Abstract
Rational
Caloric restriction increases the risk of relapse in abstinent drug users. Hormones involved in the regulation of energy balance and food intake, such as leptin and ghrelin, are implicated in drug-related behaviors.
Objectives
We investigated the role of leptin and ghrelin in the augmentation of heroin seeking induced by chronic food restriction.
Methods
Rats self-administered heroin (0.1 mg/kg/infusion) for 10 days followed by 14 days of drug withdrawal. During withdrawal, rats were food restricted to 90% of their original body weight or were given free access to food. In experiment 1, we measured the plasma concentrations of leptin and ghrelin following heroin self-administration and withdrawal. In experiment 2, leptin was administered centrally (2.0 or 4.0 μg; i.c.v.) prior to a heroin-seeking test under extinction conditions. High density of both leptin and ghrelin receptors was previously identified in the ventral tegmental area (VTA), suggesting a direct effect on reward and motivation. Hence, we administered leptin (experiment 3; 0.125 or 0.250 μg/side), or ghrelin receptor antagonist JMV 2959 (experiment 4; 2.0 or 10.0 μg/side) directly into the VTA prior to the heroin-seeking test.
Results
Chronic food restriction significantly decreased plasma levels of leptin and elevated plasma levels of ghrelin. Central administration of leptin had no statistically significant effect on heroin seeking. Intra-VTA administration of either leptin or JMV 2959 dose-dependently and selectively decreased heroin seeking in the food-restricted rats.
Conclusions
Leptin and ghrelin transmission in the VTA can modulate the augmentation of heroin seeking induced by chronic food restriction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dependent drug users are characterized by compulsive drug seeking, and cycling through phases of drug use, abstinence, and relapse (O’Brien 1997; O’Brien and McLellan 1996). In particular, relapse is considered a major obstacle in the successful treatment of addiction. For example, in dependent heroin users followed for three decades, 25% had relapsed even after 15 years of abstinence (Hser et al. 2001). Relapse to drug use is triggered by three factors: exposure to the self-administered drug (de Wit 1996), cues associated with drug availability and drug effects (Carter and Tiffany 1999; Childress et al. 1993), and stress (Sinha 2001).
These triggers to relapse are modulated by restricted food intake, or caloric restriction. For example, the risk for relapse in abstinent smokers was remarkably higher when subjects underwent concurrent caloric restriction (Hall et al. 1992). The effects of caloric restriction on increased drug seeking are also evident in animal models of relapse. We found that chronic food restriction following extinction training (Shalev 2012), or during 2 weeks of drug withdrawal (D’Cunha et al. 2013) augmented heroin seeking in rats that previously self-administered heroin.
Earlier work from our laboratory was focused on the brain mechanisms that underlie acute food deprivation-induced reinstatement of extinguished drug seeking. We have previously reported that acute food deprivation can reinstate heroin and cocaine seeking, an effect that is mediated by extra-hypothalamic corticotropin-releasing factor (CRF), and involves dopamine (DA) transmission in the mesolimbic pathway (e.g., Shalev et al. 2006; Tobin et al. 2013). However, our recent work suggests that the brain mechanisms that underlie the effects of chronic food restriction on drug seeking are not entirely identical to the ones described for acute food deprivation (Sedki et al. 2013a). Thus, the neural mechanisms underlying the augmentation of heroin seeking induced by chronic food restriction are not fully elucidated.
We have previously demonstrated that DA in the mesolimbic pathway plays a key role in the augmentation of heroin seeking induced by chronic food restriction. Specifically, chronic food restriction increased levels of extracellular DA in the nucleus accumbens, and blocking DA receptors in the nucleus accumbens reduced the augmentation of heroin seeking induced by chronic food restriction (D’Cunha et al. 2017). Dopamine in the mesolimbic pathway can be modulated by the hormones leptin and ghrelin, which regulate food intake and energy homeostasis (Abizaid et al. 2006; Fulton et al. 2006; Hommel et al. 2006). Leptin is released by peripheral adipocytes (Friedman and Halaas 1998), and central administration of leptin decreases food intake and increases energy expenditure (Ahima et al. 2000). Additionally, central administration of leptin attenuates the reinstatement of heroin seeking induced by acute food deprivation (Shalev et al. 2001). Leptin receptors can be found on DA neurons in the ventral tegmental area (VTA; Figlewicz et al. 2003; Hommel et al. 2006), but leptin’s effects on DA neurons function may also be mediated presynaptically (Thompson and Borgland 2013). Intra-VTA leptin reduces cocaine-induced DA release in the mesolimbic pathway terminals, specifically in the nucleus accumbens (NAc), and decreases the expression of cocaine conditioned place preference, suggesting an attenuation in cocaine reward (You et al. 2016). Taken together, these findings suggest that leptin is a likely modulator of drug reward and the incentive value of drug-associated cues.
Ghrelin has the reciprocal actions as leptin. It is released by the gut and binds to the growth hormone secretagogue receptor (GHS-R1a; Kojima et al. 1999). Plasma ghrelin is elevated during food restriction and rapidly decreases following meal intake (Toshinai et al. 2001). As with leptin, the effects of ghrelin extend beyond energy balance and food intake and can affect drug use and drug seeking. Central administration of ghrelin increases alcohol intake (Jerlhag et al. 2009), and increases the breakpoint for heroin under a progressive ratio schedule of reinforcement (Maric et al. 2012). Like leptin, ghrelin receptors are abundant in the VTA, and the VTA is a target for ghrelin as shown in binding studies (Abizaid et al. 2006). Ghrelin in the VTA increases DA neuronal activity, and intra-VTA infusions of ghrelin increase food intake in rats (Abizaid et al. 2006). Therefore, ghrelin may mediate heroin seeking induced by chronic food restriction.
The current study was designed to elucidate the role of leptin and ghrelin in chronic food restriction-induced augmentation of heroin seeking. In experiment 1, we characterized basal plasma levels of circulating leptin and ghrelin in sated and food-restricted rats, as well as changes in their levels following re-feeding. Moreover, since it is demonstrated that ghrelin levels fluctuate throughout the day, and peak prior to meals (Drazen et al. 2006), we wanted to assess if the increase in plasma ghrelin, that usually accompanies the onset of the dark phase, would be shifted in the chronically food-restricted rats to precede their daily meal that was delivered in the middle of the dark phase. Next, in experiment 2, we tested the hypothesis that centrally administered leptin will attenuate food restriction-induced augmentation of heroin seeking. As a follow-up to experiment 2, in experiment 3, we used intra-VTA injections of leptin to locally target leptin receptors and investigate their involvement in heroin seeking, and also assessed changes in leptin receptor-mediated signaling in the VTA in food-restricted rats. Finally, experiment 4 examined whether ghrelin receptors (GHS-R1a) in the VTA are involved in the augmentation of heroin seeking induced by chronic food restriction.
Materials and methods
Subjects
Male Long Evans rats (325–350 g on arrival; Charles River, St. Constant, Quebec, Canada or Raleigh, North Carolina, USA) were used in the four experiments. Rats were kept on a reverse 12 h light-dark cycle (lights OFF 9:30 am) at 21 °C. Following recovery from surgery (described below), rats were individually housed in operant conditioning chambers with free access to food and water during heroin self-administration. At the end of heroin self-administration phase, rats were returned to the animal colony for the withdrawal and food restriction phase (described below). On the last day of the withdrawal phase, rats were brought back to the operant conditioning chambers for the heroin-seeking test. All animals were treated in accordance with the guidelines of the Canadian Council on Animal Care and the approval for all procedures was granted by the Concordia University Animal Research Ethics Committee.
