Abstract
Rationale
Cannabidiol (CBD), a non-intoxicating component of cannabis, or the psychoactive Δ9-tetrahydrocannabiol (THC), shows anti-hyperalgesia and anti-inflammatory properties.
Objectives
The present study evaluates the anti-inflammatory and anti-hyperalgesia effects of CBD’s potent acidic precursor, cannabidiolic acid (CBDA), in a rodent model of carrageenan-induced acute inflammation in the rat hind paw, when administered systemically (intraperitoneal, i.p.) or orally before and/or after carrageenan. In addition, we assess the effects of oral administration of THC or CBDA, their mechanism of action, and the efficacy of combined ineffective doses of THC and CBDA in this model. Finally, we compare the efficacy of CBD and CBDA.
Results
CBDA given i.p. 60 min prior to carrageenan (but not 60 min after carrageenan) produced dose-dependent anti-hyperalgesia and anti-inflammatory effects. In addition, THC or CBDA given by oral gavage 60 min prior to carrageenan produced anti-hyperalgesia effects, and THC reduced inflammation. The anti-hyperalgesia effects of THC were blocked by SR141716 (a cannabinoid 1 receptor antagonist), while CBDA’s effects were blocked by AMG9810 (a transient receptor potential cation channel subfamily V member 1 antagonist). In comparison to CBDA, an equivalent low dose of CBD did not reduce hyperalgesia, suggesting that CBDA is more potent than CBD for this indication. Interestingly, when ineffective doses of CBDA or THC alone were combined, this combination produced an anti-hyperalgesia effect and reduced inflammation.
Conclusion
CBDA or THC alone, as well as very low doses of combined CBDA and THC, has anti-inflammatory and anti-hyperalgesia effects in this animal model of acute inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis sativa has been used medicinally for centuries. The cannabis plant consists of over 100 cannabinoid compounds, the primary ones being the psychoactive component Δ9-tetrahydrocannabinol (THC), and the non-intoxicating component cannabidiol (CBD). The most frequently reported use of medical cannabis is for pain relief (e.g., Ogborne et al. 2000; Reiman et al. 2017). Indeed, cannabis has been shown to decrease pain intensity in healthy participants (Cooper et al. 2013) and in chronic non-cancer pain patients (Ware et al. 2015). These findings are supported by experimental results from preclinical models in which cannabinoids consistently reduce pain and inflammation (see Lötsch et al. 2018 for a recent review).
Significantly enhanced pain sensation (hyperalgesia) is one of the most common signs of an inflammatory disorder. Intra-plantar injection of carrageenan (or other irritating substances) into a rat’s hind paw (plantar surface) leads to thermal hyperalgesia (increased sensitivity to pain demonstrated by a shorter paw withdrawal latency when stimulated by a heat source) and paw edema (swelling of the injected paw), providing a model to evaluate the ability of compounds to alleviate this response (Winter et al. 1962; Hargreaves et al. 1988), and both THC and CBD have been evaluated using this animal model.
Nabilone (0.75, 1.5, 2.5 mg/kg, orally) or THC (3.75–100 mg/kg, orally), given 1 h pre-carrageenan in rats, has been shown to reduce the development of edema (Sofia et al. 1973a, 1973b; Conti et al. 2002) and the associated hyperalgesia (Conti et al. 2002). Like with carrageenan, injection of complete Freund’s adjuvant (CFA) into the hind paw produces hyperalgesia, and THC (0.32–3.2 mg/kg, intraperitoneally (i.p.), or locally 250–500 μg), when administered 24 h post-CFA produces anti-hyperalgesia effects in rats (Craft et al. 2013). Furthermore, in the tail-flick or by hot-plate test, when assessed 60 min post-THC administration, THC (10 mg/kg, intraperitoneally, i.p.), showed an increase in pain threshold in rats (Novelli et al. 1983). High doses of THC have been shown to produce hypoactivity (≥ 10 mg/kg, i.p., e.g., Katsidoni et al. 2013; Rock et al. 2015; Taffe et al. 2015) and catalepsy (≥ 4 mg/kg, i.p., e.g., Prescott et al. 1992) in rats, suggesting sedative effects of THC at these higher doses. These psychoactive side effects of THC may greatly limit its therapeutic efficacy for some patients.
CBD (5–40 mg/kg, orally, once/day for 3 days) when administered 2 h post-carrageenan has been shown to reduce hyperalgesia after a single injection in rats (Costa et al. 2004a, b). Furthermore, a single dose of CBD (7.5–40 mg/kg, orally) reduces edema and subsequent daily doses cause further edema reductions (Costa et al. 2004a). When administered 1 h pre-carrageenan, CBD (20–40 mg/kg, orally) reduces paw edema in rats (Sofia et al. 1973a). In addition, when applied as a transdermal patch 19 h pre-carrageenan (100 mg CBD), or after the administration of CFA in the CFA-induced monoarthritic knee joint model (6.2 and 62 mg/day), topical CBD application has therapeutic potential for relief of arthritis pain-related behaviors and inflammation without evident side effects in mice (Lodzki et al. 2003; Hammell et al. 2016). Recently, the acidic precursor of CBD, cannabidiolic acid (CBDA), has been shown to be 100–1000 times more potent than CBD in reducing nausea in rat models (Bolognini et al. 2013; Rock and Parker 2013), suggesting that CBDA may also be more potent than CBD in the carrageenan-induced inflammation and/or hyperalgesia model. Therefore, we aimed to test the efficacy of CBDA in this rodent model of inflammatory pain. In addition, because CBD’s anti-hyperalgesia effects have been shown to be mediated by transient receptor potential cation channel subfamily V member 1 (TRPV1; Costa et al. 2004b), and CBDA has also been shown to bind to this receptor (Ligresti et al. 2006); we also tested whether CBDA’s effects in this model were mediated by TRPV1.
Recently, a cohort study of the long-term safety of medical cannabis (12.5 ± 1.5% THC) showed that it was safe for patients, but its use was associated with an increased risk of non-serious adverse events such as somnolence and euphoria, and a greater rate of developing non-serious respiratory adverse events (Ware et al. 2015). It is likely that some of the non-serious adverse events experienced by medical cannabis patients may be due to the psychoactive effects of THC activating the cannabinoid 1 (CB1) receptor. As various cannabis strains differ in their amount of relative cannabinoids (THC:CBD), strains with higher CBD—a cannabis constituent that has low affinity for the CB1 receptor—may be a better therapeutic option. In addition, oral, rather than smoked administration, may reduce respiratory-related adverse events.
