Abstract
Rationale and objectives
Cannabinoid receptor 2 (CB2R) signaling in the brain is associated with the pathophysiology of depression. Sickness behavior, characterized by lessened mobility, social interaction, and depressive behavior, is linked with neuroinflammation, oxidative stress, and immune system. The present study was aimed at evaluating 1-phenylisatin (PI), a CB2R agonist, in sickness behavior.
Methods
Influence of acute and 7-day activation of CB2R using PI in lipopolysaccharide (LPS)-induced sickness behavior was assessed in mice. An acute injection of LPS (1.5 mg/kg) produced a fully developed sickness behavior in animals within 1 h of administration. The behavioral paradigm was assessed by open field test, forced swim test, and tail suspension test. Further, tumor necrosis factor-α (TNF-α), antioxidant enzymes, and lipid peroxidation were measured in the brain to correlate neuroinflammation and oxidative stress with sickness behavior. Both treatments, PI (20 mg/kg) and imipramine (15 mg/kg), were administered orally (once for acute and once daily for 7-day protocols).
Results
LPS elevated the brain TNF-α level, augmented oxidative stress, and induced the sickness behavior in mice. Acute and 7-day treatment of mice with PI significantly reduced the LPS-induced sickness behavior. In addition, PI inhibited the neuroinflammation evidenced by a reduction in brain TNF-α and oxidative stress.
Conclusion
Our data propose that acute and long-term activation of CB2R might prevent neuroinflammation and oxidative stress-associated sickness behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endocannabinoid system acts through two receptors, cannabinoid receptors 1 and 2, i.e., CB1R and CB2R respectively (Howlett 2005; Pertwee et al. 2010). CB1Rs are G protein-coupled receptors ubiquitously distributed in brain regions like hippocampus, cortex, and basal ganglia. Interestingly, CB2R is selectively expressed in conditions of neuroinflammation (Lou et al. 2011; Benito et al. 2008). Increasing evidence points towards the importance of CB2Rs in the brain, where they are primarily located on the microglia and thereby involved in neuro-immune modulation. Though the expression of CB2Rs in the neurons has been a topic of debate, accruing proof and current research specify that CB2Rs in dopaminergic neurons are involved in the pathogenesis of psychosis, depression, and anxiety (Perdikaris et al. 2018; Liu et al. 2017).
Recently, in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) model of Parkinson’s disease, CB2R activation was shown to prevent the production of neurotoxic mediators from glial cells and infiltration of peripheral immune cells (Chung et al. 2016). In a transgenic mouse model of Alzheimer’s disease and intracerebroventricular (ICV) streptozotocin-induced dementia model, CB2R agonist has been shown to alleviate cognitive deficits (Wu et al. 2017; Jayant et al. 2016). Further, in an animal model of schizophrenia, CB2R agonist potentiated hyperactivity (Kruk-Slomka et al. 2017). Thus, in the present study, we pursued to explore the effect of CB2 agonist, 1-phenylisatin (PI), on lipopolysaccharide (LPS)-induced sickness behavior in mice.
LPS is a bacterial endotoxin primarily obtained from the gram-negative bacteria which is widely used to study the effect of systemic endotoxemia and inflammatory response on the tissue. When LPS is injected into the mice, it binds to the endogenous receptor of LPS (CD14) and activates p38MAPK resulting in the release of tumor necrosis factor-α (TNF-α) in the periphery by NF-κB signaling (Bluthe et al. 2000; Mallik et al. 2016). When the concentration of TNF-α increases in the periphery, it crosses the BBB and thus activates NF-κB signaling in the microglial cells. This results in the release of pro-inflammatory cytokines and reactive oxygen species (ROS) leading to increased oxidative stress (Varatharaj and Galea 2017; Kurosawa et al. 2016). Increased oxidative stress leads to neuroinflammation and neuronal cell death which ultimately results in sickness type behavior and depression (O’connor et al. 2009; Ren et al. 2018). We hypothesized that treatment with PI might reduce neuroinflammation, thereby ameliorates LPS-induced sickness behavior in mice.
Materials and methods
Animals
Male Swiss Albino mice (8–10 weeks, 20–30 g) were obtained from the inbred strains of Central Animal Research Facility (CARF), Manipal Academy of Higher Education, India. Institutional Animal Ethics Committee approved the experimental protocol. The experiments were performed following the CPCSEA guidelines. All the procedures were performed under controlled laboratory conditions. Animals were maintained at controlled temperature, humidity, and 12-h day and night cycle. Food and water were accessible ad libitum. Two days before the experiment, mice were handled individually daily for acclimatization.
