Abstract
Obesity causes inflammation in the adipose tissue and can affect the central nervous system, leading to oxidative stress and mitochondrial dysfunction. Therefore, it becomes necessary to seek new therapeutic alternatives. Gold nanoparticles (GNPs) could take carnitine to the adipose tissue, thus increasing fatty acid oxidation, reducing inflammation, and, consequently, restoring brain homeostasis. The objective of this study was to investigate the effects of GNPs associated with carnitine on the neurochemical parameters of obesity-induced mice. Eighty male Swiss mice that received a normal lipid diet (control group) or a high-fat diet (obese group) for 10 weeks were used. At the end of the sixth week, the groups were divided for daily treatment with saline, GNPs (70 µg/kg), carnitine (500 mg/kg), or GNPs associated with carnitine, respectively. Body weight was monitored weekly. At the end of the tenth week, the animals were euthanized and the mesenteric fat removed and weighed; the brain structures were separated for biochemical analysis. It was found that obesity caused oxidative damage and mitochondrial dysfunction in brain structures. Treatment with GNPs isolated reduced oxidative stress in the hippocampus. Carnitine isolated decreased the accumulation of mesenteric fat and oxidative stress in the hippocampus. The combination of treatments reduced the accumulation of mesenteric fat and mitochondrial dysfunction in the striatum. Therefore, these treatments in isolation, become a promising option for the treatment of obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is considered a low grade chronic inflammatory disease, of multifactorial cause, and characterized by abnormal or excessive accumulation of fat [1]. This condition has become a global epidemic due to its growing prevalence; data from the World Health Organization (WHO) indicate that in 2016 more than 1.9 billion adults were overweight, of which 650 thousand were obese [2]. Hence, significant impacts on health and medical costs have occurred, making obesity and its associated comorbidities a public health problem [3].
Initially, the greatest accumulation of fat occurs in the subcutaneous adipose tissue, being the largest and least harmful, where the expansion of this tissue occurs predominantly through hyperplasia [4]. However, when the capacity for hyperplasia reaches its limit, hypertrophy occurs, where excess fat is routed to more harmful adipose tissue depots such as visceral adipose tissue [5]. The expansion of visceral adipose tissue occurs almost exclusively through adipocyte hypertrophy [6]. Adipocyte hypertrophy in obesity is a complex phenomenon expressed not only by an increase in the size of individual cells but also by remodeling of adipose tissue, leading to tissue hypoxia [7].
In addition, this remodeling of adipose tissue leads to the recruitment from the bloodstream and infiltration into the tissue of monocytes, giving rise to tissue macrophages [8]. These macrophages express the M1 phenotype, i.e., present a pro-inflammatory profile which are responsible for the increase in the production of pro-inflammatory cytokines such as the alpha tumor necrosis factor (TNF-α), interleukin (IL)-1β, and IL-6, which can block the effects of insulin action in the adipocytes and generate reactive oxygen species (ROS), such as nitric oxide (NO), via nitric oxide synthase (iNOS) activation, leading to a pro-inflammatory condition [9, 10].
The inflammation resulting from obesity does not only occur at the systemic level, but can affect the central nervous system (CNS). Therefore, this inflammation can cause damage to the blood–brain barrier (BBB), allowing the passage of pro-inflammatory cytokines from the periphery to the CNS, generating neuroinflammation [11]. This neuroinflammation initially affects the hypothalamus causing the interruption of hypothalamic signals of satiety and perpetuation of excessive eating [12]. Consequently, this inflammation affects other brain structures, causing damage to the prefrontal cortex, the hippocampus, and the striatum; these are regions participating in the reward system, as well as cognition and memory [13].
In obesity, the signaling of these pro-inflammatory cytokines, as well as the excess nutrients ingested, leads to the formation of ROS, through different intracellular pathways. Indeed, oxidative stress is present in obesity, which can lead to oxidation of biomolecules, such as lipids, proteins, and DNA, with consequent loss of their biological functions [14]. Furthermore, oxidative stress is associated with mitochondrial dysfunction, affecting production of adenosine triphosphate (ATP) [15]. Therefore, oxidative stress and compromised oxidative phosphorylation may be linked to the development of obesity and related metabolic disorders, which bear implications for the obesity treatment [16].
The initial treatment of obesity is based on lifestyle changes, that is, changes in dietary patterns, such as a reduction in calories ingested, associated with regular physical activity [17]. However, lifestyle changes alone are not always effective. For this reason, pharmacological options are commonly associated in the treatment. However, current pharmacological treatments for obesity are known to cause several side effects, and their continuous use for long periods is not recommended [18]. Therefore, it is necessary to implement new therapeutic options, which aim not only at weight loss but also at restoring metabolic homeostasis. To this end, new therapies have shown to be of interest, specifically nanotechnology, in which materials with sizes from 1 to 100 nm are used to serve as diagnostic tools for diseases, or to deliver therapeutic agents to specific locations in a controlled manner [19].
Among the nanoparticles, gold nanoparticles (GNPs) stand out for their antioxidant and anti-inflammatory properties [20]. In this connection, a study indicated that in obese Swiss mice, intraperitoneal administration of GNPs for 14 days led to a significant concentration of GNPs in the adipose tissue, which may be useful for the treatment of inflammation associated with obesity [21].
The use of GNPs In animal models of obesity has been shown to be effective in preventing weight gain, reducing fat deposition, reducing metabolic inflammation, and improving glucose tolerance and insulin sensitivity [22, 23]. Furthermore, the peripheral effects of GNPs can be found at the CNS level, since GNPs easily cross the BBB [24]. That said, a study demonstrated that the administration of GNPs was effective in reducing the damage caused by a high-fat diet, reducing oxidative stress and brain inflammation, mainly evidenced by the reduction in IL-1β and TNF-α levels in brain structures of mice with obesity treated with GNPs [21]. Thus suggesting an effect of GNPs in reducing neuroinflammation caused by obesity [25].
In addition, GNPs have the ability to carry drugs and biomolecules to target tissues and cells, easily conjugating to oligonucleotides, proteins, and peptides [26]. Carnitine, as it is a dipeptide, has a high potential for binding to GNPs. Furthermore, carnitine’s main function is to transport long-chain fatty acids from the cytosol to the mitochondrial matrix, where β-oxidation occurs [27]. Furthermore, carnitine may work as an antioxidant and anti-inflammatory compound [28]. Thus, it is expected that the combination of GNPs with carnitine can impact adipose tissue inflammation, and, therefore, reestablish brain functionality.
Materials and Methods
Experimental Model
The present work is an experimental obesity animal model study in which mice were fed a high-fat diet and treated with GNPs associated with carnitine. Eighty male Swiss mice (Mus musculus species) with 40 days of life, weighing around 30 to 40 g each, were used. The animals were kept in cages lined with wood shavings, with a maximum capacity of 5 mice that were kept in light–dark cycles of 12 h and temperature of 23 ± 1 °C. They had free access to water and feed. The animals were supplied by the Animal Facility of the Universidade Federal de Santa Catarina (UFSC, Federal University of Santa Catarina) and were housed in the Experimental Animal Facility of the Universidade do Sul de Santa Catarina (UNISUL, University of Southern Santa Catarina), Tubarão campus.
Study Design
Initially, the animals were weighed and separated into two groups: the control group (n = 40) who received a standard diet, and the obese group (n = 40) who received a high-fat diet. Then, the protocol for the induction of obesity in the animal model began and lasted 10 weeks. During that period, the animals were fed a high-fat diet. The protocol for inducing obesity was based on previous studies [29]. Thus, to confirm the animal model of obesity, the body weight was checked weekly throughout the experiment.
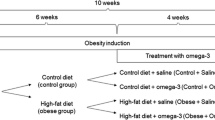
At the end of the sixth week, treatment with GNPs associated with carnitine was started. The animals were again separated into the following groups: control group + saline (Control—Sal); control group + GNPs (Control—GNPs); control group + carnitine (Control—Carn); control group + GNPs + carnitine (Control—GNPs + Carn); obese group + saline (Obese—Sal); obese group + GNPs (Obese—GNPs); obese group + carnitine (Obese—Carn); obese group + GNPs + carnitine (Obese—GNPs + Carn); all groups included n = 10 each. The groups received, intraperitoneally, once a day, a treatment with the following concentrations: the GNP groups received 70 µg/kg [21] of GNPs (20 nm); the carnitine groups received 500 mg/kg [30] carnitine diluted in saline; the GNPs + carnitine groups received 500 mg/mL of GNPs + carnitine. In the control and obese groups, only saline was administered in parallel to the treatment groups. Treatment lasted 4 weeks until the end of the experiment (tenth week).