Surgical procedures
Intravenous catheterization was completed under ketamine and xylazine (90.0 and 13.0 mg/kg, i.p.) anesthesia as previously described (Sedki et al. 2013b). Briefly, silastic catheters (Dow Corning, Midland, MI, USA) were inserted into the right jugular vein and secured with silk sutures. During the intravenous catheter surgery, rats were also implanted with either a unilateral guide cannula (23 gauge; Plastics One, Roanoke, VA) aimed 2 mm above one of the lateral ventricles: AP = − 0.5 mm, ML = ± 1.4 mm, DV = − 3.0 mm relative to Bregma (experiments 1A and 1B), or bilateral guide cannulae (23 gauge) targeting the VTA: AP = − 5.8 mm, ML = ± 2.2 mm, DV = − 7.5 mm with a 12° angle. Following surgery, rats were given penicillin (450,000 IU/rat, s.c.) and analgesic ketoprofen (5.0 mg/kg, s.c.; CDMV, Quebec, Canada). Throughout self-administration, rats were flushed daily with heparin and gentamicin in sterile saline (7.5 IU + 12.0 μg per day per rat) to prevent catheter blockage. Following recovery from surgery in experiment 2, intracerebroventricular (i.c.v.) guide cannula placement was verified by demonstrating a short latency (< 60 s) vigorous drinking response to angiotensin II (100.0 nmol, i.c.v.).
Apparatus
Experiments were conducted in operant conditioning chambers (Colburn Instruments, Whitehall, PA, USA; 29.0 cm × 29.0 cm × 25.5 cm), placed in individual sound-attenuating cubicles. Each chamber contained two retractable levers located 9 cm above the grid floor. Responses on the “active” lever activated the infusion pump (Colburn Instruments), whereas responses on the “inactive” lever were recorded but had no programmed consequences. Rats were attached to the infusion pump via a liquid swivel (Lomir Biomedical, QC, Canada) and Tygon tubing shielded with a metal spring.
Drugs
Heroin (diacetylmorphine HCl; National Institute on Drug Abuse, Baltimore, MD, USA) was dissolved in physiological saline and delivered at a concentration of 0.1 mg/kg per infusion. Recombinant murine leptin (Peprotech; Roanoke, VA) was dissolved in sterile water to concentrations of 0.25 μg/μl, 0.50 μg/μl, 1.0 μg/μl, or 2.0 μg/μl. Doses of hormone administered were based on previous reports (Fulton et al. 2000; Shalev et al. 2001; You et al. 2016). GHS-R1a antagonist JMV 2959 (EMD Millipore, Billerica, Massachusetts, USA) was dissolved in 0.9% sterile saline to a concentration of 4.0 μg/μl or 20.0 μg/μl. Administered doses were based on previous work (Skibicka et al. 2011).
Procedure
Experiments 2–4 all consisted of three phases: heroin self-administration, withdrawal and food restriction, and finally the heroin-seeking test.
Training
Following recovery from surgery, rats were housed in the operant conditioning chambers for a 24-h habituation period and 10 days of heroin self-administration training. Rats were given three 3-h training sessions per day separated by a 3-h period under a fixed interval (FI) 20 s schedule of reinforcement. The first self-administration session started at the onset of the dark phase and was marked by the insertion of both levers, the illumination of a red houselight and the activation of a white-light/tone complex cue (2.9 kHz; 10 dB above background level) above the active lever. The white-light/tone complex remained on for 30 s or until the active lever was pressed. A response on the active lever resulted in the activation of the infusion pump (5 s; 0.13 ml/infusion) and the white-light/tone complex, and the houselight was turned off. Subsequent responses on the active lever for the following 20 s were recorded but not reinforced. Responses on the inactive lever had no programmed consequences. At the end of each 3-h session, the active lever was retracted and the houselight was turned off. To help with lever discrimination, the inactive lever remained extended until 1 h before the 1st session on the following day.
Withdrawal and food restriction
After the heroin self-administration training, rats were transferred back to the animal colony and individually housed in standard Plexiglass cages. Following a 24-h drug washout period, rats were matched for average number of infusions, active lever responses, and body weight during the last 5 days of training and assigned to either the food restricted (FDR) or sated group. FDR rats were given approximately 15 g of standard rat chow daily at 1:30 pm. The food ration was titrated daily to bring the FDR rats to 90% of their original body weight at the beginning of the withdrawal period. “Sated” rats had unrestricted access to food throughout the withdrawal period.
Intracranial injections
Intracranial injections were made using a syringe pump (Harvard Apparatus, Holliston, MA, USA) connected to a 10-μl Hamilton syringe. The syringe was attached via polyethylene-20 tubing to a 28-gauge injector (Plastics One) that extended either 2 mm (experiment 2), or 1 mm beyond the guide cannula (experiments 3 and 4). In all experiments, rats received mock injections on the 2 days preceding the heroin-seeking test to habituate them to the procedure, using short injectors, not extending beyond the guide cannulae.
Experiment 1A: characterization of plasma levels of leptin and acylated ghrelin following chronic food restriction and re-feeding
Blood was collected from groups of rats that went through the phases of heroin self-administration for 10 days, 14 days of withdrawal/food restriction, and a heroin-seeking test. A small nip was made at the tip of the tail, and blood was collected in Eppendorf tubes which contained 20 μl of heparin per 1 ml of blood collected. A baseline measure of blood was collected in FDR and sated rats at the onset of the dark phase (9:30 am). Next, FDR rats were allowed 2 h of free access to rat chow in their home cage, followed by another tail blood collection from both 2-h re-fed and sated rats. Finally, 24 h following ad libitum access to food, a third and final tail blood collection was taken from the 24-h re-fed and sated rats. In another group of rats, we assessed plasma fluctuations of acylated ghrelin. The first sample was collected at the onset of the dark phase (9:30 am), then again prior to the chronically food-restricted rats’ meal time (1:30 pm), and lastly at the onset of the light phase (9:30 pm) in both FDR and sated rats.
Plasma was separated by centrifugation at 10,000 rpm for 10 min. Following centrifugation, blood plasma was aliquoted and stored at − 80 °C until processed. To protect the acylated ghrelin molecule, 1.0 μl of 1.0 N HCl and 1.0 μl of the protease inhibitor phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich) were added per 100 μl of blood plasma. Plasma leptin and ghrelin were determined in separate enzyme-linked immunosorbent assay (ELISA) kits (Millipore, MA, USA). The reported detection sensitivity for the ELISA kits was 0.04 ng/ml for leptin and 7.9 pg/ml for acylated ghrelin.
Experiment 2A: the effect of a single central administration of leptin on the augmentation of heroin seeking induced by chronic food restriction
In a different group of rats, on the 14th day of food restriction, rats were injected with vehicle or leptin (2.0 μg/rat, i.c.v.) approximately 30 min before the 3-h heroin-seeking test. Leptin was administered over 2 min at a rate of 1.0 μl/min and the injector was left in place for an additional 1 min. Sterile water was used as the vehicle.
Experiment 2B: the effect of repeated central administration of leptin on the augmentation of heroin seeking induced by chronic food restriction
We have previously reported that 24 h of re-feeding the FDR rats eliminates the augmentation of heroin seeking induced by chronic food restriction (D’Cunha et al. 2013). In experiment 1 in the current study, we found a statistically significant increase in plasma leptin 24 h following re-feeding. Consequently, we wanted to investigate if we could mimic the re-feeding effects with leptin administration approximately 24 h and 30 min prior to the 3-h heroin-seeking test. Moreover, we have previously demonstrated that a similar repeated treatment with leptin was highly effective in blocking acute food deprivation-induced reinstatement of heroin seeking (Shalev et al. 2001). Rats received either vehicle, 2.0, or 4.0 μg of leptin into the ventricles on the 13th day of food restriction and the same dose on the 14th day of food restriction, approximately 22 h and 30 min prior to the 3-h heroin-seeking test, respectively. Administration of leptin was identical to experiment 2A and sterile water was used as the vehicle.