Chemicals and reagents
All the chemicals used in the study were of analytical grade. Lipopolysaccharide (Escherichia coli 0111:B4), 2-thiobarbituric acid, l-glutathione reduced, 5,5-dithio-bis (2-nitrobenzoic acid), and 1-phenylisatin were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Carboxymethyl cellulose, sodium dihydrogenphosphate anhydrous, disodium hydrogen phosphate anhydrous, and trichloroacetic acid were obtained from Merck Millipore Corporation (Merck KGaA, Darmstadt, Germany).
Drug treatments
Single-dose pre-treatment protocol
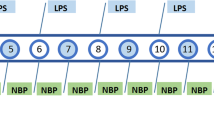
Animals were randomly assigned to four groups: group I—vehicle + saline, group II—vehicle + LPS, group III—imipramine + LPS, and group IV—PI + LPS. All the treatments were given orally (p.o.) while LPS (1.5 mg/kg) was administered intraperitoneally. Carboxy methyl cellulose (CMC; 0.25%w/v) was given as vehicle for both saline and LPS group at a dosing volume of 10 ml/kg. Imipramine + LPS group and PI + LPS group were treated with imipramine (30 mg/kg) and PI (20) mg/kg respectively 60 min before LPS challenge as described in Fig. 1. Behavioral assays were performed within 1–2 h of LPS administration and were video recorded. The recorded data was analyzed by blind observers to reduce the bias. Animals were sacrificed by cervical dislocation 3 h post LPS injections, with brain samples rapidly isolated and stored at − 20 °C until further estimations. Brains were homogenized in cold phosphate buffer (0.1 M, 7.4 pH) for antioxidant and cytokine estimations.
Seven-day pre-treatment protocol
For the 7-day pre-treatment study, animals were randomized and were assigned into four groups (n = 6) similar to single-dose pre-treatment protocol. Saline and LPS groups were administered with 0.25% w/v CMC for 7 days at a dosing volume of 10 ml/kg. LPS was administered at a dose of 1.5 mg/kg intraperitoneally on the 7th day of the treatment. Imipramine + LPS and PI + LPS were treated with imipramine (15 mg/kg) and PI (20 mg/kg) p.o. respectively for 7 days. The selection of treatment doses was based on our preliminary and published studies with supporting literature evidence. The per-se treatments within the doses used were found to be having no significant effect (Teixeira et al. 2000; Kumar et al. 2014; Jayant et al. 2016; Mallik et al. 2016). Therefore, the control groups consisting of saline + PI and saline + imipramine were not employed. On day 7, animals were treated with LPS 60 min after the treatment schedule as described in Fig. 1. Behavioral assays and sampling were as explained in the “Single-dose pre-treatment protocol” section.
Open field test
Open field test (OFT) was performed to evaluate the effect of treatment groups and LPS on locomotor activity of the animals. Locomotor activity was assessed in mice by individually placing them into a clean, novel glass arena (30 × 30 × 60 cm) at 150–200 lx. The open field arena was divided into nine virtual quadrants (10 × 10 cm each). Parameters checked were the number of crossings, center square entries, groomings, and rearings over a period of 6 min (Mallik et al. 2016).
Forced swim test
Effect of LPS and treatments on the behavioral despair of the animals was assessed by forced swim test (FST). The mice were placed individually in a square glass container (30 × 30 × 60 cm) containing water at a level of 30 cm. A mouse is thought to be immobile when it continues to float on water with minimal movement of limbs to keep the head above water. Immobility time was assessed in seconds for 6 min. Minimum effort employed by the animal to keep himself floating was taken into consideration for immobility (Porsolt et al. 1978, Mallik et al. 2016).
Tail suspension test
Briefly, mice were hung individually by placing a small piece of medical adhesive tape approximately 1–1.5 cm from the tip of the tail 30 cm above the surface. To prevent back climbing, the plastic tubing was placed around the tail earlier to applying the tape. The animal was said to be immobile when it ceased all its movement and dropped his head down. Immobility time in seconds was noted for 6 min (Mallik et al. 2016).
