Abstract
Purpose
The recent release of a medical cannabis strain has given a new impulse for the study of cannabis in Italy. The National Health Service advises to consume medical cannabis by vaporizing, in decoction or oil form. This is the first study that explores the pharmacokinetics and tolerability of a single oral dose of cannabis as decoction (200 ml) or in olive oil (1 ml), as a first step to improve the prescriptive recommendations.
Methods
This is a single-center, open-label, two-period crossover study designed to assess the pharmacokinetics and tolerability of oral cannabis administered to 13 patients with medication overuse headache (MOH). A liquid chromatography tandem-mass spectrometry (LC-MS/MS) method was conducted for the quantification of THC, CBD, 11-OH-THC, THC-COOH, THC-COOH-glucuronide, THCA-A, and CBDA. Blood pressure, heart rate, and a short list of symptoms by numerical rating scale (NRS) were assessed.
Results
Decoctions of cannabis showed high variability in cannabinoids content, compared to cannabis oil. For both preparations, THCA-A and CBDA were the most widely absorbed cannabinoids, while THC and CBD were less absorbed. The most important differences concern the bioavailability of THC, higher in oil (AUC0–24 7.44, 95% CI 5.19, 9.68) than in decoction (AUC0–24 3.34, 95% CI 2.07, 4.60), and the bioavailability of CBDA. No serious adverse events were reported.
Conclusions
Cannabis decoction and cannabis oil showed different pharmacokinetic properties, as well as distinct consequences on patients. This study was performed in a limited number of patients; future studies should be performed to investigate the clinical efficacy in larger populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant genus Cannabis, a member of the family Cannabaceae, has a long history as an herbal medicine and has been used for medical purposes in a variety of forms [1]. Cannabis plant contains a large number of cannabinoids, pharmacologically active ingredients found in three recognized cannabis species (Cannabis sativa, Cannabis indica, and Cannabis ruderalis). In order to obtain an in vivo effect, a heating stage is a critical factor for all formulations, which is essential for the conversion of carboxylic acids into their decarboxylated analogs. The psychotropic component, Δ9-tetrahydrocannabinol (Δ9-THC, or simply THC), is the result of the decarboxylation of tetrahydrocannabinolic acid (THCA-A); the non psychotropic cannabidiol (CBD) is obtained in a similar way from its acid precursor, cannabidiolic acid (CBDA). Conversions also take place at room temperature, but they are slower. Once absorbed, THC and CBD interact with endocannabinoid receptors (CB1 and CB2) in the central nervous system and periphery, demonstrating improvement in several chronic conditions, such as neuropathic pain, rheumatoid arthritis, spasticity, and sleep disorders [2, 3]. Their acid precursors are less familiar and are described as pharmacologically inactive [4].
The use of cannabis as medicine is not immediately available, being disciplined by several government restrictions and regulations worldwide. Similar to most medicines that act on the central nervous system, cannabis is burdened with a series of side effects, including an increased risk of psychosis [5] and motor vehicle crashes [6]. Commonly, medical cannabis is inhaled, but the recent expansion of the legal cannabis market has increased the variety of products not designed for inhalation, including many edible foods and beverages, oils, and various kinds of extracts [7]. The edible products are considered more suitable for therapeutic applications, due to a longer duration of effects and a reduced exposure to inhaled carcinogens, but limited research has evaluated the attributes of oral cannabis preparations. As example, their absorption is erratic and unpredictable; hence, it is difficult to define a therapeutic dose acceptable for many patients. It has been suggested that higher bioavailability is obtained in an oil formulation [8], but no in-depth studies have been completed on the topic. Oral absorption of THC has been investigated in a chocolate cookie or a gelatin capsule, resulting in maximal plasma concentration around 10 ng/ml, usually after 60–120 min [9, 10]. The acid of the stomach degrades THC, and a subsequent first-pass liver metabolism reduces its absorption, ranging between 2 and 14%. Metabolism occurs in the liver, by enzymes of the cytochrome P450 complex; hydroxylation results in the active metabolite 11-hydroxy-THC (11-OH-THC), and further oxidation in the inactive THC-COOH, which is in turn glucuronated to 11-nor-9-carboxy-THC glucuronide (or THC-COOH-glucuronide) [11]. Less information is available about cannabidiol (CBD): after oral administration, the plasmatic concentrations, the metabolic pattern, and the excretion rates appear in the same range as THC [4, 12]. The oral absorption of THCA-A and CBDA in humans has never been documented. When THCA-A is inhaled, it does not seem to convert to THC in vivo; it displays its own metabolic and elimination pathways [13] and can be found, together with THC, in the blood serum and urine of Cannabis consumers [14, 15].