Experiment 3A: the effects of intra-VTA leptin on the augmentation of heroin seeking induced by chronic food restriction
As previously mentioned, leptin receptors are found in high density in the VTA, and intra-VTA leptin can modulate DA activity (Fulton et al. 2006; Hommel et al. 2006; Thompson and Borgland 2013). The purpose of experiment 3 was to investigate the role of leptin administration directly into the VTA. Rats received a bilateral infusion of vehicle or leptin (0.125 or 0.250 μg/side) into the VTA approximately 30 min prior to the heroin-seeking test on the 14th day of food restriction. Solutions were administered over 1 min at a flow rate of 0.5 μl/min. The injector was left in place for 1 min following each microinjection.
Experiment 3B: the effects of chronic food restriction on leptin-receptor signaling in the VTA
Leptin induces rapid phosphorylation of signal-transducer-and-activator-of-transcription-3 (STAT3), a key intracellular signaling pathway of leptin receptor activation (Banks et al. 2000) that is found in DA and GABA neurons in the VTA (Fulton et al. 2006). In a separate group of rats, we assessed the levels of STAT3 proteins in VTA tissue obtained from flash frozen brains collected immediately following a 30-min heroin-seeking test in FDR (14 days) or sated rats. Tissue punches (0.75 mm diameter), one for each hemisphere, were collected from 1 mm thick frozen coronal slices and homogenized in lysis buffer (20 mM Tris, pH 7.5; 150 mM NaCl; 1 mM Na2EDTA; 1 mM EGTA; 1% Triton; 2.5 mM sodium pyrophosphate; 1 mM β-glycerophosphate; 1 mM sodium orthovanadate; 1 μg/ml leupeptin; 1 mM phenylmethylsulfonyl fluoride; 1 μl phosphatase inhibitors cocktails 2, and 1 μl phosphatase inhibitors cocktails 3; Sigma-Aldrich). Proteins (40 μg) were loaded into 10% polyacrylamide gel, and separated under 90 V electrophoresis. Proteins on each gel were then transferred to nitrocellulose membrane, non-specific biding was blocked with 5% dry skim milk in TBS-Tween 0.1% and incubated with the primary antibodies for pSTAT3 (1:1000; Cell Signaling, 9145). Membranes were then washed and incubated with secondary antibody (1:3000 for pSTAT3 and 1:10,000 for GAPDH; Goat-anti-rabbit-HRP, Bio-Rad, 1721019), washed and developed by enhanced chemiluminescence. Bands were revealed on a film and analyzed using ImageJ software (free software available from NIH at http://rsbweb.nih.gov/ij/). For total STAT3 assessment, membranes were stripped with 0.2 N NaOH, reprobed with an anti-STAT3 antibody (1:1000; Cell Signaling, 4904), and analyzed as described above. GAPDH was used as loading and transfer control, and data were standardized according to GAPDH values.
Experiment 4: the effects of intra-VTA GHS-R1a antagonist, JMV 2959, administration on the augmentation of heroin seeking induced by chronic food restriction
On the 14th day of food restriction, rats were administered with vehicle or the GHS-R1a receptor antagonist JMV 2959 (2.0 or 10.0 μg/side) bilaterally into the VTA approximately 10 min prior to the 3-h heroin-seeking test. JMV 2959 was administered at a flow rate of 0.5 μl/min over 1 min. Injector was left in place for an additional minute and 0.9% sterile saline was used as a vehicle.
Histology
At the end of experiments 3 and 4, rats were euthanized with carbon dioxide and decapitated. Brains were fixed with 4% paraformaldehyde solution for a week before being sliced in 40 μm coronal sections. Slices were then stained with cresyl violet and cannula placements were determined under a light microscope with reference to a brain atlas (Paxinos and Watson 2005).
Statistical analysis
To characterize the changes of plasma levels of leptin and acylated ghrelin, separate mixed factorial analyses of variance (ANOVA) were used, with the between subjects factor of feeding condition (FDR, sated) and the within subjects factor of time (baseline, 2-h re-fed, 24-h re-fed; or the following sample times: 9:30 am, 1:30 pm, 9:30 pm). To assess the effects of leptin or JMV 2959 on the augmentation of heroin seeking induced by chronic food restriction, active and inactive lever responses made during the 3-h heroin-seeking test were analyzed separately using a two-way ANOVA with the between subjects factor of feeding condition (FDR, sated) and either: experiment 2A leptin dose (vehicle or 2.0 μg/rat); experiment 2B leptin dose (vehicle, 2.0 or 4.0 μg/rat); experiment 3 leptin dose (vehicle, 0.125, 0.250 μg/side); experiment 4 JMV 2959 dose (vehicle, 2.0 or 10.0 μg/side). Repeated measures ANOVAs were preceded by Mauchly’s sphericity test, and a Greenhouse-Geisser correction was used when statistical significance was found. Statistically significant main effects and interactions are reported for p ≤ 0.05, and were followed up with post hoc analyses with a Bonferroni correction. STAT3 and pSTAT3 optical density data were adjusted to GAPDH expression, and feeding condition groups were compared using independent samples t tests.
Results
All rats acquired reliable heroin self-administration behavior. Mean ± SEM number of infusions and number of active and inactive lever responses made on the last day of heroin self-administration training for each experiment are shown in Table 1. For experiments 3A and 4, only rats that had correct histological placements and were included in the analysis (Fig. 2). In all experiments, on test day, the FDR rats were approximately 90% of their original body weight at the start of the withdrawal phase, or approximately 75–80% of the sated rats’ body weight (Table 1).
Experiment 1A: characterization of plasma levels of leptin and acylated ghrelin following chronic food restriction and re-feeding
Following the heroin-seeking test, rats were maintained as either food restricted (FDR; n = 6) or sated (n = 6) and assessed for plasma hormone concentration levels at 3 time-points.
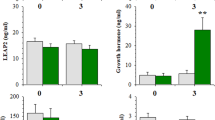
As seen in Fig. 1a, plasma concentrations of leptin were significantly reduced in chronically food-restricted rats (feeding condition, F(1,10) = 13.484, p = 0.004, η2 = 0.574). Plasma levels in FDR rats increased gradually following re-feeding (time, F(2,20) = 7.303, p = 0.004, η2 = 0.337; feeding condition × time, F(2,20) = 4.397, p = 0.026, η2 = 0.203). Within the FDR rats, there was a trend difference in plasma leptin levels from baseline compared to 2 h following re-feeding (p = 0.064), and a statistically significant increase from baseline to 24 h following re-feeding (p = 0.003). There were no statistically significant changes in plasma leptin concentrations in the sated rats over time.