In a recent study by Baron et al. (2018), the cannabinoid content was analyzed in the top 15 preferred cannabis strains (identified among patients with migraine, headache, arthritis, and chronic pain). CBDA was detected in 13 strains with the percentage ranging from 0.1–12.6%, whereas CBD was detected in only 4 strains with the percentage ranging from 0.1–11.4%. Wang et al. (2018) detected trace amounts of CBDA in leaf samples and 0.015 – 11.2% in flower bud samples, while Ibrahim et al. (2018) detected CBDA in flower bud samples ranging from 0.57–2.3%. Recently, 200 cannabis oils were analyzed and CBDA concentrations ranging from 0 to 6 mg/ml (Carcieri et al. 2018) were detected.
Although the pharmacokinetics for THC and CBD have been well documented (see Ujváry and Hanuš 2016 for a recent review), little is known about CBDA. A most recent study (Pellesi et al. 2018) evaluated the pharmacokinetics of oral cannabis administered in a decoction (4.4 mg of CBDA) or olive oil (8.8 mg of CBDA). CBDA was detectable in blood in the range of 30 min to 1 h after consumption, achieving higher blood concentrations compared to the other cannabinoids. CBDA was no longer present in blood 4–8 h after intake. In fact, CBDA had a peak serum concentration of 74.61 ng/ml while CBD’s peak was only 4.39 ng/ml, suggesting much higher levels of CBDA than CBD being present in vivo after oral consumption. Clearly, more research is needed on CBDA.
Therefore, using the carrageenan model of inflammatory pain, the aims of our study were (1) to examine the efficacy of CBDA to reduce hyperalgesia and edema when administered i.p. pre- or post-carrageenan; (2) to examine the efficacy of THC or CBDA to reduce hyperalgesia and edema when administered orally pre-carrageenan; and (3) to examine the efficacy of combined ineffective doses of THC and CBDA to reduce hyperalgesia and edema when administered orally pre-carrageenan. We also aimed to compare the efficacy of CBDA with CBD in this model, as well as to investigate the mechanism of action for CBDA and THC’s effects.
Materials and methods
Animals
All animal procedures complied with the Canadian Council on Animal Care and were approved by the Institutional Animal Care Committee (accredited by the Canadian Council on Animal Care). Naïve male Sprague-Dawley rats, weighing between 260 and 334 g on the day of the plantar test, obtained from Charles River Laboratories (St Constant, Quebec), were used for assessment of hyperalgesia and edema. The rats were pair-housed in opaque plastic shoebox cages (48 × 26 × 20 cm), containing bed-o-cob bedding from Harlan Laboratories, Inc. (Mississauga, Ontario), a brown paper towel, and Crink-l’Nest™ (The Andersons, Inc., Maumee, Ohio). Additionally, the rats were provided with a soft white paper container that was 14 cm long and 12 cm in diameter. The colony room was kept at an ambient temperature of 21 °C with a 12/12 h light-dark schedule (lights off at 7 am). The rats were tested in their dark cycle and were maintained on chow (Highland Rat Choc [8640]) and water ad libitum.
Drugs
THC, CBDA (kindly provided by Prairie Plant Systems Inc.), and CBD (kindly provided by Professor Raphael Mechoulam) were administered i.p. or orally (by oral gavage) at a volume of 1 ml/kg. The CB1 receptor antagonist SR141716 (SR; Sequoia Research Products, Pangbourne, UK) and TPRV1 receptor antagonist AMG9810 (AMG; Tocris, Minneapolis, MN, USA) were administered i.p. at a volume of 1 ml/kg (1 mg/kg). These doses for SR and AMG were selected because they have been previously shown to have no effect on their own in pain-related rat paradigms (Nackley et al. 2003; Gavva et al. 2005; Tékus et al. 2010). The compounds were first dissolved in ethanol. The drugs were prepared in a graduated cylinder to ensure the appropriate final concentration of drug. The ethanol/drug solution was measured into the graduated cylinder, the Tween80 was added and the mixture vortexed. The ethanol was evaporated using a nitrogen stream (complete evaporation determined by volume of Tween80 left in the cylinder) after which saline was added. The final vehicle (VEH) solution consisted of 1:9 Tween80:saline. For combined doses of CBDA and THC, the drugs were mixed in a cocktail in VEH. λ-Carrageenan (Sigma Aldrich) was mixed in saline (1% w/v in saline, 10 mg/ml), with a volume of 0.1 ml injected into the plantar surface of the hind paw.
Apparatus
Infrared heat hypersensitivity testing was based on the Hargreaves procedure (Hargreaves et al. 1988) using the plantar test. An Ugo Basile 37370 plantar test apparatus (Italy) was used to measure the withdrawal latency of the paw that received the carrageenan injection (inflamed paw) or the non-inflamed paw. The plantar test apparatus consisted of a movable infrared light generator sitting below a glass pane, upon which the operator deposited the rat. The operator positioned the infrared generator underneath the hind paw (midplantar area), and activated both the infrared source and a reaction time counter via a start key. When the rat withdraws and licks the paw, the infrared beam (8-mm-diameter spot) is automatically switched off and the timer stops. This automatically determines the withdrawal latency to the nearest 0.1 s. The infrared intensity was set at 60 W/cm2 and the cutoff time was 20 s. Electronic digital calipers (Control Company, Fisher Scientific) were used to measure the paw thickness (mm).
Procedures
Thermal hyperalgesia was evaluated on the same animals used to determine edema, employing the radiant heat method (Hargreaves et al. 1988). The experimenters were blind to the treatment conditions.
Twenty-four hours prior to testing, rats received two 10-min habituation sessions during which the thickness of each hind paw (mm) was measured and the rats were then placed in the clear Plexiglas box of the plantar test apparatus and allowed to acclimatize.