In Italy, the recent release of a national medical cannabis strain, named FM2, has given a new impulse for the study of medical cannabis. In November 2015, the Italian Ministry of Health authorized the production of cannabis plants to the Military Pharmaceutical Chemical Works of Florence (Italy) [16]. The cannabis strain FM2 contains 5–8% of THC and 7–12% of CBD, similar to the Dutch pharmaceutical product Bediol® [3]. The Ministerial Decree advises to consume medicinal cannabis by vaporizing or in the form of a decoction: the indications for the preparation of the medicinal products were indicated [16]. Subsequently, the use of cannabis-based oil was indicated by a regional deliberation, in a territory with more than 4 million inhabitants [17]. Having regard to the latest measures taken by the Italian Ministry of Health, it is necessary to study the pharmacokinetic properties of cannabinoids after oral administration. This study explores the non-compartmental pharmacokinetic parameters and tolerability of a single oral dose of cannabis FM2 assumed as decoction (200 ml) and in olive oil (1 ml), as a first step to improve the knowledge of oral cannabis preparations and the prescriptive recommendations.
Material and methods
Oral cannabis preparations
The cannabis flowering tops (5–8% THC, 7–12% CBD) originated from the Military Pharmaceutical Chemical Works of Florence, where they were cultivated under standardized conditions. The oral cannabis products were prescribed in accordance with the current legislation. Cannabis decoction was prepared from 500 mg of cannabis plant preparation, according to the indications of the Ministerial Decree [16]. A single decoction was prepared for each patient, 2 h before administration, in order to evaluate the reproducibility of the method; a small sample (1 ml) was collected for the quantification of cannabinoids, for each decoction. A cannabis-based oily solution was prepared by a territorial pharmacy, according to the indications reported by Romano and Hazekamp [18]. Again, a small sample was collected for the quantification of cannabinoids, before administration, for each patient. Every sample was analyzed using a liquid chromatography tandem-mass spectrometry (LC-MS/MS) method, already proposed by Pacifici et al. [19]
Study design
This is a single-center, open-label, two-period crossover study designed to assess the pharmacokinetics and tolerability of cannabis FM2 administered as a decoction or in olive oil, to patients with medication overuse headache (MOH). The major criteria for patient eligibility was a diagnosis of MOH, according to the ICHD-3 beta criteria [20], resistant to at least three prophylactic therapies. Other inclusion criteria were as follows: 18 years of age or older, able to tolerate oral intake, adequate liver and renal function, non-smokers, and on stable doses of any concomitant medication (≥ 12 weeks for all medications). Exclusion criteria were as follows: pregnancy, history of psychiatric disorders, drug or alcohol abuse, and a history of cannabis or other illicit substances usage. This is an exploratory study; no sample size calculation was performed: 13 patients were enrolled; the study was conducted at the Day Hospital of Medical Toxicology and Headache Centre, Modena (Italy). The study was approved by the Ethical Committee of Modena (protocol n. 41/2017) and completed in accordance with the ethical standards of the Helsinki Declaration. Written informed consent was obtained from all patients prior to study participation.