Plasma concentrations of leptin and acylated ghrelin in chronically food restricted (FDR) and sated rats. a Concentration of plasma leptin in FDR rats (n = 6) was significantly lower than in sated rats (n = 6, p = 0.004) and gradually increased following 2 h and 24 h of re-feeding. #p = 0.003, 24 h re-feeding significantly higher than baseline within the FDR group. b Plasma concentration of acylated ghrelin in FDR rats (n = 6) was significantly higher than in the sated rats (n = 6) under baseline conditions, but not following 2 h and 24 h of re-feeding. &p = 0.001, FDR significantly different from sated group at baseline. c Plasma concentration of acylated ghrelin in FDR (n = 6) was generally higher compared to the sated rats (n = 6, p = 0.001). Within the FDR group, plasma ghrelin increased significantly between the onset of the dark phase (9:30 am) to prior to meal time in the FDR group (1:30 pm), and decreased at the onset of the light phase (9:30 pm). *p < 0.02, compared to 9:30 pm within the FDR group. @p < 0.001, compared to 1:30 pm within the FDR group. There were no statistically significant changes in plasma ghrelin levels in the sated rats. All data presented as mean ± SEM
For the analysis of plasma acylated ghrelin levels following re-feeding, Mauchly’s sphericity test was significant (p < 0.05); therefore, data were analyzed using the Greenhouse-Geisser correction. Plasma ghrelin levels in FDR rats were higher than in sated rats but decreased rapidly following re-feeding (Fig. 1b). The main effect of feeding condition was not statistically significant, although there was a large effect size, F(1,10) = 3.246, p = 0.102, η2 = 0.245. The effect of feeding status on plasma ghrelin levels was reflected in the statistically significant main effect of time (F(2,20) = 19.859, p = 0.001, η2 = 0.490) and feeding condition × time interaction (F(2,20) = 10.688, p = 0.007, η2 = 0.264). Pairwise comparisons with a Bonferroni correction revealed that within the FDR rats, there was a statistically significant decrease in plasma acylated ghrelin levels from baseline compared to 2 h following re-feeding (p = 0.001) and to 24 h following re-feeding (p = 0.001). There were no statistically significant changes in plasma acylated ghrelin levels in the sated rats over time.
For the analysis of changes in plasma acylated ghrelin levels at different time-points throughout the day, Mauchly’s test of sphericity was significant (p < 0.05); therefore, data were analyzed using the Greenhouse-Geisser correction. As seen in Fig. 1c, plasma ghrelin levels were higher in FDR rats compared to the sated group (feeding condition, F(1,10) = 37.822, p < 0.001, η2 = 0.791). Plasma ghrelin levels in FDR rats fluctuated dramatically over time (time, F(2,20) = 25.263, p < 0.001, η2 = 0.366; feeding condition × time, F(2,20) = 33.673, p < 0.001, η2 = 0.488). Pairwise comparisons with a Bonferroni correction revealed that within the FDR rats, there was a statistically significant increase in ghrelin from the onset of the dark phase at 9:30 am to prior to meal time at 1:30 pm (p < 0.001). There was also a significant decrease in plasma ghrelin from 1:30 pm to the onset of the light phase at 9:30 pm (p < 0.001). Plasma ghrelin levels at the onset of the dark phase at 9:30 am were also significantly higher than at the onset of the light phase at 9:30 pm, (p = 0.020). There were no statistically significant changes in plasma ghrelin levels in the sated rats.
Experiment 2A: the effect of a single central administration of leptin on the augmentation of heroin seeking induced by chronic food restriction
The final analysis included 20 rats divided into four groups: FDR–vehicle (n = 4), FDR–2.0 μg (n = 6), sated–vehicle (n = 4), sated–2.0 μg (n = 6). As seen in Fig. 2a, overall the FDR groups pressed more on the active lever during the 3-h heroin-seeking test compared to the sated groups (feeding condition, F(1,16) = 7.083, p = 0.017, η2 = 0.304). A single pre-test i.c.v. administration of leptin had no effect on active lever responses (leptin dose, F(1,16) = 0.244, p = 0.628, η2 = 0.010), and there was no statistically significant interaction for feeding condition × leptin dose (F(1,16) = 0.010, p = 0.922, η2 = 0.000421).
The effect of i.c.v. leptin injections on heroin seeking in food restricted (FDR) and sated rats. a The number of active lever presses (left) did not significantly change following a single leptin (2.0 μg) or vehicle administration, 30 min prior to the heroin-seeking test. The number of inactive lever presses (right) increased significantly (p = 0.041) in leptin injected rats. b The number of active (left) and inactive (right) lever presses did not significantly change when leptin (2.0 or 4.0 μg) or vehicle injections were given twice, 24 h and 30 min prior to the heroin-seeking test. *p < 0.05, compared to the sated groups. Data presented as mean ± SEM. Group size is indicated by the number located in the bar
Leptin injections resulted in a statistically significant increase in inactive lever responses (leptin dose, F(1,16) = 4.955, p = 0.041, η2 = 0.184). It should be noted that inactive lever responses were still much lower than active lever responses. There were no other significant effects observed for inactive lever responses during the heroin-seeking test (feeding condition, F(1,16) = 4.165, p = 0.058, η2 = 0.155; feeding condition × leptin dose F(1,16) = 1.773, p = 0.202, η2 = 0.066).
Experiment 2B: the effect of repeated central administration of leptin on the augmentation of heroin seeking induced by chronic food restriction
The final analysis included 60 rats divided into six groups: FDR–vehicle (n = 15), FDR–2.0 μg (n = 8), FDR–4.0 μg (n = 8), sated–vehicle (n = 13), sated–2.0 μg (n = 7), sated–4.0 μg (n = 9). As previously demonstrated, the FDR groups pressed significantly more on the active lever than the sated groups, (feeding condition, F(1,54) = 11.329, p = 0.001, η2 = 0.165; Fig. 2b). Although both feeding condition groups showed a decrease in active lever responses following leptin administration, there was no statistically significant main effect of leptin dose (F(2,54) = 1.421, p = 0.250, η2 = 0.042) or an interaction of feeding condition × leptin dose (F(2,54) = 0.153, p = 0.858, η2 = 0.0044). Inactive lever responses were not statistically significantly affected by feeding condition, (F(1,54) = 1.484, p = 0.228, η2 = 0.024), treatment with leptin dose (F(2,54) = 0.931, p = 0.401, η2 = 0.030) or interaction of feeding condition × leptin dose (F(2,54) = 2.020, p = 0.142, η2 = 0.066).
Both 2.0 μg and 4.0 μg of leptin administered on FDR 13 and FDR 14 significantly reduced 24 h food intake in the sated rats as compared to vehicle. A one-way ANOVA performed on FDR 14 food consumption data revealed a main effect of leptin dose (F(2,26) = 5.006, p = 0.014, η2 = 0.278; Fig. 3). Post hoc analysis revealed that both the sated–2.0 μg and the sated–4.0 μg groups consumed less food than the sated–vehicle group (p = 0.034, p = 0.055, respectively). Similarly, a one-way ANOVA performed on FDR 15 food consumption data (~ 24 h after the last leptin injection) revealed a statistically significant main effect of leptin dose (F(2,26) = 5.665, p = 0.009, η2 = 0.304). Post hoc analysis revealed that both the sated–2.0 μg and the sated–4.0 μg groups consumed less food than the sated–vehicle group (p = 0.031, p = 0.027, respectively).
The effects of i.c.v. administration of leptin on food intake in sated rats. Leptin (2.0 or 4.0 μg) or vehicle was injected twice, once on day 13, and again on day 14 of withdrawal approximately 30 min before the heroin-seeking test. Leptin administration significantly reduced food intake on days 14 and 15. *p < 0.05 compared to either the 2.0 or 4.0 μg treated groups. Data presented as mean ± SEM. Group sizes as in Fig. 2b (sated)
Experiment 3A: the effect of intra-VTA leptin administration on the augmentation of heroin seeking induced by chronic food restriction
Only rats with correct cannula placements located in the VTA were included in the statistical analysis (Fig. 4a). The final analysis included the following groups of rats: FDR–vehicle (n = 9), FDR–0.125 μg (n = 8), FDR–0.250 μg (n = 9), sated–vehicle (n = 9), sated–0.125 μg (n = 9), and sated–0.250 μg (n = 8).
