Abstract
Rationale
Acute 1-day food deprivation reinstates heroin seeking in rats via a leptin-dependent mechanism. However, leptin has no effect on footshock- or heroin-priming-induced reinstatement of drug seeking. These data may indicate that the neuronal systems underlying food-deprivation-induced reinstatement are dissociable from those involved in reinstatement induced by footshock stress.
Objectives
We used the reinstatement procedure to examine the roles of the adrenal stress hormone, corticosterone, and brain corticotropin-releasing factor (CRF) in acute food-deprivation-induced reinstatement of extinguished heroin seeking in rats.
Materials and methods
The rats were trained to press a lever for heroin (0.05−0.1 mg/kg/infusion, i.v.) for 10 days. Experiment 1: After heroin self-administration training, the rats were divided into two groups, which received either bilateral adrenalectomy surgery or sham surgery. Next, the rats were given 7–10 days of extinction training (during which lever presses were not reinforced with heroin). The rats were subsequently tested for reinstatement after acute (21 h) food deprivation. Experiment 2: After heroin self-administration and extinction training, the rats were tested for reinstatement induced by acute food deprivation. Before the test session, the rats were given intracerebroventricular injections of the CRF receptor antagonist α-helical CRF (0, 3, or 10 μg/rat).
Results
Adrenalectomy had no effect on the extinction behavior or acute food-deprivation-induced reinstatement of heroin seeking. The CRF receptor antagonist, α-helical CRF, dose-dependently blocked food-deprivation-induced reinstatement.
Conclusions
The present data suggest that, as demonstrated for footshock-induced reinstatement of drug seeking, brain CRF, but not corticosterone, plays a critical role in acute food-deprivation-induced reinstatement of heroin seeking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to stressful life events is thought to be important for the induction of craving and relapse to drugs in humans. Thus, drug abusers often cite stress and negative affect as reasons for relapse to drug use (Sinha 2001). Using a reinstatement model of drug relapse (de Wit and Stewart 1981; Stewart and de Wit 1987) it was demonstrated that exposure to footshock stress reinstates extinguished drug-seeking behavior in rats trained to self-administer heroin, cocaine, nicotine, and alcohol (Shaham et al. 2003), and that administration of the anxiogenic drug, yohimbine, reinstates methamphetamine seeking (Shepard et al. 2004).
Food deprivation is an environmental stressor and, in laboratory animals, there is considerable evidence that acute and chronic food restriction or food deprivation facilitate the initiation and maintenance of drug-reinforced behavior (Carroll and Meisch 1984; Piazza and Le Moal 1998; Lu et al. 2003), and potentiate opiate and psychostimulant reward (Carr and Simon 1984; Bell et al. 1997; Carr 2002; Stuber et al. 2002). These findings seem relevant to the human condition as food deprivation has been anecdotally linked to increased drug intake and relapse (Hanna and Hornick 1977; Carroll 1985). Similarly, caloric restriction has been reported to increase cigarette smoking, and the risk for relapse in abstinent smokers (Hall et al. 1992; Cheskin et al. 2005), but see Zacny and de Wit (1992) for different results.
We have previously demonstrated reliable reinstatement of heroin and cocaine seeking after exposure to acute, 1-day food deprivation (Shalev et al. 2000, 2003) via a leptin-dependent mechanism. Thus, food deprivation-induced reinstatement of heroin seeking was completely blocked by intracerebroventricular administration of leptin (Shalev et al. 2001). The latter finding is in agreement with a previous study showing that central administration of leptin reverses chronic food restriction-induced sensitization of lateral hypothalamic brain stimulation reward (Fulton et al. 2000). Interestingly, leptin administration had no effect on footshock- or heroin priming-induced reinstatement of heroin seeking (Shalev et al. 2001). We have, therefore, suggested that the neuronal mechanisms that underlie food deprivation-induced reinstatement may be dissociable from those involved in reinstatement induced by footshock stress or drug priming (Shalev et al. 2002). However, to confirm (or refute) this hypothesis, “double dissociation” should be demonstrated. That is, pharmacological agents known to block footshock- or drug priming-induced reinstatement should not attenuate or block reinstatement induced by food deprivation. It is now well established that the adrenal stress-hormone, corticosterone, has a permissive (modulatory) role in footshock-induced reinstatement of cocaine seeking, which is dissociated from the stressor-induced increases in corticosterone levels, and, furthermore, corticosterone is not involved in heroin reinstatement (Shaham et al. 1997a; Erb et al. 1998, 2001a). On the other hand, extrahypothalamic corticotropin-releasing factor (CRF) is critically involved in footshock-induced reinstatement. Thus, the administration of CRF-receptor antagonists attenuates footshock-induced reinstatement in heroin-, cocaine-, and alcohol-trained rats (Shaham et al. 1997a, 1998, 2000a; Erb et al. 1998, 2001b; Le et al. 2000; Wang et al. 2005). In addition, central administration of CRF reinstates drug seeking in heroin- (Shaham et al. 1997a) and cocaine-trained (Erb and Stewart 1999) rats. Finally, CRF-receptor antagonists have only minimal effects on cocaine, and heroin-priming-induced reinstatement of drug seeking (Shaham et al. 1997a; Erb et al. 1998).