Study procedures
The first day of the study, the patients arrived at the center at 7.40 a.m. The patients were fasting from midnight and had not taken acute medication for migraine (NSAIDs or triptans) for the previous 8 h. The other medications were assumed regularly during the day. Baseline assessments included physical examination (height, weight, and vital signs), self-report ratings, urine drug screening for illicit substances (opiates, cannabinoids, amphetamines, cocaine, methadone, benzodiazepines, and barbiturates), and blood sampling. At 8.00 a.m., each patient received a single dose of cannabis decoction. Everyone was allowed a low-fat snack at 10.00 a.m., the same for all, to minimize differences in drug absorption. Water intake was allowed, as needed. Blood samples and safety data were collected until 4.00 p.m., when the patients returned home accompanied by a family member. Each patient was asked to abstain from physical activity and/or alcohol until the following day. They returned at 8.00 a.m., to complete the 24-h follow-up. The same procedures were implemented for the intake of cannabis oil, after a wash-out period of at least 2 weeks. Driving of any vehicles and operating potentially dangerous machines were not recommended until 1 week after each treatment.
Blood specimens
Blood specimens were collected in sodium citrate-coated tubes (2.7 mL) before cannabis intake and 0.5 (T1), 1 (T2), 1.5 (T3), 2 (T4), 3 (T5), 4 (T6), 6 (T7), 8 (T8), and 24 h (T9) after administration. The samples were instantly deep frozen and stored at − 20 °C. The samples were analyzed within the next 48 h. LC-MS/MS method was conducted for the quantification of THC, CBD, 11-OH-THC, THC-COOH, and THC-COOH-glucuronide, in whole blood, according to the methodology described by Palazzoli et al. [21]. In this study, the methodology was also extended and validated to the determination of THCA-A and CBDA. Sample preparation consisted in deproteinization with a mixture of 0.1% formic acid solution in acetonitrile:methanol, 70:30 (v/v), and purification onto a Phree™ phospholipid removal tube of 200 μl whole blood. Liquid chromatography analysis were performed with an Agilent 1200 LC system (Agilent, Waldbronn, Germany). Separation was achieved on a Kinetex EVO C18 column (100 × 2.1 mm; 5 μm particle size) (Phenomenex, Bologna, Italy) using gradient elution with flow rate 0.35 ml/min, mobil phase A (2.0 mM aqueous ammonium acetate) and mobile phase B (acetonitrile). Total run time was 20 min. Tandem mass spectrometry was performed using a SCIEX API 4000 QTRAP mass analyzer, equipped with a Turbo Ion Spray source (SCIEX Toronto, Canada) operating in electrospray ionization (ESI) positive/negative mode. The Analyst Software (version 1.5.2) was used for instrument control, data acquisition, and qualitative and quantitative data analysis. The limit of quantitation (LOQ) and upper limit of linearity (ULOL), respectively, for blood analysis, were the following: THC, 0.25, 100 ng/mL; CBD, 0.25, 50 ng/mL; 11-OH-THC, 0.25, 100 ng/mL; THC-COOH, 0.5, 100 ng/mL; THC-COOH-glucuronide, 0.5, 100 ng/mL; THCA-A, 0.5, 100 ng/mL; and CBDA, 0.5, 100 ng/mL. Method validation parameters (selectivity, sensitivity, linearity, precision, extraction efficiency, matrix effect, stability, and carry over) and validation results are reported in Online Resource 1.
Safety and tolerability assessment
Medical investigators were instructed to collect all adverse events regardless the oral cannabis formulations. Blood pressure and heart rate were detected before each blood sampling. In addition, a short list of symptoms was assessed at baseline, T2, T4, T6, T8, and T9, by numerical rating scale (NRS) measurements (drowsiness, euphoria, anxiety, aggression, confusion, dizziness, hallucinations, pain, and nausea). The NRS is an 11-item unidimensional measurement of self-reported intensity, in which a patient selects a whole number (0–10) that best reflects the intensity of the symptom. The numeric scale ranges from ‘0,’ representing the absence of the effect, to ‘10,’ representing the maximum imaginable perception of the effect. The NRS instrument has already been used in the past, when the pharmacokinetic properties of cannabis taken orally, smoked, or intravenously were compared [10]. At the baseline, the mean value reported by the patients was considered as a benchmark. The mean values reported by the patients in the following times were considered as positive or negative variations with respect to the mean value, measured before administering the drug.