At the end of the experiment (tenth week), the animals were weighed for the last time, and were then euthanized by decapitation. The decapitation method of death is justified by the fact that chemical methods, using drugs, interfere with the biochemical analyses to be carried out in this study [31] (Fig. 1).
Experimental design of the study. Experimental procedure to verify the effect of treatment with GNPs, carnitine, or a combination of GNPs and carnitine on obesity. Initially, the animals were divided into a control group and an obese group to begin the obesity animal model induction protocol, which lasted 10 weeks. At the end of the sixth week, treatment began, where the animals were divided again into the following groups: Control—Sal; Control—GNPs; Control—Carn; Control—GNPs + Carn; Obese—Sal; Obese—GNPs; Obese—Carn; Obese—GNPs + Carn. The treatment lasted 4 weeks, until the end of the experiment (tenth week). At the end of the experiment, the animals were euthanized, and the mesenteric fat and brain structures were removed
Diets
The control-diet groups were offered a purified low-fat diet. In the groups that received a high-fat diet, a purified high-fat diet was offered. The diet food was purchased from a company specialized in the production of standardized diets for animal studies (PragSoluções Biociências, Jaú, SP, Brazil). The high-fat diet supplied significantly more calories and a higher amount of saturated fat than the control diet. Diet composition was based on a previous study by Cintra et al. and is described in Table 1 [32].
Synthesis and Characterization of Gold Nanoparticles
GNPs with an average size of 20 nm were synthesized from the chemical reduction of the metallic precursor tetrachloroauric acid (HAuCl4) (Sigma-Aldrich, MO, USA) with the reducing agent and stabilizer sodium citrate (Nuclear, SP, Brazil). Initially, 100 mL of tetrachloroauric acid was transferred to a round bottom flask; the solution was heated to 95 °C under stirring. The previously prepared sodium citrate solution was then added, and the system was maintained at the described temperature, under stirring for 20 min; according to Turkerich et al. [33], the solution acquired a color corresponding to the size of the solution.
The GNP solution was immediately characterized employing ultraviolet–visible (UV–Vis) spectroscopy techniques, via monitoring of the resonant plasmon surface band, using a model UV-1800 spectrophotometer. For UV–visible spectrometry, the measurement of the resonant plasmon surface band was performed at room temperature in a spectrophotometer, using a quartz cuvette with 1 cm optical path. The electronic spectrum of the solution was in the range of 532 nm for 20 nm GNPs.
For the characterization of the nanoparticles, the zeta potential was measured using a NanoBrook Omni equipment (Brookhaven Instruments Corporation, NY, USA), with temperature control and standard 40 mW red laser 640 nm; measurements of electrophoretic mobility were performed and the results were converted into potential value (mV) by the equipment’s actual software; measurements were taken in twenty cycles with 1-s interval for each cycle. Measurements were performed on samples dissolved in deionized water, for synthesized samples. The samples had their concentration adjusted to 1.0 mg/mL (mother solution), and then diluted in the proportion of 1/30 for later reading. Readings were taken at 25 °C.
Conjugation of Carnitine and Gold Nanoparticles
The conjugation of GNPs with carnitine was performed by adding carnitine to GNPs; this mixture was stirred for 1 h at room temperature, in order for the reaction to occur. The carnitine concentration was 500 mg/mL in GNPs, which was the previously tested minimum concentration capable of binding to the nanoparticles [30]. This concentration corresponded to the same amounts as the individual treatments, that is, the final solution was diluted with 500 mg of carnitine for each milliliter of GNPs, corresponding to 500 mg of carnitine and 70 µg of GNPs for each milliliter of solution.
Removal, Weighing, and Storage of Tissues
After the animals’ euthanasia, the abdominal cavity was opened and the adipose tissue of the mesenteric region, located along the intestinal tract, was removed and weighed on a high precision scale. Results were expressed in grams. The animals’ brains were quickly removed, and the hypothalamus, prefrontal cortex, hippocampus, and striatum brain structures were separated forthwith. Then, the samples were stored at − 80 °C for use in the biochemical analyses.
Analysis of Oxidant Production
The ROS production was determined by the intracellular formation of 2′,7′-dichlorofluorescein (DCF). Initially, the samples were homogenized, then 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was added and incubated for 30 min at 37 °C. Fluorescence was determined using a wavelength of 488 nm (excitation) and 525 nm (emission), and the calibration curve was plotted using standard DCF (10 µM) [34]. The results were expressed in fluorescence/mg of protein.
The stable metabolite nitrite was evaluated as an indicator of nitric oxide (NO) formation. In order to measure the nitrite content, the samples were incubated with Griess reagent (1% sulfanilamide and 0.1% N-1(naphthylethylenediamine) at room temperature for 10 min and the absorbance measured at 540 nm. The nitrite content was calculated based on a standard curve from 0 to 100 nM performed with the metabolite sodium nitrite (NaNO2). The results were calculated in µmol nitrite/mg protein [35].
Analyses were normalized by protein content. Proteins were determined by the method of Lowry et al. [36], and bovine serum albumin was used as standard.
Oxidative Damage Analysis
Oxidative damage to proteins was assessed by determining the carbonyl groups in oxidized proteins, based on the reaction with dinitrophenylhydrazine, following the method described by Levine et al. [37]. In this technique, proteins were precipitated by adding 20% trichloroacetic acid and dissolved in dinitrophenylhydrazine. Absorbance was read at 340 nm. The results were expressed in nmol of carbonylated proteins per mg of protein (nmol/mg protein).
Antioxidant Enzyme Activity
The activity of the antioxidant enzyme superoxide dismutase (SOD) was determined by the inhibition of adrenaline auto-oxidation measured spectrophotometrically, as described by Bannister and Calabrese [38]. The technique is based on the inhibition of the reaction of the superoxide anion radical (O2•-) with adrenaline, a compound that self-oxidizes with pH variation. Adrenaline oxidation causes adrenochrome formation; SOD activity was determined by measuring the speed of adrenochrome formation, determined spectrophotometrically at 480 nm, in a reaction medium containing glycine–NaOH (50 mM at pH 10.2) and 60 mM adrenaline. The results were expressed in U/mg of protein.
Glutathione (GSH) levels were determined as described by Hissin and Hilf [39]. GSH was measured after protein precipitation with 1 mL 10% trichloroacetic acid. Next, 800 mM phosphate buffer, pH 7.4 and 500 μm of DTNB were added. The resulting color development from the reaction between DTNB and the thiols peaked within 5 min and remained stable for over 30 min. Absorbance was read at 412 nm after 10 min. A reduced glutathione standard curve was used to calculate the GSH levels in the samples. The results were expressed in μmol/mg of protein.
SOD and GSH analyses were normalized by protein content. Proteins were determined by the method of Lowry et al. [36], and bovine serum albumin was used as standard.
Activity of Succinate Dehydrogenase Enzyme
The succinate dehydrogenase (SDH) enzyme activity was determined using the method described by Fischer et al. [40]. An incubation medium was added with: 62.4 mM pH7.4 potassium phosphate buffer, 250 mM sodium succinate, 2,6-DCIP, and sample. The systems were preincubated for 20 min at 30 °C in water bath; subsequently, 100 mM sodium azide, 1 mM rotenone, 2,6-DCIP, and phenazine methosulfate were added. The reduction of 2,6-DCIP was determined at 600 nm for 5 min in a spectrophotometer. Results were expressed in nmol/min × mg protein. They were normalized by protein content. Proteins were determined by the method of Lowry et al. [36], and bovine serum albumin was used as standard.
Activity of Mitochondrial Respiratory Chain Complexes
Complex I activity was evaluated by the method described by Cassina and Radi [41], by the NADH-dependent rate of ferricyanide reduction. One hundred millimolars potassium phosphate buffer, 10 mM ferricyanide, 14 mM NADH, 2 mM rotenone, and sample were added to the reaction medium. After adding all the reagents and the sample, the reading was performed in a spectrophotometer every 1 min, for 3 min, at 420 nm.
Complex II activity was assessed using the method described by Fischer et al. [40] by the reduction of 2,6-DCIP absorbance. The sample was added to an incubation medium containing 62.5 mM potassium phosphate buffer, 250 mM sodium succinate, and 0.5 mM 2,6-DCIP; the solution was then incubated for 20 min at 30 °C in water bath. After incubation, 100 mM sodium azide, 2 mM rotenone, and again 0.5 mM 2,6-DCIP were added; a spectrophotometer reading was taken every 1 min, for 5 min, at 600 nm.