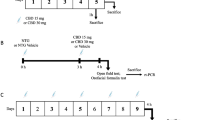
On the following day, baseline measures of hind paw thickness were taken, along with baseline hind paw withdrawal latencies (s). Following baseline measures, for those animals in the CBDA pre-carrageenan (i.p.) group (experiment 1a; n = 8/group), a pretreatment injection of VEH or CBDA (10, 1000 μg/kg) was delivered i.p., or for those animals in the oral CBDA, THC, or CBD pre-carrageenan group (experiments 2, 3, 5; n = 8/group), VEH, CBDA (0.1, 1, 10, 100, 1000 μg/kg), THC (100, 1000 μg/kg), or CBD (100, 10,000 μg/kg) was administered by oral gavage. Sixty minutes later, acute inflammation was induced by intra-plantar injection of 0.1 ml of 1% λ-carrageenan into the plantar surface of the hind paw (left or right paw injection counterbalanced) with a 27-G needle. Alternatively, for those animals in the CBDA post-carrageenan group (experiment 1b; n = 8/group), CBDA (10, 1000 μg/kg) or VEH was delivered i.p. 60 min after carrageenan. In experiment 4, to investigate the mechanism of action of the anti-hyperalgesia effects of CBDA and THC, additional groups of rats were coadministered the TRPV1 antagonist AMG (1 mg/kg, i.p.) or the CB1 receptor antagonist SR (1 mg/kg, i.p.) 30 min prior to 100 μg/kg CBDA or 1000 μg/kg THC (orally), respectively.
Carrageenan-induced hyperalgesia was measured at 30, 60, 180, and 360 min after carrageenan administration and expressed as a mean difference in paw withdrawal latency in seconds (ipsilateral paw withdrawal latency at each time point minus baseline ipsilateral paw withdrawal latency; Costa et al. 2004b).
Carrageenan-induced paw edema was measured at the time of carrageenan injection (0) and 30, 60, 180, and 360 min after carrageenan administration and expressed as a mean paw thickness difference in millimeters (ipsilateral paw thickness at each time point minus baseline ipsilateral paw thickness; Conti et al. 2002).
Statistical analysis
In each experiment, the mean difference in paw withdrawal latencies and the mean paw thickness differences were entered into separate factorial analysis of variance (ANOVA) as appropriate. When sphericity (determined by Mauchly’s sphericity test) was not assumed, the Greenhouse–Geisser correction factor (when ε < .75) or the Huynh-Feldt correction (when ε > .75) was applied to all repeated factors to avoid spurious significance due to lack of homogeneity of variance and covariance in repeated factors (Keselman et al. 2001). One-way ANOVAs for each time point were also conducted, with LSD post hoc analyses. For all statistical analyses, significance was defined as p < 0.05.
Results
Experiment 1a: CBDA pre-carrageenan (i.p.)
Carrageenan produced hyperalgesia and pretreatment with 10 μg/kg CBDA (i.p.) abolishing this effect at 180 and 360 min after carrageenan, but 1000 μg/kg CBDA did not. Figure 1a, b presents the mean (± S.E.M.) difference in paw withdrawal latency (s) for each paw treatment at the 180 and 360 time points. A 4 (time) × 4 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (2.8, 79.2) = 2.7, p = 0.05, a significant main effect of paw treatment F (3, 28) = 8.5, p < 0.001, but a non-significant time × paw treatment interaction F (8.5, 79.2) = 0.4, p = 0.9. Subsequent one-way ANOVAs for each time point revealed differences only at the 60 time point F (3, 28) = 3.3, p < 0.05, the 180 time point, F (3, 28) = 5.1, p < 0.01, and the 360 time point, F (3, 28) = 7.2, p = 0.01. LSD post hoc analyses revealed that at the 180 and 360 time points, in comparison to the VEH-inflamed (VEH-I) group, withdrawal latencies were significantly longer for the 10 CBDA-inflamed (10 CBDA-I) group, (p’s < 0.01).
Effect of VEH or CBDA (10, 1000 μg/kg, i.p.) on paw withdrawal latency (s) at 180 (a) or 360 min (b) after carrageenan and paw thickness at 360 min after carrageenan (c), when administered 60 min pre-carrageenan (n = 8/group). The mean (± S.E.M.) difference in paw withdrawal latency from baseline for each paw treatment was measured. The mean (± S.E.M.) difference in paw thickness (mm; ipsilateral paw thickness at each time interval minus baseline paw thickness) for each paw treatment was measured. The asterisks indicate a significant difference from the VEH-I group (**p < 0.01; *p < 0.05)
Carrageenan produced increased paw thickness and pretreatment with 10 μg/kg CBDA (i.p.) exerting anti-inflammatory effects at 360 min after carrageenan. Figure 1c presents the mean (± S.E.M.) difference in paw thickness (mm) for each paw treatment at the 360 time point. A 5 (time) × 4 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (2.8, 77.5) = 68.6, p < 0.001, a significant main effect of paw treatment F (3, 28) = 79.7, p < 0.001, and a significant time × paw treatment interaction F (8.3, 77.5) = 8.8, p < 0.001. Subsequent one-way ANOVAs for each time point revealed differences at every time point (p’s < 0.001). LSD post hoc analyses revealed that at all time points, the VEH-I group had significantly thicker paws than the VEH-non-inflamed (VEH-Non I) group (p’s < 0.001). In addition, at the 360 min time point, in comparison to the VEH-I group, paw thicknesses were significantly less for the 10 CBDA-I group (p < 0.05), indicating that at 360 min, 10 μg/kg CBDA (i.p.) when administered 60 min prior to carrageenan had an anti-inflammatory effect.
Experiment 1b: CBDA post-carrageenan (i.p.)
Carrageenan produced hyperalgesia and post-treatment with CBDA (i.p.) did not reduce hyperalgesia. Figure 2a, b presents the mean (± S.E.M.) difference in paw withdrawal latency (s) for each paw treatment at the 180 and 360 time points. A 4 (time) × 4 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (2.8, 79.1) = 4.0, p = 0.01, a significant main effect of paw treatment F (3, 28) = 9.8, p < 0.001, and a significant time × paw treatment interaction F (8.5, 79.1) = 2.0, p < 0.05. Subsequent one-way ANOVAs for each time point revealed differences at all time points (p’s < 0.05). LSD post hoc analyses revealed that at all time points, only the VEH-I group had a significantly quicker withdrawal than the VEH/non-inflamed paw (p’s < 0.001).