Pharmacokinetics and statistical evaluations
Participant demographics and quantifications of cannabinoids are presented using descriptive statistics. The pharmacokinetic parameters were determined by non-compartmental analysis, following a single oral dose, using PK solver software (version 1.0.1, China) [22]. The detailed comparison of PKSolver estimates with the most utilized pharmacokinetic softwares (WinNonlin and Scientist) are reported by Zhang et al. [22]. On the basis of the model used, the pharmacokinetic parameters calculated were the area under the concentration-time curve from 0 to 24 h after drug administration (AUC0–24), time of onset of drug absorption (Tlag), peak plasma concentration (Cmax), time to reach Cmax (Tmax), and plasma half-life (t1/2). The stated Tlag was when the observed concentration was ≥ Lower Limit of Quantification (LLOQ). After dosing, point observations with undetectable or detectable cannabinoid levels below LLOQ were counted as 0. For all estimates of pharmacokinetic parameters, data were explored for plausibility of normal distribution using histograms and Shapiro–Wilk test. Comparisons for non-normally distributed parameters were performed using Wilcoxon matched-pairs signed-rank test. Comparisons of normally distributed parameters were performed using paired t test. Safety data were analyzed by analysis of variance (ANOVA) with post-hoc Tukey multi-comparison test. Statistical comparisons were performed using STATAIC-13 software; the level of significance was p < 0.05. Figures were created with OriginPro 2017 software, OriginLab Corporation, Northampton, MA 01060 USA.
Results
Patients
A total of 13 Caucasian patients (7 females and 6 males, mean age 51.08 years, mean disease duration 12.38 years) were enrolled and completed the study; their characteristics are presented in Table 1. Among them, four patients were excluded from the statistical analysis: cannabis decoction was incorrectly prepared for two patients, a patient had eaten before taking cannabis oil, and another patient was positive to cannabinoids urinary screening at the baseline. The characteristics of the nine patients included in the data analysis (5 females and 4 males, mean age 51.13 years, mean disease duration 10.56 years) are also presented in Table 1. Most of them used triptans to treat migraine attacks (78%). Oral concomitant medications assumed by each patient, at stable dose for at least 12 weeks, are presented in Online Resource 2.
Cannabis decoction and oil
Decoctions of cannabis showed high variability of cannabinoids, especially for THC (1.85 ± 1.6 mg/200 ml) and CBD (1.93 ± 1.17 mg/200 ml). THCA-A and CBDA were dosed at higher concentrations, with less variability between decoctions (results are presented in Table 2). About the nine patients included in the data analysis, 4 out of 9 (44%) assumed a cannabis decoction containing less than 1 mg of THC, whereas 2 out of 9 (22%) assumed a decoction containing less than 1 mg of CBD. Analysis performed on cannabis oil confirmed the same quantities of cannabinoids administered for each patient (Table 2).
Cannabinoids determination
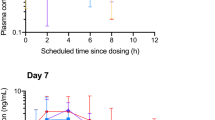
All baseline specimens were below the LOD value for each cannabinoid, both for decoctions and olive oil formulations. This was in accordance with the requirements for participation in the study. THCA-A, as well as CBDA, became detectable in blood in the range of 0.5–1 h, achieving higher blood concentrations compared to the other cannabinoids. The formulation, decoction or oil, did not change significantly the concentrations over time of THCA-A and CBDA: the latter declined faster and was no longer present in blood 4–8 h after intake, whereas THCA-A decreased more slowly, within 24 h. THC was detectable 0.5–1 h after the administration of oral cannabis preparations, it never exceeded 6 ng/ml and declined subsequently over 6–10 h. Three patients taking cannabis decoction had THC concentrations that never reached 1 ng/ml (patients 1, 7, and 9); for two of these, it was not possible to calculate t1/2. Interestingly, 11-OH-THC reached significantly lower concentrations than THC: two patients taking cannabis decoction (patients 6 and 7) had no detectable blood 11-OH-THC at any time point. Moreover, its blood concentrations had never reached 1 ng/ml, except for one patient taking decoction (patient 3) and three patients taking oil (patients 2, 3, and 8). Due to these reasons, it was not possible to calculate t1/2 of 11-OH-THC in several patients (patients 5, 6, 7, 8, and 9 taking decoction and patients 1, 4, 6, and 9 taking oil). In the other patients, blood 11-OH-THC was detectable in 0.5–1 h and decreased in the following 2–8 h; apparently, there were no differences between decoction and oil formulations. Blood THC-COOH was found in all patients; its detection was more consistent and significantly longer than THC. These considerations are even more pronounced for THC-COOH-glucuronide, which had always been identified in the blood after 24 h of decoction or oil administration. However, neither THC-COOH nor THC-COOH-glucuronide reached blood concentrations comparable to THCA-A and CBDA. In one patient taking decoction (patient 4), t1/2 of THC-COOH could not be calculated. Differently from THC, CBD was detectable in a high concentration within 1 h the decoction administration, not exceeding 9 ng/ml, and decreased over the next 4 h. When it was administered in oil, CBD appeared slightly later, persisting for a longer period of time. In one patient taking oil (patient 9), t1/2 of CBD could not be calculated. Aggregate data on THC, CBD, 11-OH-THC, THC-COOH, THC-COOH-glucuronide, THCA-A, and CBDA concentrations over time by cannabis decoction and cannabis oil are provided in Figs. 1, 2, and 3 and in Online Resource 4. Complete qualitative and quantitative results of blood analysis, for each patient, are summarized in Online Resource 3.
