J, Winkle J, Doukas D et al (2014) Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol Psychiatry 19(10):1106–1114
Osafo N, Biney RP, Obiri DD (2016) Aqueous ethanol fruit extract of Xylopia aethiopica and xylopic acid exhibit anti-inflammatory activity through inhibition of the arachidonic acid pathway. UK J Pharm Biosci 4:35–41
Osafo N, Obiri DD, Antwi AO, Yeboah OK (2018) The acute anti-inflammatory action of xylopic acid isolated from Xylopia aethiopica. J Basic Clin Physiol Pharmacol 29(6):659–669
Osafo N, Obiri DD, Danquah KO, Essel LB, Antwi AO (2019) Potential effects of xylopic acid on acetic acid-induced ulcerative colitis in rats. Turk J Gastroenterol 30(8):732–744
Ostadhadi S, Khan MI, Norouzi-Javidan A, Chamanara M, Jazaeri F, Zolfaghari S et al (2016) Involvement of NMDA receptors and L-arginine/nitric oxide/cyclic guanosine monophosphate pathway in the antidepressant-like effects of topiramate in mice forced swimming test. Brain Res Bull 122:62–70
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M et al (2016) Major depressive disorder. Nat Rev Dis Primers 2(1):1–20
Pehrson AL, Sanchez C (2014) Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectrums 19(2):121–133
Piato ÂL, Capiotti KM, Tamborski AR, Oses JP, Barcellos LJ, Bogo MR et al (2011) Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog Neuro-Psychopharmacol Biol Psychiatry 35(2):561–567
Poleszak E, Wlaź P, Szewczyk B, Wlaź A, Kasperek R, Wróbel A et al (2011) A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J Neural Transm 118(11):1535–1546
Poleszak E, Stasiuk W, Szopa A, Wyska E, Serefko A, Oniszczuk A et al (2016) Traxoprodil, a selective antagonist of the NR2B subunit of the NMDA receptor, potentiates the antidepressant-like effects of certain antidepressant drugs in the forced swim test in mice. Metab Brain Dis 31(4):803–814
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266(5604):730–732
Ramaker M, Dulawa S (2017) Identifying fast-onset antidepressants using rodent models. Mol Psychiatry 22(5):656–665
Réus GZ, Abelaira HM, Tuon T, Titus SE, Ignácio ZM, Rodrigues ALS et al (2016) Glutamatergic NMDA receptor as therapeutic target for depression. In: Advances in protein chemistry and structural biology, vol 103. Elsevier, pp 169–202
Ripoll N, David DJP, Dailly E, Hascoët M, Bourin M (2003) Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res 143(2):193–200
Rossi S, Studer V, Motta C, Polidoro S, Perugini J, Macchiarulo G et al (2017) Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology 89(13):1338–1347
Ruhé HG, Mason NS, Schene AH (2007) Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry 12(4):331–359
Schröter K, Brum M, Brunkhorst-Kanaan N, Tole F, Ziegler C, Domschke K et al (2020) Longitudinal multi-level biomarker analysis of BDNF in major depression and bipolar disorder. Eur Arch Psychiatry Clin Neurosci 270(2):169–181
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Smolinsky AN, Bergner CL, LaPorte JL, Kalueff AV (2009) Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression. In: Mood and anxiety related phenotypes in mice. Springer, pp 21–36
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370
Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV (2014) Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 37(5):264–278
Stone EA, Lin Y (2011) Open-space forced swim model of depression for mice. Curr Protoc Neurosci 54(1):9.36. 31–39.36. 38
Strawn JR, Mills JA, Sauley BA, Welge JA (2018) The impact of antidepressant dose and class on treatment response in pediatric anxiety disorders: a meta-analysis. J Am Acad Child Adolesc Psychiatry 57(4):235–244 e232
Sulakhiya K, Keshavlal GP, Bezbaruah BB, Dwivedi S, Gurjar SS, Munde N et al (2016) Lipopolysaccharide induced anxiety-and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett 611:106–111
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Takahashi JA, Boaventura MAD, de Carvalho BJ (1995) Frutoic acid, a dimeric kaurane diterpene from Xylopia frutescens. Phytochemistry 40(2):607–609
Tomaz V, Cordeiro R, Costa A, De Lucena D, Júnior HN, De Sousa F et al (2014) Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience 268:236–246
Vane J (1959) The relative activities of some tryptamine analogues on the isolated rat stomach strip preparation. Br J Pharmacol Chemother 14(1):87–98
Vidal R, Castro E, Pilar-Cuellar F, Pascual-Brazo J, Diaz A, Luisa Rojo M et al (2014) Serotonin 5-HT4 receptors: a new strategy for developing fast acting antidepressants? Curr Pharm Des 20(23):3751–3762
Wang J, Jing L, Toledo-Salas J-C, Xu L (2015) Rapid-onset antidepressant efficacy of glutamatergic system modulators: the neural plasticity hypothesis of depression. Neurosci Bull 31(1):75–86
Wang X, Zhu L, Hu J, Guo R, Ye S, Liu F et al (2020) FGF21 attenuated LPS-induced depressive-like behavior via inhibiting the inflammatory pathway. Front Pharmacol 11:154
Whooley MA, Wong JM (2013) Depression and cardiovascular disorders. Annu Rev Clin Psychol 9:327–354
Woelfer M, Kasties V, Kahlfuss S, Walter M (2019) The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder. Neuroscience 403:93–110
Xu J, Guo P, Liu C, Sun Z, Gui L, Guo Y et al (2011) Neuroprotective kaurane diterpenes from Fritillaria ebeiensis. Biosci Biotechnol Biochem 75(7):1386–1388
Yalcin I, Aksu F, Bodard S, Chalon S, Belzung C (2007) Antidepressant-like effect of tramadol in the unpredictable chronic mild stress procedure: possible involvement of the noradrenergic system. Behav Pharmacol 18(7):623–631
Zanos P, Thompson SM, Duman RS, Zarate CA, Gould TD (2018) Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32(3):197–227
Zhang J, Yi S, Li Y, Xiao C, Liu C, Jiang W et al (2020) The antidepressant effects of asperosaponin VI are mediated by the suppression of microglial activation and reduction of TLR4/NF-κB-induced IDO expression. Psychopharmacology 237:2531–2545
Zhou Q-G, Zhu X-H, Nemes AD, Zhu D-Y (2018) Neuronal nitric oxide synthase and affective disorders. IBRO Rep 5:116–132
Acknowledgements
The authors are grateful to technicians of the Departments of Pharmacology in Kwame Nkrumah University of Science and Technology and in University of Cape Coast for their technical support at various stages of the project.
Funding
This work was funded using personal funds of all contributing authors.
Author information
Authors and Affiliations
Contributions
RPB conducted all experiments, analysed the data and wrote the first draft of manuscript. CKB participated in the carrying experiments on mechanism of action and zebrafish assays. DWA participated in chronic depression experiments and analyses of the data. EW and EOA conceptualised the work, provided supervision of the experiments and reviewed the analyses. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biney, R.P., Benneh, C.K., Adongo, D.W. et al. Evidence of an antidepressant-like effect of xylopic acid mediated by serotonergic mechanisms. Psychopharmacology 238, 2105–2120 (2021). https://doi.org/10.1007/s00213-021-05835-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05835-6