Food deprivation is an aversive event that is known to activate the hypothalamic–pituitary–adrenal (HPA) axis in animals, resulting in an increased secretion of corticosterone (Marinelli et al. 1996; Dallman et al. 1999). We have recently demonstrated that a food-deprivation-induced increase in corticosterone release is not necessary for reinstatement of cocaine seeking by acute food deprivation (Shalev et al. 2003). However, the role of corticosterone and CRF in food-deprivation-induced reinstatement of heroin seeking is unknown.
In the present study, we examined the involvement of corticosterone in acute food-deprivation-induced reinstatement of extinguished heroin seeking using adrenalectomized (ADX) and sham-operated rats. In addition, we tested the effect of blocking the CRF receptors in the brain by pretreatment with the CRF antagonist α-helical CRF on reinstatement induced by acute food deprivation. Our hypotheses were that food-deprivation-induced release of corticosterone is not necessary for the reinstatement of heroin seeking and that CRF is not involved in food deprivation-induced reinstatement. We therefore expected to see no effect for adrenalectomy or the blocking of CRF receptors on food-deprivation-induced reinstatement of heroin seeking.
Materials and methods
Experiment 1: adrenalectomy
Subjects
Ten male Long–Evans rats (Charles River, Raleigh, NC, USA; 350–400 g) were used. At 5 to 7 days after surgery for implantation of the intravenous (IV) catheters, the rats were transferred to the self-administration chambers where they were chronically housed under a reversed 12-h:12-h light–dark cycle (lights on at 10:00 P.M.). Water and food were freely available, except when food deprivation conditions were applied (see below). The body weight of the rats was measured daily. The experimental procedures followed the “Principles of laboratory animal care” (NIH publication no. 85-23, 1996) and were approved by the Animal Care and Use Committee of IRP/NIDA.
Surgery
The rats were implanted under anesthesia (a mixture of xylazine + ketamine, 10 + 100 mg/kg, i.p.) with IV Silastic catheters (Dow Corning, Midland, MI, USA) into the right jugular vein as previously described (Shalev et al. 2000). In brief, the catheter was secured to the vein with a silk suture and passed subcutaneously to the top of the skull, where it was connected to a modified 22-gauge cannula (Plastics One, Roanoke, VA, USA). The cannula was mounted to the skull with jeweler screws and dental cement. After surgery, the catheters were flushed every 24–48 h with 0.08 mg/ml gentamicin in sterile saline (0.1 ml). If catheter patency was suspected, a very small dose of xylazine + ketamine (0.25+2.5 mg/kg) was injected through the catheter. This caused an immediate and short-lasting (1−2 min) anesthetic effect.
Bilateral ADX was rapidly (3−5 min) performed via the dorsal approach with the rats under isoflurane (Abbot Laboratories, North Chicago, IL, USA) anesthesia, between 10:00 P.M. and 12:00 A.M. The time of surgery and surgical procedure for ADX followed the procedure described by Marinelli et al. (1997). Sham-operated rats were exposed to the same procedure as the ADX rats, with the exception that the adrenal glands were not removed. After surgery, the ADX rats were given physiological saline in their drinking bottles.
Apparatus
The experiment was conducted in self-administration chambers. Each chamber had two levers located 9 cm above the floor, but only one lever (an “active”, retractable lever) activated the infusion pump (Razel, Stamford, CT, USA). Presses on the other lever (an “inactive”, stationary, non-retractable lever) were recorded but did not activate the pump. The cannula on the rat’s skull was connected to a liquid swivel with polyethylene-50 tubing that was protected by a metal spring. Outside of the chamber, the swivel was connected to a 20-ml syringe on the infusion pump.
Procedure
The experiment included three phases: self-administration training, extinction training, and tests for reinstatement. Self-administration training was conducted over a period of 10 days, three 3-h sessions/day that were separated by 3 h. The first session of each day started at the onset of the dark cycle. Each session began with the insertion of the active lever into the chamber and the illumination of a cue light above this lever for 30 s. A red houselight was turned on for the entire session. Each response on the active lever resulted in the delivery of 0.1 mg/kg (first 6 days) or 0.05 mg/kg (last 4 days) of heroin and the initiation of a 20-s timeout period. During this period, lever presses were not reinforced, and the cue light was turned on. At the end of each session, the houselight was turned off and the active lever was retracted. The dose of heroin was reduced to 50% of the initial training dose during the last 4 days of training to verify that the rats acquired robust heroin-taking behavior as indicated by an increase in responding to compensate for the decrease in the drug dose (see, for a review, Yokel 1987).