Non-compartmental pharmacokinetic parameters
In patients taking cannabis decoction, THCA-A, CBDA, and CBD showed lowest Tlag and were always identified 30 min after administration, while THC appeared shortly afterwards (Tlag: 0.61 ± 0.22 h). The same trend was observed with the administration of cannabis oil, albeit at a slightly longer time. CBD reached Cmax quickly, both in decoction (Tmax 0.56 ± 0.17 h) and in oil (Tmax 1.00 ± 0.25 h), with a significant time lag (p = 0.018). Tmax of CBDA, THCA-A, and THC was not influenced by the administering method; all of them reached the peak plasma concentration later, within 2 h of administration. THCA-A (mean AUC0–24 206.76 in decoction, mean AUC0–24 247.89 in oil) and CBDA (mean AUC0–24 96.05 in decoction, mean AUC0–24 62.72 in oil) were the most widely absorbed cannabinoids, while THC and CBD were less absorbed, although present at dosable concentrations. Nevertheless, THCA-A and CBD reached higher Cmax than THC and CBD. Among the most important differences between the two oral formulations, THC is absorbed more in cannabis oil (mean Cmax 3.29 ng/ml, 95% CI 2.38, 4.20; mean AUC0–24 7.44, 95% CI 5.19, 9.68) than in decoction (mean Cmax 1.38 ng/ml, 95% CI 0.89, 1.88; AUC0–24 3.34, 95% CI 2.07, 4.60). This is confirmed by an increased exposure of 11-OH-THC and THC-COOH in patients who have taken cannabis oil, comparing Cmax of the two metabolites between cannabis formulations. On the other hand, CBDA is absorbed more in decoction (AUC0–24 96.05, 95% CI 73.523, 118.568) than in oil (AUC0–24 62.72, 95% CI 43.269, 82.173). The different cannabis formulations did not affect the pharmacokinetics of THC-COOH-glucuronide, the cannabinoid with the highest AUC0–24, and the longest t1/2 (AUC0–24 382.2 vs. 452.53, t1/2 19.75 vs. 23.32 h, decoction vs. oil). CBD and CBDA had a mean t1/2 less than 1 h, while t1/2 of THCA-A, THC, 11-OH-THC, and THC-COOH were all above 1 h. Non-compartmental pharmacokinetic outcomes are summarized in Table 3.
Tolerability and adverse events
No serious adverse events were reported after the administration of oral cannabis. No clinically relevant changes in blood pressure and heart rate were found. In Tables 4 and 5, the intensity of subjective effects measured by NRS scale were similarly distributed with both decoction and oil formulations, with the exception of drowsiness, 1 h (vs. baseline p < 0.01, vs. 8 h p < 0.01, vs. 24 h p < 0.01) and 2 h (vs. 8 h p < 0.01, vs. 24 h p < 0.05) after the oil administration.