Effect of VEH or CBDA (10, 1000 μg/kg, i.p.) on paw withdrawal latency (s) at 180 (a) or 360 min (b) after carrageenan and paw thickness at 360 min after carrageenan (c) when administered 60 min post-carrageenan (n = 8/group). The mean (± S.E.M.) difference in paw withdrawal latencies from baseline for each paw treatment was measured. The mean (± S.E.M.) difference in paw thickness (mm; ipsilateral paw thickness at each time interval minus baseline paw thickness) for each paw treatment was measured
Carrageenan produced increased paw thickness that was not reduced by post-treatment with CBDA (i.p.). Figure 2c presents the mean (± S.E.M.) difference in paw thickness (mm) for each paw treatment at the 360 min time point post-carrageenan. A 5 (time) × 4 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (4, 112) = 146.9, p < 0.001, a significant main effect of paw treatment F (3, 28) = 164.7, p < 0.001, and a significant time × paw treatment interaction F (12, 112) = 16.4, p < 0.001. Subsequent one-way ANOVAs for each time point revealed differences at every time point (p’s < 0.001). LSD post hoc analyses revealed that at all time points, only the VEH-I group had paws significantly thicker than the VEH-Non I group (p’s < 0.001).
Experiment 2: Oral CBDA or THC pre-carrageenan
Carrageenan produced hyperalgesia and pretreatment with 1000 μg/kg THC, or 1, 100 μg/kg CBDA (delivered by oral gavage) reduced this effect. Figure 3a, b presents the mean (± S.E.M.) difference in paw withdrawal latency (s) for each paw treatment. A 4 (time) × 7 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (3, 147) = 12.3 p < 0.001, a significant main effect of paw treatment F (6, 49) = 4.3, p = 0.002, but a non-significant time × paw treatment interaction F (18, 147) = 0.8, p = 0.7. Subsequent one-way ANOVAs for each time point revealed significant differences at all time points (p’s < 0.05). LSD post hoc analyses revealed that the latency to lift the inflamed paw was significantly longer for 1000 THC-inflamed (1000 THC-I) group in comparison to the VEH-I group at the 30, 180, and 360 min time points (p’s < 0.05), and were marginally longer at the 60 min time point (p = 0.09), suggesting an analgesic effect for THC at 1000 μg/kg, given orally. In addition, only at 360 min did the CBDA-inflamed groups differ from VEH-I group in the latency to withdraw the inflamed paw. In comparison to the VEH-I group, withdrawal latencies were significantly longer for the 1 CBDA-inflamed (1 CBDA-I) group (p < 0.05), 100 CBDA/inflamed (100 CBDA-I) group (p < 0.05), and marginally longer for the 10 CBDA-inflamed (10 CBDA-I) group (p = 0.06), and the 1000 CBDA-inflamed (1000 CBDA-I) group (p = 0.09). These results indicate that at 360 min post-carrageenan, pretreatment with CBDA (orally) reduces hyperalgesia.
Effect of CBDA (1, 10, 100, 1000 μg/kg, oral gavage), THC (1000 μg/kg, oral gavage) or VEH on paw withdrawal latency (s) at 180 (a) 360 min (b) after carrageenan and paw thickness at 180 (c) and 360 min (d) after carrageenan, when administered 60 min pre-carrageenan (n = 8/group). The mean (± S.E.M.) difference in paw withdrawal latencies from baseline for each paw treatment was measured at 30, 60, 180, and 360 min following carrageenan treatment. The mean (± S.E.M.) difference in paw thickness (mm; ipsilateral paw thickness at each time interval minus baseline paw thickness) for each paw treatment was measured. The asterisks indicate a significant difference from the VEH-I group (**p < 0.01; *p < 0.05)
Carrageenan produced increased paw thickness that was reduced by THC (1000 μg/kg), but not by CBDA (when administered by oral gavage). Figure 3c, d presents the mean (± S.E.M.) difference in paw thickness (mm) for each paw treatment. A 5 (time) × 7 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (2.3, 111.5) = 165.6, p < 0.001, a significant main effect of paw treatment F (6, 49) = 47.9, p < 0.001, and a significant time × paw treatment interaction F (13.6, 111.5) = 5.0, p < 0.001. Subsequent one-way ANOVAs for each time point revealed differences at every time point (p’s < 0.001). LSD post hoc analyses revealed that at the 180 and 360 min time points, in comparison to the VEH-I group, the 1000 THC-I group paw thicknesses were significantly smaller (p’s < 0.05).
Experiment 3: Oral combined ineffective doses of CBDA and THC pre-carrageenan
Carrageenan produced hyperalgesia and pretreatment with 100 μg/kg THC + 0.1 μg/kg CBDA by oral gavage (but not either of these doses alone) reduced this effect. Figure 4a–d presents the mean (± S.E.M.) difference in paw withdrawal latency (s) for each paw treatment. A 4 (time) × 5 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (3, 105) = 16.2 p < 0.001, a significant main effect of paw treatment F (4, 35) = 6.8, p < 0.001, and a significant time × paw treatment interaction F (12, 105) = 2.2, p < 0.02. Subsequent one-way ANOVAs for each time point revealed significant differences at all time points (p’s < 0.05). LSD post hoc analyses revealed that in comparison to the VEH-I paw, the latency to lift the inflamed paw was significantly longer for the 100 THC + 0.1 CBDA-inflamed (100 THC + 0.1 CBDA-I) group at all time points (p’s < 0.05), but did not differ from the CBDA or THC alone groups. These results indicate that when ineffective doses alone are combined, CBDA and THC given orally can reduce hyperalgesia.