Cannula placements for experiments 3 and 4. Approximate anatomical position for microinjector tips of guide cannula targeting the VTA for a experiment 3 (n = 52; open circles) or b experiment 4 (n = 50; open triangles). Images modified from the brain atlas of Paxinos and Watson (2005), Figures 78–82 (− 5.40 to − 5.88 mm posterior to Bregma)
Food restriction augmented heroin seeking on test day, as indicted by the higher number of active lever responses compared to the sated rats (feeding condition, F(1,46) = 21.181, p < 0.001, η2 = 0.261; Fig. 5). Intra-VTA leptin injections reversed the food restriction effect, without changing drug seeking in the sated groups (leptin dose F(2,46) = 1.688, p = 0.196, η2 = 0.042; feeding condition × leptin, F(2,46) = 5.328, p = 0.008, η2 = 0.131). Post hoc analysis revealed that the FDR–vehicle had statistically significantly more active lever presses than all of the sated groups during the heroin-seeking test (p < 0.002). Furthermore, the FDR–vehicle group had significantly more active lever presses than the FDR–0.250 μg group (p = 0.012) but not the FDR–0.125 μg group (p = 0.507). There were no statistically significant effects on inactive lever responses (feeding condition, F(1,46) = 1.158, p = 0.288, η2 = 0.021; leptin dose F(2,46) = 1.802, p = 0.176, η2 = 0.066; feeding condition × leptin, F(2,46) = 1.783, p = 0.180, η2 = 0.066).
The effect of intra-VTA leptin injections on heroin seeking in food restricted (FDR) and sated rats. Leptin (0.125 or 0.250 μg/side) or vehicle was injected 30 min before the heroin-seeking test. The number of active (left), but not inactive (right) lever presses was significantly reduced following leptin administration. *p < 0.05, compared to sated-vehicle rats. #p < 0.05, FDR–vehicle vs. FDR–0.250 μg leptin. Data presented as mean ± SEM. Group size is indicated by the number located in the bar
Experiment 3B: the effects of chronic food restriction on leptin-receptor signaling in the VTA
Four rats were removed from the final analyses (1 sated, 3 FDR) because of low self-administration training, insufficient protein in sample, or lack of protein expression signal. The mean ± SEM number of active lever response made during the 30-min heroin-seeking test by the FDR rats (95.0 ± 16.9, n = 6) was statistically significantly higher compared to the sated rats (46.3 ± 10.3, n = 7; t(11) = 2.54, p = 0.03; d = 1.53). Inactive lever responses in the two feeding condition groups were not statistically significantly different (data not shown). Levels of STAT3 or pSTAT3 were not statistically significantly different between the groups (t(11) = 0.56, p = 0.58, d = 0.35; t(11) = 0.63, p = 0.54; d = 0.37, respectively; Fig. 6a). The pSTAT3/STAT3 ratios also showed no statistically significant difference between FDR and sated rats (t(11) = 0.71, p = 0.49; d = 0.44; Fig. 6b). A strong, although not statistically significant, inverse correlation was found only in the FDR group between pSTAT3/STAT ratio and the number of active lever response made during the heroin-seeking test (FDR: r = − 0.78, p = 0.06; sated: r = − 0.14, p = 0.76).
Expression of STAT3 and pSTAT3 in the VTA in food restricted (FDR; n = 6) and sated (n = 7) rats. a Food restriction did not change the expression of pSTAT3 (left) or STAT3 (right). Data presented as mean ± SEM fold-change from sated group. b Food restriction did not significantly change pSTAT3/STAT3 ratio compared to sated rats (mean ± SEM). Data presented as mean ± SEM. Group size is indicated by the number located in the bar
Experiment 4: the effects of intra-VTA ghrelin receptor antagonist (JMV 2959) administration on the augmentation of heroin seeking induced by chronic food restriction
Only rats with correct cannula placements located in the VTA as assessed by histological verification were included in the final statistical analyses (Fig. 4b). The final analyses included the following groups of rats: FDR–vehicle (n = 9), FDR–2.0 μg (n = 8), FDR–10.0 μg (n = 9), sated–vehicle (n = 8), sated–2.0 μg (n = 8), and sated–10.0 μg (n = 8). Food restriction significantly augmented heroin seeking, as the FDR groups pressed more on the active lever than the sated groups (feeding condition, F(1,44) = 8.834, p = 0.005, η2 = 0.140; Fig. 7). Intra-VTA injections of JMV 2959 reversed the food restriction effect, without affecting drug seeking in the sated groups (JMV 2959 dose, F(2,44) = 1.116, p = 0.337, η2 = 0.048; feeding condition × JMV 2959 dose, F(2,44) = 4.095, p = 0.023, η2 = 0.129). Post hoc analyses revealed that the FDR–vehicle group had significantly more active lever presses than the sated–vehicle (p = 0.009), the sated–2.0 μg (p = 0.015), and the FDR–10.0 μg (p = 0.046) groups. Analysis of the inactive lever responses found only a significant effect of JMV 2959 dose (F(2,44) = 3.45, p = 0.041, η2 = 0.136), driven by a small overall increase in inactive lever response in rats treated with the 10.0 μg dose. No other significant effects were observed for inactive lever responses during the heroin-seeking test (feeding condition, F(1,44) = 0.003, p = 0.953, η2 = 0.0000638; feeding condition × JMV 2959 dose, F(2,44) = 1.936, p = 0.156, η2 = 0.071).
The effect of intra-VTA injections of the ghrelin receptor antagonist, JMV 2959, on heroin seeking in food restricted (FDR) and sated rats. JMV 2959 (2.0 or 10.0 μg/side) or vehicle was injected 30 min before the heroin-seeking test. The number of active (left), but not inactive (right) lever presses was significantly reduced following JMV 2959 administration. *p < 0.05, FDR–vehicle vs. sated–vehicle, and sated–2.0 μg JMV 2959. #p < 0.05, FDR–vehicle vs. FDR–10.0 μg JMV 2959. Data presented as mean ± SEM. Group size is indicated by the number located in the bar
Discussion
As we have previously demonstrated (D’Cunha et al. 2013), chronic food restriction over a period of drug withdrawal significantly augmented heroin seeking in rats with a history of heroin self-administration. Central administration of leptin, either as single or repeated infusions, had no selective effect on food restriction-induced augmentation of heroin seeking. Most importantly, administration of leptin directly into the VTA decreased heroin seeking selectively in the FDR rats. Lastly, administration of the ghrelin receptor antagonist JMV 2959 directly into the VTA selectively decreased heroin seeking in the FDR rats.
We also found that, as expected, plasma leptin and ghrelin levels in rats with a history of heroin self-administration are modulated by chronic food restriction; however, we did not identify food restriction-induced changes in STAT3 signaling in the VTA.
Changes in plasma concentrations of leptin and acylated ghrelin in the FDR and sated rats
In agreement with previous reports (Johansson et al. 2008; Kinzig et al. 2009), 14 days of chronic food restriction following 10 days of heroin self-administration significantly decreased plasma leptin levels. Absolute basal levels of plasma leptin in the current study were higher than previously reported. Possible reasons for these discrepancies include acute food deprivation of sated rats prior to blood collection, differences in time of blood collection (light vs. dark phase in the current study), and lower body weight of the rats at baseline in the previous studies. Nevertheless, the pattern of change in plasma leptin levels that we recorded is consistent with the effects of chronic food restriction that were observed in earlier studies. In addition, plasma leptin levels significantly increased following 2 or 24 h of free access to chow, supporting leptin’s potential role as a short-term satiety signal (Johansson et al. 2008).