Discussion
Two years after the Ministerial Decree [16], thousands of patients have already been treated with medical cannabis provided and financially covered by the Italian Health Service, but there is still a lack of information on preparation protocols and dosages. In the present study, we are interested in pharmacokinetics and tolerability of a single dose of cannabis decoction (200 ml) and a single dose of cannabis oil (1 ml), in patients with MOH, resistant to standard therapies. The first objective was to observe whether the cannabis formulations were reproducible and standardized, to provide an ideal long-term treatment for patients with chronic disorders. Prepared decoctions showed high variability in the cannabinoid recovery: this occurred especially for THC, a key substance for a therapeutic effect [23]. About 44% of patients assumed a cannabis decoction containing less than 1 mg of THC, and 33% of the decoction-taking patients reached blood concentrations of THC lower than 1 ng/ml, which is considered the minimum effective blood concentration. Among the main reasons, the low temperatures used in the heating process originated a conversion into decarboxylated analogs unpredictable and incomplete, with the presence of THCA-A and CBDA in decoction preparations [16, 24]. Moreover, the conversion of THCA-A into THC is limited in boiling water, also due to the result of saturation of the water phase with THC, whilst THCA-A is more hydrophilic and soluble [25]. Concerns remain whether stable and therapeutic cannabinoid levels are achievable in real-life situations, when the preparation of the decoction is performed by the patient at home, in non-standard conditions. Regarding cannabis oil, the single dose was administered from a one solution of olive oil, ensuring the same dose for all patients. However, with the absence of a unique preparation method, Italian galenic preparations are actually not standardized, showing a wide variability in cannabinoids concentrations [26]. Thus, the absence of a clear methodology for the standardization of oral cannabis preparations currently limits their potential in clinical application. The daily prescription of a stable amount of medical cannabis will be an additional stimulus for its study and to further clarify the role of each main cannabinoid of the plant in its clinical effect.
Pharmacokinetic parameters and the tolerability observed are consistent with prior reports of oral cannabis administration [4, 27]. Vandrey and colleagues reported a mean Cmax of 1, 3.5, and 3.3 ng/ml in whole blood, for cannabis brownies containing 10, 25, or 50 mg of THC, respectively. Subjective drug effects were increased according to the dosage, which peaked at 1.5–3 h after administration and were significantly correlated with cannabinoids concentrations [28]. However, the pharmacokinetic investigation revealed some important differences between the cannabis preparations: patients taking cannabis decoction had a higher bioavailability of CBDA. At the same time, when the same patients assumed cannabis oil, showing a higher concentration and bioavailability of THC, as well as a higher concentration of its metabolites (11-OH-THC and THC-COOH). As a result, the two oral cannabis preparations cannot be considered equivalent medications, because patients absorbed different amounts of cannabinoids. The subjective effects are also dissimilar: cannabis oil is associated with an increased drowsiness, from 1 to 2 h after administration, compared to cannabis decoction. The differences in cannabinoids recovery may be due to the higher hydrophilicity of CBDA, compared to THC, as well as to the different methods of administration, but this does not fully explain why both THCA-A and CBDA are well absorbed in both decoction and oil, compared to THC. With the data at hand, we are not able to identify the causes of these differences. In addition, we found that blood 11-OH-THC reached significantly lower concentrations than THC, not exceeding 1 ng/ml in 14 out of 18 administrations (78%); in literature, it was reported that blood 11-OH-THC even exceeded THC concentrations, when the dose is administered orally [4]. Some authors suggested that the concomitant presence of CBD and its metabolites could inhibit the liver metabolism of THC. Few studies on this subject were performed in animals: in one of them, rats were acutely pre-treated with CBD, prior to THC administration [29]. Structural differences between THC and CBD may lead to a CYP2C19-mediated preferential oxidation of the methyl groups of CBD [30]. Anyway, the heating stage of cannabis extracts could contribute even more to the low concentration of 11-OH-THC: the unheated extracts of Cannabis sativa showed a separate metabolic profile compared to heated extracts in healthy male volunteers, showing low concentrations of 11-OH-THC [31]. Due to the nature of the study, it was not possible to identify the precise cause(s) of this result. The second singularity concerning THCA-A and CBDA: they were identified in both cannabis preparations, and they were well absorbed, reaching blood concentrations up to 100 ng/ml in the first 2 h after administration. Although it is preferable to optimize the decarboxylation process of cannabis, with a consequent reduction of THCA-A and CBDA in favor of THC and CBD, the presence of the precursors may have some advantages. First, they could differentiate between the intake of cannabis products and the prescribed medications, which contain only pure THC and/or CBD, whereas both have shown interesting pharmacological properties, not yet studied in vivo. THCA-A is not a psychoactive compound, but binds some targets of specific interest for pain and headaches: it is an inhibitor of cyclooxygenase enzymes (COX-1 and COX-2) [32], a transient receptor potential ankyrin 1 (TRPA1) agonist and a transient receptor potential vanilloid 1 (TRPV1) antagonist [33]. Moreover, it was capable of attenuating nausea and vomiting with a CB1-mediated mechanism [34]. CBDA was otherwise successfully investigated in pre-clinical anticipatory nausea models [35, 36].