Effect of CBDA (0.1 μg/kg, oral gavage), THC (100 μg/kg, oral gavage), their combination, or VEH on paw withdrawal latency (s) at 30 (a), 60 (b), 180 (c), or 360 min (d) after carrageenan and paw thickness at 0 (e), 180 (f), or 360 min (g) after carrageenan, when administered 60 min pre-carrageenan (n = 8/group). The mean (± S.E.M.) difference in paw withdrawal latencies from baseline for each paw treatment was measured. The mean (± S.E.M.) difference in paw thickness (mm; ipsilateral paw thickness at each time interval minus baseline paw thickness) for each paw treatment was measured. The asterisks indicate a significant difference from the VEH-I group (***p ≤ 0.001; **p < 0.01; *p < 0.05)
Carrageenan produced increased paw thickness that was reduced by 100 THC + 0.1 CBDA by oral gavage (but not either of these doses alone) when administered by oral gavage. Figure 4e–g presents the mean (± S.E.M.) difference in paw thickness (mm) for each paw treatment. A 5 (time) × 5 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (2.2, 75.8) = 213.9, p < 0.001, a significant main effect of paw treatment F (4, 35) = 98.3, p < 0.001, and a significant time × paw treatment interaction F (8.7, 75.8) = 13.4, p < 0.001. Subsequent one-way ANOVAs for each time point revealed differences at every time point (p’s < 0.001). LSD post hoc analyses revealed that in comparison to group VEH-I, the 100 THC + 0.1 CBDA-I group had significantly smaller paw thicknesses at the 0, 180, and 360 min time points, (p’s < 0.05), but these CBDA or THC alone doses did not.
Experiment 4: Mechanism of action for oral administration of CBDA or THC
Coadministration of SR with THC or AMG with CBDA blocked the anti-hyperalgesia effects. Figure 5a presents the mean (± S.E.M.) difference in paw withdrawal latency (s) for each paw treatment. A 4 (time) × 5 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (3, 105) = 13.6 p = 0.001, a non-significant main effect of paw treatment F (4, 35) = 1.6, p > 0.05, and a significant time × paw treatment interaction F (12, 105) = 2.6, p < 0.01. Subsequent one-way ANOVAs for each time point revealed significant differences only at the 360 time point (p < 0.01). LSD post hoc analyses revealed that at the 360 min time point, in comparison to the VEH-I group, the latency to lift the inflamed paw was significantly longer for the 1000 THC-I and the 100 CBDA-I groups (p’s < 0.05). Group AMG + 100 CBDA-inflamed (AMG + 100 CBD-/I) significantly differed from group 100 CBDA-I, and group SR + 1000 THC-inflamed (SR + 1000 THC-I) significantly differed from group 1000 THC-I (p’s < 0.05). Administration of either AMG or SR with CBDA or THC (respectively) blocked the anti-hyperalgesia effects, suggesting that CBDA is exerting its effects via TRPV1 receptors, while THC is activating CB1 receptors.
Effect of CBDA (100 μg/kg, oral gavage) alone and in combination with AMG (1 mg/kg, i.p.), and THC (1000 μg/kg, oral gavage) alone and in combination with SR (1 mg/kg, i.p.) on paw withdrawal latency (s) at 360 (a) after carrageenan and paw thickness at 360 min (b) after carrageenan (n = 8/group). The mean (± S.E.M.) difference in paw withdrawal latencies from baseline for each paw treatment was measured. The mean (± S.E.M.) difference in paw thickness (mm; ipsilateral paw thickness at each time interval minus baseline paw thickness) for each paw treatment was measured. The asterisks indicate a significant difference from the VEH-I group (**p < 0.01; *p < 0.05). The pound sign indicates a significant difference from the 100 CBDA-I group (p < 0.05). The ampersand indicates a significant difference from the 1000 THC-I group (p < 0.05)
No pretreatment reduced edema produced by carrageenan. Figure 5b presents the mean (± S.E.M.) difference in paw thickness (mm) for each paw treatment. A 5 (time) × 4 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (2.2, 76.4) = 125.8, p < 0.001, a non-significant main effect of paw treatment F (4, 35) = 1.9, p > 0.05, and a non-significant time × paw treatment interaction F (8.7, 76.4) = 0.5, p > 0.05.
Experiment 5: Comparison of oral administration of CBD with CBDA
Pretreatment with 10,000 μg/kg CBD or 100 μg/kg CBDA (but not 100 μg/kg CBD) by oral gavage reduced hyperalgesia. Figure 6a, b presents the mean (± S.E.M.) difference in paw withdrawal latency (s) for each paw treatment. A 4 (time) × 5 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (3, 84) = 6.4 p = 0.001, a significant main effect of paw treatment F (3, 28) = 3.7, p < 0.05, and a non-significant time × paw treatment interaction F (9, 84) = 1.1, p > 0.05. Subsequent one-way ANOVAs for each time point revealed significant differences at the 180 and 360 time points (p’s < 0.05). LSD post hoc analyses revealed that at the 180 min time point, in comparison to the VEH-I group, the latency to lift the inflamed paw was significantly longer for group 10,000 CBD-inflamed (10,000 CBD-I; p’s < 0.01). In addition, at the 360 min time point, in comparison to the VEH-I paw, the latency to lift the inflamed paw was significantly longer for both group 100 CBDA-I and 10,000 CBD-I (p’s < 0.05). As CBDA is effective at a much lower dose than CBD, these results demonstrate that CBDA is more potent in this hyperalgesia model.
Effect of CBD (100, 10,000 μg/kg, oral gavage), CBDA (100 μg/kg, oral gavage), or VEH on paw withdrawal latency (s) at 180 (a) and 360 min (b) after carrageenan and paw thickness at 360 min (c) after carrageenan, when administered 60 min pre-carrageenan (n = 8/group). The mean (± S.E.M.) difference in paw withdrawal latencies from baseline for each paw treatment was measured. The mean (± S.E.M.) difference in paw thickness (mm; ipsilateral paw thickness at each time interval minus baseline paw thickness) for each paw treatment was measured. The asterisks indicate a significant difference from the VEH-I group (**p < 0.01; *p < 0.05)
When administered by oral gavage, no dose of CBD (or CBDA) tested reduced edema. Figure 6c presents the mean (± S.E.M.) difference in paw thickness (mm) for each paw treatment. A 5 (time) × 4 (paw treatment) mixed factors ANOVA revealed a significant main effect of time F (2.2, 62.8) = 121.4, p < 0.001, a non-significant main effect of paw treatment F (3, 28) = 0.4, p > 0.05, and a non-significant time × paw treatment interaction F (6.7, 62.8) = 0.5, p > 0.05.
