Abstract
The sensorimotor cortex and the cerebellum are interconnected by the corticopontocerebellar (CPC) pathway and by neuronal groups such as the serotonergic system. Our aims were to determine the levels of cerebellar serotonin (5-HT) and lipid peroxidation (LP) after cortical iron injection and to analyze the motor function produced by the injury. Rats were divided into the following three groups: control, injured and recovering. Motor function was evaluated using the beam-walking test as an assessment of overall locomotor function and the footprint test as an assessment of gait. We also determined the levels of 5-HT and LP two and twenty days post-lesion. We found an increase in cerebellar 5-HT and a concomitant increase in LP in the pons and cerebellum of injured rats, which correlated with their motor deficits. Recovering rats showed normal 5-HT and LP levels. The increase of 5-HT in injured rats could be a result of serotonergic axonal injury after cortical iron injection. The LP and motor deficits could be due to impairments in neuronal connectivity affecting the corticospinal and CPC tracts and dysmetric stride could be indicative of an ataxic gait that involves the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In mammals, there is a large connectivity between the cortex and the cerebellum [1–3]. Studies in adult human patients with cortical brain injury showed a reorganization of GABA-mediated mechanisms and glucose metabolism in the cerebellum [4]. Furthermore, anesthetized rats present changes in cerebellar activity following acute cortical injury [5]. Other reports have suggested that cortical injuries induce biochemical changes in terms of noradrenaline levels and lipid peroxidation (LP) products in the cerebellum and pons, respectively [6, 7]. These results support, in part, an interruption of the corticopontocerebellar (CPC), cerebellothalamocortical and noradrenergic pathways following brain injury induced by cortical axotomy and neuronal loss [2, 3, 7, 8].
In certain pathological conditions, there is an accumulation of iron that takes place in specific brain areas that is related to neurodegenerative disorders, including Huntington disease, Alzheimer’s disease and Parkinson’s disease [9]. An injection of iron in the brain of animal models is associated with oxidative injury, neurodegeneration and neuron death [9–14]. The effect of iron accumulation is mediated by the formation of reactive species, including hydrogen peroxide (H2O2) and hydroxyl radical (•OH) [15, 16]. Both •OH and the products of •OH can react with polyunsaturated fatty acids in cell membranes, increase rates of peroxidation in the brain and contribute to neuronal damage [10, 16, 17].
In addition, it is well known that cortical iron injection is associated with epileptic discharges resulting from specific changes in both excitatory and inhibitory synapses [9, 18]. In our recent research, we have observed remote noradrenergic inhibition after iron injection into cortex, which involves LP affecting both sides of the pons [7]. However, the effects in the cerebellar serotonergic system after brain injury have not been completely elucidated. It is important to note that the iron injection model is well accepted as an inducer of oxidative stress. Although some researchers contend that it is not a typical cortical brain injury, especially from the viewpoint of disease, we believe that these models mimic the effects of an intracortical hemorrhage because of the free radical formation induced by the iron contained in hemoglobin. The described changes occur under specific conditions of experimental injury. In this framework, it has been proposed that recovery of motor function after focal cortical injury would accompany the functional restoration of neuronal groups located in regions that are remote from, but anatomically related to, the injured site [7, 19–22].
Another neuronal group that projects ascending fibers to all brain areas, including the cortex and cerebellum, is the rostral group (B5-B9), which contains serotonergic neurons [1, 23]. In rats, the rostral group includes the pontine, dorsal and median raphe nuclei [1, 23]. In addition, all the components of the cerebellar system are innervated by serotonergic afferents [24]. However, the effects of cerebral cortical injury on the cerebellum have received relatively little attention. Finally, some findings suggest that axotomy induced by transection of the medial forebrain bundle increases the levels of serotonin (5-HT) in the substantia nigra [25]. This lesion can subsequently lead to 5-HT hyperinnervation in the adult rat ventral mesencephalon [26]. These results suggest that a compensatory response was generated by the axotomy. However, the changes in the cerebellar serotonergic system after cortical brain injury have not been thoroughly delineated.
In this study, we determine the 5-HT content, LP and motor deficit in the cerebellum after cortical iron injection.