After training, the rats were left undisturbed for 2 days. The rats were divided into two corticosterone manipulation groups: ADX or sham surgery. The groups were matched for a similar heroin infusion rate at the end of the training phase.
After ADX surgery and 2 days of recovery, the extinction phase started. During the extinction phase, the conditions were identical to those of the training phase, with the exception that the heroin syringes were removed. For the first 3 days, the rats were given three 3-h extinction sessions/day. The number of sessions was subsequently reduced to one 3-h session/day, and the rats were given four to six daily extinction sessions until they reached the extinction criterion of 20 responses or less on the previously active lever. At this point, the rats were tested for food-deprivation-induced reinstatement under extinction conditions. Food deprivation was accomplished by the removal of food from the food hoppers for 21 h before the 3-h test session. The food hoppers were refilled at the end of the test session.
Plasma corticosterone determination
At the end of the experiment, the rats were rapidly decapitated during the first hour of the dark cycle (the time of onset for the first daily training session and for the reinstatement test). Trunk blood was collected into heparinized vials, and plasma was removed and stored at −70 C. Plasma samples were then analyzed for corticosterone levels by a radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA, USA). The assay was carried out in Dr. Michael H. Baumann’s laboratory at the Medication Discovery Research Branch, IRP/NIDA/NIH. The intra- and interassay coefficients of variation commonly observed in Dr. Baumann’s laboratory for corticosterone assays are 3.0 and 10.0%, respectively.
Experiment 2: α-helical CRF
Subjects and apparatus
Due to the setting of the laboratory in a new location (Concordia University, Montreal, Quebec, Canada), several minor changes have been introduced to the apparatus and procedure. Thirty-three male Long–Evans rats (Charles River, Canada; 350–400 g) were used. The rats were handled as described above, but the self-administration chambers (Coulbourn Instruments, Allentown, PA, USA) had two retractable levers. The experimental procedures followed the principles established in the Canadian Council on Animal Care’s Guide to the Care and Use of Experimental Animals and were approved by the Animal Research Ethics Committee of Concordia University.
Surgery
The rats were implanted with IV Silastic catheters into the right jugular vein as described above. For each rat, a guide cannula (23-gauge, Plastics One, Roanoke, VA, USA) was also implanted during the IV surgery. The cannula was aimed 2 mm above one of the lateral ventricles: −0.9 mm posterior, 1.4 mm lateral, and 2.0 mm ventral to bregma (Paxinos and Watson 2005). After surgery, the catheters were flushed every 24–48 h with 0.08 mg/ml gentamicin in sterile saline (0.1 ml). At the end of the experiments, cannulae placement was verified by demonstrating a short-latency (<60 s) drinking response to angiotensin II (100 ng, ICV).
Procedure
The training and extinction procedures for heroin self-administration differed to some degree from those used in experiment 1. Three groups of rats (n=10–12 per group) were trained to self-administer heroin (0.1 and 0.05 mg/kg per infusion), as described above, over a period of 10 days, with three 3-h sessions/day that were separated by 4 h. Extinction training was initiated immediately after the conclusion of the training phase and was conducted over a period of 10–12 days, with three 3-h session/day for the first 5 days followed by 5–7 days of one 3-h session/day, until the rats reached the extinction criterion of 15 responses or less on the previously active lever. The rats were tested twice, 48 h apart, for reinstatement of drug seeking: after 21-h food deprivation and under food-sated conditions, in a counterbalanced order. The rats were tested 10 min after ICV injection of one of three doses of α-helical CRF (vehicle, 3 or 10 μg), so that each rat was tested with only one dose of the antagonist. In between the two test sessions, a regular extinction session was conducted.
Drugs and injection procedure
Heroin (diacetylmorphine HCl; experiment 1: NIDA, NIH, experiment 2: Almat Pharma Chem, Concord, Ontario, Canada) was dissolved in sterile physiological saline. α-Helical CRF was obtained from Sigma (Canada) and dissolved in distilled water. The drug was injected ICV at a volume of 2 μl over a period of 2 min, using an injector (28 gauge) that extended 2 mm below the tip of the guide cannula. The injector was retained in position for an additional 60 s after the injection. The doses of α-helical CRF are based on previous reports (Shaham et al. 1997a).
