Abstract
Rationale
Social rank has been shown to influence dopamine (DA) D2 receptor function and vulnerability to cocaine self-administration in cynomolgus monkeys. The present studies were designed to extend these findings to maintenance of cocaine reinforcement and to DA D1 receptors.
Objective
Examine the effects of a high-efficacy D1 agonist on an unconditioned behavior (eyeblinking) and a low-efficacy D1 agonist on cocaine self-administration, as well as the effects of cocaine exposure on D2 receptor function across social ranks, as determined by positron emission tomography (PET).
Methods
Effects of the high-efficacy D1 agonist SKF 81297 and cocaine (0.3–3.0 mg/kg) on spontaneous blinking were characterized in eight monkeys during 15-min observation periods. Next, the ability of the low-efficacy D1 agonist SKF 38393 (0.1–17 mg/kg) to decrease cocaine self-administration (0.003–0.1 mg/kg per injection, IV) was assessed in 11 monkeys responding under a fixed-ratio 50 schedule. Finally, D2 receptor levels in the caudate and putamen were assessed in nineteen monkeys using PET.
Results
SKF 81297, but not cocaine, significantly increased blinking in all monkeys, with slightly greater potency in dominant monkeys. SKF 38393 dose-dependently decreased cocaine-maintained response rates with similar behavioral potency and efficacy across social rank. After an extensive cocaine self-administration history, D2 receptor levels did not differ across social ranks.
Conclusions
These results suggest that D1 receptor function is not substantially influenced by social rank in monkeys from well-established social groups. While an earlier study showed that dominant monkeys had higher D2 receptor levels and were less sensitive to the reinforcing effects of cocaine during initial exposure, the present findings indicate that long-term cocaine use changed D2 receptor levels such that D2 receptor function and cocaine reinforcement were not different between social ranks. These findings suggest that cocaine exposure attenuated the impact of social housing on DA receptor function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence from animal studies clearly indicates that the reinforcing effects of cocaine can be modulated by environmental variables including rearing conditions, food restriction, the availability of alternative reinforcers and the presence of environmental stressors (reviewed in LeSage et al. 1999). Exposure to social stressors such as defeat stress can facilitate cocaine self-administration in rodents (Miczek and Mutschler 1996), an effect that is particularly prominent during initial exposure to low (0.25 or 0.32 mg/kg per injection) but not higher (0.75 mg/kg per injection) doses of the drug (Haney et al. 1995; Covington and Miczek 2001; Kabbaj et al. 2001). Factors related to vulnerability and maintenance in human cocaine abusers (Gawin 1991) can be studied in nonhuman primate models of chronic social stress. In one such model, socially housed cynomolgus monkeys are exposed to continuous, inescapable stress resulting from the agonistic interactions that contribute to formation of the linear dominance hierarchies that characterize their social organization (Bernstein 1981). A profound impact of social status on nonhuman primate physiology has been consistently reported (e.g. Kaplan and Manuck 1999, Kaplan et al. 2002). Moreover, it is clear that the effects of dopaminergic drugs can differ according to social rank. For example, in squirrel monkeys, the effects of d-amphetamine on aggressive behavior were more profound in dominant monkeys (Miczek and Gold 1983; Martin et al. 1990).
Environmentally induced changes in brain dopamine (DA) systems are particularly relevant to vulnerability to the abuse-related effects of cocaine, because considerable evidence suggests that the ability of cocaine to act as a reinforcer is due largely to its ability to elevate extracellular DA (e.g. Pettit and Justice 1989, 1991; Bradberry 2000; Czoty et al. 2002). Miczek and colleagues have demonstrated that the ability of social defeat stress to increase DA levels in the nucleus accumbens is closely related to its ability to enhance the reinforcing effects of cocaine (Tidey and Miczek 1997) and reproduce the discriminative stimulus produced by cocaine or amphetamine (Miczek et al. 1999). In a recent study in cynomolgus monkeys, positron emission tomography (PET) was used to measure D2 receptor binding before and after monkeys were group housed (Morgan et al. 2002). When individually housed, D2 receptor binding did not differ across monkeys and was not predictive of eventual social rank. However, after 3 months of social housing, D2 receptor binding increased significantly in monkeys who became dominant, but was unchanged in monkeys that became subordinate. Consistent with a major role for D2 receptors in cocaine self-administration (Goldstein and Volkow 2002), cocaine functioned as a reinforcer in subordinate monkeys. In contrast, cocaine failed to maintain self-administration in dominant monkeys. These findings suggest that exposure to environmental variables associated with the attainment of dominance produced alterations in D2 receptor levels that impacted the reinforcing effects of cocaine. Consistent with these findings in monkeys, others have reported similar alterations in the physiology of brain DA systems in rats raised in social groups (“enriched” environments) versus those that were individually housed (“isolated”; Bowling et al. 1993; Rilke et al. 1995, 1998). Moreover, “enriched” rodents are less sensitive to the behavioral effects of cocaine and amphetamine, including locomotor-stimulant (Bardo et al. 1995), discriminative stimulus (Fowler et al. 1993) and reinforcing effects (Green et al. 2002). Taken together, these results raise the possibility that social housing of nonhuman primates provides a continuum of effects from chronic stress in subordinate monkeys to environmental enrichment in dominant animals, and that these environmental variables impact extracellular DA, the function of DA receptors and, ultimately, vulnerability to cocaine abuse.