Experimental Procedure
Animals
We used male Wistar rats (290–320 g) provided by the animal facility of the Instituto Nacional de Rehabilitacion. The rats were maintained on a 12-h/12-h light/dark cycle and were acclimatized to the laboratory conditions for at least 1 week prior to surgery. During this time, the rats were handled daily to accustom them to the experimental manipulations. The rats were treated according to the Guide for the Care and Use of Experimental Animals [27]. We used the minimum possible number of animals following bioethical and statistical criteria, according to Festing [28].
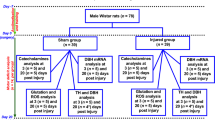
The animals were allocated to three groups (n = 37). (1) Control group: rats received an intracortical injection (10 μL) of artificial cerebrospinal fluid (CSF, in mM: 125 NaCl, 3 KCl, 1.3 CaCl2, 1 MgCl2 and 2.3 NaHCO3, pH 7.2) in free-moving conditions and their motor deficits were evaluated for 20 days. (2) Injured group: CSF containing ferrous chloride (FeCl2, 50 mM) was injected into the cortex and this group was decapitated two days after intracortical injection. (3) Recovering group: This group received the same treatment as the injured group, and the motor deficits resulting from the cortical injury were evaluated for 20 days.
Surgery
The surgical method was described by Bueno-Nava et al. [7]. The rats were mounted onto a stereotaxic frame (Stoelting Corp., Wood Dale, IL). A cannula made with a stainless steel syringe needle without its beveled edge (no. 18 bore) was placed on the meninges over the motor cortical representation of the hind limb [29] and fixed with dental acrylic.
Injection of Solutions
Fluids were administered by inserting a syringe needle through the cannula to a depth of 1 mm into the cortical parenchyma. This needle was connected to a Hamilton syringe (1 mL) with a micro-hose (0.6-mm i.d., FEP tubing, BAS). The solutions were injected at a rate of 0.2 μL/min using an injection pump (CMA/Microdialysis AB, Sweden: Mod CMA/400). The correct positioning of the site of injection of iron into the right cortex was described by Bueno-Nava et al. [7].
Motor Deficit and Stride Analysis
At 24 h post-injection, motor deficits were evaluated using the beam-walking test (Fig. 1) described in Brailowsky et al. [30], and the stride deficits were evaluated using the wooden walkway test described by Gonzalez-Pina et al. [31]. Measurements were made every 24 h for 20 days. The beam-walking test was used to assess overall locomotor function and the footprint test to assess gait.
Scale used for the quantification of the motor deficits using the beam-walking test according to Brailowsky et al. (1986). The motor deficit was evaluated daily on four sections of the beam (2 m long, 2.5 cm wide). Rats with no apparent deficit obtained a motor score of 0 (a). An animal that widened its base and presented all four toes off the beam bilaterally received a score = 1, but a score = 2 if the animal limped with one hind limb (hypotonus). An animal with at least 3 slips and/or 4 toes off the beam (unilaterally) received a score of 3 (b). Animals with falls or at least 3 slips received a score of 4 (c). Rats that dragged a limb obtained a score of 5 (d) and those that were unable to run received a score of 6 (f). Finally, the scores received on four sections were summed to generate the final motor score (example, final motor score of 3 + 3 + 4 + 4 = 14). Behaviors attaining a score of 1 or 2 are not shown as they are not apparent in a still frame
HPLC-EC Analysis of 5-HT Content
The rats (n = 16) were decapitated and the pons and cerebellum extracted on different days depending on group (2 or 20 days), but always between 18:00 and 19:00 h in order to eliminate circadian variations in 5-HT. Extraction of the brains and analysis of 5-HT were performed according to Gonzalez-Pina and Paz [32]. 5-HT content was analyzed by high performance liquid chromatography (Alltech, HPLC Pump, Model: 626) coupled to a coulometric electrochemical detector (ESA, Model: Coulochem III). Detection conditions were as follows: analytical cell (ESA 5011A) with the potentials = +350 mV, E1 = +200 mV and E2 = −200 mV. The peaks were sent to a computer with the EZCrom SI (version 3.2.1) program. An analytical column for catecholamines was used (Alltech, Adsorbosphere Catecholamine, 100 × 4.6 mm, 3 μm particle size). The flow rate used was 1.6 mL/min.
Lipid Peroxidation Analysis
LP was estimated by measuring the formation of lipid fluorescent products (LFP, n = 7/group) using the technique described by Triggs and Willmore [33] as modified by Santamaria and Rios [34].