Chronically food-restricted rats also had significantly higher plasma acylated ghrelin levels compared to the sated rats, a finding that is consistent with previous reports (Kinzig et al. 2009; Toshinai et al. 2001), but see (Johansson et al. 2008). In contrast to a previous report (Drazen et al. 2006), we did not see a peak in plasma ghrelin in the sated rats at the onset of the dark phase. This intriguing discrepancy could be the result of the plasma ghrelin analysis method that targeted acylated ghrelin in the current study, while no information is available for the method used by Drazen et al. (2006). We did however observe that plasma ghrelin levels were the highest prior to the delivery of the daily meal in the FDR rats, similar to the findings reported by Drazen et al. (2006). Finally, re-feeding the previously food-restricted rats for 2 h or 24 h statistically significantly decreased plasma ghrelin levels.
We therefore have demonstrated a considerable modulation of circulating levels of leptin and ghrelin in our experimental subjects that coincided with the augmentation of heroin seeking in FDR rats.
Effects of central administration of leptin on heroin seeking in the FDR and sated rats
Contrary to our hypothesis, central administration of leptin did not statistically significantly decrease heroin seeking in the FDR rats. Although repeated i.c.v. administration of the high dose of leptin (4.0 μg) attenuated overall heroin seeking with a considerable effect size (vehicle vs. 4.0 μg leptin-treated rats: Cohen’s d = 0.61; 95%CI [−30.19, 31.4]), the treatment effect was not statistically significant, and small attenuation in heroin seeking was also observed in the sated group. These results contrast with our previous report that the same doses of leptin attenuated acute food deprivation-induced reinstatement of heroin seeking (Shalev et al. 2001). A possible explanation is the different types of caloric restriction challenges. Not only are there distinctions in the metabolic consequences between acute food deprivation and chronic food restriction (Bi et al. 2003), but the neural mechanisms impacted by these manipulations are distinct. For example, administration of a corticotrophin releasing factor (CRF) antagonist attenuated acute food deprivation-induced reinstatement of heroin seeking (Shalev et al. 2006) but had no effect on food restriction-induced augmentation of heroin seeking (Sedki et al. 2013a). Alternatively, the current study investigated heroin seeking following an “abstinence” period, whereas Shalev et al. (2001) investigated reinstatement of heroin seeking following extinction training. The neural mechanisms mediating these procedures are, at least partially, distinct (Fuchs et al. 2006).
Interestingly, intravenous leptin attenuated cocaine seeking in rats with unlimited access to food under “surprising” extinction conditions (You et al. 2016). The discrepancy between our results and You et al.’s could be explained by the different procedures used (prolonged withdrawal versus a “surprise extinction” during the self-administration phase, respectively). Alternative explanations could be the different classes of self-administered drugs (opiate versus psychostimulant drugs, respectively), or the different route of leptin administration (i.c.v. vs. intravenous).
The small effect for repeated leptin treatment on heroin seeking could be explained by an ineffective dose or reduced leptin sensitivity of the rats in our study. However, in accordance with the well-established effect of central leptin administration on food intake ((Bruijnzeel et al. 2011; Halaas et al. 1997; Morton et al. 2006), we have found that both doses of leptin significantly reduced food intake in the sated rats; therefore, the non-significant effects on heroin seeking are not due to a lack of efficacy of the leptin treatment.
Effects of intra-VTA leptin administration on heroin seeking in FDR and sated rats
We found that intra-VTA leptin significantly decreased the augmentation of heroin seeking induced by chronic food restriction. Although leptin’s primary role is in the hypothalamus to regulate food intake and energy metabolism (Ahima et al. 2000), it also acts on extra-hypothalamic targets, specifically the VTA to affect food intake and reward function (Bruijnzeel et al. 2011). Within the VTA, there is high expression of leptin receptors located on dopaminergic neurons, on gamma-aminobutyric acid-ergic (GABAergic) neurons (Figlewicz et al. 2003; Fulton et al. 2006; Hommel et al. 2006), and putatively, on presynaptic glutamatergic terminals (Thompson and Borgland 2013). Leptin application to the midbrain in vitro and systemic administration in anesthetized rats in vivo both significantly decrease the firing rate of DA neurons in the VTA (Hommel et al. 2006). These inhibitory actions of leptin on VTA DA neurons are thought to be mediated through a change in VTA DA neurons intrinsic properties (Hommel et al. 2006), and by presynaptic inhibition of glutamatergic release onto DA neurons (Thompson and Borgland 2013). More recently, Shen et al. (2016) reported that selective knockdown of leptin receptors in the VTA resulted in increased DA levels in the NAc. In addition, intra-VTA administration of leptin inhibited the increase in extracellular DA in the NAc induced by cocaine in rats (You et al. 2016), while i.c.v. injections of a leptin antagonist, superactive mouse leptin antagonist (SMLA), resulted in upregulation of DA and its metabolites in the NAc in mice (Shen et al. 2016). Importantly, the leptin-signal driven changes in DA transmission described above were associated with altered reward-related behaviors. Thus, You et al. (2016) found that intra-VTA leptin decreased conditioned place preference for cocaine, and central administration of a leptin receptor antagonist increased cocaine conditioned place preference (Shen et al. 2016).
We have recently identified a selective increase in extracellular DA levels in the NAc of food-restricted rats during heroin seeking tests (D’Cunha et al. 2017). Moreover, we have found that blocking DA D1-like receptors in the NAc decreased the augmentation of heroin seeking induced by chronic food restriction (D’Cunha et al. 2017). Taken together with the previous findings described above, our current findings suggest that chronic food restriction-induced reduction in plasma leptin levels results in a decrease in the inhibitory signal of leptin on VTA DA neurons’ activity and increases excitability in these neurons. This, in turn, would make the FDR rats more responsive to drug-associated cues, leading to increased dopaminergic transmission in the terminals of the mesolimbic pathway, such as the NAc, and to augmented heroin seeking. Accordingly, when leptin is administered directly into the VTA of FDR rats, it reduces DA neurons excitability, effectively attenuating the impact of re-exposure to the heroin-taking context and cues. It has been previously suggested that under food restriction, the DA system becomes highly responsive to leptin and its effects on appetitive motivation (Fernandes et al. 2015). Such an adaptation would help explain why we only see decreased heroin seeking in the FDR, but not sated, rats following intra-VTA leptin administration.
The same mechanism could explain our previous findings that acute re-feeding (2 or 24 h before testing) reversed the augmentation of heroin seeking in food-restricted rats (D’Cunha et al. 2013). Thus, as demonstrated here, re-feeding increases plasma leptin in FDR rats, which would result in reduced/normalized excitability of VTA DA neurons and a reduction in DA levels in the NAc, eventually leading to decreased heroin seeking. Although plasma leptin levels did not recover to baseline levels following 24 h re-feeding, the robust behavioral effect reported by D’Cunha et al. (2013) can be explained by an increased sensitivity to leptin that follows exposure to low circulating leptin levels (Zhao et al. 2019). Interestingly, with both the re-feeding and intra-VTA leptin injections approaches, heroin seeking was reduced to the level demonstrated by the sated rats, but not completely eliminated. This suggests a “ceiling effect” or a limit to the inhibitory signal of leptin in the VTA, maybe due to receptor saturation.