Results from our earlier study demonstrated differences in vulnerability to self-administer cocaine as a function of social rank. However, continued exposure to cocaine eventually resulted in the drug functioning as a reinforcer in dominant monkeys. Data from other studies demonstrate that environmental variables that influence vulnerability (e.g. behavioral history) do not influence rates of responding during maintenance (Nader and Bowen 1995). In addition, the effects of cocaine on DA receptor densities can differ in monkeys during initial versus long-term cocaine exposure (Nader et al. 2002). One goal of the present study was to examine D2 receptor binding, with PET, in socially housed cynomolgus monkeys after an extensive history of cocaine self-administration. Since cocaine has been shown to decrease D2 receptor levels (e.g. Volkow et al. 1990; Nader et al. 2002), we hypothesized that the pharmacology of cocaine would “reverse” the effects of social housing on D2 receptor levels in dominant monkeys.
While there is a growing database on the role of D2 receptors in modulating the effects of the environment on cocaine self-administration, little information exists to address potential involvement of D1 receptors which also mediate the effects of cocaine. Both D1 and D2 receptor agonists can maintain self-administration in monkeys previously trained to self-administer cocaine (Woolverton et al. 1984; Weed and Woolverton 1995; Grech et al. 1996) and agonists and antagonists at these receptors can affect rates of cocaine-maintained responding (e.g. Woolverton and Virus 1989; Bergman et al. 1990; Nader et al. 1999b; Caine et al. 2000). Furthermore, studies using rats reared in isolation have demonstrated that environmental variables can alter D1 receptor number and function (Guisado et al. 1980; Gariepy et al. 1995). Thus, D1 receptor systems could potentially play an important role in the ability of environmental variables to alter the reinforcing effects of cocaine in monkeys.
To further characterize the neurobiological consequences of social housing in nonhuman primates, the present studies assessed the unconditioned effects of D1 receptor stimulation (spontaneous blinking) and the ability of a D1 agonist to decrease cocaine self-administration in group-housed cynomolgus monkeys. First, the effects of the high-efficacy D1 receptor agonist SKF 81297 on spontaneous blinking were studied in dominant and subordinate monkeys. High-efficacy D1 agonists have been demonstrated to increase spontaneous blinking in monkeys (Elsworth et al. 1991). Next, the ability of the low-efficacy D1 agonist SKF 38393 to alter cocaine self-administration was examined. Low-efficacy D1 agonists have been shown to decrease the reinforcing effects of cocaine in a manner consistent with pharmacological antagonism (Bergman and Rosenzweig-Lipson 1992; Katz and Witkin 1992; Platt et al. 2001). These two tools were used to provide measures of unconditioned and conditioned effects, respectively, of D1 receptor function in socially housed monkeys.
Materials and methods
Subjects
Twenty-one adult male cynomolgus monkeys (Macaca fascicularis) served as subjects. Monkeys lived in social groups, with each pen consisting of a stainless steel cage (Allentown Caging Inc, Allentown, N.J., USA) with removable wire mesh partitions to separate the monkeys into quadrants of the cage when necessary. When the partitions were removed, the living space was approximately 80 ft3. Each quadrant, which was approximately 12 ft3, was equipped with a water spout from which water was continuously available. Monkeys lived in social groups of four monkeys/pen during experiment 1 (blinking study) and three monkeys/pen during experiments 2 (cocaine self-administration studies) and 3 (D2 PET studies). Monkeys were weighed weekly and fed enough food daily (Purina Monkey Chow and fresh fruit) to maintain body weights at approximately 95% of free-feeding levels.