Statistical Analysis
The results of the beam-walking test and footprint analysis were analyzed with a non-correlated Mann–Whitney U test. Statistical analysis of the 5-HT and LP measurements was performed with a one-way ANOVA and a post hoc Tukey test. In all cases, the significance levels were set to P ≤ 0.05.
Results
In the cerebellum, the 5-HT concentration in the right hemisphere of the injured group was 3.6 times higher than that of the respective control group (F 2,13 = 7.22, P = 0.0042), and the left hemisphere 5-HT concentration was 1.1 times higher than the respective control (F 2,13 = 5.55, P = 0.0092; Fig. 2). The 5-HT content of the injured group was also higher in both hemispheres of the cerebellum (1.01 times, right hemisphere, P = 0.0443; 0.8 times, left hemisphere, P = 0.0227) in comparison with the recovering group. In contrast, the cerebellar 5-HT concentration did not show a significant difference (P > 0.05) between the recovering and control groups in either hemisphere of the cerebellum.
Cerebellar 5-HT content. The cerebellum showed an increase in 5-HT levels in both hemispheres in injured rats at 2 days post-lesion. In contrast, serotonin levels were similar in controls and recovering rats at 20 days. The mean values are plotted. The error bars indicate the standard error. ** P < 0.05, * P < 0.01
Two days after cortical iron injection, LP in the injured groups was elevated in both the right (F 2,18 = 4.63, P = 0.0129) and left (F 2,18 = 5.46, P = 0.0136) cerebellar hemispheres when compared to their respective control group (Fig. 3a). This represented an increase of 1.4 and 1.5 times, respectively. Similarly, cerebellar LP in the injured rats was significantly elevated in both the right (F 2,18 = 4.63, P = 0.0129) and left (F 2,18 = 5.46, P = 0.0065) hemispheres in comparison to the recovering groups. Twenty days after the iron injection into the cortex, the cerebellar LP did not show a significant difference between the recovering and control groups in either hemisphere (P > 0.05).
Lipid fluorescence products (LFPs). a LFPs were found to be significantly enhanced in both hemispheres of the cerebellum in rats at 2 days post-lesion. In contrast, no significant differences were observed in the recovering and control rats at 20 days. b LFPs in the pons increased in both hemispheres in the injured rats at two days post-lesion. No significant changes were observed between the control and recovered rats at 20 days. The mean values are plotted. The error bars indicate the standard error. ** P < 0.01, * P < 0.02
We observed that LP was elevated in both sides of the pons in rats from the injured group compared with their respective control groups (right side, F 2,13 = 4.35, P = 0.0135; left side, F 2,13 = 6.52, P = 0.0048; Fig. 3b). Specifically, LP was ~1.5 times higher than that of the controls in the right side and ~1.8 times higher in the left side. LP in the recovering group returned to levels similar to the respective control in the 20 days after cortical iron injection.
Motor deficits as measured by the beam-walking test were significantly higher in the recovering group than in the control group at days 1 (P = 0.0033), 2 (P = 0.0156), 3 (P = 0.0072), 6 (P = 0.0053) and 8 (P = 0.0063) (Fig. 4). The motor deficit decreased to normal levels by 9 days after the cortical lesion in the recovering group, and this recovery was sustained for the remaining 11 days.
Scores obtained from the beam-walking test in control (filled circles) and recovering rats (filled triangles). The motor deficit increased from the first day after injury in the recovering rats, with recovery achieved by the ninth day. This recovery was sustained for the next 11 days. Values are the mean ± standard error. ** P < 0.01, * P < 0.02
The footprint analysis revealed that the FeCl2 injection into the cortex increased stride length in the recovering group for up 20 days post-injury compared with its basal level at day 0 before surgery (P < 0.0001). Similarly, we observed that injection of CSF increased stride length in the control group relative to its basal level, but only at 15 days post-injury (P < 0.0001). A comparison of the recovering group with the control group showed significant changes in stride length at days 13 (P = 0.0314), 15 (P = 0.0002) and 16 (P = 0.0015) post-injection, in this case suggesting that stride length decreased in the control group (Fig. 5a). Finally, at 20 days after the iron injection, the stride length was not significantly different between the control and recovering groups.