The limitation of the present study is the low number of patients and the lack of a pharmacokinetic-pharmacodynamic study model. More patients and people suffering from distinct chronic disorders, who could benefit from medical cannabis, would be able to confirm and further develop these results in the future. Gender issue is another potential topic to be expanded, due to the small sample size enrolled for this research. At the same time, the present results are relevant because they represent the first pharmacokinetic comparison among oral cannabis preparations, specifically prescribed to patients with MOH. New efforts are now needed to assess whether the pharmacokinetic properties are associated with a different clinical effect, especially on long-term treatments.
References
Baron EP (2015) Comprehensive review of medicinal marijuana, cannabinoids, and therapeutic implications in medicine and headache: what a long strange trip it’s been …. Headache 55:885–916
Russo E, Guy GW (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66:234–246
Whiting PF, Wolff RF, Deshpande S et al (2015) Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 357:2456–2473
Grotenhermen F (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42:327–360
Andrade C (2016) Cannabis and neuropsychiatry, 2: the longitudinal risk of psychosis as an adverse outcome. J Clin Psychiatry 77:e739–e742
Salomonsen-Sautel S, Min SJ, Sakai JT, Thurstone C, Hopfer C (2014) Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend 140:137–144
Grella CE, Rodriguez L, Kim T (2014) Patterns of medical marijuana use among individuals sampled from medical marijuana dispensaries in Los Angeles. J Psychoactive Drugs 46:263–272
Harvey DJ (1991) Metabolism and pharmacokinetics of the cannabinoids. In: Watson RR (ed) Biochemistry and physiology of substance abuse, Vol III. CRC Press, Boca Raton, pp 279–365
Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M (1983) Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol, in men and women. Clin Pharmacol Ther 34:352–363
Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK (1980) Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther 28:409–416
Huestis MA (2007) Human cannabinoid pharmacokinetics. Chem Biodivers 4:1770–1804
Harvey DJ, Mechoulam R (1990) Metabolites of cannabidiol identified in human urine. Xenobiotica 20:303–320
Jung J, Meyer MR, Maurer HH, Neusüss C, Weinmann W, Auwärter V (2009) Studies on the metabolism of the Delta9-tetrahydrocannabinol precursor Delta9-tetrahydrocannabinolic acid A (Delta9-THCA-A) in rat using LC-MS/MS, LC-QTOF MS and GC-MS techniques. J Mass Spectrom 44:1423–1433
Dussy FE, Hamberg C, Luginbühl M, Schwerzmann T, Briellmann TA (2005) Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products. Forensic Sci Int 149:3–10
Jung J, Kempf J, Mahler H, Weinmann W (2007) Detection of Delta9-tetrahydrocannabinolic acid A in human urine and blood serum by LC-MS/MS. J Mass Spectrom 42:354–360
Website: Decreto 9 Novembre 2015: Funzioni di Organismo statale per la cannabis previsto dagli articoli 23 e 28 della convenzione unica sugli stupefacenti del 1961, come modificata nel 1972. Available at: http://www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg;jsessionid=p1rnwNujUKlqQ5azhAQ95A__.NTC-AS3-GURI2A. Accessed 30 Dec 2017
Website: Attuazione della legge regionale n. 11 del 17 luglio 2014 e del Decreto Ministeriale 9 novembre 2015 inerenti l’uso medico dei preparati vegetali a base di cannabis sativa. Available at: http://salute.regione.emilia-romagna.it/documentazione/leggi/regionali/dgr-2127-2016/dgr-1250-2016/at_download/file/dgr-1250-2016-cannabis.pdf. Accessed 30 Dec 2017
Romano LL, Hazekamp A (2013) Cannabis oil: chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids 1:1–11