Discussion
CBDA (10 μg/kg, i.p. or 1, 100 μg/kg orally) administered 60 min prior to carrageenan produced anti-hyperalgesia effects. These findings are in agreement with those for CBD, as CBD (5–40 mg/kg, orally) has been reported to reduce hyperalgesia and/or edema (Sofia et al. 1973a; Costa et al. 2004a, b). And indeed, when tested in our model, CBD (10 mg/kg, orally) reduced hyperalgesia when administered 60 min prior to carrageenan, but when CBD was administered at a similar low dose to CBDA (100 μg/kg, orally), this dose of CBD was ineffective (unlike CBDA). These results suggest that CBDA is more potent than CBD in this model. This enhanced potency of CBDA has been previously demonstrated in the conditioned gaping model (a rat model of acute nausea; Rock and Parker 2013) and contextually elicited conditioned gaping model (a rat model of anticipatory model; Rock et al. 2014). Interestingly, CBDA (10 μg/kg, i.p.) administered 60 min prior to carrageenan produced dose-dependent anti-inflammatory effects, but oral administration of CBDA (at the doses tested) did not. These routes of administration differences in the anti-inflammatory ability of CBDA are likely due to differences in drug metabolism, with oral administration being greatly impacted by first-pass liver metabolism.
Consistent with previous literature (Sofia et al. 1973a, b; Conti et al. 2002), we also showed that THC (1000 μg/kg, orally) administered 60 min prior to carrageenan produced dose-dependent anti-hyperalgesia and anti-inflammatory effects. Most interestingly though, we also demonstrated that ineffective doses alone of THC (100 μg/kg, orally) or CBDA (0.1 μg/kg, orally), when combined, effectively reduced hyperalgesia and inflammation, suggesting a synergistic effect of combining THC and CBDA. Our group has previously shown (Rock et al. 2016) that when administered orally, doses as high as 10 mg/kg THC or 10 μg/kg CBDA do not interfere with locomotor activity, suggesting that these anti-hyperalgesia effects are not simply due to sedation, and rather are indicative of reduced pain sensitivity.
In comparison to the i.p. administered VEH/inflamed group, the orally administered VEH/inflamed group showed somewhat more hyperalgesia (indicating a greater sensitivity to pain and a faster paw withdrawal latency) at 30 min after carrageenan administration. This difference in paw withdrawal latency between the two routes of drug administration may be due to the stress involved with oral gavage. Indeed, stress, fear, and anxiety can modulate pain (as discussed by Woodhams et al. 2017). Certainly, stressed rats show significantly reduced pain thresholds (e.g., Uesugi et al. 2011; Korczeniewska et al. 2017) and human patients subjected to stress also show reduced pain thresholds, which are not accompanied simply by a reduction in the threshold of sensitivity to tactile stimulation in general (Ashkinazi and Vershinina 1999). It is noteworthy, though, that our reported mean difference in paw withdrawal latency for the oral VEH/inflamed group at 180 min (~ 7 s) is consistent with the mean for a similar group treated by oral gavage at 180 min (~ 6 s) reported by Costa and colleagues (2004), suggesting good consistency between research groups.
Although little research has focused on CBDA (in comparison to other cannabinoids), there is evidence to suggest that CBDA is an agonist at transient receptor potential channels of ankyrin type-1 (TRPA1; De Petrocellis et al. 2008), an agonist at transient receptor potential cation channel subfamily V member 1 (TRPV1; Ligresti et al. 2006), and an antagonist at transient receptor potential channels of melastatin type-8 (TRPM8; De Petrocellis et al. 2008). In addition, CBDA has been shown to enhance the activation of serotonin 1A receptors (Bolognini et al. 2013). Here, we show that coadministration of the TRPV1 antagonist AMG9810 blocked the anti-hyperalgesic effect of CBDA, suggesting that the analgesic properties of CBDA may be mediated by activation of TRPV1 receptors. Furthermore, administration of SR blocked the anti-hyperalgesic effect of THC, providing further evidence that THC’s ability to inhibit pain-like behaviors is likely due to its action at peripheral and central CB1 receptors, although cannabinoid 2 (CB2) receptor activation may also be inhibiting the pro-inflammatory effects of immune cells and microglia to reduce pain (Romero-Sandoval et al. 2008). Furthermore, the enhanced effectiveness of combined THC and CBD is most likely due the ability of CBD to inhibit cytochrome P450 enzymes for liver metabolism (Bornheim and Correia 1990, 1991), resulting in higher blood THC levels (Reid and Bornheim 2001; Klein et al. 2011; Hložek et al. 2017), but this has not been assessed for CBDA.
The literature is replete with evidence of beneficial interactions between CBD and THC in both preclinical and clinical studies (for a recent review, see Boggs et al. 2017). For example, CBD enhances the pain-relieving actions of THC in a mouse chronic constriction injury (CCI) model of neuropathic pain (Casey et al. 2017), it enhances the reduction of mechanical allodynia in mice treated with paclitaxel, a mouse model of chemotherapy-induced peripheral neuropathy (Ward et al. 2011, 2014; King et al. 2017) and it enhances the pain-relieving actions of THC in the tail-flick test in mice (Varvel et al. 2006). Multiple studies have demonstrated efficacy of Sativex (an oromucosal spray containing a 1:1 ratio of THC:CBD) in multiple sclerosis-related pain (Rog et al. 2005, 2007; Langford et al. 2013; Ferrè et al. 2016; Russo et al. 2016; Vermersch and Trojano 2016), cancer-related pain (Portenoy et al. 2012; Johnson et al. 2013), and pain caused by rheumatoid arthritis (Blake et al. 2006). To date, very few studies have explored the interaction between CBDA and THC, suggesting that much more research is needed. As well as in the current opioid epidemic, it would be valuable to learn about the conditions under which CBD, CBDA, and other cannabinoids can be considered as alternatives to the opioid analgesics. Future work is planned to address this issue.
The use of the very liberal LSD test for post hoc comparisons is a limitation of the present study; however, here we have independently replicated results previously reported in the literature, adding internal consistency for our results and partially overcoming this potential limitation. As well, future work needs to address the mechanisms of the inverted U-shaped dose-response curve observed in experiment 1.
