Abstract
Rationale
Gypenosides have been reported to produce neuroprotective effects and increase monoamine neurotransmitter levels in the brain.
Objective
Considering that depression is involved in monoamine reduction, this study evaluated the antidepressant-like effects of gypenosides in mice exposed to chronic unpredictable mild stress (CUMS).
Methods
The sucrose preference test and forced swimming test were performed after administration of gypenosides (at 25, 50, or 100 mg/kg) for 4 weeks. Hippocampal brain-derived neurotrophic factor (BDNF) and its downstream targets were analyzed by western blot. Additionally, hippocampal neuronal proliferation was measured by immunohistochemistry.
Results
Four-week treatment with fluoxetine (20 mg/kg) and gypenosides (at either 50 or 100 mg/kg) increased sucrose preference and decreased the immobility time in mice exposed to CUMS. In addition, gypenosides (at either 50 or 100 mg/kg) also increased BDNF expression and neuronal proliferation in the hippocampus of CUMS animals. Further, we showed that treating CUMS mice with K252a, which is an inhibitor of the BDNF receptor TrkB, blocked the effects of gypenosides (100 mg/kg), including behavioral improvements, neuronal proliferation, and up-regulation of p-TrkB, p-ERK, and p-Akt proteins.
Conclusions
This study demonstrates that gypenosides exhibit antidepressant-like effects in mice, which may be mediated by activation of the BDNF-ERK/Akt signaling pathway in the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
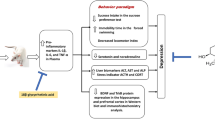
Depression is a mood disorder with a high lifetime prevalence and a high rate of suicide, and depression results in substantial personal suffering and is a major economic burden (Pincus and Pettit 2001; Kessler et al. 2003). However, the neurobiology of depression is not clear. Monoaminergic deficiency and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis have been shown to be involved in early observations of the pathogenesis of depression, but recent studies have indicated that neurotrophin may also play a critical role in the pathogenesis and treatment of depression (Angelucci et al. 2005).
Brain-derived neurotrophic factor (BDNF), which is the most prominent neurotrophic factor in the brain, can bind with its cognate receptor, tropomyosin-related kinase B (TrkB), to activate BDNF-TrkB signaling (Lee and Kim 2010). Early studies have indicated that BDNF can produce an antidepressant response and promote neuronal differentiation, maturation, and survival (Mattson et al. 2004). Clear evidence has demonstrated that serum BDNF levels can be significantly decreased in major depressive patients; however, antidepressant treatment has been shown to up-regulate BDNF in the hippocampus (Duman 2004; Monteleone et al. 2008; Sen et al. 2008). Additionally, BDNF-TrkB downstream signaling, including MEK/ERK and PI3K-Akt pathways, can modulate neurotransmitter release and postsynaptic responses, and these functions are closely associated with antidepressant exposure (Duman and Voleti 2012; Li and Keifer 2012; Numakawa et al. 2013). However, behavioral responses to antidepressants have been abolished in animals in which BDNF-TrkB signaling is inhibited (Autry et al. 2011). Thus, these results suggest that BDNF plays a critical role in the molecular mechanisms of antidepressants.
Gypenosides, which are the saponin extracts isolated from the Gynostemma pentaphyllum plant, effect many pharmacological activities in vivo and in vitro. Gypenosides are an attractive natural product because no mortality or abnormalities have been observed after acute and 90-day subchronic treatment with gypenosides in rats (Chiranthanut et al. 2013). Evidence has indicated that diseases involving the central nervous system can be alleviated by treatment with gypenosides. For example, gypenosides have had neuroprotective effects on dopaminergic neurons and cortical cells in vitro (Wang et al. 2010b). Studies in vivo have also revealed that gypenosides can increase cell proliferation in the subventricular zone of rats induced by middle cerebral artery occlusion or ethanol (Wang et al. 2014). Gypenosides have also been shown to reduce the activation of inflammatory astrocytes in cerebral hypoperfusion rats (Zhang et al. 2011) and to exhibit anti-stress properties in rodents induced by chronic electric footshock stress or 6-hydroxydopamine (Choi et al. 2013). Notably, gypenosides have been found to increase the levels of monoamine neurotransmitter in the brain (Shin et al. 2014). Studies have also shown that gypenoside administration rescues the diminished levels of serotonin and dopamine that are induced by the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease and induced by the chronic electric footshock stress model of anxiety disorder. Because monoamine neurotransmitter concentrations are reduced in depression and because the known clinical antidepressants can increase the levels of monoamine neurotransmitters (Wang et al. 2013), it is implied that the monoamine up-regulation activity of gypenosides might exert potent antidepressant-like effects.
