Abstract
Rationale
The effect of atypical antipsychotic drugs (AAPDs), e.g., lurasidone, to improve cognitive impairment associated with schizophrenia (CIAS), has been suggested to be due, in part, to enhancing release of dopamine (DA), acetylcholine (ACh), and glutamate (Glu) in cortex and hippocampus.
Results
The present study found acute lurasidone reversed the cognitive deficit in novel object recognition (NOR) in subchronic (sc) phencyclidine (PCP)-treated mice, an animal model for CIAS. This effect of lurasidone was blocked by pretreatment with the 5-HT1AR antagonist, WAY-100635, or the 5-HT7R agonist, AS 19. Lurasidone significantly increased medial prefrontal cortex (mPFC) ACh, DA, and Glu efflux, all of which were blocked by WAY-100635, with similar effects in the dorsal striatum (dSTR), except for the absence of an effect on Glu increase. AS 19 inhibited Glu, but not DA efflux, in the dSTR. The selective 5-HT7R antagonist, SB-26970, increased mPFC DA, 5-HT, Glu, and, importantly, also GABA efflux and striatal DA, NE, 5-HT, and Glu efflux, indicating tonic inhibition of the release of these neurotransmitters by 5-HT7R stimulation. These results provide new evidence that GABA release in the mPFC is tonically inhibited by 5-HT7R stimulation and suggest that a selective 5-HT7R antagonist might be clinically useful to enhance cortical GABAergic release. All SB-269970 effects were blocked by AS 19 or WAY-100635, suggesting 5-HT1AR agonism is necessary for the release of these neurotransmitters by SB-269970. Lurasidone increased ACh, DA, and NE but not Glu efflux in mPFC and dSTR DA and Glu efflux in 5-HT7 KO mice.
Conclusion
We conclude that lurasidone-induced Glu efflux in mPFC requires 5-HT7R antagonism while its effects on cortical ACh and DA efflux are mainly due to 5-HT1AR stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA), norepinephrine (NE), acetylcholine (ACh), serotonin (5-HT), glutamate (Glu), and GABA are among the most important neurotransmitters involved in cognition. There is extensive evidence that abnormalities in these neurotransmitters contribute to the cognitive impairment associated with schizophrenia (CIAS). Preclinical studies are consistent with the now robust clinical evidence that atypical antipsychotic drugs (AAPDs) improve some domains of cognition in some patients with schizophrenia (Désaméricq et al. 2014; Meltzer 2015). Consistent with the clinical findings, AAPDs have been shown to rescue episodic memory and cognitive flexibility deficits produced by subchronic (sc) administration of the N-methyl-d-aspartate receptor (NMDAR) antagonist, phencyclidine (PCP; Meltzer et al. 2013; Grayson et al. 2016). It has been suggested that this rescue is due, in part, to increased cortical and hippocampal DA and ACh release and that these mechanisms also contribute to their efficacy in treating CIAS (Kuroki et al. 2008; Masana et al. 2012; Meltzer and Huang 2008; Meltzer et al. 2013). The mechanisms by which the AAPDs affect the release of monoamines, including DA, NE, ACh, 5-HT, Glu, and GABA, which affect the activity of pyramidal neurons and the multiple types of GABAergic interneurons that are essential for cognition (Fritschy and Panzanelli 2014) are only partially understood (Masana et al. 2012; Huang et al. 2014; Kamińska et al. 2013).
The ability of AAPDs to enhance brain DA and ACh efflux may be due, in part, to 5-HT1A partial agonism and 5-HT7 antagonism (Assié et al. 2005; Ichikawa et al. 2001; Meltzer and Huang 2008; Ohoyama et al. 2011; Rollema et al. 2000). Some AAPDs, e.g., clozapine, asenapine, lurasidone, quetiapine, and ziprasidone, are direct acting 5-HT1A partial agonists, as indicated by affinities for 5-HT1ARs and the ability of selective 5-HT1AR antagonists, e.g., N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY-100635), to block the effects of AAPDs on cortical DA and ACh efflux (Ichikawa et al. 2001; Sato et al. 2007), as well as rescue sc NMDAR antagonist, e.g., PCP or (5S,10R)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cycloheptan-5,10-imine (MK-801)-induced cognitive deficits in rodents (Horiguchi and Meltzer 2012, 2013). Similar evidence indicates that the AAPDs olanzapine and risperidone are indirect 5-HT1A agonists (Meltzer and Huang 2008). Cortical DA efflux following systemic administration of clozapine and other atypical APDs is absent in 5-HT1A knockout mice (Bortolozzi et al. 2010).
5-HT7 antagonists, e.g., (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine (SB-269970), significantly increase cortical DA and 5-HT release (Bonaventure et al. 2011; Kusek et al. 2015; Wesołowska and Kowalska, 2008). Lurasidone, an AAPD which fits the canonical model of more potent 5-HT2A than D2 antagonism (Meltzer et al. 1989), is also a potent 5-HT7R antagonist and 5-HT1AR partial agonist (Ishibashi et al. 2010). We have previously reported lurasidone increased medial prefrontal cortex (mPFC) ACh, DA, and Glu efflux and ventral striatal DA and Glu efflux in awake, freely moving rats (Huang et al. 2014). We also found that the selective 5-HT1AR partial agonist, 3a alpha, 4 beta, 7 beta, 7a alpha-hexahydro-2-(4-(4-(2-pyrimidinyl)-1-piperazinyl)-butyl)-4,7-methano-1H-isoindole-1,3(2H)-dione dihydrogen citrate (tandospirone; Shimizu et al., 1987), and SB-269970 potentiated sub-effective dose (SED) lurasidone-induced cortical DA efflux in rats (Huang et al. 2012) and rescued novel object recognition (NOR), a measure of episodic memory (Horiguchi and Meltzer 2012; Horiguchi et al. 2011), as well as reversal learning in scPCP-treated rodents (Rajagopal et al. 2016b). There is other evidence that 5-HT7 blockade by SB-269970 or lurasidone, or 5-HT1AR stimulation by tandospirone, improved cognitive deficits in rodent models relevant to CIAS based on the NMDAR antagonists, PCP, and ketamine (Bonaventure et al. 2011; Horisawa et al. 2013; Millan 2000; Nikiforuk et al. 2013; Schreiber and Newman-Tancredi 2014; Sumiyoshi et al. 2001; Uehara et al. 2014; Yuen et al. 2012). We found that SB-269970 mimicked the effect of lurasidone to enhance NMDAR-mediated synaptic response and surface expression of NR2A and NR2B subunits in PFC of rats (Yuen et al. 2012). Moreover, tandospirone increased mPFC and dorsal striatal (dSTR) DA, while SB-269970 slightly increased DA, 5-HT, and Glu efflux, in both regions (Huang et al. 2014; Kusek et al. 2015; Wesołowska and Kowalska 2008). Systemic administration of tandospirone did not increase ACh efflux in rat PFC (Huang et al. 2014); however, stimulation of cortical 5-HT1ARs by 8-OH-DPAT or NAN-190, a 5-HT1A antagonist, was reported to increase cortical ACh release (Fujii et al. 1997; Koyama et al. 1999; Nakai et al. 1998). The atypical APDs, clozapine, quetiapine, and zotepine, which also increase cortical Glu efflux (Yamamoto et al. 1994; Yamamura et al. 2009), have comparable binding affinities for 5-HT7 and 5-HT2ARs (Meltzer 2012). Moreover, 5-HT7Rs and 5-HT1ARs form hetero- and homodimers. Functionally, heterodimerization decreases 5-HT1AR-mediated activation of Gi protein without affecting 5-HT7R-mediated signaling (Béïque et al. 2004; Renner et al. 2012). Thus, 5-HT1A and 5-HT7R mechanisms may contribute to the lurasidone or SB-269970-induced neurotransmitter efflux. However, additional mechanisms may also be involved because 5-HT7R are important for the co-expression of many other receptors and proteins important for synaptic plasticity that contribute to learning and memory (Amargós-Bosch et al. 2004; Benarroch 2010; Leiser et al. 2015; Li, 2017; Lladó-Pelfort et al. 2012; Renner et al. 2012; Stiedl et al. 2015; Tokarski et al. 2011). It has previously been reported that the 5-HT1AR antagonist, NAN-190, increased GABA release in rat dentate gyrus (DG) slices and that the 5-HT1A agonist, 8-OH-DPAT, blocked this effect, indicating an inhibitory effect of 5-HT1AR on GABA release in this region (Matsuyama et al. 1997). The glutamate agonist, NMDA, also increased the release of DG GABA, an effect blocked by either MK-801 or 8-OH-DPAT alone or synergistically. It has been concluded that NMDARs and 5-HT1ARs on DG GABA neurons together regulate the acting of GABA neurons and the release of GABA in this region (Matsuyama et al. 1997).