Percent change with respect to basal values obtained from footprint analysis in control (filled circles) and recovering rats (filled triangles). Values are the mean ± standard error. a Stride length: a P < 0.05, a significant change from the basal level in the recovering group. b P < 0.05, a significant change from the basal level in the control group. c P < 0.05, significantly different between the recovering and control groups. b Stride width: a P < 0.05, a significant change from the basal level in the recovering group. b P < 0.05, a significant change from the basal level in the control group. c Stride angle: a P < 0.05, significantly different between the recovering and control groups
The stride width analysis revealed a significant increase in the control group over the basal level at days 2 (P = 0.0066), 5(P = 0.0128), 6(P = 0.0233), 7(P = 0.0204), 8(P = 0.0271) and 9 (P = 0.0125) post-injection (Fig. 5b). Similarly, we observed that injection of FeCl2 into the cortex increased the stride width of the recovering group relative to their respective basal levels, but only at days 1(P < 0.0001), 6(P = 0.0123), 9(P = 0.0278), 12(P = 0.0084) and 13(P = 0.0210). However, comparison of the recovering group with the control group with respect to stride width did not reveal a significant difference.
Finally, the stride angles of the control and recovering groups were not significantly different at their respective basal levels (P > 0.05). In contrast, the control and recovering groups showed a significant difference in stride angle on the second test day after cortical iron injection (P = 0.0089), suggesting that stride angle decreased in the recovering group (Fig. 5c).
Discussion
Iron accumulation has been described in the sensorimotor cortex and cerebellum of Alzheimer’s disease patients [35]. Furthermore, free iron could contribute to epileptogenesis after the injection of iron into rat cortex and to the initial direct injury after head trauma [9]. The volume and concentration of FeCl2 injected into the rat sensorimotor cortex in this study are directly related to the quantity of extravasated blood deposited within the brain following intracerebral hemorrhage in humans. According to Triggs and Willmore [33], the iron injected into the cortex in this study is equivalent to a 40-mL hemorrhage in an average human brain.
In the rat, the pons receives input from collaterals of the corticospinal axons, and these represent the first link in the large pathways connecting the sensorimotor cortex to the cerebellum [2]. Our findings show a bilateral increase in pontine LP that could be an indicator of changes to the pontine projection neurons including the serotonergic and noradrenergic neurons after injection of FeCl2 into the cortex [7, 23, 24].
In a previous study, the cerebellar LP after iron injection in the cortex did not show significant differences between the control and recovering groups compared to the injured group, but the injured group with iron did show a tendency for increased LP in comparison to the control and recovering groups [7]. In the present study, we increased the number of animals to further explore this issue. With this larger sample, we observed that the cerebellar LP levels were elevated in both hemispheres compared with their respective control groups 2 days after cortical FeCl2 injection. The discrepancy between the two studies could be due to the difference in sample sizes. The cerebellar LP after cortical iron injection supports the theory that brain injury can lead to damage of remote areas, distant from the initial site of injury [9, 20]. Another finding that supports this remote effect is the observation of increased LP in the injured area [33] and in both sides of the pons [7] at 2 h and 48 h after the injection of FeCl2 into the cortex. Furthermore, in another animal model, remote ipsilateral and contralateral LP in the cortex and cerebellum of the rat occur at 0-60 min after occlusion of the middle cerebral artery [8]. These findings support the interruption of the CPC pathway as the mechanism of cerebellar LP [7, 8]. Uchino et al. [36] described various types of remote secondary degeneration in the brainstem and cerebellum including the corticopontine tract in the brainstem after frontal lobe infarction. These findings are in the context of oxidative stress and its relationship to neurodegeneration [37]. In addition, our results show that at 20 days post-injury, the cerebellar and pontine LP levels were similar between the recovering group and the control group. These findings suggest that the increase in cerebellar LP is transitory, as it is in the pons [7]. Finally, one could raise the possibility of having some element of the stainless steel cannula inducing oxidation, which could result in a variety of physiological changes. However, one of the properties of stainless steel is resistance to corrosion, and it is only used for temporary implants [38]; therefore, such a non-specific effect is unlikely. In addition, the results of our study indicate that the response to the cannula in terms of LP was not significantly different between the control group and recovering groups. In contrast, comparison of these groups with the iron-injured group did show significant differences. Our results suggest that, in terms of LP and motor deficit in the control group, the effect of the metal ions released by the cannula is minimal [39].