Social status was determined for each monkey according to the outcomes of agonistic encounters as described previously (Kaplan et al. 1982; Morgan et al. 2000). Initially, aggressive, submissive and affiliative behaviors were recorded for individual monkeys during 45-min observation sessions. The animal that aggressed towards, and elicited submissive behaviors from, all others was designated the dominant monkey. The subordinate monkey received aggression from all others and rarely aggressed. Stability of the hierarchies in each pen was confirmed daily by visual inspection. For example, when a peanut was offered by the technician, the dominant animal typically threatened the other monkeys in the pen and retrieved the peanut, whereas the subordinate animal typically moved to the rear of the pen and engaged in submissive behaviors (e.g. lip-smacking) and vocalizations. Ranks did not change during each experiment. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Wake Forest University.
General procedure
Each monkey was fitted with a nylon collar (Primate Products, Redwood City, Calif., USA) and trained to approach the front of the cage when the investigator was present. Monkeys were guided into a restraint chair (Primate Products) using a specially designed stainless steel pole (Primate Products). All subjects were acclimated to this procedure and trained to sit calmly in the chair.
Experiment 1: social rank and SKF 81297-induced eyeblinking
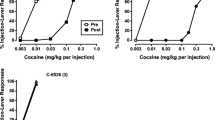
Eight socially housed monkeys (four dominant, four subordinate) were seated in a primate chair and sessions were videotaped for later scoring. Following a 30-min baseline period, saline was administered (IV) and blinking was counted during the last 2.5 min of the following 15-min period. Subsequently, cumulative doses of SKF 81297 or cocaine (0.3–3.0 mg/kg IV) were administered. Blinking was counted in the last 2.5 min of the 15-min period following each dosing increment and is expressed in terms of individual monkeys’ blink rates (total blinks/2.5 min). The total session length was approximately 90 min.
Experiment 2: effects of SKF 38393 on cocaine self-administration
Catheter implantation
Nineteen monkeys were surgically prepared with a chronic indwelling venous catheter and subcutaneous vascular access port (Access Technologies, Skokie, Ill., USA) under sterile surgical conditions. Anesthesia was induced with ketamine (15 mg/kg) and butorphanol (0.025 mg/kg) and maintained with ketamine supplements. A catheter was inserted into a major vein (femoral, internal or external jugular, brachial) and passed to the level of the vena cava. The proximal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was attached to the vascular access port, which was placed in a pocket formed by blunt dissection. Post-operative antibiotics (25 mg/kg kefzol, cefazolin sodium; Marsam Pharmaceuticals, Cherry Hill, N.J., USA) were administered for 5–7 days. To prolong patency, each port was flushed with a solution of heparinized saline (500 IU/ml) at the end of each experimental session.
Self-administration procedures
Each day, monkeys were separated by partitioning the cage into quadrants. Next, each monkey was seated in a restraint chair and placed into a ventilated, sound-attenuating chamber (1.5×0.74×0.76 m; Med Associates, East Fairfield, Vt., USA). The back of the animal was cleaned with 95% ethyl alcohol and betadine, and the port was connected to an infusion pump (Cole-Parmer, Inc. Chicago, Ill., USA) located outside the chamber via a 20 gauge Huber Point Needle (Access Technologies). The pump was operated for approximately 3 s to fill the port and catheter with the dose of cocaine available for the session. During the session, 50 responses on the operant lever (FR50) activated the pump for 10 s (during training, completion of an FR50 produced a food pellet). Sessions lasted until 30 reinforcers had been obtained or 60 min had elapsed, whichever came first. Several doses of cocaine (saline, 0.001–0.1 mg/kg per injection) were made available; a dose was studied for at least three consecutive sessions and until responding was deemed stable (±15% of the mean of three consecutive sessions). After all sessions had been completed each day, monkeys were fed and allowed 2 h to eat before partitions were removed and animals were group housed again. Self-administration sessions were conducted 5 days per week.