The mPFC, hippocampus, and dSTR are central to the pathophysiology of some of the domains of cognition impaired in CIAS (Barch and Ceaser 2012; Devan et al. 2011) and have substantially different 5-HTR inputs. 5-HT1A and 5-HT2ARs are highly expressed in PFC pyramidal neurons, parvalbumin immunoreactive interneurons (PV-IR IN), and non-PV-IR IN, but expression is low in dSTR. 5-HT7Rs are highly expressed in frontal cortex, hippocampus, thalamus, and hypothalamus in pyramidal and some GABA interneurons (reviewed in Hedlund and Sutcliffe, 2004) and dSTR choline acetyltransferase IR interneurons (ChAT-IR IN) (de Almeida and Mengod 2007, 2008; Leiser et al. 2015; Pehrson et al. 2015). The dSTR has also been implicated in the positive symptoms of schizophrenia, leading to the suggestion that AAPDs, because of alleged ventral striatal selectivity, may have limited effect on CIAS (Kegeles et al. 2010).
The present study examines the effect of lurasidone and SB-269970 on neurotransmitter release in the cortex and dSTR, an important component of cognition that has received little previous study, in relation to AAPD action. We also investigated if constitutively eliminating 5-HT7 receptors would influence the effect of lurasidone or SB-269970 on neurotransmitters’ efflux. Thus, we tested both compounds in both wild-type (WT) and 5-HT7 knock out (KO) mice. The 5-HT1A selective antagonist, WAY-100635, and the selective 5-HT7 agonist, (2S)-(+)-8-(1,3,5-trimethylpyrazolin-4-yl)-2-(dimethylamino) (AS 19), were used to examine the relative importance of the 5-HT1AR and 5-HT7R s for neurotransmitter efflux in these regions. The importance of both 5-HT1ARs and 5-HT7Rs for the ability of SB-269970 to enhance GABA efflux in mPFC was demonstrated.
Materials and methods
Animals and drugs
Male wild-type C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine, USA) and 5-HT7RKO (constitutive KO) mice, 5-HT7−/−, C57Bl/6J background breeding pairs, were generously provided by Dr. Hedlund from The Scripps Research Institute and bred at Northwestern University for over 20 generations. For mouse background details, please refer to Sarkisyan and Hedlund (2009). Two- to three-month-old young adult mice, of similar weight, were used throughout the study. They were housed four per cage in a controlled 14:10-h light-dark cycle with free access to food and water.
Lurasidone (Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) and AS 19 (Tocris Bioscience, Ellisville, MO, USA) were suspended in 0.5% methylcellulose and 0.2% Tween 80 solution. WAY-100635 and SB-269970 (Tocris) were dissolved in saline. PCP was supplied as a generous gift from the National Institute of Drug Abuse (Bethesda, MD) and dissolved in saline. Vehicle or drugs were administered intraperitoneally (i.p.) in a volume of 0.1 ml/10 g body weight to randomly assigned animals. The dose of drugs is selected by referring from our previous studies in rats (Horiguchi and Meltzer 2012; Horiguchi et al. 2011; Huang et al. 2012) and mice (Rajagopal et al. 2016b).
Novel object recognition task
The method has been described in detail elsewhere (Rajagopal et al. 2016a). Male C57BL/6J mice were randomly assigned to treatment groups of ten each for treatment saline (i.p.) or PCP (10 mg/kg, i.p.) twice a day for 7 days in each experiment, followed by a 7-day washout period. Drugs were administered 30 min prior to the NOR acquisition trial. This was followed by a 24-h inter-trial interval, which the mice were returned to the home cage, until the retention trial. The discrimination index (DI) [(time spent exploring the novel object − time spent exploring the familiar object) / total exploration time] was then calculated for retention trials. All data are expressed as mean ± SEM (N = 8–10 per group). Exploration data were analyzed by a repeated-measures two-way ANOVA followed by the pair-wise comparison when a significant effect was detected by the ANOVA. DI data were analyzed by one-way ANOVA followed by the Bonferroni test when a significant effect was detected by the ANOVA.
Microdialysis and neurotransmitter assays
The method has been described in greater detail previously (Huang et al. 2015). Dual cannulas for both mPFC and dSTR were implanted in the same animal. The stereotaxic coordinate of the implanted probe was A + 2.0, L + 0.5 (10° inclination), and V − 3.0 mm for the mPFC and A + 1.0, L − 1.5, and V − 4.5 mm for the dSTR, relative to the bregma. The details of the mass spectrometric/UHPLC (Ultra-HPLC) procedure for measuring neurotransmitter concentrations are described elsewhere (Huang et al. 2014).
Mean pre-drug baseline levels (time points − 90, − 60, − 30, and 0 min before drug or vehicle injection) were averaged and designated as 100%. Output levels for neurotransmitters were then expressed as a percentage of baselines. AUC (% of area under the curve, 0–180 min) were calculated and used for one-way ANOVA-LSD test to determine the group differences in each region (IBM SPSS statistics 20, IBM Co., NY, USA). Two-way ANOVA tests were used to determine the interaction of genotype (WT × KO) on lurasidone-induced neurotransmitter efflux. Two-way repeated measure ANOVAs followed by Bonferroni were used for the time points in time-response curves. A probability of less than 0.05 was considered significant. All results are given as mean ± standard error of mean (SEM).
Results
WAY-100635 and AS 19 block lurasidone-induced restoration of NOR in scPCP-treated mice
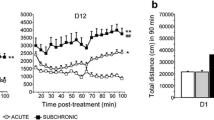
There was no significant effect of any of the drug treatments on object exploration during the acquisition trials (F6, 61 = 0.77; P = 0.19). In the retention trials, a significant interaction between drug treatment and object exploration times was found (F6, 61 = 14.16, P < 0.001). Post hoc analysis revealed that the saline but not the scPCP-treated mice explored the novel significantly more than the familiar object (P < 0.01). Lurasidone, 0.3 mg/kg, pretreatment restored exploration of the novel object to control levels (P < 0.001). WAY-100635 (0.6 mg/kg) and AS 19 (10 mg/kg), by themselves, did not reverse the scPCP-induced NOR deficit, but each, alone, significantly blocked the ameliorating effect of lurasidone, wherein the animals explored both novel and familiar objects similarly. The DI (Fig. 1) showed significant interaction between groups (F6, 61 = 16.52, P < 0.001). The DI for scPCP-treated animals given saline was significantly reduced compared to the saline-treated control animals (***P < 0.001); lurasidone, 0.3 mg/kg, pretreatment restored DI to normal levels (0.3 mg/kg; ###P < 0.001). WAY-100635 (0.6 mg/kg) and AS 19 (10 mg/kg), by themselves, did not reverse the scPCP-induced NOR deficit; Pretreatment with either drug 30 min before lurasidone blocked the ameliorating effect of lurasidone ($$P < 0.01).