The serotonergic neurons of the rostral group project ascending fibers to the cerebral cortex and cerebellum [1, 23]. Schweinghofer et al. [24] suggest that the serotonergic input to the cerebellum arises from a fairly restricted nucleus in the medullary and pontine reticular formation. Our results show that two days after FeCl2 injection, there are significant increases in cerebellar 5-HT in both hemispheres. This increase could be the result of serotonergic axonal injury from FeCl2 in the cortex. In relation to this effect, it has been shown that iron (Fe2+) catalyzes the formation of •OH via Fenton’s reaction [15]. Both the •OH and the products of •OH can initiate the propagation of LP [40]. In this context, some axotomy-induced alterations in the electrophysiological features of neurons have been reported [41]. It has also been shown that axotomy of the ascending serotonergic pathways of the raphe nuclei increases the levels of 5-HT and its metabolite 5-hydroxyindolacetic acid in the substantia nigra and also causes a serotonergic hyperinnervation in the adult rat ventral mesencephalon [25, 26]. Our results suggest that the increase in cerebellar 5-HT levels in injured rats could be a compensatory response to neuronal excitability after axonal injury of serotonergic terminals in the brain cortex. It has been previously shown that direct lesions to serotonergic neurons can produce a compensatory response such as the reorganization of the serotonergic plexus in the area dentata after lesions of the median raphe nucleus [42]. In our results, we observed that the elevated cerebellar 5-HT concentrations reverted to normal levels in the recovering group at 20 days post-lesion. This recovery period is consistent with our previous results [7].
The cerebellar neuronal networks are involved in online error correction mechanisms that are important for coordination, visual guidance and velocity control during movement [43]. These body movements are present in the locomotor function of the rat [44] and may be qualitatively measured by the beam-walking test [30, 45, 46]. It has been documented that focal cortical damage in rats induces a temporary motor deficit [7, 22, 30, 31, 47]. The results of the present work revealed that cortical iron injection resulted in a motor deficit for 8 days. These findings are consistent with the behavioral findings of our previous study [7]. Our findings show that LP and motor deficit may indicate impaired neuronal connectivity in the corticospinal and CPC tracts resulting from cortical damage from iron in the cortex. Previously, it had been suggested that the noradrenergic system facilitates recovery in injured animals [6, 7, 22, 32, 47, 48] because noradrenergic drugs facilitate motor recovery in injured rats [6, 18, 48]. However, evidence linking cerebellar 5-HT dysfunction to behavioral deficits remains scarce. In this context, previous evidence has suggested that disturbances in cerebellar serotonergic modulation are involved in cerebellar ataxia [24]. In addition, it has also been suggested that treatment with the selective 5-HT reuptake inhibitor fluoxetine could increase the serotonergic transmission, thus stimulating motor function and possibly also promoting the restorative processes that follow a brain injury [49].
Goldstein [50] mentioned that the tests used to assess the sensorimotor functions after cortical injury, such as the beam walking and the footprint tests, can provide a comprehensive assessment of lesion-related deficits. Work by Hruska et al. [44] supported the measurement of footprint-based stride length, width and angle as quantitative aspects of normal locomotion in rats. The footprint test is specific to certain types of deficits that are not observable in the beam-walking [44, 50]. Specifically, stride width and stride length measurements are a common test for ataxic gait in rodents [51]. Our results indicate that both stride length and width increased significantly after cortical iron injection, possibly indicative of an ataxic gait [51]. The difference between the control and recovering groups was in the duration of the increased stride length in the injured rats compared to the control group. Presumably, these results were induced by the loss of neurons and axons at the injury site and indicate error or damage in the cerebello-spinal and spino-cerebellar connectivity [52] and possibly other systems such as the basal ganglia [53].
In conclusion, local brain damage could result in the activation of remote inhibitory mechanisms through the elevation of oxidative stress affecting mainly the pons and the cerebellar 5-HT levels. Reversal of the oxidative stress is concomitant with functional recovery. However, we cannot exclude the possibility that the increase in lipid products may be independent of motor recovery. The motor deficits, especially gait and beam walking, are transient, and it may be that the spontaneous recovery just happens to be occurring simultaneously with the reduction in stress. Further studies on the role of free radicals in brain injury are needed to clarify this issue. In this context, it is also necessary to further our understanding of the role of antioxidant enzymes during recovery from brain injury. The use of antioxidant therapies may represent an important step in the development of therapeutic strategies for human patients after stroke or trauma.