Of the 19 catheterized monkeys, 11 were used in the SKF 38393 study (four dominant, three intermediate and four subordinate). Following determination of cocaine dose-response curves, animals received an injection of SKF 38393 (0.1–17.0 mg/kg, IV) 5 min prior to the cocaine self-administration session in a volume of approximately 1 ml/10 kg. SKF doses were typically tested on Tuesdays and Fridays; each dose of SKF 38393 was tested at least twice in each monkey in combination with several doses of cocaine (0.003–0.1 mg/kg per injection). For data analysis, cocaine dose-response curves are presented as mean (±SEM) response rate of all days before SKF 38393 pretreatment for the respective cocaine dose, while SKF 38393 pretreatment data are presented as mean of all determinations.
Drugs
(−)Cocaine HCl (National Institute on Drug Abuse, Bethesda, Md., USA) was dissolved in sterile saline. Different doses were studied by changing the drug concentration; all drug concentrations were prepared in 250 ml of sterile saline. During sessions, responding on the lever delivered approximately 1.0 ml of drug solution over 10 s. SKF 81297 and SKF 38393 (Research Biochemicals International, Natick, Mass., USA) were diluted in sterile water and sonicated and/or heated to aid solubilizing if necessary.
Experiment 3: effects of cocaine self-administration on D2 receptor DVR determined with PET
PET imaging studies were conducted in all 19 monkeys that were self-administering cocaine and living in social groups. Methodological details regarding the data acquisition protocol, blood sampling and metabolite analysis for [18F]fluoroclebopride (FCP) have been described previously (Mach et al. 1996; Nader et al. 1999a). Image acquisition occurred on a GE Advance NXi PET Scanner (General Electric Systems, Milwaukee, Wisc.). This device has 18 detector rings that provide 35 contiguous image planes over a 15.2 cm axial field of view and the center-to-center spacing between slices is 4.25 mm. The spatial resolution of the scanner is approximately 4.8 mm in all three dimensions and the sensitivity is approximately 200 kcps/μCi per ml. Twenty-six frames were acquired over 3 h (5×1 min, 5×2 min, 5×5 min, 8×10 min, 3×20 min). The first five frames of each study’s PET image data were then added together. This summed image represents tracer uptake in the early part of the study and approximates a blood flow image. This image was then registered to the animal’s MRI (see below) using the AIR algorithm (Woods et al. 1993) after extracting the brain from the MRI using the method of Smith (2002). This method provides excellent registration of cortical and subcortical regions.
Regions of interest (ROI) for the caudate, putamen and cerebellum (used as an index of nonspecific binding due to its low density of D2 receptors) were then drawn on each subject’s MRI and transferred to their registered PET scans. Time-activity curves for [18F]FCP were generated, and distribution volumes were obtained for ROIs in each hemisphere using the linear portion of the Logan plot (Logan et al. 1990). For all regions, the right and left sides were averaged and the ratio of distribution volume in caudate and putamen to the distribution volume in the cerebellum (the distribution volume ratio, DVR) was calculated. The DVR thus provided a measure of specific binding to D2 receptors and served as the dependent measure for data analysis.
Prior to the start of the PET study, monkeys were initially anesthetized with 8 mg/kg ketamine, intubated and maintained throughout the scan by inhaled isoflurane (1.5%). This induction protocol has no effect on [18F]FCP DVRs (Nader et al. 1999a). Catheters (22-gauge angiocath; Becton Dickinson Vascular Access, Sandy, Utah, USA) were placed in an external artery and vein by percutaneous sticks and lactated ringer’s solution (IV) was delivered to the monkey throughout the study. A paralytic (0.07 mg/kg vecuronium bromide) was administered and respiration was maintained by a ventilator. Supplemental doses of vecuronium bromide (0.1 mg/hr) were administered throughout the study. At the start of the scan, approximately 4 mCi [18F]FCP was injected, followed by 3 ml heparinized saline. Arterial blood samples were collected into preheparinized tubes for analysis.
MRI studies
On separate days, and prior to any PET studies, T1-weighted volume magnetic resonance images (MRI) were also obtained on each animal for registration with PET scan images (1.5 T GE Signa; GE Medical Systems). This type of scan provides excellent tissue contrast allowing easy identification of the brain regions. Studies were conducted while the monkey was anesthesized with approximately 15 mg/kg ketamine.