Estimation of brain cytokine levels
Brain TNF-α was estimated using Cusabio mouse tumor necrosis factor-α (Cusabio Biotech CO., Ltd., USA). The assay was performed as per manufacturer’s instructions. The results were interpolated from the standard curve derived from TNF-α provided by the manufacturer. The samples were diluted four times with the sample diluent while analyzing the TNF-α levels (pg/ml) in the brain. The same samples were subjected for protein estimation (mg/ml) using Bradford’s method (Bradford 1976). The final brain TNF-α levels (pg/mg of protein) were obtained by dividing the brain TNF-α levels by respective protein concentration of the samples.
Antioxidant enzyme estimation
Superoxide dismutase (SOD) was quantified in the brain homogenate according to the previously published method (Bhattacharya et al. 2000). Formation of adenochrome was measured spectrophotometrically at 480 nm and was expressed as SOD unit (U)/min/mg of protein. Amount of GSH formed was measured spectrophotometrically at 412 nm and was expressed as millimoles/mg of protein (Moron et al. 1979). Brain catalase activity was determined by measuring the rate of decomposition of H2O2 and was expressed as millimoles/min/mg of protein as described previously (Aebi 1984). Protein estimation was carried out using Bradford’s method (Bradford 1976).
Lipid peroxidation
Lipid peroxidation in the brain homogenates was quantified according to the method of Janero (1990). Malonaldehyde (MDA) was formed due to the reaction of the thiobarbituric acid and trichloroacetic acid. MDA formed was estimated spectrophotometrically at 532 nm and was expressed as micromoles/mg of protein.
Statistical analysis
Statistical analysis was performed using the software GraphPadPrism 5.01 (Graph Pad Software Inc., San Diego, CA, USA). Results are expressed as mean ± S.E.M. Experimental and control groups were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. The difference was determined to be significant if the p value was p < 0.05.
Results
Effect of PI and imipramine on LPS-induced sickness behavior in mice
Open field test
In the acute and 7-day study, exposure of single dose of LPS to mice significantly decreased the number of line crossings (16.67 ± 3.14 vs. 110.8 ± 24.33 of saline-treated group Fig. 2a, 8.5 ± 1.61 vs. 101.5 ± 8.27; Fig. 3a) in the open field arena when compared to that of the saline control group indicating significantly decreased locomotor activity. Furthermore, the center square entries (0.17 ± 0.17 vs. 5.33 ± 2.22, Fig. 2b; 0.17 ± 0.17 vs. 4.5 ± 1.54, Fig. 3b), grooming (1.50 ± 0.22 vs. 14.33 ± 1.99, Fig. 2c; 3.83 ± 0.75 vs. 17.00 ± 1.55, Fig. 3c), and rearing (27.60 ± 5.89 vs. 4.5 ± 0.56, Fig. 2d; 25.33 ± 3.02 vs. 2.33 ± 0.99, Fig. 3d) behavior were also remarkably affected. A single dose of imipramine could not reverse the effect of LPS in OFT whereas 7-day pre-treatment significantly increased the number of line crossings. Acute PI treatment significantly increased grooming (7.33 ± 0.76, F [3, 20] = 17.97, p < 0.001) and rearings (15 ± 2.24, F [3, 19] = 11.27, p < 0.001) in LPS-treated animals and prophylactic 7-day administration of PI significantly increased number of line crossings (37.50 ± 1.71, F [3, 20] = 81.51, p < 0.001), center square entry (6.33 ± 1.50, F [3, 20] = 7.16, p < 0.01), grooming (6.33 ± 1.50, F [3, 20] = 32.89, p < 0.001), and rearings (13.00 ± 1.90, F [3, 19] = 29.28, p < 0.001) compared to LPS group.
Effect of acute administration of imipramine and PI on LPS-induced changes in locomotor activity parameters. (A) Number of line crossings, (B) number of center square entries, (C) number of grooming, and (D) number of rearing in the open field test (OFT). * and # represent p < 0.05 compared with saline and lipopolysaccharide (LPS), respectively. Experimental and control groups were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test
Forced swim test
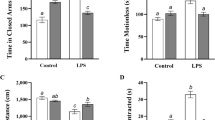
In the acute study, in FST paradigm, mice treated with LPS showed significant increase (198.6 ± 14.15 vs. 56.50 ± 6.22, Fig. 3a) in the immobility time compared to normal control, which was significantly reduced by imipramine (58.83 ± 1.62) and PI (80.83 ± 5.90, F [3, 19] = 72.14, p < 0.001), suggestive of anti-depressant activities of both the drugs (Fig. 3a). Seven days of pre-treatment with PI (95.17 ± 2.26 vs. 222.00 ± 8.88, Fig. 5a) and imipramine (52.00 ± 3.05vs. 222.00 ± 8.88, Fig. 5a) significantly reduced the effect of LPS treatment (F [3, 20] = 20.11, p < 0.001).