References
Kandel ER, Schwartz JH, Jessel TM (2000) Principles of neural science. McGraw-Hill, New York
Leergaard TB (2003) Clustered and laminar topographic patterns in rat cerebro-pontine pathways. Anat Embryol (Berl) 206:149–162
Daskalakis ZJ, Paradiso GO, Christensen KB et al (2004) Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 557:689–700
Niimura K, Chugani DC, Muzik O et al (1999) Cerebellar reorganization following cortical injury in humans: effects of lesion size and age. Neurology 52:792–797
Culic M, Blanusa ML, Grbic G et al (2005) Spectral analysis of cerebellar activity after acute brain injury in anesthetized rats. Acta Neurobiol Exp (Wars) 65:11–17
Krobert KA, Sutton RL, Feeney DM (1994) Spontaneous and amphetamine-evoked release of cerebellar noradrenaline after sensorimotor cortex contusion: an in vivo microdialysis study in the awake rat. J Neurochem 62:2233–2240
Bueno-Nava A, Montes S, DelaGarza-Montano P et al (2008) Reversal of noradrenergic depletion and lipid peroxidation in the pons after brain injury correlates with motor function recovery in rats. Neurosci Lett 443:32–36
Serteser M, Özben T, Gümüşlü S et al (2001) Biochemical evidence of crossed cerebellar diaschisis in terms of nitric oxide indicators and lipid peroxidation products in rats during focal cerebral ischemia. Acta Neurol Scand 103:43–48
Thompson KJ, Shoham S, Connor JR (2001) Iron and neurodegenerative disorders. Brain Res Bull 55:155–164
Subbarao KV, Richardson JS (1990) Iron-dependent peroxidation of rat brain: a regional study. J Neurosci Res 26:224–232
Sziráki I, Mohanakumar KP, Rauhala P et al (1998) Manganese: a transition metal protects nigrostriatal neurons from oxidative stress in the iron-induced animal model of parkinsonism. Neuroscience 85:1101–1111
Lin AM (2001) Coexistence of zinc and iron augmented oxidative injuries in the nigrostriatral dopaminergic system of SD rats. Free Radic Biol Med 30:225–231
Lin AM, Chen CF, Ho LT (2002) Neuroprotective effect of intermittent hypoxia on iron-induced oxidative injury in rat brain. Exp Neurol 176:328–335
Gregory A, Hayflick SJ (2005) Neurodegeneration with brain iron accumulation. Folia Neuropathol 43:286–296
Davies KJ (1995) Oxidative stress: the paradox of aerobic life. Biochem Soc Symp 61:1–31
Mosley RL, Benner EJ, Kadiu I et al (2006) Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6:261–281
Yoshida K, Kaneto K, Miyajima H et al (2000) Increased lipid peroxidation in the brains of aceruloplasminemia patients. J Neurol Sci 175:91–95
D’Ambrosio R, Perucca E (2004) Epilepsy after head injury. Curr Opin Neurol 17:731–735
Feeney DM, Sutton RL, Boyeson MG et al (1985) The locus coeruleus and cerebral metabolism: recovery of function after cortical injury. Physiol Psychol 13:197–203
Feeney DM, Baron JC (1986) Diaschisis. Stroke 17:817–820
Boyeson MG, Feeney DM (1990) Intraventricular norepinephrine facilitates motor recovery following sensorimotor cortex injury. Pharmacol Biochem Behav 35:497–501
Goldstein LB (2006) Neurotransmitters and motor activity: effects on functional recovery after brain injury. NeuroRx 3:451–457
Ciranna L (2006) Serotonin as a modulator of glutamate-and GABA-mediated neurotransmission: implicationsin physiological functions and in pathology. Curr Neuropharmacol 4:101–114
Schweighofer N, Doya K, Kuroda S (2004) Cerebellar aminergic neuromodulation: towards a functional understanding. Brain Res Brain Res Rev 44:103–116
Venero JL, Revuelta M, Cano J et al (1997) Time course changes in the dopaminergic nigrostriatal system following transection of the medial forebrain bundle: detection of oxidatively modified proteins in substantia nigra. J Neurochem 68:2458–2468
Revuelta M, Venero JL, Machado A et al (1999) Serotonin hyperinnervation in the adult rat ventral mesencephalon following unilateral transection of the medial forebrain bundle. Correlation with reactive microgial and astroglial populations. Neuroscience 91:567–577