Pacifici R, Marchei E, Salvatore F, Guandalini L, Busardò FP, Pichini S (2017) Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin Chem Lab Med
Headache Classification Committee of the International Headache Society (IHS) (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Palazzoli F, Citti C, Licata M, Vilella A, Manca L, Zoli M, Vandelli MA, Forni F, Cannazza G (2017) Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC-MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J Pharm Biomed Anal 150:25–32
Zhang Y, Huo M, Zhou J, Xie S (2010) PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Prog Biomed 99:306–314
Kandasamy R, Dawson CT, Craft RM, Morgan MM (2018) Anti-migraine effect of ∆9-tetrahydrocannabinol in the female rat. Eur J Pharmacol 818:271–277
Hazekamp A, Bastola K, Rashidi H, Bender J, Verpoorte R (2007) Cannabis tea revisited: a systematic evaluation of the cannabinoid composition of cannabis tea. J Ethnopharmacol 113:85–90
Hazekamp A, Verpoorte R (2006) Structure elucidation of the tetrahydrocannabinol complex with randomly methylated beta-cyclodextrin. Eur J Pharm Sci 29:340–347
Carcieri C, Tomasello C, Simiele M, De Nicolò A, Avataneo V, Canzoneri L, Cusato J, Di Perri G, D'Avolio A (2018) Cannabinoids concentration variability in cannabis olive oil galenic preparations. J Pharm Pharmacol 70(1):143–149
Pini LA, Guerzoni S, Cainazzo MM, Ferrari A, Sarchielli P, Tiraferri I, Ciccarese M, Zappaterra M (2012) Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial. J Headache Pain 13(8):677–684
Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, Cone EJ (2017) Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol 41:83–99
Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS (2011) Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 218:443–457
Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K (2011) Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci 89:165–170
Eichler M, Spinedi L, Unfer-Grauwiler S, Bodmer M, Surber C, Luedi M, Drewe J (2012) Heat exposure of Cannabis sativa extracts affects the pharmacokinetic and metabolic profile in healthy male subjects. Planta Med 78:686–691
Ruhaak LR, Felth J, Karlsson PC, Rafter JJ, Verpoorte R, Bohlin L (2011) Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol Pharm Bull 34:774–778
De Petrocellis L, Ligresti A, Schiano Moriello A, Iappelli M, Verde R, Stott CG, Cristino L, Orlando P, Di Marzo V (2013) Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. Br J Pharmacol 168:79–102
Rock EM, Kopstick RL, Limebeer CL, Parker LA (2013) Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br J Pharmacol 170:641–648
Bolognini D, Rock EM, Cluny NL, Cascio MG, Limebeer CL, Duncan M, Stott CG, Javid FA, Parker LA, Pertwee RG (2013) Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br J Pharmacol 168:1456–1470
Brierley DI, Samuels J, Duncan M, Whalley BJ, Williams CM (2016) Neuromotor tolerability and behavioural characterisation of cannabidiolic acid, a phytocannabinoid with therapeutic potential for anticipatory nausea. Psychopharmacology 233:243–254
Acknowledgments
We sincerely thank the staff and patients of Medical Toxicology and Headache Centre of Modena, who made possible the realization of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Ethical Committee of Modena (protocol n. 41/2017) and completed in accordance with the ethical standards of the Helsinki Declaration. Written informed consent was obtained from all patients prior to study participation.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pellesi, L., Licata, M., Verri, P. et al. Pharmacokinetics and tolerability of oral cannabis preparations in patients with medication overuse headache (MOH)—a pilot study. Eur J Clin Pharmacol 74, 1427–1436 (2018). https://doi.org/10.1007/s00228-018-2516-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2516-3