Statistical analysis
Experiment 1
The number of responses on the previously active lever and the number of responses on the inactive lever made during the first 3 days of extinction (9 h/day) were analyzed separately with repeated measures ANOVA using a between-subject factor of group (sham, ADX) and a within-subject factor of day (1–3). Data from the tests for reinstatement were analyzed separately for responses on the active lever and responses on the inactive lever. The last day of extinction served as the baseline sated condition, against which the effect of food deprivation on lever pressing behavior was compared. Data were analyzed with a repeated-measures ANOVA, using a between-subjects factor of group (sham, ADX) and a within-subjects factor of food deprivation (food deprived and food sated). Significant differences are reported for p≤0.05.
Experiment 2
Active and inactive responses made during the tests for reinstatement were analyzed separately, as described above. The numbers of responses made under food deprivation or sated conditions, after α-helical CRF administration, were compared. Data were analyzed with repeated-measures ANOVA using a between-subject factor of α-helical CRF dose (vehicle, 3, or 10 μg) and a within-subject factor of food deprivation (food deprived and food sated). ANOVA was followed by post-hoc tests (Fisher’s protected least-significant difference) where appropriate. Significant results are reported for p≤0.05.
Results
Experiment 1: adrenalectomy
One rat from the sham group was excluded from the analyses due to an extremely high number of responses on the active lever during the food deprivation test (552 responses compared to a mean of 183 lever presses in the sham group).
Training and extinction phases
The rats demonstrated reliable heroin self-administration as indicated by an increase in the number of infusions when the unit dose was reduced from 0.1 to 0.05 mg/kg/infusion. The mean±SEM numbers of infusions taken on the last day (9 h) with a unit dose 0.1 mg/kg/infusion in the ADX (n=5) and sham (n=4) groups were 62.2±6.4 and 56.2±6.8, respectively. The mean±SEM numbers of infusions taken on the last day with 0.05 mg/kg/infusion unit−dose in the ADX and sham groups were 94.4±12.2 and 80.5±12.8, respectively. Figure 1a shows the mean±SEM number of lever presses on the previously active lever made during the extinction phase for rats from the ADX and sham groups. The number of active lever responses decreased rapidly after the first day of extinction. An analysis performed on the number of active lever responses during the first 3 days of extinction showed a significant effect of day (F (2,14)=22.6, p<0.01), but no significant group or group × day effects. The number of responses on the inactive lever was very low in both groups: a mean of 2.8 and 0.0 for the ADX and sham groups, respectively, on the first extinction day.
Extinction and reinstatement of heroin seeking in adrenalectomized (ADX, n=5) and sham-operated (n=4) rats. aExtinction: mean±SEM number of lever presses on the previously active and inactive lever during extinction of heroin-seeking behavior. Extinction training duration was 9 h (three 3 h sessions/day) for extinction days 1–3 and 3 h (one 3 h session/day) for extinction days 4–7. b Test for reinstatement: mean±SEM number of responses on the previously active lever during 3-h test sessions under sated condition and after 21 h of food deprivation. *p<0.01, significantly different from sated condition
Test for reinstatement
Figure 1b shows the mean±SEM number of presses on the previously active lever made during the 3-h test for reinstatement after 21 h of food deprivation in the ADX and sham groups, and the mean±SEM number of active lever responses made during the last day of extinction (baseline). Adrenalectomy had no effect on 21-h food-deprivation-induced reinstatement of heroin seeking. ANOVA performed on the number of active lever responses revealed a significant effect of food deprivation (F (1,7)=20.8, p=0.003). No significant differences were observed for inactive lever presses and the number of responses was very low (all group means below four responses/3 h).
Complete adrenalectomy was verified by radioimmunoassay which showed levels of plasma corticosterone that were below the detection level of the assay (10 ng/ml).
Experiment 2: α-helical CRF
Seven out of the 33 rats were excluded from the analyses due to poor health (four rats) and incorrect placement of the guide cannula (three rats). Thus, the number of rats in each α-helical CRF dose group that were included in the final analyses was n=7, n=10, and n=9 for the vehicle, 3-μg-treatment, and 10-μg-treatment groups, respectively.
Training and extinction phases
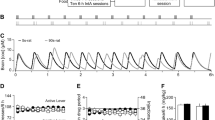
The rats demonstrated reliable heroin self-administration as indicated by an increase in the number of infusions when the unit dose was reduced from 0.1 to 0.05 mg/kg/infusion. The mean±SEM numbers of infusions taken on the last day (9 h) with a unit dose 0.1 mg/kg/infusion in the vehicle, 3-μg-treatment, and 10-μg-treatment groups were 29.7±2.9, 20.4±2.2, and 24.0±4.5, respectively. The mean±SEM numbers of infusions taken on the last day with 0.05 mg/kg/infusion unit-dose in the vehicle, 3-μg-treatment, and 10-μg-treatment groups were 49.7±8.8, 35.5±3.3, and 40.3±4.1 respectively. Figure 2a shows the mean±SEM number of lever presses on the previously active and inactive levers made during the extinction phase. The active and inactive lever presses decreased significantly over the first 5 days with three 3 h/day sessions (F (4,100)=23.2, p<0.01; F (4,100)=10.6, p<0.01, respectively) and remained low over the following five 3-h sessions (p>0.1).