Olfert ED, Cross BM, McWilliam AA (1993) Guide to the care and use of experimental animals. Can Council Anim Care
Festing MF (1994) Reduction of animal use: experimental design and quality of experiments. Lab Anim 28:212–221
Hall RD, Lindholm EP (1974) Organization of motor and somatosensory neocortex in the albino rat. Brain Res 66:23–38
Brailowsky S, Knight RT, Blood K et al (1986) Gamma-aminobutyric acid-induced potentiation of cortical hemiplegia. Brain Res 362:322–330
Gonzalez-Pina R, Bueno-Nava A, Montes S et al (2005) Pontine norepinephrine content after motor cortical ablation in rats. Proc West Pharmacol Soc 48:73–76
Gonzalez-Pina R, Paz C (1997) Brain monoamine changes in rats after short periods of ozone exposure. Neurochem Res 22:63–66
Triggs WJ, Willmore LJ (1984) In vivo lipid peroxidation in rat brain following intracortical Fe2+ injection. J Neurochem 42:976–980
Santamaría A, Ríos C (1993) MK-801, an N-methyl-d-aspartate receptor antagonist, blocks quinolinic acid-induced lipid peroxidation in rat corpus striatum. Neurosci Lett 159:51–54
Kala SV, Hasinoff BB, Richardson JS (1996) Brain samples from Alzheimer’s patients have elevated levels of loosely bound iron. Int J Neurosci 86:263–269
Uchino A, Takase Y, Nomiyama K et al (2006) Brainstem and cerebellar changes after cerebrovascular accidents: magnetic resonance imaging. Eur Radiol 16:592–597
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Gotman I (1997) Characteristics of metals used in implants. J Endourol 11:383–389
Tomizawa Y, Hanawa T, Kuroda D et al (2006) Corrosion of stainless steel sternal wire after long-term implantation. J Artif Organs 9:61–66
Halliwell B (1987) Oxidants and human disease: some new concepts. FASEB J 1:358–364
Titmus MJ, Faber DS (1990) Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol 35:1–51
Haring JH (1991) Reorganization of the area dentata serotoninergic plexus after lesions of the median raphe nucleus. J Comp Neurol 306:576–584
Johnson MT, Ebner TJ (2000) Processing of multiple kinematic signals in the cerebellum and motor cortices. Brain Res Brain Res Rev 33:155–168
Hruska RE, Kennedy S, Silbergeld EK (1979) Quantitative aspects of normal locomotion in rats. Life Sci 25:171–180
Feeney DM, Gonzalez A, Law WA (1982) Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217:855–857
Boyeson MG, Krobert K (1992) Cerebellar norepinephrine infusions facilitate recovery after sensorimotor cortex injury. Brain Res Bull 29:435–439
Gonzalez-Pina R, Bueno-Nava A, Montes S et al (2006) Pontine and cerebellar norepinephrine content in adult rats recovering from focal cortical injury. Neurochem Res 31:1443–1449
Feeney DM, Weisend MP, Kline AE (1993) Noradrenergic pharmacotherapy, intracerebral infusion and adrenal transplantation promote functional recovery after cortical damage. J Neural Transplant Plast 4:199–203
Dam M, Tonin P, De Boni A et al (1996) Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke 27:1211–1214
Goldstein LB (2003) Model of recovery of locomotor ability after sensorimotor cortex injury in rats. ILAR J 44:125–129
Crawley J (1999) Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835:18–26
Garwicz M (2002) Spinal reflexes provide motor error signals to cerebellar modules-relevance for motor coordination. Brain Res Brain Res Rev 40:152–165
Groenewegen HJ (2003) The basal ganglia and motor control. Neural Plast 10:107–120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bueno-Nava, A., Gonzalez-Pina, R., Alfaro-Rodriguez, A. et al. Recovery of Motor Deficit, Cerebellar Serotonin and Lipid Peroxidation Levels in the Cortex of Injured Rats. Neurochem Res 35, 1538–1545 (2010). https://doi.org/10.1007/s11064-010-0213-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0213-4