Data analysis
The primary dependent variables examined in the present studies were: blink rate (expt 1), response rate (responses/s) and cocaine intake (expt 2) and DVR for [18F]FCP at the D2 receptor (expt 3). Data were analyzed using repeated-measures one- or two-way analyses of variance (ANOVA), with post-hoc Dunnett’s or Bonferroni tests when significant main effects were indicated by the ANOVA. In all cases, differences were considered significant at the 95% level of confidence (P<0.05).
Results
Experiment 1
Baseline rates of spontaneous blinking did not differ between dominant and subordinate monkeys in either the SKF 81297 or cocaine study, and ranged from group means of 10.5–12.3 blinks/min. Administration of SKF 81297 (0.3–3.0 mg/kg) produced dose-dependent increases in spontaneous blinking in all monkeys (Fig. 1). Increases in blinking reached statistical significance in both dominant [F(4,12)=23.07] and subordinate monkeys [F(4,12)=10.61]. Post-hoc Dunnett’s multiple comparisons tests indicated that blink rates were significantly increased above baseline by 0.3, 1.0 and 3.0 mg/kg SKF 81297 in dominant monkeys and by 1.0 and 3.0 mg/kg SKF 81297 in subordinates. A two-way ANOVA revealed a significant effect of treatment [F(4,15)=15.30] but not rank, with no significant interaction. Blink rates were not significantly altered by cocaine in dominant or subordinate monkeys (data not shown). A two-way ANOVA indicated no main effects of cocaine treatment or rank and no significant interaction.
Experiment 2
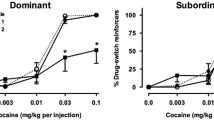
Cocaine-maintained responding varied significantly [F(4,24)=5.20] as a function of dose (saline, 0.003–0.1 mg/kg per injection) and was characterized as an inverted U-shaped function in most monkeys. The highest response rates were maintained by 0.01 mg/kg per injection cocaine in subordinates, while higher rates of responding were maintained by 0.003 mg/kg per injection in dominant and intermediate-ranked monkeys (Fig. 2A). The ANOVA revealed neither a significant effect of rank nor an interaction between rank and cocaine dose. Cocaine intake increased monotonically as a function of dose (Fig. 2B). A two-way ANOVA revealed a significant effect of cocaine dose [F(3,18)=35.39] but not of rank on cocaine intakes, with no significant interaction.
Representative data following SKF 38393 administration are shown from a dominant, intermediate and subordinate monkey (Fig. 3). Administration of SKF 38393 decreased cocaine-maintained response rates in ten of 11 monkeys in a manner that was largely dose-dependent. Prominent effects were observed on responding maintained by the cocaine dose at the peak of the individual’s dose-effect curve, whereas SKF 38393 decreased or did not affect rates maintained by higher cocaine doses. ED50 values for SKF 38393-induced decreases in responding maintained by the dose of cocaine at the peak of the dose-effect curve varied across individuals and did not differ according to social rank (Table 1). Administration of 17 mg/kg SKF 38393 had lethal effects in one subject (C-6524), despite previous exposure to that dose on two occasions.
Experiment 3
Regions of interest were drawn around the caudate and putamen and DVRs were calculated using the cerebellum as the reference region. D2 receptor DVRs were not significantly different between regions (i.e. caudate versus putamen). Comparisons between ranks indicated that D2 receptor DVRs did not differ between dominant, intermediate and subordinate monkeys (Table 2) in the caudate nucleus or putamen.
Discussion
One purpose of the present studies was to extend earlier investigations of the neurobiological consequences of social housing in cynomolgus monkeys by examining D1 receptor function. The sensitivity of monkeys from different social ranks to the effects of D1 agonists was assessed using in vivo assays involving an unconditioned behavior (spontaneous blinking) and a conditioned behavior (cocaine self-administration). These studies were carried out in monkeys from well-established social groups and found modest effects of D1 receptor stimulation on blinking. The effects of the low-efficacy D1 agonist SKF 38393 on rates of cocaine self-administration were not different in dominant, intermediate and subordinate monkeys. Because D1 receptor function was not assessed prior to group housing, we cannot determine whether the functional status of D1 receptors was differentially altered as a consequence of social rank. Nonetheless, it appears that dominant and subordinate cynomolgus monkeys from well-established social groups do not exhibit profound differences in D1 receptor function. A second purpose of the present studies was to examine D2 receptor function in monkeys from well-established social groups that also had an extensive history of cocaine self-administration. In contrast to our earlier observation that social rank influenced D2 receptor function and vulnerability to self-administer cocaine during initial exposure (Morgan et al. 2002), there were no significant differences between social ranks in either D2 function or the reinforcing effects of cocaine in socially housed cynomolgus monkeys with extensive histories of cocaine self-administration. These findings suggest that continued exposure to cocaine can reverse or attenuate the powerful interactions of environmental variables with DA receptor function.