Extinction and reinstatement of heroin seeking. a Extinction: mean±SEM number of lever presses on the previously active lever and on the inactive lever during extinction of heroin-seeking behavior. Extinction training duration was 9 h (three 3 h sessions/day) for extinction days 1–5, and 3 h (one 3 h session/day) for extinction days 6–10. Test for reinstatement in the vehicle (Veh, n=7), 3-μg-treatment (n=10), and 10-μg-treatment (n=9) groups: mean±SEM number of responses b on the previously active lever and c on the inactive lever during 3-h test sessions under sated condition and after 21 h of food deprivation. α-Helical CRF or vehicle was injected ICV 10 min before the 3-h test session. *p≤0.05, significantly different from sated condition; **p<0.05, significantly different from the 3- and 10-μg-treatment groups under food deprivation condition; *** p<0.01, significantly different from the 3-μg-treatment group under sated condition
Test for reinstatement
Pretreatment with both the 3 and 10 μg doses of α-helical CRF substantially attenuated the food-deprivation-induced increase in responding on the previously active lever that was observed in the vehicle group (Fig. 2b). ANOVA performed on the number of active lever responses during the 3-h test session revealed significant effects of food deprivation (F (1,23)=15.7, p=0.0006), α-helical CRF dose (F (2,23)=4.2, p=0.03), and food deprivation × α-helical CRF dose (F (2,23)=5.1, p=0.01). The number of presses on the inactive lever was low in all groups and was not affected by the experimental manipulation (Fig. 2c). The statistical analysis indicates that pretreatment with α-helical CRF attenuated food-deprivation-induced reinstatement of heroin seeking in a dose-dependent way. Post-hoc tests revealed significant differences between the number of active lever responses under food deprivation compared to food-sated condition for the rats pretreated with vehicle and 3 μg α-helical CRF (p≤0.05), but not the 10-μg-treatment pretreated rats (p>0.1). In addition, within the food deprivation condition, the number of active lever responses in the vehicle-pretreated rats was significantly higher than in the 3- and 10-μg α-helical CRF pretreated rats (p<0.05). Finally, within the sated condition, the number of active lever presses in the 10-μg-treatment group was slightly, though significantly, higher than in the 3-μg-treatment group (p<0.01). The reasons for this effect are unknown.
Discussion
The first major finding in this study is that corticosterone is not involved in reinstatement of heroin seeking by acute, 1-day food deprivation. Although acute food deprivation is known to activate the HPA axis, resulting in an enhanced secretion of corticosterone (Dallman et al. 1999), abolishing the food-deprivation-induced increase in plasma corticosterone by adrenalectomy did not interfere with the ability of food deprivation to reinstate heroin seeking. This observation is somewhat different from our findings with cocaine-trained rats (Shalev et al. 2003) but is in accordance with the effect of adrenalectomy on footshock-induced reinstatement of heroin seeking (Shaham et al. 1997a). In our previous study (Shalev et al. 2003), we found that adrenalectomy significantly attenuated the food-deprivation-induced reinstatement of cocaine seeking, and that this effect was completely reversed by restoring corticosterone plasma to a basal level with corticosterone replacement. We argued that these results support the suggested permissive role of corticosterone in stress-induced reinstatement of cocaine seeking, where a minimal level of corticosterone is necessary for reinstatement, but the stressor-induced increase in corticosterone levels does not play a role in the stressor’s effect on reinstatement (Erb et al. 1998; Shalev et al. 2003). In heroin-trained rats, however, adrenalectomy does not attenuate reinstatement induced by footshock (Shaham et al. 1997a) or acute food deprivation (present study). Thus, corticosterone is not necessary for footshock- or food-deprivation-induced reinstatement of heroin seeking. Additional support for a minor role for corticosterone in stress-induced reinstatement of drug seeking comes from studies showing that the activation of the HPA axis is not sufficient to induce reinstatement. For example, we have previously shown that exposing rats to footshock in an environment that was not associated with drug self-administration does not result in reinstatement of extinguished heroin seeking (Shalev et al. 2000). Exposure to a different stressor, restraint, which potently activates the HPA axis (Kant et al. 1985) also failed to induce reinstatement (Shalev et al. 2000). Finally, exposing male rats to highly arousing stimulus, sexually active females, which increases plasma corticosterone levels (Bonilla-Jaime et al. 2006), had no effect on the reinstatement of drug seeking (Shaham et al. 1997b).