We did not identify changes in pSTAT3 or pSTAT3/STAT3 ratio in the VTA that paralleled the changes in plasma leptin in the FDR rats. Intra-cellular leptin receptor signaling in the VTA is complex, and involves multiple pathways, i.e., the JAK-STAT (Morton et al. 2009), PI3K (Thompson and Borgland 2013), and the ERK 1/2 (Trinko et al. 2011) pathways. Thus, a critical involvement of a different intra-cellular leptin receptor signaling pathway should be considered. In addition, the STAT3 pathway can be activated by signals other than leptin (Frias et al. 2007), which could make the identification of a reduction in activation of leptin receptors through the expression of pSTAT more challenging. The lack of difference between FDR and sated in STAT3 activation, and the partial recovery of leptin levels following 24 h re-feeding could also be interpreted as a local pharmacological effect of intra-VTA leptin, while under physiological conditions, circulating leptin is not involved. However, we believe that our data do not support this interpretation. We suggest a “disinhibition process” by which the lower presence of leptin removes a tonic inhibition of VTA DA neurons, but this might not be readily observed by changes in pSTAT3. The only report of a parallel reduction in plasma leptin and VTA pSTAT3 (You et al. 2016) involved concomitant exposure to cocaine. We are not aware of a similar report in naïve or drug abstinent food-restricted animals. Interestingly, although the result did not quite reach statistical significance (p = 0.06), data from the FDR rats demonstrated a strong inverse correlation (r = − 0.78) between the pSTAT3/STAT3 ratio and heroin seeking during the test. This strong correlation suggests a modulation of heroin seeking by VTA leptin receptor activation, in food-restricted rats.
It is unclear why central administration and direct infusion of leptin into the VTA resulted in different behavioral responses. One possibility is that leptin administered into the ventricles is unable to reach the local concentration for sufficient receptor activation in the VTA, and in turn drive significant behavioral effects. It has been reported that i.c.v. administration of leptin results in limited diffusion into the brain tissue, and that the affected brain areas are the ones close to a periventricular space, e.g., the hypothalamus (Maness et al. 1998). If indeed most i.c.v. leptin targets the hypothalamic nuclei, we would predict that these nuclei play a minimal role in drug seeking as their primary function is to regulate feeding and energy homeostasis (Acquas et al. 1993). Although speculative, we would predict that intra-hypothalamic leptin administration would have little or no effect on heroin seeking in the FDR rats.
Effects of blocking ghrelin receptors in the VTA on heroin seeking in FDR and sated rats
Administration of the ghrelin receptor antagonist, JMV 2959, into the VTA selectively decreased heroin seeking in chronically food-restricted rats, suggesting an important role for ghrelin receptor activation in the augmentation of heroin seeking induced by chronic food restriction. This is in contrast to previous findings in our laboratory that central administration of a ghrelin receptor antagonist had no effect on heroin self-administration, or on the reinstatement of heroin seeking induced by acute food deprivation (Maric et al. 2012). This may be explained by, first, the use of different ghrelin receptor antagonists: Maric et al. (2012) used the peptide-based GHS-R1a antagonist D-Lys3-GHRP-6 (Traebert et al. 2002), whereas here we used the small molecule-type antagonist JMV 2959 (Moulin et al. 2013). Second, Maric et al. (2012) administered the antagonist centrally, whereas in the current study, we directly targeted the VTA. Finally, as mentioned above, the neural adaptations associated with acute food deprivation, used in the previous study, versus chronic food restriction, used in this study, are distinct (Bi et al. 2003); as are the differences between the neural mechanisms mediating the reinstatement of extinguished drug seeking versus drug seeking following drug withdrawal and abstinence (Fuchs et al. 2006).
We propose that intra-VTA administration of JMV 2959 directly altered dopaminergic transmission in the downstream terminals of the mesolimbic circuit, consequentially affecting heroin seeking. It is well documented that ghrelin transmission in the VTA modulates DA activity in the mesolimbic pathway (Abizaid et al. 2006). Approximately 35–60% of neurons in the VTA that express GHS-R1a are dopaminergic, with the remainder being GABAergic (Abizaid et al. 2006; van Zessen et al. 2012). However, it seems that ghrelin also increases the activation of VTA DA neurons by increasing excitatory presynaptic glutamatergic input and decreasing inhibitory GABAergic input through rearrangement of the synaptic input (Abizaid et al. 2006). Since chronic food restriction increases plasma ghrelin levels, it is possible that this lowering of the activation threshold in VTA neurons could increase DA levels in the terminal regions of the mesolimbic pathway, such as the NAc, upon exposure to drug cues. Indeed, we have recently reported that FDR rats are more responsive to drug-associated cues, leading to increased DA transmission in the NAc and augmented heroin seeking (D’Cunha et al. 2017). Therefore, we suggest that administration of JMV 2959 blocked this ghrelin-mediated increase in VTA DA firing rates, subsequently reducing DA levels in the NAc and heroin seeking in the FDR rats. This process would also explain why JMV 2959 had no effect on heroin seeking in the sated rats, which demonstrated lower levels of circulating ghrelin.
An important consideration when interpreting the current results is that the ghrelin receptor, GHS-R1a, has constitutive activity (Holst et al. 2003; Holst and Schwartz 2004). The physiological role of this activity remains unclear, although it is hypothesized to be related to ligand-independent release of growth hormone (Holst and Schwartz 2004). Importantly, recent evidence suggests that the GHS-R1a receptor antagonist that we used, JMV 2959, does not affect growth hormone secretion, yet effectively reduces ghrelin-induced food intake (M’Kadmi et al. 2015). These findings suggest dissociated pathways that are involved in ghrelin actions on its receptor and the constitutive activity of the GHS-R1a.
Conclusion
We have demonstrated here that changes in leptin and ghrelin transmission in the VTA can modulate heroin seeking in food-restricted rats. Moreover, the rapid decrease in plasma ghrelin to baseline levels following re-feeding, concomitantly with the fast increase in plasma leptin described above, coincide nicely with the attenuation of heroin seeking in food-restricted rats re-fed for 2 h or 24 h that we have previously reported (D’Cunha et al. 2013). We suggest that chronic food restriction-induced changes in both leptin and ghrelin levels in the VTA could lead to altered DA transmission in the VTA and downstream mesolimbic pathway targets, following exposure to heroin-associated cues. These changes in leptin and ghrelin signaling in the VTA may be directly linked to the dopaminergic changes that we have observed in the NAc of food-restricted rats during the heroin-seeking test (D’Cunha et al. 2017). Future studies should investigate this putative link between leptin and ghrelin in the VTA and downstream regulation of DA in the NAc in chronically food-restricted rats.