Effect of AS 19 and WAY-100635 on lurasidone-induced restoration of NOR deficit in scPCP-treat mice. Saline but not scPCP-treated mice explored novel object significantly more than the familiar object. The DI showed significant interaction between the groups (F6, 61 = 16.52, P < 0.001). The DI for scPCP-treated animals given saline was significantly reduced compared to the saline-treated control animals (***P < 0.001), such effect was blocked by lurasidone (0.3 mg/kg; ###P < 0.001). WAY-100635 (0.6 mg/kg) and AS 19 (10 mg/kg) by themselves did not reverse scPCP-induced NOR deficit but significantly blocked the ameliorating effect of lurasidone ($$P < 0.01). N = 8–10 for each group
Effect of WAY-100635 and AS 19 on lurasidone-induced neurotransmitter efflux in mPFC and dSTR
One-way ANOVA indicated significant neurotransmitter increases induced by treatments, as summarized in Supplemental Table 1. Lurasidone, 1.0 mg/kg, ip, significantly increased cortical ACh (***P < 0.001, vs vehicle), DA (***P < 0.001), and Glu (*P = 0.022) efflux, without producing significant changes in NE, 5-HT, or GABA efflux (Fig. 2a). AS 19, 10 mg/kg, and WAY-100635, 0.6 mg/kg, given alone, did not significantly affect efflux of these neurotransmitters in mPFC. WAY-100635 pretreatment significantly suppressed the lurasidone-induced ACh (#P = 0.20, vs lurasidone, Fig. 2c) and DA (#P = 0.026, Fig. 2d) efflux. Following pretreatment with either WAY-100635 or AS 19, lurasidone no longer increased Glu efflux (Fig. 2e). However, post hoc testing indicated that the efflux of Glu after WAY-100635 pretreatment was also not significantly less than that of the veh-lurasidone group (P = 0.217). AS 19 did not significantly affect the lurasidone-induced increase in DA (P = 0.924) or ACh (P = 0.442 vs lurasidone group). The combination of AS 19 and lurasidone produced a significant increase in NE efflux in the mPFC not produced by lurasidone alone. In dSTR (Fig. 2b), lurasidone significantly increased DA (**P = 0.007) and Glu (*P = 0.041) efflux. AS 19 and WAY-100635 alone did not affect dSTR NT efflux. AS 19 pretreatment significantly diminished lurasidone-induced Glu efflux (#P = 0.050, Fig. 2f). WAY-100635 significantly decreased dSTR lurasidone-induced DA efflux (Fig. 2g); the increase in dSTR DA efflux was not significant when compared to vehicle (Fig. 2b, g). Although AS 19 diminished dSTR Glu efflux for most of the period of study, as can be seen in Fig. 2f, a statically significant increase in Glu release was noted between 120 and 180 min. By contrast, pretreatment with WAY-100635 blocked the lurasidone-induced Glu efflux throughout the study period (Fig. 2f).
Effect of AS 19 and WAY-100635 on lurasidone-induced neurotransmitter efflux in mPFC and dSTR. a, b The AUCs (0 to 180 min) for all the neurotransmitters. c–g The time course response (X-axis for time) on neurotransmitter efflux compared to the averaged baseline (Y-axis for % of baseline). In mPFC (a), lurasidone, 1.0 mg/kg, ip, increased cortical ACh (c), DA (d), and Glu (e) efflux. WAY-100635, 0.6 mg/kg, given 30 min before lurasidone, significantly suppressed lurasidone-induced the ACh (c) and DA (d) efflux. There was no significant increase in Glu efflux by pretreatment with WAY-100635 (a). In dSTR (b), lurasidone increased DA and Glu efflux. Lurasidone-induced Glu (f) efflux was suppressed by AS 19, 10 mg/kg. WAY-100635 partially suppressed lurasidone-induced DA efflux (g), and DA increase after WAY-100635 was not significant when compared to vehicle. AS 19 significantly blocked the Glu efflux induced by lurasidone (f). *P < 0.05, **P < 0.01, and P < 0.001, vs vehicle; #P < 0.05 vs lurasidone group
Effect of WAY-100635 and AS 19 on SB-269970-induced neurotransmitter efflux in mPFC and dSTR
To further clarify the role of 5-HT7 antagonism and 5-HT1A agonism on neurotransmitter release in the mPFC and dSTR, we assessed the ability of the selective 5-HT7 antagonist, SB-269970, to stimulate NT efflux with and without pretreatment with either WAY-100635 or AS 19. One-way ANOVA test results for AUCs are summarized in Supplemental Table 2. In mPFC (Fig. 3a), SB-269970, 3.0 mg/kg, ip, increased mPFC DA (***P < 0.001), 5-HT (***P < 0.001), Glu (**P = 0.009), and GABA (*P = 0.038) efflux (Fig. 3a). mPFC NE efflux was not affected by SB-269970. WAY-100635 or AS 19, alone, administered 30 min before SB-269970, significantly inhibited the increase in mPFC 5-HT (each ##P = 0.001, Fig. 3d) efflux. AS 19, 30 min before SB-269970, also significantly inhibited the increase in DA (##P = 0.006, Fig. 3c) efflux.
Effect of WAY-100635 or AS 19 on the selective 5-HT7 antagonist SB-269970-induced neurotransmitter efflux in both regions. a, b The AUCs (0 to 180 min) for all the neurotransmitters. c–h Time course response (X-axis for time) on neurotransmitter efflux compared to the averaged baseline (Y-axis for % of baseline). In mPFC (a), SB-269970, 3.0 mg/kg, ip, increased cortical DA, 5-HT, Glu, and GABA efflux. WAY-100635 (0.6 mg/kg) significantly suppressed SB-269970-induced the 5-HT (d) efflux. AS 19 (10 mg/kg) significantly suppressed SB-269970-induced the DA (c) and 5-HT (d) efflux. WAY-100635 or AS 19 did not significantly block lurasidone-induced Glu (e) or GABA (f) efflux; however, the Glu or GABA increases were not significant when compared to the vehicle group (a). In dSTR (b), SB-269970 increased DA, 5-HT, NE, and Glu efflux. SB-269970-induced 5-HT and NE (g) efflux were suppressed by WAY-100635. AS 19 significantly blocked SB-269970-induced NE (g) and Glu (h) efflux. WAY-100635 or AS 19 did not significantly block lurasidone-induced DA efflux; however, the DA increase was not significant when compared to the vehicle group (b). *P < 0.05, **P < 0.01, and P < 0.001, vs vehicle; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs SB-269970 group
The increase in mPFC Glu efflux produced by SB-269970 was also inhibited by either WAY-100635 or AS 19 pretreatment (Fig. 3a for AUCs and Fig. 3e for time-response curves). However, there was no difference in AUC on Glu efflux between the effect of veh + SB-269970 and its effects with either pretreatment (Fig. 3a). The increases in mPFC GABA efflux were also blocked by either WAY-100635 or AS 19 (Fig. 3a, f). In summary, the increases in DA, 5-HT, Glu, and GABA efflux in mPFC following administration of SB-269970 were dependent on both 5-HT7R blockade and 5-HT1AR stimulation, secondary to the release of 5-HT. In dSTR (Fig. 3b), SB-269970 increased DA (*P = 0.024), 5-HT (**P = 0.003), NE (***P < 0.001), and Glu (**P = 0.009) efflux (Fig. 3b). Unlike the mPFC, there was no effect of SB-269970 on dSTR GABA efflux. The SB-269970-induced 5-HT (#P = 0.020), NE (##P = 0.003) efflux, and Glu (P = 0.089) efflux were suppressed by WAY-100635. AS 19 significantly blocked SB-269970-induced NE (###P < 0.001, Fig. 3g) and Glu (###P < 0.001, 3H) efflux but not that of 5-HT (P = 0.594). Further study is needed to determine the role of 5-HT1AR and 5-HT7R in the hippocampus and other brain regions critical for specific types of cognition.