No group differences were observed for lever pressing behavior during the extinction phase. These data are in agreement with previous reports from our laboratory and others’ on the lack of effect for adrenalectomy on extinction responding in rats previously trained to self-administer cocaine (Erb et al. 1998; Shalev et al. 2003), heroin (Shaham et al. 1997a), alcohol (Le et al. 2000), or non-drug reinforcers (Mason 1983). However, see Micco et al. (1979) and Thomas and Papini (2001) for findings indicating some modulation by corticosterone of extinction behavior previously reinforced by non-drug reward.
Our second major finding is that the CRF receptor antagonist α-helical CRF, administered intracerebroventricularly, suppressed acute food-deprivation-induced reinstatement of heroin seeking. These data extend previous findings on the role of CRF in footshock-induced reinstatement of heroin and cocaine seeking (Shaham et al. 1997a; Erb et al. 1998; Lu et al. 2000). In contrast to a previous report with footshock-induced reinstatement (Shaham et al. 1997a), the suppression of food-deprivation-induced reinstatement of heroin seeking was complete, indicating a critical role of CRF in this effect.
The differential role of corticosterone in food-deprivation-induced reinstatement of cocaine vs heroin seeking
The differential effect of adrenalectomy on food-deprivation-induced reinstatement of heroin and cocaine seeking was addressed by Erb et al. (1998) when discussing a similar effect after footshock-induced reinstatement in adrenalectomized rats. These authors suggested that state dependency develops over training with cocaine, as corticosterone is repeatedly released after each infusion, showing no signs of tolerance (Torres and Rivier 1992). In contrast, corticosterone response to repeated opioid agonists shows complete tolerance (Pechnick 1993). Thus, some minimal level of corticosterone may be required to maintain responding in cocaine-trained rats, especially after the extinction of cocaine-related cues. However, it should be noted that a similar scenario could be hypothesized for reinstatement induced by a priming injection of the drug in heroin- or cocaine-trained rats. Yet, adrenalectomy does not affect priming-induced reinstatement of cocaine or heroin seeking (Shaham et al. 1997a; Erb et al. 1998). At this time, the differential effect of adrenalectomy on food-deprivation-induced reinstatement (and stress-induced reinstatement in general) of heroin and cocaine seeking still awaits clarification.
The effect of CRF receptor blockade on food-deprivation-induced reinstatement of heroin seeking
As mentioned above, the current data extend previous findings with footshock-induced reinstatement of drug seeking. It has been shown that ICV administration of the nonselective CRF antagonists α-helical CRF and d-Phe-CRF and systemic injections of the specific CRF1 receptor antagonist CP-154,526 attenuate footshock-induced reinstatement of heroin, cocaine, and alcohol seeking (Shaham et al. 1997a, 1998; Erb et al. 1998; Le et al. 2000). Blockade of the CRF receptors and specifically the CRF1 receptor similarly attenuates footshock-induced reinstatement of morphine- and cocaine-conditioned place preference (Lu et al. 2000, 2001).
Data from neuroanatomical studies indicate that the effects of footshock stress on reinstatement of drug seeking involve the CRF-containing projection from the central amygdala to the bed nucleus of stria terminalis (BNST) (Erb and Stewart 1999; Erb et al. 2001a), and the noradrenergic (NA) projection from lateral tegmental NA nuclei via the ventral NA bundle (Shaham et al. 2000b). A role for CRF in the BNST in stress-induced reinstatement is further supported by recent findings from Wang et al. (2006) that demonstrated blockade of footshock-induced reinstatement of morphine-conditioned place preference with intra-BNST injections of CRF-1 receptor antagonist. In addition, a role for CRF in the ventral tegmental area (VTA) in stress-induced reinstatement was recently demonstrated in cocaine-trained rats. Thus, footshock-induced reinstatement of cocaine seeking was blocked by intra-VTA injection of the CRF receptor antagonist α-helical CRF (Wang et al. 2005). In as much as the acute food-deprivation-induced reinstatement of drug seeking is due to its stress-like properties, it is possible that this effect is mediated by the same CRF-containing pathways and NA projections, briefly reviewed above, that are involved in footshock-induced reinstatement. In support of this suggestion, it was reported that starvation (7 days) increases CRF concentration in extra-hypothalamic sites such as the midbrain, the thalamus, and the neurointermediate lobe of the pituitary (Suemaru et al. 1986). Moreover, an acute 24-h food deprivation was reported to increase NA utilization in the hypothalamus (Stanley et al. 1989), a region which receives its major NA input from the same NA projection implicated in footshock-induced reinstatement (Moore and Bloom 1979). The effect of acute food deprivation on extrahypothalamic CRF levels has not been studied.