Effect of acute administration of imipramine and PI against LPS-induced immobility in (A) forced swim test (FST) and (B) tail suspension test (TST). * and # represent p < 0.05 compared with saline and lipopolysaccharide (LPS), respectively. Experimental and control groups were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test
Effect of chronic administration of imipramine and PI on LPS-induced changes in locomotor activity parameters. (A) The number of line crossings, (B) number of center square entries, (C) number of grooming, and (D) number of rearing in the open field test (OFT). * and # represent p < 0.05 compared with saline and lipopolysaccharide (LPS), respectively. Experimental and control groups were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test
Effect of chronic administration of imipramine and PI against LPS-induced immobility in (A) forced swim test (FST) and (B) tail suspension test (TST). * and # represent p < 0.05 compared with saline and lipopolysaccharide (LPS), respectively. Experimental and control groups were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test
Tail suspension test
In TST conducted in acute study and 7-day study, LPS administration resulted in significant increase (201.2 ± 9.46 vs. 124.2 ± 12.31, Fig. 4b; 222.0 ± 8.88 vs. 116.5 ± 12.94, Fig. 5b) in the immobility time when compared to normal control. Single-dose treatment and 7-day pre-treatment with imipramine (68.17 ± 13.15; 89.67 ± 21.19) and PI (106.80 ± 16.84; 153.70 ± 10.78, F [3, 20] = 17.9 and 16.23, respectively, p < 0.001) showed a significant decrease in immobility as compared to LPS group indicating a decrease in behavioral despair nature of animals.
Effect of PI and imipramine on antioxidant enzyme and lipid peroxidation level
In the brain homogenates, LPS administration significantly deranged the antioxidant enzymes including catalase and SOD. In both acute (236.00 ± 15.58 vs. 570.40 ± 77.26, Fig. 6a) and 7-day (200.20 ± 16.75 vs. 410.90 ± 40.53, Fig. 6a) studies, catalase levels were profoundly decreased as compared to the normal control group. Pre-treatment of the animals with imipramine significantly improved the catalase activity in both acute (472.70 ± 80.85, F [3, 16] = 5.15, p < 0.05) and 7-day (422.80 ± 28.97, F [3, 16] = 5.79, p < 0.01) groups. Interestingly, only 7-day pre-treatment with PI produced a significant increase in catalase levels (471.50 ± 71.14, F [3, 16] = 5.79, p < 0.01). Similarly, SOD levels were also significantly reduced by LPS administration (504.90 ± 178.2 vs. 2113 ± 531.80 acute; 466.5 ± 118.2 vs.1485 ± 413.60 chronic, respectively, Fig. 6b) as compared to the normal control. Acute pre-treatment of PI significantly increased the SOD (3646.00 ± 466.5, F [3, 16] = 10.01, p < 0.001) activity as compared to LPS, but no significant changes in SOD activity were observed after 7 days of PI treatment. Imipramine induced a significant increase in SOD after acute administration; however, no change was observed after 7-day treatment.
Effect of imipramine and PI on brain (A) catalase, (B) superoxide dismutase (SOD), (C) glutathione (GSH), and (D) malondialdehyde levels. * and # represent p < 0.05 compared with saline and lipopolysaccharide (LPS), respectively. Experimental and control groups were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test
In both acute and 7-day pre-treatment studies, brain GSH levels were significantly reduced (0.006 ± 0.002 vs. 0.102 ± 0.01, 0.164 ± 0.01 vs. 0.08 ± 0.01, Fig. 6c) by LPS administration. Pre-treatment of animals with both imipramine and PI significantly improved the GSH levels in both acute (0.049 ± 0.01; 0.054 ± 0.01, respectively, F [3, 20] = 21.26, p < 0.001) and 7-day treatment groups (0.046 ± 0.01; 0.051 ± 0.01, respectively, F [3, 20] = 22.95, p < 0.001) in the brain as compared to LPS-treated animals.