Effect of lurasidone on neurotransmitter efflux in 5-HT7R KO mice mPFC and dSTR
The ability of lurasidone to induce neurotransmitter efflux was also tested in 5-HT7R KO mice. There were no differences in basal levels of any neurotransmitter in the mPFC or dSTR between the WT and 5-HT7 knockout mice. Lurasidone significantly increased neurotransmitter efflux in both regions of both type of mice, and one-way ANOVA test results for AUCs were summarized in Supplemental Table 3. As previously noted, lurasidone, 1.0 mg/kg, significantly increased ACh (P < 0.001), DA (P = 0.001), and Glu (P = 0.012) efflux in mPFC (Fig. 4a) and DA (P = 0.086) and Glu (P = 0.041) efflux in dSTR (Fig. 4b) of WT mice. In 5-HT7R KO mice, lurasidone increased cortical DA (P < 0.001) and striatal DA (P = 0.02) efflux; however, lurasidone failed to increase the cortical or striatal Glu (P = 0.190 and 0.250, Fig. 4c and d, respectively, vs KO vehicle group) in the KO mice. However, two-way ANOVA test suggested there was no interaction of genotype (WT × KO) on lurasidone-induced any neurotransmitter efflux.
Effect of lurasidone on NT efflux in 5-HT7R KO mice mPFC and dSTR. a, b AUCs (0 to 180 min) for all the neurotransmitters. c, d Time course response (X-axis for time) on neurotransmitter efflux compared to the averaged baseline (Y-axis for % of baseline). Lurasidone significantly increased ACh, DA, and Glu efflux in mPFC (a) and DA and Glu efflux in dSTR (b) of WT mice. In 5-HT7R KO mice, lurasidone-induced a similar efflux on cortical DA and striatal DA efflux; however, the increase-induced by lurasidone on Glu efflux were not significant by different to that of KO vehicle group in either mPFC or dSTR (c and d, respectively). *P < 0.05, **P < 0.01, and P < 0.001, vs WT or KO vehicle groups
Discussion
The present study found that acute treatment with lurasidone restored NOR function in scPCP-treated mice, in agreement with our previous studies in rats (Horiguchi et al. 2011; Horiguchi and Meltzer 2012). Furthermore, WAY-100635 and AS 19 prevented the ability of lurasidone to ameliorate the deficit in NOR produced by scPCP in both rodent species. The effects of lurasidone and these selective agents on DA, ACh, Glu, GABA, 5-HT, and NE efflux in WT, scPCP, and 5-HT7 KO mice help to clarify the contributions of these neurotransmitters in these two regions to the amelioration by lurasidone of the NOR deficit in scPCP-treated mice and possibly CIAS as well.
As in rats (Horiguchi et al. 2011; Horiguchi and Meltzer 2012), the ability of lurasidone to restore NOR in scPCP-treated mice was blocked by either the 5-HT1A antagonist WAY-100635 or the 5-HT7 agonist, AS 19. This is the first report to our knowledge of this effect in mice, which enables studies of transgenic mice and utilization of the extensive literature of scPCP treatment in rats to interpret the murine findings. Thus, 5-HT1A agonism contributes to lurasidone-induced cortical ACh and DA efflux in both rodent species. The effect of lurasidone to increase DA, ACh, and Glu efflux was not significantly different in the 5-HT7 KO compared to WT mice, indicating that constitutively eliminating 5-HT7Rs did not influence the ability of lurasidone to increase neurotransmitters, suggesting there must be the result of actions on non-5-HT7 receptors. However, neurodevelopmental effects of germline deletion of the 5-HT7R may have contributed to the results reported here, due to compensatory mechanisms during development. Studies in which the 5-HT7R is deleted or inactivated in adult mice, e.g., by a DREADD or viral mechanism, could clarify this issue.
Administration of the selective 5-HT7 antagonist, SB-269970, to WT mice produced significant increases in efflux of DA, 5-HT, Glu, and GABA in the mPFC and DA, 5-HT, NE, and Glu, but not GABA, in the dSTR. Bonaventure et al. (2011) previously reported that SB-269970 suppressed MK-801-induced cortical Glu efflux without affecting basal levels of Glu efflux. The reason for this difference is unclear and requires further study. Our data suggests tonic inhibition, in a regionally selective manner, of the activity of cortical glutamatergic neurons, and DA, 5-HT, and NE projection neurons by 5-HT7R stimulation. The increase in cortical GABA in these mice by SB-269970 is of particular interest because of the extensive evidence for diminished activity of some GABA interneurons in both schizophrenia and depression (Volk et al. 2016; Sibille 2017). We found that the increase in GABA in mPFC by SB-269970 was blocked by either WAY-100635 or AS 19, indicating that 5-HT1AR stimulation is necessary for enhancement of GABA efflux. These results extend the finding that activation of 5-HT7R results in an enhancement of GABAergic transmission in the hippocampal CA1 area, possibly by enhancement of excitatory glutamatergic input to GABAergic interneurons by presynaptic 5-HT7Rs or by activation of 5-HT7R on GABA interneurons (Renner et al. 2012; Tokarski et al. 2011). The effect of SB-269970 on GABA efflux in the mPFC reported here is consistent with the results reported in the rat hippocampus (Tokarski et al. 2011). This effect has been postulated to be important for the rapid antidepressant effect of 5-HT7 antidepressants in rodents (Tokarski et al. 2012). SB-269970 has been reported to inhibit the locomotor activity (LMA) produced by amphetamine and PCP in rats (Waters et al. 2012). The effect was greater for amphetamine LMA, which is different from that of AAPDs (Millan et al. 1999) and lurasidone (Meltzer et al., unpublished data). These data support previous suggestions that 5-HT7 R antagonism could be important for the antipsychotic action of lurasidone. The effect of AS-19 and WAY-100635 to block GABA efflux in SB-269970-treated mice is in accord with the inhibitory effect of 5-HT7R and the stimulatory effect of 5-HT1AR in rat DG previously mentioned (Matsuyama et al. 1997). The ability of 5-HT7Rs to regulate GABA release is not evident in the dSTR. It should be noted that the GABA released in the mPFC and dSTR as measured here may be released from vesicular GABA that arises from terminals of DA neurons from the substantia nigra (Tritsch et al. 2012, 2014). GABA can be co-released with DA and ACh, as well as other neurotransmitters in this manner (Tritsch et al. 2016). As pointed out by Tritsch et al. (2016), synergistic actions of GABA, DA, and ACh may have an important effect on synaptic plasticity mechanisms, important for cognition and social interaction.