It could be argued that the effect of α-helical CRF on reinstatement of drug seeking is due to some generalized behavioral effect. However, this seems unlikely, as administration of the CRF antagonist does not affect locomotor activity (Diamant and de Wied 1991). Moreover, a low dose (3 μg) of α-helical CRF had only a small attenuating effect on the reinstatement of heroin seeking, in the self-administration procedure, after a priming injection of heroin, while the high dose (10 μg) had no effect on priming-induced reinstatement (Shaham et al. 1997a).
An alternative explanation for the effects of α-helical CRF on food-deprivation-induced reinstatement might involve a direct effect on appetite or the level of hunger perceived by the food-deprived rats. CRF is known to affect appetite through CRF receptors in the hypothalamus (Lawrence et al. 1999). However, this reasoning also seems unlikely as CRF is a potent anorexigen (Lawrence et al. 1999), and the administration of CRF-receptor antagonists results in orexigenic effect (Heinrichs and Koob 1992), which would likely act to enhance the food deprivation impact on reinstatement rather than block its effect.
Conclusion
We found that abolishing the food-deprivation-induced increase in corticosterone release has no effect on food-deprivation-induced reinstatement of heroin seeking. We also demonstrated that the administration of a CRF antagonist blocks the effects of acute food deprivation on reinstatement of heroin seeking. These findings extend previous results on the critical role of CRF in stress-induced reinstatement of drug seeking and the minor role for corticosterone release (Shaham et al. 2003). Contrary to our hypothesis, our present findings seem to suggest that both footshock-induced reinstatement of drug seeking (Shaham et al. 2003) and reinstatement induced by acute food deprivation are mediated by common stress-related neuronal pathways. Nevertheless, as most recent neuroanatomical findings indicate, there is a large degree of overlap among brain sites that mediate reinstatement induced by drug priming, drug-associated cues, and stress (Bossert et al. 2005). It is therefore possible that, in addition to the demonstrated activation of stress-related neuronal systems, food deprivation might also trigger reinstatement of drug seeking by accessing neuronal pathways other than those implicated in stress-induced reinstatement. For example, altering the internal need state of the organism by dietary manipulation can change the incentive motivating effects of external stimuli. This effect has been shown to generalize to rewards other than food, such as drugs and electrical brain stimulation (Carroll 1985; Carr 1996). Thus, as previously suggested by Stewart et al. (1984), it is possible that food deprivation “reinstates” the incentive motivational value of the extinguished drug-related cues, resulting in the reinstatement of drug seeking behavior.
References
Bell SM, Stewart RB, Thompson SC, Meisch RA (1997) Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 131:1–8
Bonilla-Jaime H, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S (2006) Hormonal responses to different sexually related conditions in male rats. Horm Behav 49:376–382
Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y (2005) Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol 526:36–50
Carr KD (1996) Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res 21:1455–1467
Carr KD (2002) Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 76:353–364
Carr KD, Simon EJ (1984) Potentiation of reward by hunger is opioid mediated. Brain Res 297:369–373
Carroll ME (1985) The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend 16:95–109
Carroll ME, Meisch ME (1984) Increased drug-reinforced behavior due to food deprivation. In: Thompson T, Dews PB, Barrett JE (eds) Advances in behavioral pharmacology. Academic, New York, pp 47–88
Cheskin LJ, Hess JM, Henningfield J, Gorelick DA (2005) Calorie restriction increases cigarette use in adult smokers. Psychopharmacology (Berl) 179:430–436
Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A et al (1999) Starvation: early signals, sensors, and sequelae. Endocrinology 140:4015–4023
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143
Diamant M, de Wied D (1991) Autonomic and behavioral effects of centrally administered corticotropin-releasing factor in rats. Endocrinology 129:446–454
Erb S, Stewart J (1999) A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19(RC35):1–6
Erb S, Shaham Y, Stewart J (1998) The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 14:5529–5536
Erb S, Salmaso N, Rodaros D, Stewart J (2001a) A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 158:360–365
Erb S, Shaham Y, Stewart J (2001b) Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress 4:289–303
Fulton S, Woodside B, Shizgal P (2000) Modulation of brain reward circuitry by leptin. Science 287:125–128
Hall SM, Tunstall CD, Vila KL, Duffy J (1992) Weight gain prevention and smoking cessation: cautionary findings. Am J Public Health 82:799–803
Hanna JM, Hornick CA (1977) Use of coca leaf in southern Peru: adaptation or addiction. Bull Narc 29:63–74
Heinrichs SC, Koob GF (1992) Corticotropin-releasing factor modulates dietary preference in nutritionally and physically stressed rats. Psychopharmacology (Berl) 109:177–184
Kant GJ, Eggleston T, Landman-Rorbers L, Kenion CC, Driver GC, Meyerhoff JL (1985) Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav 22:631–634