Initial investigation of the effects of social hierarchy on D1 receptor function involved examination of increases in spontaneous eyeblinking elicited by SKF 81297. Under baseline conditions, blink rates did not differ between dominant and subordinate monkeys. Blink rates were significantly increased by 0.3 mg/kg SKF 81297 in dominant monkeys, whereas higher doses were required to significantly increase blinking in subordinates. These results suggested that subtle differences in D1 receptor function may exist between dominant and subordinate monkeys. In contrast to SKF 81297, cocaine did not consistently or significantly alter blink rates. These effects are consistent with previous studies of the effects of direct and indirect DA agonists on spontaneous blinking in monkeys (Elsworth et al. 1991; Kleven and Koek 1996).
To further examine differences in D1 receptor function in socially housed monkeys, we assessed the effects of SKF 38393 on rates of cocaine self-administration. Previously, we had shown that dominant monkeys were less sensitive to the reinforcing effects of cocaine compared to subordinates (Morgan et al. 2002). Our earlier study examined initial cocaine exposure, which we believe models vulnerability to the reinforcing effects of cocaine in the “acquisition” phase. However, continued exposure to cocaine resulted in increases in response rates and cocaine intakes by dominant monkeys to the point that the drug functioned as a reinforcer in all monkeys (unpublished observations), thus modeling the “maintenance” phase of drug use. It is certainly possible that variables that influence vulnerability do not similarly influence maintenance. In support of this hypothesis, neuronal adaptations are different in monkeys with a brief versus extensive history of cocaine self-administration (Nader et al. 2002; present study).
Regardless of social rank, SKF 38393 dose-dependently decreased response rates maintained by the cocaine dose at the peak of the cocaine dose-effect curve, and decreased or did not alter response rates maintained by doses of cocaine on the descending limb of the cocaine dose-effect curve. These results are consistent with previous observations of the effects of SKF 38393 and other low efficacy D1 agonists on cocaine self-administration in individually housed nonhuman primates (Bergman and Rosenzweig-Lipson 1992; Katz and Witkin 1992; Caine et al. 2000; Platt et al. 2001). Individual differences were observed in potency of SKF 38393; however, similar to the results of the blinking studies, these differences were unrelated to social rank, as evidenced by the variability and lack of significant differences in ED50 values across social rank.
In the present PET studies of D2 receptor function, no differences were observed between dominant, intermediate and subordinate monkeys’ D2 receptor DVRs in specific regions of the basal ganglia (i.e. the caudate and putamen). In our earlier study using a separate group of cynomolgus monkeys (Morgan et al. 2002) we found significant differences between cocaine-naive dominant and subordinate cynomolgus monkeys in basal ganglia D2 receptor DVRs, with dominant animals having an approximately 20% higher DVR compared to subordinates. While quantitative comparisons between studies are not possible because of the use of different PET scanners, potential confounding variables include subject factors (e.g. age, weight, origin), neuroadaptation following long-term social housing and social group size. Assessment of the potential influence of subject factors will require additional research to fully address. However, it is unlikely that the lack of rank-related differences in D2 DVRs represents differential neuroadaptation to long-term social housing. Although our earlier study documented changes in DVRs within 3 months of group formation while the present study involved well-established social groups, a previous study documented differences in D2 receptor binding between dominant and subordinate cynomolgus monkeys from social groups that had been stable for over 3 years (Grant et al. 1998). Regarding social group size, it is important to note that during the self-administration phase of the current studies monkeys were housed in pens of three as opposed to pens of four during our previous study. The influence of group size on environmentally induced alterations in dopaminergic physiology and the reinforcing effects of cocaine has not been studied, but it is possible that social rank may influence physiology to a lesser degree in a smaller social group. In a group of three, subordinate monkeys have fewer opportunities to experience physical and psychological stress and dominant monkeys have fewer opportunities to experience the enrichment associated with behaviors that reinforce their dominant status. This hypothesis is supported by a recent meta-analysis of nonhuman primate species indicating that subordinate monkeys exhibit relatively higher circulating levels of cortisol in species which experience higher rates of exposure to stressors (Abbott et al. 2003).