The effects of WAY-100635 and AS 19 themselves and on lurasidone-induced neurotransmitter release provide functional information about the role of 5-HT1ARs and 5-HT7Rs on the activity of subcortical and cortical neurons. Neither WAY-100635 nor AS 19 alone affected the release of any neurotransmitter during the collection period indicating stimulation of 5-HT1AR does not inhibit basal release of these neurotransmitters. The absence of an effect of AS 19 by itself indicates endogenous 5-HT7 tone leave as is or does not affect neurotransmitter release. By contrast, both drugs blocked the increase in cortical Glu produced by lurasidone, indicating 5-HT1AR stimulation and 5-HT7R antagonism contribute to this effect of lurasidone. Only WAY-100635 blocked the lurasidone-induced increases in cortical DA, ACh, and DA metabolites, indicating that 5-HT1AR stimulation by lurasidone is necessary for its ability to activate DA and ACh neurons. These findings are consistent with the ability of SB-269970 to increase DA, 5-HT, Glu, and GABA efflux in the mPFC, all of which were blocked by either AS 19 or WAY-100635.
Role of 5-HT1A and 5-HT7 receptors on GABAA interneurons in cortical neurotransmitter release.
Various subtypes of GABA neurons and GABAAR on interneurons and principal neurons play a critical role in the regulation of neurotransmitter release during cognitive function. Systemic administration of 5-HT1A agonists, e.g., 8-OH-DPAT, selectively stimulates 5-HT1ARs located on GABA interneurons in the PFC (Lladó-Pelfort et al. 2012), leading to disinhibition of Glu neurons and activation of DA neurons in the ventral tegmental area (VTA) which project to mPFC. Local blockade of GABAA inputs with the GABAA antagonist, gabazine, prevented 8-OH-DPAT-induced excitation of pyramidal neurons (Lladó-Pelfort et al. 2012). Moreover, the highly selective 5-HT1A agonist, BAY x 3702, increased the firing rate and burst firing of DA neurons in the VTA and DA release in the VTA and mPFC, which was blocked by WAY-100635. Intracortical BAY x 3702 in both rat and mouse increased local extracellular DA in WT but not 5-HT1A KO mice or in the presence of the GABAA antagonist, bicuculline (Díaz-Mataix et al. 2005), indicating that this effect was mediated by GABA interneurons. Furthermore, administration of the AAPDs, clozapine and olanzapine, locally and systemically, increased cortical DA efflux in the wild type, and 5-HT2A KO, but not in, 5-HT1A KO, mice (Bortolozzi et al. 2010; Díaz-Mataix et al. 2005). Local mPFC perfusion of WAY-100635 or bicuculline prevented mPFC DA efflux induced by local or systemic administration of clozapine, risperidone, and olanzapine (Díaz-Mataix et al. 2005; Li et al. 2009). Thus, the direct and indirect effects of AAPDs on 5-HT1ARs could be a crucial component of the efficacy of AAPDs to treat CIAS and variations in the genetics of 5-HT1ARs within patients are likely to affect cognitive and other response to AAPDs (Bosia et al. 2011).
WAY-100135 completely blocked the enhancement of ACh release induced by locally applied 8-OH-DPAT and partially reduced the effects of systemic 8-OH-DPAT (Nakai et al. 1998). This suggests that lurasidone activates mPFC 5-HT1ARs located on GABAergic interneurons regulating glutamatergic neurons, via inhibition-enhanced cortical GABA neurons, disinhibiting Glu neurons, leading cortical DA and ACh release.
5-HT1AR on GABA interneurons and regulation of cortical neurotransmitter release
The PFC receives serotonergic inputs from raphe nuclei (Pehrson et al. 2015); 5-HT1AR agonists suppress the firing of 5-HT neurons in raphe nuclei, increase the firing of cortical pyramidal neurons, and reduce that of fast-spiking GABA interneurons (Celada et al. 2013). The inhibitory responses elicited in mPFC pyramidal neurons by raphe stimulation are blocked by the GABAA antagonist, picrotoxin (Puig et al. 2005). As with pyramidal neurons, endogenous 5-HT1AR stimulation inhibits, and 5-HT2AR stimulation excites, PFC PV-expressing fast-spiking interneurons in vivo (Puig et al. 2010). Local administration of bicuculline disinhibits GABA interneurons, increasing DA and ACh efflux in PFC and STR, an effect blocked by the GABAA agonist, muscimol (Deboer and Westerink 1994; Kommalage and Höglund 2005; Santiago and Westerink, 1992). Moreover, intracortical bicuculline increased dSTR DA efflux, whereas muscimol reduced DA efflux, suggesting the PFC regulates DA efflux from the STR projections to dSTR GABA interneurons (Matsumoto et al. 2003). 5-HT1A and 5-HT2AR are heavily co-expressed in at least 80% of pyramidal neurons and GABAergic interneurons in rat and mouse PFC (Celada et al. 2013), enabling integrated inhibitory (5-HT1A) and excitatory (5-HT2A) stimuli to these cells (Avesar and Gulledge 2012). Presynaptic and postsynaptic reduction of GABAergic transmission due to 5-HT1A- and 5-HT2A-mediated inhibition or stimulation, respectively, has been postulated (García-Oscos et al. 2015). In addition to 5-HT1A stimulation, cortical ACh efflux secondary to DA D1R stimulation to DA efflux in NAC or STR has must be considered, since 8-OH-DPAT-induced cortical ACh efflux is blocked by WAY-100635 and the D1 antagonist, SCH 23390 (Consolo et al. 1996).
5-HT7Rs and 5-HT1ARs heterodimerization decreases 5-HT1AR-mediated activation of Gi protein and markedly decreased the ability of the 5-HT1AR to activate potassium channels in hippocampal neurons in vivo (Renner et al. 2012). We have recently found that the top hit predicting response to lurasidone in acutely psychotic schizophrenia patients at genome wide significance level is the two-pore potassium channel gene, KCNK9 (Li et al. 2018). Here we report that SB-269970 increases the efflux of GABA in the mPFC, in agreement with the results of Tokarski et al. (2011). Taken together with the electrophysiological data from our group (Yuen et al. 2012), it can be suggested that stimulation of pyramidal neurons in PFC produced by lurasidone is causally related to its 5-HT7R antagonism.
Our finding that the effect of lurasidone on mPFC DA efflux was not blocked by AS 19 suggests that 5-HT7 blockade contributes relatively little to DA efflux by lurasidone, compared to its effect on Glu efflux, and compared to its importance for the effect of SB-269970, on cortical DA, 5-HT, Glu, and GABA efflux. WAY-100635 had no significant effect on lurasidone-induced NT efflux in the dSTR, perhaps, consistent with the low level of expression of 5-HT1ARs in this region (Leiser et al. 2015; Pehrson et al. 2015). However, AS 19 significantly blocked dSTR lurasidone- and SB-269970-induced Glu efflux. The principle neurons in the dSTR are GABAergic medium spiny neurons (MSN, > 80% of striatal neurons), some of which are PV-immunoreactive (IR) interneurons and others choline acetyltransferase (ChAT-IR) interneurons. 5-HT7R are highly expressed in the latter group of interneurons (Leiser et al. 2015; Pehrson et al. 2015). Because the ChAT-IR neurons have a complex mix of excitatory and inhibitory effects on the activity of both PV-IR interneurons and MSNs (Pehrson et al. 2015) and contain depolarizing 5-HT2AR as well, lurasidone may, through 5-HT7 and 5-HT2AR antagonism, diminish the activity of the inhibitory MSNs, resulting in increased DA and Glu efflux. Since all compounds were given systemically in this study, the effects reported here reflect both local and external inputs.