Lawrence CB, Turnbull AV, Rothwell NJ (1999) Hypothalamic control of feeding. Curr Opin Neurobiol 9:778–783
Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y (2000) The role of corticotropin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150:317–324
Lu L, Ceng X, Huang M (2000) Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence in rats. Neuroreport 11:2373–2378
Lu L, Liu D, Ceng X (2001) Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol 415:203–208
Lu L, Shepard JD, Scott Hall F, Shaham Y (2003) Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev 27:457–491
Marinelli M, Le Moal M, Piazza PV (1996) Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res 724:251–255
Marinelli M, Rouge-Pont F, Deroche V, Barrot M, De Jesus-Oliveira C, Le Moal M et al (1997) Glucocorticoids and behavioral effects of psychostimulants. I: locomotor response to cocaine depends on basal levels of glucocorticoids. J Pharmacol Exp Ther 281:1392–1400
Mason ST (1983) The neurochemistry and pharmacology of extinction behavior. Neurosci Biobehav Rev 7:325–347
Micco DJ Jr, McEwen BS, Shein W (1979) Modulation of behavioral inhibition in appetitive extinction following manipulation of adrenal steroids in rats: implications for involvement of the hippocampus. J Comp Physiol Psychol 93:323–329
Moore RY, Bloom FE (1979) Central catecholaimine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci 2:113–168
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier Academic, Burlington
Pechnick RN (1993) Effects of opioids on the hypothalamo–pituitary–adrenal axis. Annu Rev Pharmacol Toxicol 33:353–382
Piazza PV, Le Moal M (1998) The role of stress in drug self-administration. Trends Pharmacol Sci 19:67–74
Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J (1997a) Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17:2605–2614
Shaham Y, Puddicombe J, Stewart J (1997b) Sexually arousing events do not induce relapse to heroin-seeking in sexually experienced male rats. Physiol Behav 61:337–341
Shaham Y, Erb S, Leung S, Buczek Y, Stewart J (1998) CP-154,526, a selective, non peptide antagonist of the corticotropin-releasing factor type 1 receptor attenuates stress-induced relapse to drug seeking in cocaine-and heroin-trained rats. Psychopharmacology (Berl) 137:184–190
Shaham Y, Erb S, Stewart J (2000a) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33:13–33
Shaham Y, Highfield D, Delfs J, Leung S, Stewart J (2000b) Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 12:292–302
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3–20
Shalev U, Highfield D, Yap J, Shaham Y (2000) Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 150:337–346
Shalev U, Yap J, Shaham Y (2001) Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci 21(RC129):1–5
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y (2003) The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 168:170–176
Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55:1082–1089
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359
Stanley BG, Schwartz DH, Hernandez L, Hoebel BG, Leibowitz SF (1989) Patterns of extracellular norepinephrine in the paraventricular hypothalamus: relationship to circadian rhythm and deprivation-induced eating behavior. Life Sci 45:275–282
Stewart J, de Wit H (1987) Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA (ed) Methods of assessing the reinforcing properties of abused drugs. Springer, Berlin Heidelberg New York, pp 211–227
Stewart J, de Wit H, Eikelboom R (1984) Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91:251–268
Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP (2002) Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse 46:83–90
Suemaru S, Hashimoto K, Hattori T, Inoue H, Kageyama J, Ota Z (1986) Starvation-induced changes in rat brain corticotropin-releasing factor (CRF) and pituitary–adrenocortical response. Life Sci 39:1161–1166
Thomas BL, Papini MR (2001) Adrenalectomy eliminates the extinction spike in autoshaping with rats. Physiol Behav 72:543–547
Torres G, Rivier C (1992) Differential effects of intermittent or continuous exposure to cocaine on the hypothalamic–pituitary–adrenal axis and c-fos expression. Brain Res 571:204–211
Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB (2005) Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci 25:5389–5396
Wang J, Fang Q, Liu Z, Lu L (2006) Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 185:19–28
Yokel RA (1987) Intravenous self-administration: response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA (ed) Methods of assessing the reinforcing properties of abused drugs. Springer, Berlin Heidelberg New York, pp 1–34
Zacny JP, de Wit H (1992) The effects of a restricted feeding regimen on cigarette smoking in humans. Addict Behav 17:149–157
Acknowledgements
Experiment 1 was supported by funds of the intramural program of NIDA/NIH (PI: Yavin Shaham). Experiment 2 was supported by grants from the Canada Research Chair Program and Canada Foundation for Innovation (PI, Uri Shalev). We thank Dr. Michael H. Baumann of the Medication Discovery Research Branch, IRP/NIDA/NIH for his help with plasma corticosterone determination and Dr. Yavin Shaham for helpful comments on a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shalev, U., Finnie, P.S., Quinn, T. et al. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology 187, 376–384 (2006). https://doi.org/10.1007/s00213-006-0427-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0427-y