Complex IV activity was determined according to the technique described by Rustin et al. [42] and calculated by the absorbance reduction caused by the oxidation of reduced cytochrome c. Potassium phosphate buffer 62.5 mM, lauryl maltoside 125 mM, the sample diluted with SETH buffer (Sucrose, EDTA, Trizma base, and Heparin), and cytochrome c 1% were added to an incubation medium, and then, the solution was read in a spectrophotometer every 1 min, for 10 min, at 550 nm.
All activity analyses of mitochondrial respiratory chain complexes were normalized for protein content. Proteins were determined by the method of Lowry et al. [36], and bovine serum albumin was used as standard. Activity results of all mitochondrial respiratory chain complexes were expressed in nmol/min × mg protein.
Statistical Analyses
Data analyses were performed using InStat Statistical Software (GraphPad, La Jolla, CA, USA). Comparisons between experimental groups were performed using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc. Results were presented as mean ± standard error of the mean (SEM), and statistical significance was considered with p < 0.05.
Results
GNPs were synthesized and characterized, and the solution of GNP produced assumed a purple coloring, which is characteristic of GNP to 20 nm. The size of the GNP was characterized by measuring absorbance with wavelength scan (λ) from 400 to 700 nm in a spectrophotometer in the UV–vis band. The GNP of 20 nm reached Absmax 440 and λmax of 525 nm. With the association of carnitine to GNP, they reached Absmax 259 and λmax offset of 527 nm, proving a change in structure and suggesting the interaction of molecules. The association of GNP and carnitine was verified by the diameter of the molecule and with the zeta potential identified, which calculates the surface electrical potential, important to predict molecule stability. Based on the results, GNP and GNP + carnitine are stables and there was an association of GNP due to differences in zeta potential and diameter.
Throughout the experiment, the animals’ body weight was measured weekly. The animals began the experiment with body weight without statistical difference. From the beginning of the obesity induction protocol, the animals in the Obese group showed greater body weight when compared to the Control group until the sixth week (p < 0.0001). From the sixth week onwards, the animals were divided again for treatment, the animals in the groups that received a high-fat diet showed greater body weight when compared to the Control—Sal group from the seventh to tenth week of treatment, ending the experiment in the tenth week with the Obese—Sal (p < 0.0001), Obese—GNPs (p < 0.0001), Obese—Carn (p = 0.0011), and Obese—GNPs + Carn (p = 0.0002) groups with body weight significantly greater than the Control—Sal group. On the other hand, the groups that received a control diet did not show a significant difference in body weight compared to the Control—Sal group (Fig. 2).
Weekly weight monitoring of mice submitted to a high fat diet and treated with GNPs, carnitine, or combination of GNPs and carnitine. *Significant difference compared to the Control group; &significant difference compared to the control + saline (Control—Sal) group. Data were represented as mean ± SEM, p < 0.05 (two-way ANOVA followed by Tukey’s post hoc test)
At the end of the experiment, the animals’ mesenteric fat was removed and weighed. A two-way ANOVA analysis showed a significant effect of diet (F (1, 84) = 154.0; p < 0.001) and intervention (F (3, 84) = 3.589; p = 0.02) in the weight of mesenteric fat. Post hoc analysis revealed that the animals in the groups that received a high-fat diet showed greater accumulation of mesenteric fat, when compared to the Control—Sal group (p = 0.001). On the other hand, the Obese—Carn and Obese—GNPs + Carn groups showed a significant reduction in the accumulation of mesenteric fat, when compared to the Obese—Sal group (p = 0.02). The Control—GNPs, Control—Carn, and Control—GNPs + Carn groups did not show a significant difference in mesenteric fat weight compared to the Control—Sal group (Fig. 3). To analyze the production of oxidants, the intracellular formation of DCF was initially evaluated. The two-way ANOVA analysis demonstrated that there was a significant effect of diet on the prefrontal cortex (F (1, 34) = 11.54; p = 0.002) and in the hippocampus (F (1, 23) = 7.441; p = 0.0120) and effect of intervention on the prefrontal cortex (F (3, 34) = 4.22; p = 0.01) and in the hippocampus (F (3, 23) = 12.81; p < 0.0001), as well as a significant interaction (F (3, 23) = 23.04; p < 0.0001) between diet and intervention in the hippocampus. The post hoc test showed in the prefrontal cortex the production of DCF was reduced in the Obese—Carn group, compared to the Control—Sal (p = 0.008) and Obese—Sal (p = 0.01) groups; in the Obese—GNPs + Carn group, the production of DCF was reduced in relation to the Control—Sal group (p = 0.05). The other groups did not show a significant difference in prefrontal cortex compared to the Control—Sal group. When examining the hippocampus, it was noticed that the Control—GNPs, Control—GNPs + Carn, Obese – Sal, and Obese—GNPs + Carn groups showed greater production of DCF compared to the Control—Sal group (p = 0.0100, 0.0154, 0.0033, 0.0036, respectively). While the Obese—GNPs and Obese—Carn groups showed a significant reduction in the production of DCF compared to the Obese—Sal group (p = 0.0005 and < 0.0001, respectively). Finally, in the hypothalamus and striatum, no significant difference in DCF production between the groups was found (Fig. 4A).
Mesenteric fat weight of mice submitted to a high fat diet and treated with GNPs, carnitine, or combination of GNPs and carnitine. *Significant difference compared to the control + saline (Control—Sal) group; #Significant difference compared to the obese + saline (Obese—Sal) group. Data were represented as mean ± SEM, p < 0.05 (two-way ANOVA followed by Tukey’s post hoc test)
Analysis of oxidative stress parameters in brain structures of mice submitted to a high-fat diet and treated with GNPs, carnitine or combination of GNPs and carnitine. DCF oxidation (A), nitrite (B), protein carbonyls (C), SOD activity (D), glutathione (E). *Significant difference compared to the control + saline (Control—Sal) group; #significant difference compared to the obese + saline (Obese—Sal) group. Data were represented as mean ± SEM, p < 0.05 (two-way ANOVA followed by Tukey’s post hoc test)
Still evaluating the production of oxidants, the stable metabolite nitrite was assessed as an indicator of NO formation. It was possible to observe that there was no significant difference in the structures, hypothalamus, prefrontal cortex, hippocampus, and striatum between the groups (Fig. 4B).
The analysis of oxidative damage in proteins was evaluated by the determination of carbonyl groups in oxidized proteins, where in the hippocampus there was a significant effect of the intervention (F (3, 25) = 14.83; p < 0.0001) as well as a significant interaction (F (3, 25) = 6.723; p = 0.0018) between diet and intervention in the hippocampus. Still in the hippocampus, post hoc analysis revealed the Control—GNPs + Carn and Obese—Sal groups presented greater protein carbonylation, when compared to the Control—Sal group (p = 0.0358 and 0.0147, respectively). On the other hand, in the Obese—GNPs and Obese—Carn groups, protein carbonylation was lower compared to the Obese—Sal group (p = 0.0150 and < 0.0001, respectively); the Control – GNPs, Control—Carn, and Obese—GNPs + Carn groups did not show a significant difference in relation to the Control—Sal. In the structures hypothalamus, prefrontal cortex, and striatum, there was no significant difference in protein carbonylation between groups (Fig. 4C).
To evaluate the antioxidant defenses, the activity of the SOD enzyme was initially. The two-way ANOVA analysis demonstrated significant effect of intervention on the hippocampus (F (3, 30) = 4.442; p = 0.0107) and striatum (F (3, 35) = 10.24; p < 0.0001), as well as a significant interaction between diet and intervention in the prefrontal cortex (F (3, 36) = 8.842; p = 0.0002), hippocampus (F (3, 30) = 4.049; p = 0.0157), and striatum (F (3, 35) = 7.646; p = 0.0005). The post hoc test showed that in the prefrontal cortex the Obese—Carn group presented lower SOD activity when compared to the Obese—Sal group (p = 0.0270); in the other groups, there was no significant difference in relation to the Control—Sal group regarding the prefrontal cortex. However, in the hippocampus, SOD activity increased in the Obese—Carn group in relation to the Obese—Sal group (p = 0.0016); in the other groups, there was no significant difference in relation to the Control—Sal group in the hippocampus. When examining the striatum, it was noticed that the SOD activity had increased in the Obese—Sal group in relation to the Control—Sal group (p = 0.0207), while the Obese—GNPs and Obese—GNPs + Carn groups presented lower SOD activity when compared to the Obese—Sal group (p = 0.0103 and < 0.0001, respectively); in the Control—GNPs, Control—Carn, Control—GNPs + Carn, and Obese—Carn groups, there was no significant difference compared to the Control—Sal group. Finally, in the hypothalamus, there was no significant difference in SOD activity between groups (Fig. 4D).