The dSTR receives 5-HT input from raphe nuclei and Glu input from cortex and thalamus (Pehrson et al. 2015). 5-HT released by serotonergic fibers originating in the dorsal raphe nuclei (DRN) has a potent excitatory effect on striatal cholinergic interneurons. The depolarizing response to 5-HT is blocked by SB-269970, mimicked by the 5-HT7 agonist, 5-CT, and the 5-HT2R agonist 2,5-dimethoxy-4-iodoamphetamine, and antagonized by ketanserin, a 5-HT2A/2C antagonist (Bonsi et al. 2007). In DRN slices, blockade of 5-HT7Rs produced a decrease in the mean frequency of spontaneous inhibitory postsynaptic currents (sIPSCs), while activation induced an increase. This indicates ionically active 5-HT7Rs modulate the activity of GABAergic interneurons and/or other interneurons which regulate the activity of DRN serotonergic projection neurons (Kusek et al. 2015).
Lurasidone as well other atypical APDs, e.g., like asenapine, blonanserin, olanzapine, quetiapine, and risperidone, have no significant effect on extracellular GABA efflux in either mPFC or NAC (Huang et al. 2008, 2014; Ohoyama et al. 2011; Yamamura et al. 2009). However, the absence of a detectable effect on GABA release in microdialysis studies does not rule out it having an effect on GABAergic interneurons, where is not detectable by microdialysis. The ability of SEDs of lurasidone to restore NOR in scPCP-treated rodents is enhanced by SEDs of the neurosteroid pregnenolone sulfate, a negative GABAAR modulator (Rajagopal et al. 2018). We, and others (Damgaard et al. 2011), have also found that a variety of GABAAR agonists can also restore NOR in scPCP-treated rodents. This apparent paradox probably reflects the relative impact of these drugs on specific types of synaptic and extrasynaptic GABAARs and, perhaps, inverted U-shaped dose–response curves. What can be concluded from the results reported here is that 5-HT7Rs have a tonic inhibitory effect on some GABA interneurons in the mPFC and the hippocampus (see also Matsuyama et al. 1997). It has been suggested that E-I imbalance due to hypoglutamatergic activity in principal neurons and loss of PV-positive GABA interneurons in the PFC produces sustained neural firing and gamma oscillations, a possible major cause of impaired CIAS (Murray et al. 2015; Uehara et al. 2015). 5-HT7 Rs also have a key role in theta band oscillations (Zlojutro et al. 2011). A possible mechanism for lurasidone to promote neurotransmitter release in the mPFC and dSTR based on this hypothesis is given in Fig. 5 and discussed in the legend.
Hypothesis for the mechanism of action of lurasidone to increase neurotransmitter efflux in the mPFC and dSTR. The effects of lurasidone on neurotransmitter release in the mPFC may result, in part, from the stimulation of DRN 5-HT1A and/or mPFC 5-HT1A receptors located on GABAergic interneurons which project to mPFC principal (pyramidal) neurons. Inhibition of cortical GABA interneurons disinhibits the glutamatergic principal neurons. This, in turn, stimulates cortical DA and ACh neurons leading to increases cortical DA and ACh release. The latter is partially blocked by the 5-HT1A antagonist, WAY-100635. Direct 5-HT2A and 5-HT7 antagonism as well as indirect D1 agonism also contribute to the ability of lurasidone to stimulate the release of DA and ACh but are less influential than 5-HT1A agonism. In dSTR, lurasidone, through 5-HT7 antagonism, inhibits the activity of ChAT-IR interneurons which produce a complex mix of excitatory and inhibitory effects on the behavior of both PV-IR interneuron and MSNs. Lurasidone, by antagonism of 5-HT2A receptors on MSN, inhibits their activity, which leads to disinhibition of dSTR pyramidal neurons, thus enhancing DA and Glu efflux in the dSTR. These effects of lurasidone are blocked by pretreatment with the 5-HT7 agonist AS 19
The present study has some limitations as indicated here: (1) the microdialysis experiments were performed in normal animals, while scPCP mice were the bases of NOR studies. Thus, the mechanisms discussed here could be different in scPCP mice; (2) the ongoing of the glutamate and GABA in the dialysate also could be from non-neuronal pools like astrocytes as well as neurons (Del Arco et al. 2003); and (3) the 5-HT7 KO mouse data was evaluated only for mice with constitutive KO of the 5-HT7Rs.
In summary, the present study established that 5-HT1AR agonism is important to the ability of lurasidone to enhance cortical ACh and DA release, while its 5-HT7R antagonism is more important to its ability to promote Glu and GABA efflux. The effects of lurasidone on these neurotransmitters and possibly GABA were shown to contribute to the ability of lurasidone to ameliorate the scPCP-induced cognitive deficit in scPCP-treated mice; meanwhile, this ability may be relevant to its effects to improve cognitive deficits in at least some patients with schizophrenia. We hypothesize that lurasidone may reduce GABAergic inhibitory tone on various principal neurons in cortex, hippocampus, and other brain regions, through a variety of serotonergic mechanisms, including 5-HT1A agonism, 5-HT2A, and 5-HT7 antagonism, leading to an increase in the activity of glutamatergic pyramidal projection neurons which can influence ACh, DA, and Glu efflux in many brain regions (Fig. 5). The net effect in scPCP mice is to restore synaptic plasticity. This study provides additional support for current efforts to test 5-HT1A partial agonists, e.g., tandospirone and 5-HT7R antagonists, as augmentation treatment for CIAS by lurasidone and other AAPDs.
References
Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F (2004) Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex 14(3):281–299
Assié MB, Ravailhe V, Faucillon V, Newman-Tancredi A (2005) Contrasting contribution of 5-hydroxytryptamine 1A receptor activation to neurochemical profile of novel antipsychotics: frontocortical dopamine and hippocampal serotonin release in rat brain. J Pharmacol Exp Ther 315(1):265–272
Avesar D, Gulledge AT (2012) Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits 6:12
Barch DM, Ceaser A (2012) Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 16(1):27–34
Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R (2004) Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci 24(20):4807–4817
Benarroch EE (2010) Acetylcholine in the cerebral cortex: effects and clinical implications. Neurology 75(7):659–665
Bonaventure P, Aluisio L, Shoblock J, Boggs JD, Fraser IC, Lord B, Lovenberg TW, Galici R (2011) Pharmacological blockade of serotonin 5-HT7 receptor reverses working memory deficits in rats by normalizing cortical glutamate neurotransmission. PLoS One 6(6):e20210
Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A (2007) Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32(8):1840–1854