LPS administration significantly increased the lipid peroxidation, as observed by the MDA levels in both acute (41.05 ± 5.37 vs. 4.98 ± 0.20, Fig. 6d) and 7-day pre-treatment (13.92 ± 0.82 vs. 7.8 ± 0.32, Fig. 6d) groups. Pre-treatment of animals with both imipramine and PI significantly improved the MDA levels in both acute (16.30 ± 0.74; 22.96 ± 1.98, respectively, F [3, 20] = 27.37, p < 0.001) and 7-day treatment groups (7.55 ± 0.26; 9.21 ± 0.29, respectively, F [3, 20] = 37.68, p < 0.001) in the brain as compared to LPS-treated animals.
Effect of PI and imipramine on brain cytokine levels
LPS administration significantly increased TNF-α levels in both acute (383.40 ± 101.30 vs. 124.60 ± 62.93, Fig. 7a) and 7-day pre-treatment (95.88 ± 13.47 vs. 15.60 ± 0.00, Fig. 7b) groups. Acute administration of imipramine significantly reduced the TNF-α levels (84.91 ± 49.02, F [3, 20] = 6.56, p < 0.01); however, TNF-α levels in PI pre-treatment groups were not within the detectable limit. In 7-day pre-treatment groups, both imipramine and PI reduced the TNF-α levels below the detectable limit.
Effect of (A) acute and (B) chronic treatment of imipramine and PI against LPS-induced brain TNF-α levels. * and # represent p < 0.05 compared with saline and lipopolysaccharide (LPS), respectively. Experimental and control groups were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test
Discussion
We employed two different pre-treatment schedules of administration with, a single acute dose and a 7-day once daily regimen before challenging the animals with a single administration of LPS. To examine whether continuous activation of CB2R influences the LPS-induced neuroinflammation, a 7-day pre-treatment of PI at a dose of 20 mg/kg (same dose used in the acute study) was employed. According to the previous studies, 20 mg/kg of PI was shown to be neuroprotective in an animal model of Alzheimer’s disease (Jayant et al. 2016). Further, isatin derivatives also modulate the monoamine levels in the synapse by inhibiting monoamine oxidase (MAO) especially MAO-B and thus increase the dopamine release in the nigrostriatal pathway (Justo et al. 2016). During an acute injection of LPS, initially, animals demonstrate decreased movement, fever, and decrement in social communication termed as “sickness behavior” (Yirmiya 1996; Painsipp et al. 2011). In the present study, sickness behavior was evaluated by determining the immobility time in the forced swim test (FST) and tail suspension test (TST). The exploration of the animals in the open field test (OFT) was taken as an index of locomotor activity. A single dose of LPS produced significant behavioral despair in all the animals, as indicated by a significant increase in the immobility time in FST and TST. These results are in concordance with our previous data (Mallik et al. 2016).
In our study, acute administration of PI did not produce any significant change in the number of line crossings and center square entries. However, the exploratory behavior observed by the number of rearings and groomings were increased. On the contrary, 7-day continuous treatment with PI produced a significant increase in all the locomotor and exploratory behaviors. Interestingly, a similar observation is reported in the mice overexpressing CB2R, where the total distance traveled is not altered, though the central distance traveled was significantly increased (García-Gutierrez and Manzanares 2011). The behavioral despair was tremendously improved by both acute and 7-day pre-treatment with PI in FST and TST. This finding is also substantiated by a recent study by Liu et al. (2017), where the CB2R knockout mice showed a significant increase in immobility time in both of the tests we employed in our experiments. Effect of our positive control imipramine on behavioral despair was comparable to PI. However, the impact on locomotion and exploratory behavior was only apparent after 7-day pre-treatment. This effect of imipramine is consistent with our own and other reported studies (Yirmiya 1996; Teixiera et al. 2000; Mallik et al. 2016).
We have earlier reported that a single administration of LPS in the doses employed in this study produces a significant increase in peripheral cytokines including IL-6 and TNF-α (Mallik et al. 2016). The release of these pro-inflammatory factors leads to endothelial barrier compromise with a leaky blood-brain barrier, and activation of microglia produces long-term changes in the brain inflammatory markers (Buch 2013). Here, we focused only on the whole brain TNF-α levels and an array of oxidative stress markers as indicators of neuroinflammation to correlate the compounding effect of cytokines on the overall sickness behavior. LPS produced a significant increase in brain TNF-α levels and lipid peroxidation (MDA) and reduced the antioxidant defense (catalase, SOD and GSH levels). The factors implicated in inflammation-associated neurodegeneration including pro-inflammatory factors, excitotoxicity, oxidative stress, and impairment of the blood-brain barrier have been linked with CB2 stimulation (Buch 2013). Pre-treatment of the animals with CB2R agonist PI significantly reduced the lipid peroxidation and prevented the rapid decline in the antioxidant enzymes GSH and catalase. Interestingly, both the treatment schedules with PI produced comparable results. However, in our study, the 7-day pre-treatment protocol with both PI and imipramine failed to protect the loss in SOD levels.