Still with regard to antioxidant enzymes, the activity of GSH was evaluated. It was observed in the prefrontal cortex significant effect of diet [F (1, 30) = 16.50; p = 0.0003] and a significant interaction between diet and intervention [F (3, 30) = 7.167; p = 0.0009]. Furthermore, in the prefrontal cortex the Obese—GNPs + Carn group demonstrated reduced levels of GSH compared to the Control—Sal group (p = 0.0251). In the hypothalamus, hippocampus, and striatum, no difference was observed between the groups (Fig. 4E).
As for the activity of the SDH enzyme, showed a significant effect of diet on the prefrontal cortex (F (3, 30) = 7.167; p = 0.0136), hippocampus (F (1, 34) = 19.94; p < 0.0001) and striatum (F (1, 34) = 46.38; p < 0.0001), and a significant effect of intervention on the prefrontal cortex (F (3, 34) = 8.245; p = 0.0003), hippocampus (F (3, 34) = 4.837; p = 0.0066) and striatum (F (3, 34) = 3.556; p = 0.0243), as well as a significant interaction between diet and intervention in the striatum (F (3, 34) = 3.969; p = 0.0158). It was observed that in the prefrontal cortex, there was an increase in its activity in the Control—GNPs (p = 0.0205), Control—Carn (p = 0.0005), Control—GNPs + Carn (p = 0.0482), Obese—Sal (p = 0.0266), Obese—GNPs (p = 0.0083), Obese—Carn (p = 0.0016), and Obese—GNPs + Carn (p = 0.0002) groups in relation to the Control—Sal group. In the hippocampus, the Obese—Sal and Obese—Carn groups showed less SDH activity in relation to the Control—Sal group (p = 0.0171 and 0.0193, respectively); the other groups did not show significant difference in relation to the Control—Sal group. Regarding the striatum, the Obese—Sal group showed increased activity of this enzyme compared to the Control—Sal group (p = 0.0264), while the Obese—GNPs + Carn group showed greater SDH activity compared to the Control—Sal (p < 0.0001) and Obese—Sal (p = 0.0277) groups; the Control—GNPs, Control—Carn, Control—GNPs + Carn, Obese + GNPs, and Obese + Carn groups did not show a significant difference compared to the Control—Sal group. In the hypothalamus, there was no significant difference in SDH activity between the groups (Fig. 5A).
Analysis of energy metabolism in brain structures of mice submitted to a high-fat diet and treated with GNPs, carnitine, or combination of GNPs and carnitine. Succinate dehydrogenase activity (A), complex I activity (B), complex II activity (C), complex IV activity (D). *Significant difference compared to the control + saline (Control—Sal) group; #significant difference compared to the obese + saline (Obese—Sal) group. Data were represented as mean ± SEM, p < 0.05 (two-way ANOVA followed by Tukey’s post hoc test)
The mitochondrial respiratory chain complexes were examined. Initially, complex I was evaluated, where the two-way ANOVA analysis demonstrated significant effect of diet on the hippocampus (F (1, 33) = 13.65; p = 0.0008) and striatum (F (1, 31) = 40.21; p < 0.0001), effect of intervention on the prefrontal cortex (F (3, 31) = 7.721; p = 0.0005), in the hippocampus (F (3, 33) = 16.88; p < 0.0001) and striatum (F (3, 31) = 8.669; p = 0.0003), and significant interaction between diet and intervention in the prefrontal cortex (F (3, 31) = 5.973; p = 0.0025), hippocampus (F (3, 33) = 6.946; p = 0.0009), and striatum (F (3, 31) = 6.906; p = 0.0011). The post hoc test showed that complex I reduced activity in the prefrontal cortex in the Control—GNPs (p = 0.0009), Control—Carn (p = 0.0020), Control—GNPs + Carn (p = 0.0006), Obese—Sal (p = 0.0479), and Obese—Carn (p = 0.0088) groups in relation to the Control—Sal group, and the Obese—GNPs and Obese—GNPs + Carn groups were no different from the Control—Sal group. In the hippocampus, complex I activity was increased in the Control—GNPs (p = 0.0252), Control—Carn (p = 0.0002), and Obese—GNPs (p = 0.0490) groups in relation to the Control—Sal group, with no difference in the Control—GNPs + Carn, Obese—Sal, Obese—Carn and Obese—GNPs + Carn groups in relation to the Control—Sal group. In the striatum, the activity of complex I increased in the Obese—Sal (p = 0.0116), Obese—GNPs (p < 0.0001), Obese—Carn (p = 0,0005) groups when compared to the Control—Sal group; on the other hand, the Obese—GNPs + Carn group showed reduced activity of this complex in relation to the Obese—Sal group (p = 0.0042); the Control—GNPs, Control—Carn and Control—GNPs + Carn groups did not significantly differ from the Control—Sal group. In the hypothalamus, no significant difference in complex I activity between the groups was observed (Fig. 5B).
In the same way, complex II was analyzed and, showed a significant effect of diet (F (1, 34) = 65.81; p < 0.0001) and intervention (F (3, 34) = 13.99; p < 0.0001) on the prefrontal cortex, and a significant effect of diet (F (1, 33) = 55.88; p < 0.0001) on the striatum. The post hoc test showed that complex II in the prefrontal cortex, the groups Control—Carn (p = 0.0056), Control—GNPs + Carn (p = 0.0008), Obese—Sal (p < 0.0001), Obese—GNPs (p ≤ 0.0001) and Obese—Carn (p ≤ 0.0001) presented increased activity of this complex when compared to the Control—Sal group, whereas the Obese—GNPs + Carn group presented increased activity of the complex I in relation to the Control—Sal (p < 0.0001) and Obese—Sal (p = 0.0049) groups; the Control—GNPs group showed no significant difference in relation to the Control—Sal group. In the striatum, the Obese—Sal (p = 0.0033) and Obese- GNPs (p = 0.0080) groups showed greater complex I activity compared to the Control—Sal group, while the Control—GNPs, Control—Carn, Control—GNPs + Carn, Obese—Carn and Obese—GNPs + Carn groups were not significantly different from the Control—Sal group. In the hypothalamus and hippocampus, there was no significant difference in complex II activity between the groups (Fig. 5C).
Finally, the activity of the complex IV was evaluated, it was observed a significant effect of the diet on the hippocampus (F (1, 27) = 30.00; p < 0.0001), and intervention on the prefrontal cortex (F (3, 26) = 3.611; p = 0.0265), hippocampus (F (3, 27) = 4.428; p = 0.0118) and striatum (F (3, 30) = 11.12; p < 0.0001), as well as interaction between diet and intervention on the prefrontal cortex (F (3, 26) = 3.269; p = 0.0371) and striatum (F (3, 30) = 4.481; p = 0.0103). The activity of complex IV in the prefrontal cortex the Control—Carn (p = 0.0240), Obese—Sal (p = 0.0098), Obese—Carn (p = 0.0012) and Obese—GNPs + Carn (p = 0.0436) groups showed less activity when compared to the Control—Sal group, the Control—GNPs, Control—GNPs + Carn and Obese—GNPs groups did not show any evidence of being different from the Control—Sal group. In the hippocampus, the Obese—Sal and Obese—Carn groups showed reduced activity in relation to the Control—Sal group (p = 0.0062 and 0.0083, respectively); the other groups were not significantly different from the Control—Sal group. In the striatum only the Control—GNPs + Carn group showed reduced activity compared to the Control—Sal group (p = 0.0130). In the hypothalamus, there was no significant difference in complex II activity between the groups (Fig. 5D).
Discussion
In the present study, an animal model of obesity was used involving the consumption of a high-fat diet, since this model has considerable validity in human obesity [43]. Therefore, to evaluate the development of obesity, the body weight of the animals was monitored throughout the experiment; it was observed that the animals in the groups that received a high-fat diet had higher body weight, compared to the group that received a standard diet, from the first week of obesity induction. These data corroborate the study by Schraiber et al. [15], where Swiss mice fed with a high-fat diet for 10 weeks showed greater body weight gain. On the other hand, the 28-day treatment with GNPs, carnitine, or with the combination of both, was not capable of reversing the weight gain. In line with these findings, another study carried out in our laboratory, with the administration of GNPs for 14 days (70 µg/kg/day), was also unable to reverse the body weight gain and the accumulation of mesenteric fat caused by a high-fat diet [21].