Bortolozzi A, Masana M, Díaz-Mataix L, Cortés R, Scorza MC, Gingrich JA, Toth M, Artigas F (2010) Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT(1A) receptors but not 5-HT(2A) receptors. Int J Neuropsychopharmacol 13(10):1299–1314
Bosia M, Anselmetti S, Bechi M, Lorenzi C, Pirovano A, Cocchi F, Buonocore M, Bramanti P, Smeraldi E, Cavallaro R (2011) Effect of 5-HT1A-receptor functional polymorphism on theory of mind performances in schizophrenia. Psychiatry Res 188(2):187–190
Celada P, Puig MV, Artigas F (2013) Serotonin modulation of cortical neurons and networks. Front Integr Neurosci 7:25
Consolo S, Ramponi S, Ladinsky H, Baldi G (1996) A critical role for D1 receptors in the 5-HT1A-mediated facilitation of in vivo acetylcholine release in rat frontal cortex. Brain Res 707(2):320–323
Damgaard T, Plath N, Neill JC, Hansen SL (2011) Extrasynaptic GABAA receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology 214(2):403–413
de Almeida J, Mengod G (2007) Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT(2A) receptors in human and monkey prefrontal cortex. J Neurochem 103(2):475–486
de Almeida J, Mengod G (2008) Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J Neurochem 107(2):488–496
DeBoer P, Westerink BH (1994) GABAergic modulation of striatal cholinergic interneurons: an in vivo microdialysis study. J Neurochem 62(1):70–75
Del Arco A, Segovia G, Fuxe K, Mora F (2003) Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J Neurochem. 85(1): 23–33
Désaméricq G, Schurhoff F, Meary A, Szöke A, Macquin-Mavier I, Bachoud-Lévi AC, Maison P (2014) Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol 70(2):127–34
Devan BD, Hong NS, McDonald RJ (2011) Parallel associative processing in the dorsal striatum: segregation of stimulus-response and cognitive control subregions. Neurobiol Learn Mem 96(2):95–120
Díaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, Celada P, Artigas F (2005) Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci 25(47):10831–10843
Fritschy JM, Panzanelli P (2014) GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur J Neurosci 39(11):1845–1865
Fujii T, Yoshizawa M, Nakai K, Fujimoto K, Suzuki T, Kawashima K (1997) Demonstration of the facilitatory role of 8-OH-DPAT on cholinergic transmission in the rat hippocampus using in vivo microdialysis. Brain Res 761(2):244–249
García-Oscos F, Torres-Ramírez O, Dinh L, Galindo-Charles L, Pérez Padilla EA, Pineda JC, Atzori M, Salgado H (2015) Activation of 5-HT receptors inhibits GABAergic transmission by pre-and post-synaptic mechanisms in layer II/III of the juvenile rat auditory cortex. Synapse 69(3):115–127
Grayson B, Barnes SA, Markou A, Piercy C, Podda G, Neill JC (2016) Postnatal phencyclidine (PCP) as a neurodevelopmental animal model of schizophrenia pathophysiology and symptomatology: a review. Curr Top Behav Neurosci 29:403–428
Hedlund PB, Sutcliffe JG (2004) Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci 25(9):481–486
Horiguchi M, Meltzer HY (2012) The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology 221(2):205–215
Horiguchi M, Meltzer HY (2013) Blonanserin reverses the phencyclidine (PCP)-induced impairment in novel object recognition (NOR) in rats: role of indirect 5-HT1A partial agonism. Behav Brain Res 247C:158–164
Horiguchi M, Huang M, Meltzer HY (2011) The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther 338(2):605–614
Horisawa T, Nishikawa H, Toma S, Ikeda A, Horiguchi M, Ono M, Ishiyama T, Taiji M (2013) The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav Brain Res 244:66–69
Huang M, Li Z, Dai J, Shahid M, Wong EH, Meltzer HY (2008) Asenapine increases dopamine, norepinephrine, and acetylcholine efflux in the rat medial prefrontal cortex and hippocampus. Neuropsychopharmacology 33(12):2934–2945
Huang M, Horiguchi M, Felix AR, Meltzer HY (2012) 5-HT1A and 5-HT7 receptors contribute to lurasidone-induced dopamine efflux. Neuroreport 23(7):436–440
Huang M, Panos JJ, Kwon S, Oyamada Y, Rajagopal L, Meltzer HY (2014) Comparative effect of lurasidone and blonanserin on cortical glutamate, dopamine, and acetylcholine efflux: role of relative serotonin (5-HT)2A and DA D2 antagonism and 5-HT1A partial agonism. J Neurochem 128(6):938–949
Huang M, Kwon S, Oyamada Y, Rajagopal L, Miyauchi M, Meltzer HY (2015) Dopamine D3 receptor antagonism contributes to blonanserin-induced cortical dopamine and acetylcholine efflux and cognitive improvement. Pharmacol Biochem Behav 138:49–57
Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Lauqhlin IA, Meltzer HY (2001) 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 76(5):1521–1531
Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334(1):171–181
Kamińska K, Gołembiowska K, Rogóż Z (2013) Effect of risperidone on the fluoxetine-induced changes in extracellular dopamine, serotonin and noradrenaline in the rat frontal cortex. Pharmacol Rep 65(5):1144–1151
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M (2010) Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67(3):231–239
Kommalage M, Höglund AU (2005) Involvement of spinal GABA receptors in the regulation of intraspinal acetylcholine release. Eur J Pharmacol 525(1–3):69–73
Koyama T, Nkajima Y, Fujii T, Kawashima K (1999) Enhancement of cortical and hippocampal cholinergic neurotransmission through 5-HT1A receptor-mediated pathways by BAYx3702 in freely moving rats. Neurosci Lett 265:33–36
Kuroki T, Nagao N, Nakahara T (2008) Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesis. Prog Brain Res 172:199–212
Kusek M, Sowa J, Kamińska K, Gołembiowska K, Tokarski K, Hess G (2015) 5-HT7 receptor modulates GABAergic transmission in the rat dorsal raphe nucleus and controls cortical release of serotonin. Front Cell Neurosci 9:324
Leiser SC, Li Y, Pehrson AL, Dale E, Smagin G, Sanchez C (2015) Serotonergic regulation of prefrontal cortical circuitries involved in cognitive processing: a review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant Vortioxetine. ACS Chem Neurosci 6(7):970–986
Li Z, Prus AJ, Dai J, Meltzer HY (2009) Differential effects of M1 and 5-hydroxytryptamine1A receptors on atypical antipsychotic drug-induced dopamine efflux in the medial prefrontal cortex. J Pharmacol Exp Ther 330(3):948–955
Li J, Yoshikawa A, Brennan MD, Ramsey TL, Meltzer HY (2017) Genetic predictors of antipsychotic response to lurasidone identified in a genome wide association study and by schizophrenia risk genes. Schizophr Res pii: S0920–9964 (17)30196–2
Li J, Loebel A, Meltzer HY (2018) Identifying the genetic risk factors for treatment response to lurasidone by genome-wide association study: a meta-analysis of samples from three independent clinical trials. Schizophr Res. pii: S0920–9964 (18)30195–6. https://doi.org/10.1016/j.schres.2018.04.006. [Epub ahead of print].