In an LPS-induced interstitial cystitis mouse model, CB2R agonist reduced the expression of TNF-α by inhibiting leukocyte infiltration and myeloperoxidase activity (Tambaro et al. 2014). Recently, Liu et al. 2017 have demonstrated the involvement of CB2R in depression by specific deletion of CB2R dopaminergic neurons that lead to a significant increase in immobility time in both FST and TST paradigms. Dopamine neurons in the ventral tegmental area (VTA) are involved in the development of depressive-like behavior (Tye et al. 2013). In the mesolimbic dopamine circuitry involved in social interactions and mood regulation, dopaminergic neuronal signals from VTA of midbrain interact with nucleus accumbens (NAc). Further, several interconnecting pathways such as glutamatergic VTA-hippocampus-NAc, VTA-amygdala-NAc, prefrontal-NAc, norepinephrinergic/5-HT VTA-hypothalamus-NAc, and intrinsic GABAergic pathways interact with dopamine circuit in NAc (Nestler and Carlezon 2006). These findings corroborate that the putative mechanism of PI a CB2R agonist is attributed to the endocannabinoid CB2R signaling in specific regions of the brain. However, to establish the exact mechanism of PI in sickness behavior, expression of molecular markers associated with various pathways should be studied which is one of the major limitations of the study.
Conclusion
In summary, acute and long-term activation of CB2R by PI mitigated LPS-induced sickness behavior. The mechanisms for the neuroprotective potential may be its ability to prevent inflammation and oxidative stress through CB2R activation. The current study provides an insight into the novel strategy to explore the potential of CB2R signaling in the sickness/depressive-like behavior. Nevertheless, further studies to reveal the molecular pathways and neurotransmitter levels are warranted for the therapeutic role of PI in mental health.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Benito C, Tolon RM, Pazos MR, Nunez E, Castillo AI, Romero J (2008) Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol 153(2):277–285. https://doi.org/10.1038/sj.bjp.0707505
Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S (2000) Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res 14(3):174–179
Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P (2000) Role of interleukin-1β and tumour necrosis factor-α in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci 12(12):4447–4456. https://doi.org/10.1111/j.1460-9568.2000.01348.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buch SJ (2013) Cannabinoid receptor 2 activation: a means to prevent monocyte–endothelium engagement. Am J Pathol 183(5):1375–1377
Chung YC, Shin WH, Baek JY, Cho EJ, Baik HH, Kim SR, Won SY, Jin BK (2016) CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp Mol Med 48(1):e205. https://doi.org/10.1038/emm.2015.100
García-Gutierrez MS, Manzanares J (2011) Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol 25(1):111–120. https://doi.org/10.1177/0269881110379507
Howlett AC (2005) Cannabinoid receptor signaling. Handb Exp Pharmacol 168:53–79. https://doi.org/10.1007/3-540-26573-2_2
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9(6):515–540. https://doi.org/10.1016/0891-5849(90)90131-2
Jayant S, Sharma BM, Bansal R, Sharma B (2016) Pharmacological benefits of selective modulation of cannabinoid receptor type 2 (CB2) in experimental Alzheimer’s disease. Pharmacol Biochem Behav 140:39–50. https://doi.org/10.1016/j.pbb.2015.11.006
Justo LA, Durán R, Alfonso M, Fajardo D, Faro LR (2016) Effects and mechanism of action of isatin, a MAO inhibitor, on in vivo striatal dopamine release. Neurochem Int 99:147–157. https://doi.org/10.1016/j.neuint.2016.06.012
Kruk-Slomka M, Banaszkiewicz I, Biala G (2017) The impact of CB2 receptor ligands on the MK-801-induced hyperactivity in mice. Neurotox Res 31(3):410–420
Kumar N, Dhayabaran D, Nampoothiri M, Nandakumar K, Puratchikody A, Lalani N, Dawood K, Ghosh A (2014). Atypical antidepressant activity of 3,4-Bis (3,4-dimethoxyphenyl) furan-2,5-dione isolated from heart wood of cedrus deodara, in rodents. The Korean Journal of Physiology & Pharmacology 18(5):365–369