The results of the present study suggest that oral administration of CBDA potently reduces pain behavior and inflammation in rodent model of inflammatory pain. Furthermore, when ineffective doses of THC and CBDA are combined, there is an enhanced anti-hyperalgesia and anti-inflammatory effect. These results suggest that very small doses of THC and CBDA could be administered in combination to reduce inflammatory pain and may be effective against other types of pain such as multiple sclerosis-related pain.
References
Ashkinazi IY, Vershinina EA (1999) Pain sensitivity in chronic psychoemotional stress in humans. Neurosci Behav Physiol 29:333–337
Baron EP, Lucas P, Eades J, Hogue O (2018) Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain 19:37. https://doi.org/10.1186/s10194-018-0862-2
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS (2006) Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 45:50–52. https://doi.org/10.1093/rheumatology/kei183
Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M (2017) Clinical and preclinical evidence for functional interactions of cannabidiol and Δ9-tetrahydrocannabinol. Neuropsychopharmacology 43:142–154. https://doi.org/10.1038/npp.2017.209
Bolognini D, Rock EM, Cluny NL, Cascio MG, Limebeer CL, Duncan M, Stott CG, Javid FA, Parker LA, Pertwee RG (2013) Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br J Pharmacol 168:1456–1470. https://doi.org/10.1111/bph.12043
Bornheim LM, Correia MA (1990) Selective inactivation of mouse liver cytochrome P-450IIIA by cannabidiol. Mol Pharmacol 38:319–326
Bornheim LM, Correia MA (1991) Purification and characterization of the major hepatic cannabinoid hydroxylase in the mouse: a possible member of the cytochrome P-450IIC subfamily. Mol Pharmacol 40:228–234
Carcieri C, Tomasello C, Simiele M, De N, Avataneo V, Canzoneri L et al (2018) Cannabinoids concentration variability in cannabis olive oil galenic preparations. J Pharm Pharmacol 70:143–149. https://doi.org/10.1111/jphp.12845
Casey SL, Atwal N, Vaughan CW (2017) Cannabis constituent synergy in a mouse neuropathic pain model. Pain 158:2452–2460. https://doi.org/10.1097/j.pain.0000000000001051
Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G (2002) Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br J Pharmacol 135(1):181–187. https://doi.org/10.1038/sj.bjp.0704466
Cooper ZD, Comer SD, Haney M (2013) Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology 38:1984–1992. https://doi.org/10.1038/npp.2013.97
Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, Giagnoni G (2004a) Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedeberg's Arch Pharmacol 369:294–299. https://doi.org/10.1007/s00210-004-0871-3
Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M (2004b) Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol 143:247–250. https://doi.org/10.1038/sj.bjp.0705920
Craft RM, Kandasamy R, Davis SM (2013) Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Δ(9)-tetrahydrocannabinol in the rat. Pain 154:1709–1717. https://doi.org/10.1016/j.pain.2013.05.017
De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P et al (2008) Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther 325:1007–1015. https://doi.org/10.1124/jpet.107.134809
Ferrè L, Nuara A, Pavan G, Radaelli M, Moiola L, Rodegher M, Colombo B, Keller Sarmiento IJ, Martinelli V, Leocani L, Martinelli Boneschi F, Comi G, Esposito F (2016) Efficacy and safety of nabiximols (Sativex(®)) on multiple sclerosis spasticity in a real-life Italian monocentric study. Neurol Sci 37:235–242. https://doi.org/10.1007/s10072-015-2392-x
Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ (2005) AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313:474–484. https://doi.org/10.1124/jpet.104.079855
Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL et al (2016) Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain 20:936–948. https://doi.org/10.1002/ejp.818
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šíchová K, Štefková K, Tylš F, Kuchař M, Páleníček T (2017) Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol 27:1223–1237. https://doi.org/10.1016/j.euroneuro.2017.10.037
Ibrahim EA, Gul W, Gul SW, Stamper BJ, Hadad GM, Abdel Salam RA, Ibrahim A, Ahmed S, Chandra S, Lata H, Radwan M, ElSohly M (2018) Determination of acid and neutral cannabinoids in extracts of different strains of Cannabis sativa using GC-FID. Planta Med 84:250–259. https://doi.org/10.1055/s-0043-124088
Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT (2013) An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manag 46:207–218. https://doi.org/10.1016/j.jpainsymman.2012.07.014
Katsidoni V, Kastellakis A, Panagis G (2013) Biphasic effects of Delta9- tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol 16:2273–2284. https://doi.org/10.1017/S1461145713000709
Keselman HJ, Algina J, Kowalchuk RK (2001) The analysis of repeated measures designs: a review. Br J Math Stat Psychol 54:1–20
King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ (2017) Single and combined effects of Δ9 -tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol 174:2832–2841. https://doi.org/10.1111/bph.13887
Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS (2011) Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 218:443–457. https://doi.org/10.1007/s00213-011-2342-0
Korczeniewska OA, Khan J, Tao Y, Eliav E, Benoliel R (2017) Effects of sex and stress on trigeminal neuropathic pain-like behavior in rats. J Oral Facial Pain Headache 31:381–397. https://doi.org/10.11607/ofph.1807
Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, Ratcliffe S (2013) A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol 260:984–997. https://doi.org/10.1007/s00415-012-6739-4
Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L et al (2006) Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther 318:1375–1387. https://doi.org/10.1124/jpet.106.105247
Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E (2003) Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J Control Release 93:377–387
Lötsch J, Weyer-Menkhoff I, Tegeder I (2018) Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur J Pain 22:471–484. https://doi.org/10.1002/ejp.1148
Nackley AG, Makriyannis A, Hohmann AG (2003) Selective activation of cannabinoid CB(2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience 119:747–757. https://doi.org/10.1016/S0306-4522(03)00126-X