Notwithstanding the potential influence of these factors, the most compelling explanation for the present lack of rank-related differences is the monkeys’ extensive history of cocaine self-administration, which ranged from 5 to 45 months prior to the start of the present studies. Chronic elevation of DA produced by long-term self-administration of cocaine would be expected to cause down-regulation of D2 receptors to compensate for chronic hyperstimulation. This assertion is supported by autoradiographic data in rhesus monkeys demonstrating that D2 receptors are decreased following an extensive history of cocaine self-administration (Moore et al. 1998b; Nader et al. 2002). Audioradiographic studies have also documented a decrease in D1 receptor number in rhesus monkeys after long-term cocaine self-administration (Moore et al. 1998a; Nader et al. 2002). These results highlight the possibility that differences in D1 receptor function may have existed initially after social housing, but that cocaine exposure resulted in neuropharmacological changes that reversed or reduced environmentally induced alterations in D1 receptor function. Although the present results cannot address this possibility directly, other reports have suggested that environmental variables can affect D1 receptor number and function. For example, mice reared in isolation showed increased densities of D1 receptors in the striatum and were more sensitive to the behavioral effects of a D1 agonist (Gariepy et al. 1995). Future studies using individually housed, drug-naive monkeys will be necessary to address whether initial exposure to social housing can differentially alter D1 receptor function across social rank.
References
Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T Jr, Sapolsky RM (2003) Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav 43:67–82
Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP (1995) Environmental enrichment attenuates locomotor sensitization, but not in vivo dopamine release, induced by amphetamine. Pharmacol Biochem Behav 51:397–405
Bergman J, Rosenzweig-Lipson S (1992) Cocaine-antagonist effects of limited-efficacy D1 agonists. NIDA Res Monogr 119:185–189
Bergman J, Kamien JB, Spealman RD (1990) Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol 1:355–363
Bernstein IS (1981) Dominance: the baby and the bathwater. Behav Brain Sci 4:419–457
Bowling SL, Rowlett JK, Bardo MT (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32:885–893
Bradberry CW (2000) Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine abuse. J Neurosci 20:7109–7115
Caine SB, Negus SS, Mello NK (2000) Effects of dopamine D1-like and D2-like agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect curves. Psychopharmacology 148:41–51
Covington HE 3rd, Miczek KA (2001) Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges.” Psychopharmacology 158:388–398
Czoty PW, Ginsburg BC, Howell LL (2002) Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 300:831–837
Elsworth JD, Lawrence MS, Roth RH, Taylor JR, Mailman RB, Nichols DE, Lewis MH, Redmond DE Jr (1991) D1 and D2 dopamine receptors independently regulate spontaneous eyeblink rate in the vervet monkey. J Pharmacol Exp Ther 259:595–600
Fowler SC, Johnson JS, Kallman MJ, Liou JR, Wilson MC, Hikal AH (1993) In a drug discrimination procedure isolation-reared rats generalize to lower doses of amphetamine than rats reared in an enriched environment. Psychopharmacology 110:115–118
Gariepy JL, Gendreau PL, Mailman RB, Tancer M, Lewis MH (1995) Rearing conditions alter social reactivity and D1 receptors in high- and low-aggressive mice. Pharmacol Biochem Behav 51:767–773
Gawin FH (1991) Cocaine addiction: psychology and neurophysiology. Science 251:1580–1586
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH (1998) Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse 29:80–83
Grech DM, Spealman RD, Bergman J (1996) Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology 125:97–104
Green TA, Gehrke BJ, Bardo MT (2002) Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive ratio schedules. Psychopharmacology 162:373–378
Guisado E, Fernandez-Tome P, Garzon J, Del Rio J (1980) Increased dopamine receptor binding in the striatum of rats after long-term isolation. Eur J Pharmacol 65:463–464
Haney M, Maccari S, Le Moal M, Simon H, Piazza PV (1995) Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698:46–52
Kabbaj M, Norton CS, Kollack-Walter S, Watson SJ, Robinson TE, Akil H (2001) Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology 158:382–387
Kaplan JR, Manuck SB (1999) Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann N Y Acad Sci 896:145–161
Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM (1982) Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis 2:359–368
Kaplan JR, Manuck SB, Fontenot MB, Mann JJ (2002) Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis). Neuropsychopharmacology 26:431–443