Kurosawa N, Shimizu K, Seki K (2016) The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1β-induced elevated leptin levels in mice. Psychopharmacol 233(9):1725–1737. https://doi.org/10.1007/s00213-015-4084-x
Liu QR, Canseco-Alba A, Zhang HY, Tagliaferro P, Chung M, Dennis E, Sanabria B, Schanz N, Escosteguy-Neto JC, Ishiguro H, Lin Z (2017) Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci Rep 7(1):17410. https://doi.org/10.1038/s41598-017-17796-y
Lou ZY, Chen C, He Q, Zhao CB, Xiao BG (2011) Targeting CB2 receptor as a neuroinflammatory modulator in experimental autoimmune encephalomyelitis. Mol Immunol 49(3):453–461. https://doi.org/10.1016/j.molimm.2011.09.016
Mallik SB, Mudgal J, Nampoothiri M, Hall S, Anoopkumar-Dukie S, Grant G, Rao CM, Arora D (2016) Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci Lett 632:218–223. https://doi.org/10.1016/j.neulet.2016.08.044
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582(1):67–78. https://doi.org/10.1016/0304-4165(79)90289-7
Nestler EJ, Carlezon WA (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59(12):1151–1159. https://doi.org/10.1016/j.biopsych.2005.09.018
O’connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R (2009) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Mol Psychiatry 14(5):511–522. https://doi.org/10.1038/sj.mp.4002148
Painsipp E, Kofer MJ, Sinner F, Holzer P (2011) Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS One 6(6):e20719. https://doi.org/10.1371/journal.pone.0020719
Perdikaris P, Tsarouchi M, Fanarioti E, Natsaridis E, Mitsacos A, Giompres P (2018) Long lasting effects of chronic WIN55, 212-2 treatment on mesostriatal dopaminergic and cannabinoid systems in the rat brain. Neuropharmacol 129:1–5. https://doi.org/10.1016/j.neuropharm.2017.11.005
Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631. https://doi.org/10.1124/pr.110.003004
Porsolt RD, Bertin A, Jalfre M (1978) “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51(3):291–294. https://doi.org/10.1016/0014-2999(78)90414-4
Ren Z, Yan P, Zhu L, Yang H, Zhao Y, Kirby BP, Waddington JL, Zhen X (2018) Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacol 235(1):233–244. https://doi.org/10.1007/s00213-017-4761-z
Tambaro S, Casu MA, Mastinu A, Lazzari P (2014) Evaluation of selective cannabinoid CB1 and CB2 receptor agonists in a mouse model of lipopolysaccharide-induced interstitial cystitis. Eur J Pharmacol 729:67–74. https://doi.org/10.1016/j.ejphar.2014.02.013
Teixeira RC, Zangrossi H Jr, Graeff FG (2000) Behavioral effects of acute and chronic imipramine in the elevated T-maze model of anxiety. Pharmacol Biochem Behav 65(4):571–576. https://doi.org/10.1016/S0091-3057(99)00261-0
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493(7433):537–541. https://doi.org/10.1038/nature11740
Varatharaj A, Galea I (2017) The blood-brain barrier in systemic inflammation. Brain Behav Immun 60:1–2. https://doi.org/10.1016/j.bbi.2016.03.010
Wu J, Hocevar M, Foss JF, Bie B, Naguib M (2017) Activation of CB2 receptor system restores cognitive capacity and hippocampal Sox2 expression in a transgenic mouse model of Alzheimer’s disease. Eur J Pharmacol 811:12–20. https://doi.org/10.1016/j.ejphar.2017.05.044
Yirmiya R (1996) Endotoxin produces a depressive-like episode in rats. Brain Res 711(1–2):163–174. https://doi.org/10.1016/0006-8993(95)01415-2
Acknowledgments
The authors thank the Manipal Academy of Higher Education, Manipal, for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional Animal Ethics Committee approved the experimental protocol. The experiments were performed following the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahu, P., Mudgal, J., Arora, D. et al. Cannabinoid receptor 2 activation mitigates lipopolysaccharide-induced neuroinflammation and sickness behavior in mice. Psychopharmacology 236, 1829–1838 (2019). https://doi.org/10.1007/s00213-019-5166-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-5166-y