Lladó-Pelfort L, Santana N, Ghisi V, Artigas F, Celada P (2012) 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex 22(7):1487–1497
Masana M, Santana N, Artigas F, Bortolozzi A (2012) Dopamine neurotransmission and atypical antipsychotics in prefrontal cortex: a critical review. Curr Top Med Chem 12(21):2357–2374
Matsumoto M, Kanno M, Togashi H, Ueno K, Otani H, Mano Y, Yoshioka M (2003) Involvement of GABAA receptors in the regulation of the prefrontal cortex on dopamine release in the rat dorsolateral striatum. Eur J Pharmacol 482(1–3):177–184
Matsuyama S, Nei K, Tanaka C (1997) Regulation of GABA release via NMDA and 5-HT1A receptors in guinea pig dentate gyrus. Brain Res 761(1):105–112
Meltzer HY (2012) Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol 212:87–124
Meltzer HY (2015) Pharmacotherapy of cognition in schizophrenia. Curr Opin Behav Sci 4:115–121
Meltzer HY, Huang M (2008) In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res 172:177–197
Meltzer HY, Matsubara S, Lee JC (1989) The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull 25(3):390–392
Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M (2013) Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol 16(10):2181–2194
Millan MJ (2000) Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther 295(3):853–861
Millan MJ, Brocco M, Gobert A, Joly F, Bervoets K, Rivet J, Newman-Tancredi A, Audinot V, Maurel S (1999) Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci 11(12):4419–4432
Murray AJ, Woloszynowska-Fraser MU, Ansel-Bollepalli L, Cole KL, A3 F, Crouch B, Riedel G, Wulff P (2015) Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci Rep 5:16778
Nakai K, Fujii T, Fujimoto K, Suzuki T, Kawashima K (1998) Effect of WAY-100135 on the hippocampal acetylcholine release potentiated by 8-OH-DPAT, a serotonin1A receptor agonist, in normal and p-chlorophenylalanine-treated rats as measured by in vivo microdialysis. Neurosci Res 31(1):23–29
Nikiforuk A, Kos T, Fijał K, Hołuj M, Rafa D, Popik P (2013) Effects of the selective 5-HT7 receptor antagonist SB-269970 and amisulpride on ketamine-induced schizophrenia-like deficits in rats. PLoS One 8(6):e66695
Ohoyama K, Yamamura S, Hamaguchi T, Nakagawa M, Motomura E, Shiroyama T, Tanii H, Okada M (2011) Effect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortex. Eur J Pharmacol 653(1–3):47–57
Pehrson AL, Jeyarajah T, Sanchez C (2015) Regional distribution of serotonergic receptors: a systems neuroscience perspective on the downstream effects of the multimodal-acting antidepressant vortioxetine on excitatory and inhibitory neurotransmission. CNS Spectr 7:1–22
Puig MV, Artigas F, Celada P (2005) Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex 15(1):1–14
Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y (2010) Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci 30(6):2211–2222
Rajagopal L, Burgdorf JS, Moskal JR, Meltzer HY (2016a) GLYX-13 (rapastinel) ameliorates subchronic phencyclidine- and ketamine-induced declarative memory deficits in mice. Behav Brain Res 299:105–110
Rajagopal L, Massey BW, Michael E, Meltzer HY (2016b) Serotonin (5-HT)1A receptor agonism and 5-HT7 receptor antagonism ameliorate the subchronic phencyclidine-induced deficit in executive functioning in mice. Psychopharmacology 233(4):649–660
Rajagopal L, Soni D, Meltzer HY (2018) Neurosteroid pregnenolone sulfate, alone, and as augmentation of lurasidone or tandospirone, rescues phencyclidine-induced deficits in cognitive function and social interaction. Behav Brain Res 350:31–43
Renner U, Zeug A, Woehler A, Niebert M, Dityatev A, Dityateva G, Gorinski N, Guseva D, Abdel-Galil D, Fröhlich M, Döring F, Wischmeyer E, Richter DW, Neher E, Ponimaskin EG (2012) Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J Cell Sci 125(Pt 10):2486–2499
Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH (2000) 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry 48(3):229–223
Santiago M, Westerink BH (1992) The role of GABA receptors in the control of nigrostriatal dopaminergic neurons: dual-probe microdialysis study in awake rats. Eur J Pharmacol 219(2):175–181
Sarkisyan G, Hedlund PB (2009) The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav Brain Res 202(1):26–31
Sato M, Ago Y, Koda K, Nakamura S, Kawasaki T, Baba A, Matsuda T (2007) Role of postsynaptic serotonin1A receptors in risperidone-induced increase in acetylcholine release in rat prefrontal cortex. Eur J Pharmacol 559(2–3):155–160
Schreiber R, Newman-Tancredi A (2014) Improving cognition in schizophrenia with antipsychotics that elicit neurogenesis through 5-HT(1A) receptor activation. Neurobiol Learn Mem 110:72–80
Shimizu H, Hirose A, Tatsuno T, Nakamura M, Katsube J (1987) Pharmacological properties of SM-3997: a new anxioselective anxiolytic candidate. Jpn J Pharmacol 45(4):493–500
Sibille E (2017) Reduced somatostatin expression or somatostatin-positive gamma-aminobutyric acid neurons: a shared pathology across brain disorders. Biol Psychiatry 81(6):467–446
Stiedl O, Pappa E, Konradsson-Geuken Å, Ögren SO (2015) The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front Pharmacol 6:162
Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, Sumiyoshi S, Sumiyoshi C, Meltzer HY (2001) The effect of tandospirone, a serotonin(1A) agonist, on memory function in schizophrenia. Biol Psychiatry 49(10):861–868
Tokarski K, Kusek M, Hess G (2011) 5-HT7 receptors modulate GABAergic transmission in rat hippocampal CA1 area. J Physiol Pharmacol 62(5):535–540
Tokarski K, Zelek-Molik A, Duszyńska B, Satała G, Bobula B, Kusek M, Chmielarz P, Nalepa I, Hess G (2012) Acute and repeated treatment with the 5-HT7 receptor antagonist SB 269970 induces functional desensitization of 5-HT7 receptors in rat hippocampus. Pharmacol Rep 64(2):256–265
Tritsch NX, Ding JB, Sabatini BL (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490(7419):262–266
Tritsch NX, Oh WJ, Gu C, Sabatini BL (2014) Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA. not synthesis Elife 3:e01936
Tritsch NX, Granger AJ, Sabatini BL (2016) Mechanisms and functions of GABA co-release. Nat Rev Neurosci 17(3):139–145
Uehara T, Matsuoka T, Sumiyoshi T (2014) Tandospirone, a 5-HT1A partial agonist, ameliorates aberrant lactate production in the prefrontal cortex of rats exposed to blockade of N-methy-D-aspartate receptors; toward the therapeutics of cognitive impairment of schizophrenia. Front Behav Neurosci 8:291
Uehara T, Sumiyoshi T, Kurachi M (2015) New pharmacotherapy targeting cognitive dysfunction of schizophrenia via modulation of GABA neuronal function. Curr Neuropharmacol 13(6):793–801
Volk DW, Sampson AR, Zhang Y, Edelson JR, Lewis DA (2016) Cortical GABA markers identify a molecular subtype of psychotic and bipolar disorders. Psychol Med 46(12):2501–2512
Waters KA, Stean TO, Hammond B, Virley DJ, Upton N, Kew JN, Hussain I (2012) Effects of the selective 5-HT(7) receptor antagonist SB-269970 in animal models of psychosis and cognition. Behav Brain Res 228(1):211–218
Wesołowska A, Kowalska M (2008) Influence of serotonin 5-HT(7) receptor blockade on the behavioral and neurochemical effects of imipramine in rats. Pharmacol Rep 60(4):464–474
Yamamoto BK, Pehek EA, Meltzer HY (1994) Brain region effects of clozapine on amino acid and monoamine transmission. J Clin Psychiatry 55 Suppl B:8–14
Yamamura S, Ohoyama K, Hamaguchi T, Kashimoto K, Nakagawa M, Kanehara S, Suzuki D, Matsumoto T, Motomura E, Shiroyama T, Okada M (2009) Effects of quetiapine on monoamine, GABA, and glutamate release in rat prefrontal cortex. Psychopharmacology 206(2):243–258
Yuen EY, Li X, Wei J, Horiguchi M, Meltzer HY, Yan Z (2012) The novel antipsychotic drug lurasidone enhances N-methyl-D-aspartate receptor-mediated synaptic responses. Mol Pharmacol 81(2):113–119
Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, Bierut LJ, Goate A, Hesselbrock V, Kuperman S, Nurnberger J Jr, Rice JP, Schuckit MA, Foroud T, Edenberg HJ, Porjesz B, Almasy L (2011) Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 156B(1):44–58
Acknowledgements
This study was supported by grants from Sumitomo Dainippon Pharma Co., Ltd., Japan. Dr. Meltzer has been consulting and receives research fundings from Sumitomo Dainippon.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Other authors do not have any conflicts of interests.
Electronic supplementary material
ESM 1
(DOCX 17.3 kb)
Rights and permissions
About this article
Cite this article
Huang, M., Kwon, S., Rajagopal, L. et al. 5-HT1A parital agonism and 5-HT7 antagonism restore episodic memory in subchronic phencyclidine-treated mice: role of brain glutamate, dopamine, acetylcholine and GABA. Psychopharmacology 235, 2795–2808 (2018). https://doi.org/10.1007/s00213-018-4972-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4972-y