References
Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239. https://doi.org/10.1172/JCI29867
Acquas D, Meloni M, Di Chiara G (1993) Blockade of delta opioid receptors in nucleus accumbens prevent ethanol-induced stimulation of dopamine release. Eur J Pharmacol 230:239–241
Ahima RS, Saper CB, Flier JS, Elmquist JK (2000) Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263–307. https://doi.org/10.1006/frne.2000.0197
Banks AS, Davis SM, Bates SH, Myers MG (2000) Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572
Bi S, Robinson BM, Moran TH (2003) Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol 285:R1030–R1036. https://doi.org/10.1152/ajpregu.00734.2002
Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H (2011) Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav Brain Res 219:254–264. https://doi.org/10.1016/j.bbr.2011.01.020
Carter BL, Tiffany ST (1999) Meta-analysis of cue-reactivity in addiction research. Addiction 94:327–340
Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137:73–95
D’Cunha TM, Sedki F, Macri J, Casola C, Shalev U (2013) The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacology 225:241–250. https://doi.org/10.1007/s00213-012-2810-1
D’Cunha TM, Daoud E, Rizzo D, Bishop AB, Russo M, Mourra G et al (2017) Augmentation of heroin seeking following chronic food restriction in the rat: differential role for dopamine transmission in the nucleus accumbens shell and core. Neuropsychopharmacology 42:1136–1145. https://doi.org/10.1038/npp.2016.250
de Wit H (1996) Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol 4:5–10. https://doi.org/10.1037/1064-1297.4.1.5
Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC (2006) Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147:23–30. https://doi.org/10.1210/en.2005-0973
Fernandes MF, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T, Fulton S (2015) Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab 22:741–749. https://doi.org/10.1016/j.cmet.2015.08.003
Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG (2003) Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115. https://doi.org/10.1016/S0006-8993(02)04087-8
Frias MA, Rebsamen MC, Gerber-Wicht C, Lang U (2007) Prostaglandin E2 activates Stat3 in neonatal rat ventricular cardiomyocytes: a role in cardiac hypertrophy. Cardiovasc Res 73:57–65. https://doi.org/10.1016/j.cardiores.2006.09.016
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770
Fuchs RA, Branham RK, See RE (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26:3584–3588. https://doi.org/10.1523/JNEUROSCI.5146-05.2006
Fulton S, Woodside B, Shizgal P (2000) Modulation of brain reward circuitry by leptin. Science 287:125–128
Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS (2006) Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822
Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM (1997) Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A 94:8878–8883. https://doi.org/10.1073/pnas.94.16.8878
Hall SM, Tunstall CD, Vila KL, Duffy J (1992) Weight gain prevention and smoking cessation: cautionary findings. Am J Public Health 82:799–803. https://doi.org/10.2105/AJPH.82.6.799
Holst B, Schwartz TW (2004) Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci 25(3):113–117
Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW (2003) High Constitutive Signaling of the Ghrelin Receptor—Identification of a Potent Inverse Agonist. Mol Endocrinol 17(11):2201–2210
Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone R (2006) Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810. https://doi.org/10.1016/j.neuron.2006.08.023
Hser YI, Hoffman V, Grella CE, Anglin MD (2001) A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry 58:503–508
Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D et al (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A 106:11318–11323. https://doi.org/10.1073/pnas.0812809106
Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schiöth HB, Lindblom J (2008) The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides 29:1588–1595. https://doi.org/10.1016/j.peptides.2008.04.018
Kinzig KP, Hargrave SL, Tao EE (2009) Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab 296:E282–E290. https://doi.org/10.1152/ajpendo.90523.2008
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660. https://doi.org/10.1038/45230
M’Kadmi C, Leyris JP, Onfroy L, Galés C, Saulière A, Gagne D et al (2015) Agonism, antagonism, and inverse agonism bias at the ghrelin receptor signaling. J Biol Chem 290:27021–27039. https://doi.org/10.1074/jbc.M115.659250
Maness LM, Kastin AJ, Farrell CL, Banks WA (1998) Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology 139:4556–4562. https://doi.org/10.1210/endo.139.11.6319
Maric T, Sedki F, Ronfard B, Chafetz D, Shalev U (2012) A limited role for ghrelin in heroin self-administration and food deprivation-induced reinstatement of heroin seeking in rats. Addict Biol 17:613–622. https://doi.org/10.1111/j.1369-1600.2011.00396.x
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295. https://doi.org/10.1038/nature05026
Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP (2009) The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab 297:E202–E210. https://doi.org/10.1152/ajpendo.90865.2008
Moulin A, Brunel L, Boeglin D, Demange L, Ryan J, M’Kadmi C, Denoyelle S, Martinez J, Fehrentz JA (2013) The 1,2,4-triazole as a scaffold for the design of ghrelin receptor ligands: development of JMV 2959, a potent antagonist. Amino Acids 44:301–314. https://doi.org/10.1007/s00726-012-1355-2
O’Brien CP (1997) A range of research-based pharmacotherapies for addiction. Science 278:66–70. https://doi.org/10.1126/science.278.5335.66
O’Brien CP, McLellan AT (1996) Myths about the treatment of addiction. Lancet 347:237–240
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier Academic Press, San Diego
Sedki F, Abbas Z, Angelis S, Martin J, D’Cunha T, Shalev U (2013a) Is it stress? The role of stress related systems in chronic food restriction-induced augmentation of heroin seeking in the rat. Front Neurosci 7:98. https://doi.org/10.3389/fnins.2013.00098
Sedki F, D’Cunha T, Shalev U (2013b) A procedure to study the effect of prolonged food restriction on heroin seeking in abstinent rats. J Vis Exp:e50751. https://doi.org/10.3791/50751
Shalev U (2012) Chronic food restriction augments the reinstatement of extinguished heroin-seeking behavior in rats. Addict Biol 17:691–693. https://doi.org/10.1111/j.1369-1600.2010.00303.x
Shalev U, Yap J, Shaham Y (2001) Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci 21:RC129
Shalev U, Finnie PS, Quinn T, Tobin S, Wahi P (2006) A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology 187:376–384. https://doi.org/10.1007/s00213-006-0427-y
Shen M, Jiang C, Liu P, Wang F, Ma L (2016) Mesolimbic leptin signaling negatively regulates cocaine-conditioned reward. Transl Psychiatry 6:e972. https://doi.org/10.1038/tp.2016.223
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158:343–359
Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL (2011) Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180:129–137. https://doi.org/10.1016/j.neuroscience.2011.02.016
Thompson JL, Borgland SL (2013) Presynaptic leptin action suppresses excitatory synaptic transmission onto ventral tegmental area dopamine neurons. Biol Psychiatry 73:860–868. https://doi.org/10.1016/j.biopsych.2012.10.026
Tobin S, Sedki F, Abbas Z, Shalev U (2013) Antagonism of the dopamine D1-like receptor in mesocorticolimbic nuclei attenuates acute food deprivation-induced reinstatement of heroin seeking in rats. Eur J Neurosci 37:972–981. https://doi.org/10.1111/ejn.12112
Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S (2001) Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281:1220–1225. https://doi.org/10.1006/bbrc.2001.4518
Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA (2002) Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol 14:580–586
Trinko R, Gan G, Gao XB, Sears RM, Guarnieri DJ, DiLeone RJ (2011) Erk1/2 mediates leptin receptor signaling in the ventral tegmental area. PLoS One 6:e27180. https://doi.org/10.1371/journal.pone.0027180
van Zessen R, van der Plasse G, Adan RA (2012) Contribution of the mesolimbic dopamine system in mediating the effects of leptin and ghrelin on feeding. Proc Nutr Soc 71:435–445. https://doi.org/10.1017/S0029665112000614
You ZB, Wang B, Liu QR, Wu Y, Otvos L, Wise RA (2016) Reciprocal inhibitory interactions between the reward-related effects of leptin and cocaine. Neuropsychopharmacology 41:1024–1033. https://doi.org/10.1038/npp.2015.230
Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z et al (2019) Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab 30:706–719.e6. https://doi.org/10.1016/j.cmet.2019.08.005
Acknowledgments
The authors wish to thank Melissa Russo, Soraya le Noble, Damaris Rizzo, and Emilie Daoud for invaluable technical assistance throughout this project.
Funding
This work was supported by the Natural Sciences & Engineering Council Discovery Program (US: 298915), the Fonds de recherche du Quebec - Santé (CSBN), the Canada Research Chairs program (US), and a CIHR grant and FRQS research scholar award to SF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
D’Cunha, T.M., Chisholm, A., Hryhorczuk, C. et al. A role for leptin and ghrelin in the augmentation of heroin seeking induced by chronic food restriction. Psychopharmacology 237, 787–800 (2020). https://doi.org/10.1007/s00213-019-05415-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05415-9