Katz JL, Witkin JM (1992) Selective effects of the D1 dopamine receptor agonist, SKF 38393, on behavior maintained by cocaine injection in squirrel monkeys. Psychopharmacology 109:241–244
Kleven MS, Koek W (1996) Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. J Pharmacol Exp Ther 279:1211–1219
LeSage MG, Stafford D, Glowa JR (1999) Preclinical research on cocaine self-administration: environmental determinants and their interaction with pharmacological treatment. Neurosci Biobehav Rev 23:717–741
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al. (1990) Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 10:740–747
Mach RH, Nader MA, Ehrenkaufer RLE, Line SW, Smith CR, Luedtke RR, Kung M-P, Kung HF, Lyons D, Morton TE (1996) A comparison of two fluorine-18 labeled benzamide derivatives that bind reversibly to dopamine D2 receptors: in vitro binding studies and positron emission tomography. Synapse 24:322–333
Martin SP, Smith EO, Byrd LD (1990) Effects of dominance rank on d-amphetamine induced increases in aggression. Pharmacol Biochem Behav 37:493–496
Miczek KA, Gold LH (1983) d-Amphetamine in squirrel monkeys of different social status: effects on social and agonistic behavior, locomotion and stereotopies. Psychopharmacology 81:183–190
Miczek KA, Mutschler NH (1996) Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology 128:256–264
Miczek KA, Mutschler NH, van Erp AMM, Blank AD, McInerney SC (1999) d-Amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology 147:190–199
Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP (1998a) The effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse 28:1–9
Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP (1998b) The effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse 30:88–96
Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA (2000) Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol 52:115–131
Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5:169–174
Nader MA, Bowen CA (1995) The effects of different food-reinforcement histories on cocaine self-administration by rhesus monkeys. Psychopharmacology 118:287–294
Nader MA, Grant KA, Gage HD, Ehrenkaufer RL, Kaplan JR, Mach RH (1999a) PET imaging of dopamine D2 receptors with [18F]fluoroclebopride in monkeys: effects of isoflurane- and ketamine-induced anesthesia. Neuropsychopharmacology 21:589–596
Nader MA, Green KL, Luedtke RR, Mach RH (1999b) Influences of housing conditions and ethanol intake on binding characteristics of D2, 5-HT1A and benzodiazepine receptors of rats. Pharmacol Biochem Behav 52:23–928
Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ (2002) Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology 27:35–46
Pettit HO, Justice JB Jr (1989) Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav 34:899–904
Pettit HO, Justice JB Jr (1991) Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res 539:94–102
Platt DM, Rowlett JK, Spealman RD (2001) Modulation of cocaine and food self-administration by low- and high-efficacy D1 agonists in squirrel monkeys. Psychopharmacology 157:208–216
Rilke O, May T, Oehler J, Wolffgramm J (1995) Influences of housing conditions and ethanol intake on binding characteristics of D2, 5-HT1A, and benzodiazepine receptors of rats. Pharmacol Biochem Behav: 23–28
Rilke O, Jahkel M, Oehler J (1998) Dopaminergic parameters during social isolation in low and high-active mice. Pharmacol Biochem Behav 60:499–505
Smith S (2002) Fast robust automated brain extraction. Hum Brain Map 17:143–155
Tidey JW, Miczek KA (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology 130:203–212
Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzemann R, Henn F (1990) Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147:719–724
Weed MR, Woolverton WL (1995) The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 275:1367–1374
Woods RP, Mazziotta JC, Cherry SR (1993) MRI-PET registration with automated algorithm. J Comp Assist Tomogr 17:536–546
Woolverton WL, Virus RM (1989) The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav 32:691–697
Woolverton WL, Goldberg LI, Ginos JZ (1984) Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 230:678–683
Acknowledgements
This research was supported by the National Institute on Drug Abuse (DA-10584). The authors thank Matthew Dickens, Clifford Hubbard and Susan Nader for excellent technical assistance, Jay R. Kaplan for assistance with the scoring of social behavior and Robert H. Mach, Nancy Buchheimer, Kimberly Black and Michael Bounds for assistance with the PET imaging studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Czoty, P.W., Morgan, D., Shannon, E.E. et al. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology 174, 381–388 (2004). https://doi.org/10.1007/s00213-003-1752-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1752-z