Novelli GP, Peduto VA, Bertol E, Mari F, Pieraccioli E (1983) Analgesic interaction between nitrous oxide and delta-9-tetrahydrocannabinol in the rat. Br J Anaesth 55:997–1000
Ogborne AC, Smart RG, Adlaf EM (2000) Self-reported medical use of marijuana: a survey of the general population. CMAJ 162:1685–1686
Pellesi L, Licata M, Verri P, Vandelli D, Palazzoli F, Marchesi F (2018) Pharmacokinetics and tolerability of oral cannabis preparations in patients with medication overuse headache (MOH)—a pilot study. Eur J Clin Pharmacol. https://doi.org/10.1007/s00228-018-2516-3
Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, McQuade R, Wright S, Fallon MT (2012) Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain 13:438–449. https://doi.org/10.1016/j.jpain.2012.01.003
Prescott WR, Gold LH, Martin BR (1992) Evidence for separate neuronal mechanisms for the discriminative stimulus and catalepsy induced by delta 9-THC in the rat. Psychopharmacology 107:117–124
Reid MJ, Bornheim LM (2001) Cannabinoid-induced alterations in brain disposition of drugs of abuse. Biochem Pharmacol 61:1357–1367
Reiman A, Welty M, Solomon P (2017) Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res 2:160–166. https://doi.org/10.1089/can.2017.0012
Rock EM, Parker LA (2013) Effect of low doses of cannabidiolic acid and ondansetron on LiCl-induced conditioned gaping (a model of nausea-induced behaviour) in rats. Br J Pharmacol 169:685–692. https://doi.org/10.1111/bph.12162
Rock EM, Limebeer CL, Navaratnam R, Sticht MA, Bonner N, Engeland K, Downey R, Morris H, Jackson M, Parker LA (2014) A comparison of cannabidiolic acid with other treatments for anticipatory nausea using a rat model of contextually elicited conditioned gaping. Psychopharmacology 231:3207–3215. https://doi.org/10.1007/s00213-014-3498-1
Rock EM, Limebeer CL, Parker LA (2015) Effect of combined doses of Δ9-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea using rat (Sprague-Dawley) models of conditioned gaping. Psychopharmacology 232:4445–4454. https://doi.org/10.1007/s00213-015-4080-1
Rock EM, Connolly C, Limebeer CL, Parker LA (2016) Effect of combined oral doses of ∆9-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea in rat models. Psychopharmacology 233:3353–3360. https://doi.org/10.1007/s00213-016-4378-7
Rog DJ, Nurmikko TJ, Friede T, Young CA (2005) Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 65:812–819. https://doi.org/10.1212/01.wnl.0000176753.45410.8b
Rog DJ, Nurmikko TJ, Young CA (2007) Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther 29:2068–2079. https://doi.org/10.1016/j.clinthera.2007.09.013
Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA (2008) Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology 108:722–734. https://doi.org/10.1097/ALN.0b013e318167af74
Russo M, Naro A, Leo A, Sessa E, D'Aleo G, Bramanti P et al (2016) Evaluating Sativex® in neuropathic pain management: a clinical and neurophysiological assessment in multiple sclerosis. Pain Med 17:1145–1154. https://doi.org/10.1093/pm/pnv080
Sofia RD, Knobloch LC, Vassar HB (1973a) The anti-edema activity of various naturally occurring cannabinoids. Res Commun Chem Pathol Pharmacol 6:909–918
Sofia RD, Nalepa SD, Harakal JJ, Vassar HB (1973b) Anti-edema and analgesic properties of delta9-tetrahydrocannabinol (THC). J Pharmacol Exp Ther 186:646–655
Taffe MA, Creehan KM, Vandewater SA (2015) Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Delta(9) -tetrahydrocannabinol in Sprague–Dawley rats. Br J Pharmacol 172:1783–1791. https://doi.org/10.1111/bph.13024
Tékus V, Bölcskei K, Kis-Varga A, Dézsi L, Szentirmay E, Visegrády A, Horváth C, Szolcsányi J, Pethő G (2010) Effect of transient receptor potential vanilloid 1 (TRPV1) receptor antagonist compounds SB705498, BCTC and AMG9810 in rat models of thermal hyperalgesia measured with an increasing-temperature water bath. Eur J Pharmacol 641:135–141. https://doi.org/10.1016/j.ejphar.2010.05.052
Uesugi K, Sekiguchi M, Kikuchi S, Konno S (2011) The effect of repeated restraint stress in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Eur Spine J 20:1885–1891. https://doi.org/10.1007/s00586-011-1877-4
Ujváry I, Hanuš L (2016) Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res 1:90–101. https://doi.org/10.1089/can.2015.0012
Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR (2006) Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology 186:226–234. https://doi.org/10.1007/s00213-006-0356-9
Vermersch P, Trojano M (2016) Tetrahydrocannabinol:cannabidiol oromucosal spray for multiple sclerosis-related resistant spasticity in daily practice. Eur Neurol 76:216–226. https://doi.org/10.1159/000449413
Wang YH, Avula B, ElSohly MA, Radwan MM, Wang M, Wanas AS, Mehmedic Z, Khan I (2018) Quantitative determination of Δ9-THC, CBG, CBD, their acid precursors and five other neutral cannabinoids by UHPLC-UV-MS. Planta Med 84:260–266. https://doi.org/10.1055/s-0043-124873
Ward SJ, Ramirez MD, Neelakantan H, Walker EA (2011) Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth Analg 113:947–950. https://doi.org/10.1213/ANE.0b013e3182283486
Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA (2014) Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 171:636–645. https://doi.org/10.1111/bph.12439
Ware MA, Wang T, Shapiro S, Collet JP, COMPASS study team (2015) Cannabis for the management of pain: assessment of safety study (COMPASS). J Pain 16:1233–1242. https://doi.org/10.1016/j.jpain.2015.07.014
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111:544–547
Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V (2017) The cannabinoid system and pain. Neuropharmacology 124:105–120. https://doi.org/10.1016/j.neuropharm.2017.06.015
Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Collaborative Research and Development Grant (CRDPJ 476416-14) to LAP in partnership with Prairie Plant Systems Inc., as well as a grant to LAP from NSERC (92056) and from Canadian Institute of Health Research (137122).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures complied with the Canadian Council on Animal Care and were approved by the Institutional Animal Care Committee (accredited by the Canadian Council on Animal Care).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rock, E.M., Limebeer, C.L. & Parker, L.A. Effect of cannabidiolic acid and ∆9-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology 235, 3259–3271 (2018). https://doi.org/10.1007/s00213-018-5034-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5034-1