The weight of mesenteric fat was also assessed in the present study, in order to verify the accumulation of fat generated by the consumption of a high-fat diet, given that mesenteric fat is widely considered to be the most analogous to human intra-abdominal adipose tissue, both in its location and in its biology [44]. Therefore, these findings demonstrated that the consumption of a high-fat diet led to an increase in mesenteric fat, as already demonstrated in previous studies [15, 45]. On the other hand, obese animals that were administered carnitine, associated or not with GNPs, showed a reduction in mesenteric fat, in relation to the obese group without treatment. This finding can be explained by the fact that carnitine’s main function is to transport the fatty acids present in the adipose tissue into the mitochondria, thus causing their oxidation [27].
Although the reduction in mesenteric fat is not enough to be equivalent to the control group, it is known that even modest weight losses are associated with health benefits and quality of life [46]. When above 5%, weight loss has significant effects on metabolic markers such as HDL cholesterol [47]. Weight losses above 10% have important effects on steatohepatitis [48], and a 21% reduction in the primary outcome of cardiovascular events [49]. A loss of 11% in weight was associated with a reduction of almost 23% in intra-abdominal adipose tissue [50]. Furthermore, it is known that high-fat diet consumption affects the CNS even before leading to obesity. In fact, a study by Carraro et al. [51] demonstrated that inflammation is the earliest mechanism activated in the hypothalamus after the introduction of high-fat diet, generating damage after 3, 6 and 24 h of diet consumption. In the present study, did not demonstrate a reverse of mesenteric fat accumulation due to the treatment with GNPs alone; however, regardless of the reduction of adipose tissue, the action of GNPs can still be seen at the intracellular level in the CNS.
Studies demonstrate metabolic effects of consuming a high-fat diet on the mesenteric adipose tissue of animals, showing changes in oxidative stress parameters with an increase in malondialdehyde equivalents and protein carbonylation, and changes in energy metabolism with changes in Krebs cycle enzymes [52], in addition to showing increased inflammation in this tissue [53]. It is known that the metabolic dysfunctions induced by the consumption of a high-fat diet cause chronic low-grade systemic inflammation that affects the entire body, including the CNS [54]. And, all pro-inflammatory cytokine signaling resulting from obesity can stimulate the production of ROS by macrophages and monocytes [55]. In fact, in the present study, we can see higher levels of DCF resulting from the consumption of a high-fat diet in the hippocampus, with reversal after treatment with separate GNPs and carnitine. This finding corroborates the study by Prá et al. [21], where obesity induced a greater production of DCF in brain structures, including the hippocampus, and the treatment with GNPs proved to be effective, reversing this increase of DCF. The mechanism by which GNPs act in the formation of ROS has been demonstrated in some studies, either because it is considered an antioxidant agent, inhibiting ROS formation and scavenging free radicals [56], or by exhibiting mimetic activity to antioxidant enzymes, interacting directly with O2·-, and hydroxyl radicals (OH·) to form fewer reactive by-products [57]. The action of carnitine in reducing ROS corroborates the study by Lee et al. [58], where they demonstrate that carnitine was able to suppress the generation of ROS in renal tubular cells treated with perfluorooctanesulfonate.
In obesity, besides inflammation leading to increased production of ROS, mitochondrial overload also occurs due to the high electrons availability. When the rate of electron transfer along the respiratory chain is not coordinated, these electrons may escape from the complexes, resulting in increased production of ROS [59]. However, the exacerbation of reactive nitrogen species (RNS) in obesity condition is not clearly elucidated in the literature. In this study, the levels of nitrite, which is a by-product generated when NO is produced, were not altered in brain structures, suggesting that, in our study, obesity led to greater production of ROS, but not RNS. One hypothesis would be RNS are secondary metabolites of NO oxidation, and their presence is associated with their overproduction by inducible nitric oxide synthase (iNOS) or uncoupled by endothelial nitric oxide synthase (eNOS) [60]. In obesity, NO bioavailability is decreased in animal models [61] and humans [62]. However, it is also seen that the level of endogenously formed peroxynitrite (ONOO −) increases in adipose tissue mainly as a result of hyperglycemia. Once formed, ONOO − triggers a vicious cycle, further decreasing NO bioavailability and increasing nitro-oxidative stress [63].
With the greater production of ROS, there is a greater tendency to cause further damage to proteins, since these molecules seek to stabilize, leading to their oxidation, with consequent loss of their biological function [64]. In our study, to assess protein damage, we evaluated their carbonylation. Protein carbonylation is considered one of the most harmful irreversible oxidative protein modifications, and one of the main hallmarks of disorders associated with oxidative stress [65]. In this connection, it was noticed that in the hippocampus, obesity led to greater protein damage, which was significantly reversed with GNPs and carnitine separate treatment; on the other hand, regarding the group treated with GNPs and carnitine together, although we did not find a significant difference in relation to the obesity group, this group was not different from the control group, suggesting that the associated drugs treatment group is in a harm reduction process. Corroborating these findings, a study by Prá et al. [21] demonstrated that obesity led to greater protein carbonylation in brain structures that were reversed after 14-day treatment with GNPs. Since in our study we verified damage only in the hippocampus, it is suggested that this may be a response to the physiological adaptation of the animal considering 28 days of administration of the treatments, and when the condition is considered dangerous for the animal homeostasis, mechanisms are activated to control or cope with the situation [66].
To combat ROS, an active antioxidant system is necessary; such system has the SOD enzyme that reduces O2· to hydrogen peroxide (H2O2), then it requires the enzyme catalase (CAT) to reduce H2O2 to water, together with the enzyme glutathione peroxidase (GPx), which, in order to perform its function, requires adequate levels of GSH [67]. In our study, when assessing SOD levels, it was noticed that obesity led to an increase in the activity of this enzyme in the striatum, with a significant reversal in the groups that received GNPs associated or not with carnitine, and a tendency to decrease in the group that received carnitine alone, since it did not prove to be different compared to the control. It is known that the striatum regulates reward and motivation processes, and ends up being a region affected by obesity. Dopaminergic dysfunction and other neural signals in this brain structure have been considered some of the main contributors to high food intake and the development of obesity [16]. Therefore, it is suggested that this increase in SOD activity in the striatum may be due to some damage caused by obesity, which we did not address in the present study.
Corroborating with these findings, in a study by Prá et al. [21], obesity caused an increase in SOD expression that was reversed after the treatment with GNPs in the hypothalamus, prefrontal cortex, and striatum structures, but not in the hippocampus. In fact, in our study, no SOD increase was found in the hippocampus, as well as the levels of GSH were not altered in the structures. As GSH levels were not altered, it can be assumed that GPx activity is not altered either. Justifying, or even further supporting oxidative damage seen in the hippocampus, due to the lack of an efficient antioxidant defense by both SOD and GPx.
The increase in ROS can lead to damage to the mitochondria, which are responsible for energy generation through the Krebs cycle and the mitochondrial respiratory chain, which are the main areas of ATP generation. In the Krebs cycle, the enzyme SDH stands out, as it connects the cycle to the mitochondrial respiratory chain [68]. In our study, a reduction of SDH in the hippocampus could be observed in the obese group, with a tendency to reversal in the groups that received GNPs associated or not with carnitine, since these are not different from the control. In fact, in the hippocampus there was a greater production of ROS and greater damage to proteins; this damage could be reaching the SDH enzyme and compromising its function in the hippocampus. In the striatum, there was an increase in SDH activity in the obese group, with a tendency to reversal in the groups receiving GNPs or carnitine. Thus, it is assumed that although we did not verify oxidative damage in the striatum, this does not waive the need for greater energy production in this structure, due to potential inflammatory damage. Therefore, SDH dysfunction could impair mitochondrial activity, causing fewer electrons to become available for complexes I and II of the mitochondrial respiratory chain, yielding a lower generation of ATP [69].
With regard to complex I, we observed a reduction in activity in the prefrontal cortex in the groups, including the obese group. This result corroborates a previous study, where obesity has been shown to inhibit complex I in the prefrontal cortex [45]. GNP treatment for 28 days showed a tendency to reverse the inhibition of complex I caused by obesity, since the groups that receive GNPs did not present a significant difference in relation to the control, unlike the study by Prá et al. [21], where treatment with GNPs for 14 days was not able to restore complex I inhibition caused by obesity. When there is an inhibition of complex I, fewer electrons are transferred to complex III, and this reduction ends up favoring the escape of electrons and, consequently, increasing the generation of ROS [59]. Therefore, it is suggested that there may be a greater production of ROS in the prefrontal cortex, yet insufficient to be detected in our assessment. As for the striatum, greater activity of complex I can be seen in the groups that received a high-fat diet, with reversal in the group that received GNPs associated with carnitine. It is noteworthy that, the activity of SDH also increased in the striatum in an attempt to compensate ATP production and, consequently, increasing the generation of electrons, increasing the activity of complex I.
Like complex I, complex II is also a gateway for electrons from the Krebs cycle [70]. In our study, we could see an increase in complex II activity in the prefrontal cortex, and this could be an attempt to compensate for the entry of electrons into the chain, since complex I in this region was inhibited. With regard to the striatum, complex II showed greater activity in the obese group, with a tendency to reversal in the groups that received carnitine with or without GNPs, since they were not different from the control group. The increased activity of complex II in the striatum is in line with the increased activity of complex I and the enzyme SDH, demonstrating a greater need for ATP production, possibly due to a greater flow of electrons resulting from excessive fat consumption. Furthermore, with the increase of SOD in this structure, it is understood that there is an attempt to recover a damaged system; such damage may have been caused by lipid peroxidation. In fact, previous studies demonstrate lipid peroxidation in brain structures due to the consumption of a high-fat diet [45].
From complexes I and II, electrons are transported to ubiquinone; once in ubiquinone, the electrons are transferred to complex III, then they move to cytochrome c, which is in charge of transporting individual electrons from complex III to complex IV [68]. Complex IV catalyzes the final stage in the mitochondrial electron transfer chain and is considered one of the main regulatory sites of oxidative phosphorylation [71]. In our study, when evaluating complex IV activity, it was noticed that activity was inhibited in the prefrontal cortex by obesity, except in the group that received treatment with GNPs alone, assuming that complex II was unable to compensate for the low activity of complex I, because at the end of oxidative phosphorylation, this inhibition occurred. Corroborating these findings, a study by Mello et al. [45] demonstrated that consumption of a high-fat diet for 10 weeks caused inhibition of complex IV in the prefrontal cortex. Finally, there was inhibition of complex IV activity in the hippocampus caused by obesity, but this inhibition was not found in the groups treated with GNPs. Although it was not possible to observe damage to complexes I and IV, the complex IV fire electrons to oxygen, since oxygen has reduced activity; this firing of electrons does not occur in a fluid way, increasing the generation of ROS [72], justifying the increase in DCF and carbonyl proteins in the hippocampus.
The hippocampus is a brain structure associated to mood regulation, learning, and memory [73]. It has already been shown that obesity and a high-fat diet can lead to cognitive impairment, given that chronically inflamed adipose tissue causes the secretion of pro-inflammatory cytokines, which can lead to hippocampal neuroinflammation, impairing neuroplasticity in obese conditions. and diabetes [74]. Thus, both peripheral and central insulin resistance are associated with cognitive impairment and the development of neurodegenerative diseases [75]. In our study, we demonstrated that the structure most affected by the consumption of a high-fat diet was the hippocampus, corroborating the fact that obesity leads to cognitive impairment.
Regarding the treatments, a more marked effect of GNPs can be observed at the CNS level, mainly in the mitochondrial function. In a previous study, it was shown that GNPs catalyzes NADH oxidation to NAD+, which is one of the most important coenzymes in all cell types; this effect is occurring on the surface of GNPs, that is, NADH is catalyzed on the surface by GNPs [76]. This effect is potentially positive because complex I in the mitochondrial electron transport chain oxidizes NADH to NAD + , and is the main site where ROS production occurs [77]. In fact, we verified a reduction of ROS after the treatment with GNPs. Thus, modulation by GNPs of complex I could be a target for these molecules to prevent oxidative stress. On the other hand, the effect of carnitine was observed in the reduction of ROS production and protein damage, corroborating with the study by Mescka et al. [78] where treatment with carnitine was effective in improving the activity of antioxidant enzymes, and reducing lipid and protein damage, as well as the production of reactive oxygen/nitrogen species. However, although carnitine has an effect at the CNS level, its main action took place at the peripheral level, demonstrating to reduce the accumulation of fat generated by obesity.
Although we found a more marked effect of GNPs at the CNS level, when associated with carnitine, GNPs did not lose this potential effect. However, in the accumulation of mesenteric fat GNPs did not prove to be effective. On the other hand, carnitine showed its greatest effect, acting in the reduction of adipose tissue, but alone it was unable to reverse the inflammatory process installed in the tissue, which continued to damage the CNS, mainly in the hippocampus. It is noteworthy that these beneficial effects were observed even in the continuation of the high-fat diet. Therefore, more evident effects could be observed with dietary treatment associated with therapeutic alternatives, in order to be able to effectively reestablish body homeostatic control. Hence, it is suggested that the combination of GNPs with carnitine may be a promising alternative for the treatment of obesity, provided it is in conjunction with the patient’s dietary control.
The toxicity of GNPs was not evaluated in this study; however, in a previous study carried out in our laboratory, intraperitoneal administration of GNPs (18 nm) for 14 days, 70 µg/kg/day, did not lead to liver and kidney damage [21]. However, it should be noted that this study has some limitations. Firstly, we did not evaluate metabolic and inflammatory changes in the animals' adipose tissue to evaluate the effect of treatment on this tissue. Furthermore, the study was carried out only with male Swiss mice, limiting its applicability to both sexes. Finally, the treatment was carried out for just 4 weeks and continued with a diet rich in saturated fat, and it is known that treatments aimed at weight loss are generally longer and involve a change in eating pattern. The results of the present study are summarized in Fig. 6.
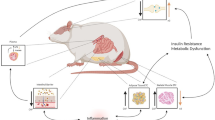
Summary of the main results found in the study. Obesity led to greater accumulation of mesenteric fat, oxidative stress in the hippocampus, and mitochondrial dysfunction in the prefrontal cortex, hippocampus, and striatum. Treatment with GNPs isolated reduced oxidative stress in the hippocampus. Carnitine isolated decreased the accumulation of mesenteric fat and oxidative stress in the hippocampus. The combination of treatments reduced the accumulation of mesenteric fat and mitochondrial dysfunction in the striatum
Conclusion
Based on this study, we can conclude that obesity led to oxidative damage and mitochondrial dysfunction in the CNS, mainly in the prefrontal cortex, striatum, and hippocampus. Treatment with GNPs demonstrated a possible beneficial effect in reducing these damages, as the result of its administration resulted, for the most part, in the equality of results compared to the control group. On the other hand, the treatment with carnitine demonstrated more efficacy peripherally, reducing the accumulation of adipose tissue. Therefore, these treatments in isolation become a promising option for the treatment of obesity. However, it is worth mentioning that this type of strategy is a complement to an adequate diet, physical exercise, and prevention of nutritional deficiencies that can contribute to energy balance and healthy body homeostasis. Thus, more studies are needed to elucidate the other mechanisms and potential side effects from the treatment with GNPs associated with carnitine.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M (2007) Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 120:S3–S8. https://doi.org/10.1016/j.amjmed.2006.11.012
World Health Organization (2016). Obesity and overweight—fact https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 15 Nov 2022
Aaseth J, Ellefsen S, Alehagen U, Sundfør TM, Alexander J (2021) Diets and drugs for weight loss and health in obesity – an update. Biomed Pharmacother 140:111789. https://doi.org/10.1016/j.biopha.2021.111789
Horwitz A, Birk R (2023) Adipose tissue hyperplasia and hypertrophy in common and syndromic obesity—the case of BBS obesity. Nutrients 15:3445. https://doi.org/10.3390/NU15153445
Hammarstedt A, Gogg S, Hedjazifar S, Nerstedt A, Smith U (2018) Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol Rev 98:1911–1941. https://doi.org/10.1152/PHYSREV.00034.2017
Wang QA, Tao C, Gupta RK, Scherer PE (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19:1338. https://doi.org/10.1038/NM.3324
Michailidou Z (2019) Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr Opin Physiol 12:39–43. https://doi.org/10.1016/j.cophys.2019.09.005
Russo L, Lumeng CN (2018) Properties and functions of adipose tissue macrophages in obesity. Immunology 155:407–417. https://doi.org/10.1111/IMM.13002
Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, Tobe K (2013) Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 56:1403–1412. https://doi.org/10.1007/s00125-013-2885-1
Gómez-Apo E, Mondragón-Maya A, Ferrari-Díaz M, Silva-Pereyra J (2021) Structural brain changes associated with overweight and obesity. J Obes 2021:6613385. https://doi.org/10.1155/2021/6613385
Van Dyken P, Lacoste B (2018) Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Front Neurosci 12:930. https://doi.org/10.3389/fnins.2018.00930
O’Brien PD, Hinder LM, Callaghan BC, Feldman EL (2017) Neurological consequences of obesity. Lancet Neurol 16:465–477. https://doi.org/10.1016/S1474-4422(17)30084-4
Guillemot-Legris O, Muccioli GG (2017) Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci 40:237–253. https://doi.org/10.1016/j.tins.2017.02.005
Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T (2014) Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci 16:378–400. https://doi.org/10.3390/ijms16010378
de Bona SR, de Mello AH, Garcez ML, de Bem SG, Zacaron RP, de Souza Goldim MP, Budni J, Silveira PCL, Petronilho F, Ferreira GK, Rezin GT (2019) Diet-induced obesity causes hypothalamic neurochemistry alterations in Swiss mice. Metab Brain Dis 34:565–573. https://doi.org/10.1007/s11011-018-0337-9
Mullins CA, Gannaban RB, Khan MS, Shah H, Siddik MAB, Hegde VK, Reddy PH, Shin AC (2020) Neural underpinnings of obesity: the role of oxidative stress and inflammation in the brain. Antioxidants 9:1018. https://doi.org/10.3390/antiox9101018
Wadden TA, Tronieri JS, Butryn ML (2020) Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol 75:235–251. https://doi.org/10.1037/AMP0000517
Krentz AJ, Fujioka K, Hompesch M (2016) Evolution of pharmacological obesity treatments: focus on adverse side-effect profiles. Diabetes Obes Metab 18:558–570. https://doi.org/10.1111/DOM.12657
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 16:71. https://doi.org/10.1186/s12951-018-0392-8
Fujita T, Zysman M, Elgrabli D, Murayama T, Haruta M, Lanone S, Ishida T, Boczkowski J (2021) Anti-inflammatory effect of gold nanoparticles supported on metal oxides. Sci Rep 11:23129. https://doi.org/10.1038/s41598-021-02419-4
Prá M, Ferreira GK, de Mello AH, Uberti MF, Engel NA, Costa AB, Zepon KM, Francisco GG, Hlavac NRC, Terra SR, Garcez ML, Zaccaron RP, Mendes C, Tschoeke ACP, Kanis LA, Budni J, Silveira PCL, Petronilho F, da Silva Paula MM, Rezin GT (2021) Treatment with isolated gold nanoparticles reverses brain damage caused by obesity. Mater Sci Eng, C 120:111392. https://doi.org/10.1016/J.MSEC.2020.111392
Sharma VK, Prateeksha GSC, Singh BN, Rao CV, Barik SK (2022) Cinnamomum verum-derived bioactives-functionalized gold nanoparticles for prevention of obesity through gut microbiota reshaping. Mater Today Bio 13:100204. https://doi.org/10.1016/J.MTBIO.2022.100204
Gao L, Hu Y, Hu D, Li Y, Yang S, Dong X, Alharbi SA, Liu H (2020) Anti-obesity activity of gold nanoparticles synthesized from Salacia chinensis modulates the biochemical alterations in high-fat diet-induced obese rat model via AMPK signaling pathway. Arab J Chem 13:6589–6597. https://doi.org/10.1016/J.ARABJC.2020.06.015
Sela H, Cohen H, Elia P, Zach R, Karpas Z, Zeiri Y (2015) Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J Nanobiotechnology 13:1–9. https://doi.org/10.1186/S12951-015-0133-1/FIGURES/3
Abel J, Silva MRD, Costa AB, Oliveira MP, Silva LED, Dela Vedova LM, Mendes TF, Tartari G, Possato JC, Ferreira GK, Machado de Avila RA, Rezin GT (2023) Therapeutic effects of the gold nanoparticle on obesity-triggered neuroinflammation: a review. J Drug Target 31:134–141. https://doi.org/10.1080/1061186X.2022.2120613
Ghosh P, Han G, De M, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60:1307–1315. https://doi.org/10.1016/J.ADDR.2008.03.016
Bremer J (1983) Carnitine–metabolism and functions. Physiol Rev 63:1420–1480. https://doi.org/10.1152/PHYSREV.1983.63.4.1420
Sawicka AK, Renzi G, Olek RA (2020) The bright and the dark sides of L-carnitine supplementation: a systematic review. J Int Soc Sports Nutr 17:49. https://doi.org/10.1186/S12970-020-00377-2
de Farias BX, Costa AB, Engel NA, de Souza Goldim MP, da Rosa TC, Cargnin-Cavalho A, Fortunato JJ, Petronilho F, Jeremias IC, Rezin GT (2020) Donepezil Prevents Inhibition of Cerebral Energetic Metabolism Without Altering Behavioral Parameters in Animal Model of Obesity. Neurochem Res 45:2487–2498. https://doi.org/10.1007/s11064-020-03107-x
Bauomy AA (2020) Zinc oxide nanoparticles and L-carnitine effects on neuro-schistosomiasis mansoni induced in mice. Environ Sci Pollut Res Int 27:18699–18707. https://doi.org/10.1007/S11356-020-08356-5
Chorilli M, Michelin DC, Salgado HRN (2007) Animais de laboratório: o camundongo. Rev Ciênc Farm Básica Apl 28:11–23
Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA (2012) Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 7:e30571. https://doi.org/10.1371/journal.pone.0030571
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75. https://doi.org/10.1039/DF9511100055
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231. https://doi.org/10.1021/TX00026A012
Chae SY, Lee M, Kim SW, Bae YH (2004) Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials 25:843–850. https://doi.org/10.1016/S0142-9612(03)00605-7
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Levine RL, Williams JA, Stadtman EP, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol Methods Enzymol 233:346–357. https://doi.org/10.1016/s0076-6879(94)33040-9
Bannister JV, Calabrese L (2006) Assays for superoxide dismutase In: Methods of biochemical analysis. Methods Biochem Anal 32:279–312. https://doi.org/10.1002/9780470110539.ch5
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226. https://doi.org/10.1016/0003-2697(76)90326-2
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36. https://doi.org/10.1016/0009-8981(85)90135-4
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316. https://doi.org/10.1006/ABBI.1996.0178
Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51. https://doi.org/10.1016/0009-8981(94)90055-8
Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Müller TD, Tschöp MH (2018) Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 14:140–162. https://doi.org/10.1038/nrendo.2017.161
Chusyd DE, Wang D, Huffman DM, Nagy TR (2016) Relationships between rodent white adipose fat pads and human white adipose fat depots. Front Nutr 3:10. https://doi.org/10.3389/fnut.2016.00010
de Mello AH, Schraiber RB, Goldim MPS, Garcez ML, Gomes ML, de Bem SG, Zaccaron RP, Schuck PF, Budni J, Silveira PCL, Petronilho F, Rezin GT (2019) Omega-3 fatty acids attenuate brain alterations in high-fat diet-induced obesity model. Mol Neurobiol 56:513–524. https://doi.org/10.1007/S12035-018-1097-6
Halpern B, Mancini MC, de Melo ME, Lamounier RN, Moreira RO, Carra MK, Kyle TK, Cercato C, Boguszewski CL (2022) Proposal of an obesity classification based on weight history: an official document by the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society for the Study of Obesity and Metabolic Syndrome (ABESO). Arch Endocrinol Metab 66:139–151. https://doi.org/10.20945/2359-3997000000465
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L (2011) Look AHEAD Research Group Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34:1481–1486. https://doi.org/10.2337/DC10-2415/-/DC1
Glass LM, Dickson RC, Anderson JC, Suriawinata AA, Putra J, Berk BS, Toor A (2015) Total body weight loss of ≥ 10 % is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci 60:1024–1030. https://doi.org/10.1007/S10620-014-3380-3
Group TLAS, Gregg EW (2016) Association of the magnitude of weight loss and physical fitness change on long-term CVD outcomes: the Look AHEAD study. Lancet Diabetes Endocrinol 4:913–921. https://doi.org/10.1016/S2213-8587(16)30162-0
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las FL, He S, Okunade AL, Patterson BW, Klein S (2016) Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 23:591–601. https://doi.org/10.1016/J.CMET.2016.02.005
Carraro RS, Souza GF, Solon C, Razolli DS, Chausse B, Barbizan R, Victorio SC, Velloso LA (2018) Hypothalamic mitochondrial abnormalities occur downstream of inflammation in diet-induced obesity. Mol Cell Endocrinol 460:238–245. https://doi.org/10.1016/J.MCE.2017.07.029
de Mello AH, Schraiber RB, Goldim MPS, Mathias K, Mendes C, Corrêa MEAB, Gomes ML, Silveira PCL, Schuck PF, Petronilho F, Rezin GT (2019) Omega-3 polyunsaturated fatty acids have beneficial effects on visceral fat in diet-induced obesity model. Biochem Cell Biol 97:693–701. https://doi.org/10.1139/bcb-2018-0361
Dinh CH, Szabo A, Yu Y, Camer D, Wang H, Huang XF (2015) Bardoxolone methyl prevents mesenteric fat deposition and inflammation in high-fat diet mice. Scientific World Journal 2015:549352. https://doi.org/10.1155/2015/549352
Crispino M, Trinchese G, Penna E, Cimmino F, Catapano A, Villano I, Perrone-Capano C, Mollica MP (2020) Interplay between peripheral and central inflammation in obesity-promoted disorders: the impact on synaptic mitochondrial functions. Int J Mol Sci 21:1–22. https://doi.org/10.3390/IJMS21175964
Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA (2011) Inflammation, oxidative stress, and obesity. Int J Mol Sci 12:3117–3132. https://doi.org/10.3390/ijms12053117
Barathmanikanth S, Kalishwaralal K, Sriram M, Pandian SR, Youn HS, Eom S, Gurunathan S (2010) Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnology 8:16. https://doi.org/10.1186/1477-3155-8-16
Zhou YT, He W, Wamer WG, Hu X, Wu X, Lo YM, Yin JJ (2013) Enzyme-mimetic effects of gold@platinum nanorods on the antioxidant activity of ascorbic acid. Nanoscale 5:1583–1591. https://doi.org/10.1039/c2nr33072e
Lee YG, Chou HC, Chen YT, Tung SY, Ko TL, Buyandelger B, Wen LL, Juan SH (2022) l-Carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells. Sci Rep 12:4673. https://doi.org/10.1038/s41598-022-08771-3
Muñoz A, Costa M (2013) Nutritionally mediated oxidative stress and inflammation. Oxid Med Cell Longev 2013:610950. https://doi.org/10.1155/2013/610950
Lancaster JR (2006) Nitroxidative, nitrosative, and nitrative stress: kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol 19:1160–1174. https://doi.org/10.1021/TX060061W
Bender SB, Herrick EK, Lott ND, Klabunde RE (2007) Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes Metab 9:688–696. https://doi.org/10.1111/J.1463-1326.2006.00650.X
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Chayama K, Oshima T (2001) Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am J Hypertens 14:1038–1045. https://doi.org/10.1016/S0895-7061(01)02191-4
Koeck T, Willard B, Crabb JW, Kinter M, Stuehr DJ, Aulak KS (2009) Glucose-mediated tyrosine nitration in adipocytes: targets and consequences. Free Radic Biol Med 46:884–892. https://doi.org/10.1016/J.FREERADBIOMED.2008.12.010
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19. https://doi.org/10.1097/WOX.0b013e3182439613
Fedorova M, Bollineni RC, Hoffmann R (2014) Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 33:79–97. https://doi.org/10.1002/MAS.21381
Andrade A, Pinto SC, Oliveira RS (2002) Animais de laboratório criação e experimentação. FIOCRUZ, Rio de Janeiro
Barbosa KBF, Costa NMB, de Alfenas R, CG, De Paula SO, Minim VPR, Bressan J, (2010) Estresse oxidativo: conceito, implicações e fatores modulatórios. Rev Nutr 23:629–643. https://doi.org/10.1590/S1415-52732010000400013
Nolfi-Donegan D, Braganza A, Shiva S (2020) Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol 37:101674. https://doi.org/10.1016/j.redox.2020.101674
Jodeiri Farshbaf M, Kiani-Esfahani A (2018) Succinate dehydrogenase: prospect for neurodegenerative diseases. Mitochondrion 42:77–83. https://doi.org/10.1016/J.MITO.2017.12.002
Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW (2008) Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr 40:445–456. https://doi.org/10.1007/s10863-008-9169-3
Li Y, Park J-S, Deng J-H, Bai Y (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr 38:283–291. https://doi.org/10.1007/s10863-006-9052-z
Mansilla N, Racca S, Gras DE, Gonzalez DH, Welchen E (2018) The complexity of mitochondrial complex IV: an update of cytochrome c oxidase biogenesis in plants. Int J Mol Sci 19:662. https://doi.org/10.3390/ijms19030662
Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC (2009) Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus 19:235–252. https://doi.org/10.1002/hipo.20499
Lee TH, Yau S (2020) From obesity to hippocampal neurodegeneration: pathogenesis and non-pharmacological interventions. Int J Mol Sci 22:201. https://doi.org/10.3390/ijms22010201
Ferreira LSS, Fernandes CS, Vieira MNN, De Felice FG (2018) Insulin resistance in Alzheimer’s disease. Front Neurosci 12:830. https://doi.org/10.3389/fnins.2018.00830
Huang X, El-Sayed IH, Yi X, El-Sayed MA (2005) Gold nanoparticles: catalyst for the oxidation of NADH to NAD+. J Photochem Photobiol B 81:76–83. https://doi.org/10.1016/j.jphotobiol.2005.05.010
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochemical Journal 417:1–13. https://doi.org/10.1042/BJ20081386
Mescka CP, Rosa AP, Schirmbeck G, da Rosa TH, Catarino F, de Souza LO, Guerreiro G, Sitta A, Vargas CR, Dutra-Filho CS (2016) L-carnitine prevents oxidative stress in the brains of rats subjected to a chemically induced chronic model of MSUD. Mol Neurobiol 53:6007–6017. https://doi.org/10.1007/S12035-015-9500-Z/FIGURES/3
Acknowledgements
We are grateful to the Instituto Anima de Educação, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ, National Council for Scientific and Technological Development), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Coordination for the Improvement of Higher Education Personnel), and the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC, Foundation for Research and Innovation Support of the State of Santa Catarina).
Author information
Authors and Affiliations
Contributions
Larissa Espindola da Silva, Jessica da Silva Abel, and Gislaine Tezza Rezin conceived and designed the experiments. Larissa Espindola da Silva, Jessica da Silva Abel, Gisele Tartari, Mariella Reinol da Silva, Mariana Pacheco de Oliveira, Larissa Marques Dela Vedova, Talita Farias Mendes, Rayane Luiz Mendes, Hevylin Jacintho Soares, and Camila Nandi Vernke performed the animal experiments. Rubya Pereira Zaccaron, Isabela da Silva Lemos, and Mariana Pacheco de Oliveira made the biochemical analyses. Fabricia Petronilho, Paulo Cesar Lock Silveira, Emilio Luiz Streck, Ricardo Andrez Machado de Ávila, and Gislaine Tezza Rezin contributed with reagents, materials, and analysis tools. Larissa Espindola da Silva, Gislaine Tezza Rezin, and Aline Haas de Mello analyzed the data. Larissa Espindola da Silva wrote the manuscript. Gislaine Tezza Rezin and Aline Haas de Mello revised the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This research project was submitted to the evaluation of UNISUL’s Comissão de Ética no Uso de Animais (CEUA, Ethics Committee on Animal Use) and approved under protocol 21.008.4.01.IV. The use of animals followed the Principles of Laboratory Animal Care (Principles of Laboratory Animal Care, National Institute of Health of the United States of America, NIH, publication number 80–23, revised in 1996), as well as the Diretriz Brasileira para o Cuidado e a Utilização de Animais para Fins Científicos e Didáticos (DBCA, Brazilian Guideline for the Care and Use of Animals for Scientific and Didactic Purposes), published in 2013 by the Conselho Nacional de Controle de Experimentação Animal (CONCEA, National Council for the Control of Animal Experiments), and complied with the arrive guidelines.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, L.E., Abel, J.S., Tartari, G. et al. Combination of Gold Nanoparticles with Carnitine Attenuates Brain Damage in an Obesity Animal Model. Mol Neurobiol 61, 6366–6382 (2024). https://doi.org/10.1007/s12035-024-03984-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-024-03984-1