Animal models are used as an effective method to study the molecular pathogenesis of depression and the effects of antidepressants (Krishnan and Nestler 2011). Chronic unpredictable mild stress (CUMS) is a well-validated animal model, which can make rodents both have depressive behavior and symptoms and reflect biochemical alterations compared with control animals. Further, mice exposed to CUMS are commonly used to evaluate the mechanisms of antidepressant drugs (Malberg et al. 2000). In the present study, we investigated whether gypenosides displayed antidepressant-like effects in CUMS mice that were analyzed by the sucrose preference test and forced swimming test. Further, to explore the potential neurotrophic role of gypenosides, BDNF and its downstream signaling pathways were also assessed.
Materials and methods
Animals
Male ICR mice (24 ± 2 g; 5 weeks old) were purchased from the Laboratory Animal Centre, Fujian Medical University in Fujian Province of PR China. Animals were housed eight per cage (320 × 180 × 160 mm) under a normal 12 h/12 h light to dark schedule with lights on at 07:00 a.m. Animals were allowed to adjust to the housing conditions before experiments began. Ambient temperature and relative humidity were maintained at 22 ± 2 °C and at 55 ± 5 %, respectively. Throughout the study, animals were given standard chow and water ad libitum. All procedures were performed in accordance with the published guidelines of the China Council on Animal Care (Regulations for the Administration of Affairs Concerning Experimental Animals, approved by the State Council on 31 October 1988 and promulgated by Decree No. 2 of the State Science and Technology Commission on 14 November 1988).
Drugs and reagents
Standardized gypenosides (purity >98%, confirmed by high-performance liquid chromatography analysis) were purchased from Ankang Chia Tai Pharmaceutical Co., Ltd (Ankang, China). Fluoxetine hydrochloride was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). K252a was purchased from Alomone Laboratories (Jerusalem, Israel).
Drug administration
To evaluate the antidepressant-like effects and BDNF-related activity of gypenosides, 100 animals were divided into two sets of 50 animals (n = 5 per group) after sucrose training. Set 2 was tested 1 day later than set 1; otherwise, the experimental procedure was the same for both animal sets: the Control/CUMS-vehicle group (0.9 % saline containing 0.3 % carboxymethyl cellulose, p.o.), the Control/CUMS-fluoxetine group (20 mg/kg of fluoxetine dissolved in 0.9 % saline containing 0.3 % carboxymethyl cellulose, p.o.), and the Control/CUMS groups that received 25, 50, or 100 mg/kg gypenosides (dissolved in 0.9 % saline containing 0.3 % carboxymethyl cellulose, p.o.).
To confirm that the BDNF signaling pathway is necessary for the antidepressant-like effects of gypenosides, the mice were randomly divided into five groups after sucrose training (n = 10): the Control-vehicle group, the CUMS-vehicle group, the CUMS-gypenosides group (100 mg/kg, p.o.), the K252a group (25 μg/kg of K252a dissolved in 0.9 % saline containing 1 % DMSO), and the CUMS-gypenosides + K252a group (100 mg/kg of gypenosides + 25 μg/kg of K252a). All animals were intraperitoneally injected first with K252a or 0.9 % saline containing 1 % DMSO 30 min before the administration of gypenosides or 0.9 % saline containing 0.3 % carboxymethyl cellulose (Wang et al. 2010a; Yi et al. 2014b). All drugs were administered at a volume of 10 ml/kg body weight once daily for the last 4 weeks of the experiment (Fig. 1).
CUMS
The CUMS procedure was obtained and followed from previous literature (Yi et al. 2014a). Briefly, the weekly stress paradigm consisted of food and water deprivation, exposure to an empty water bottle, exposure to a soiled cage, light/dark succession every 2 h, space reduction, a 45° cage tilt, overnight illumination, and predator sounds. All stressors were applied individually and continuously throughout the day and night. The control animals were housed in a separate room and had no contact with the stressed animals. To prevent habituation and to ensure the unpredictability of the stressors, all stressors were randomly scheduled over a 1-week period and were repeated throughout the duration of the experiment. The body weights of the mice were recorded once per week. On the basis of their sucrose preference following 4 weeks of CUMS, both stressed and control mice were divided into matched subgroups.
Sucrose preference test
The sucrose preference test (SPT) procedure was obtained and followed from previous literature (Yi et al. 2014a). Briefly, before the test, mice were trained to adapt to a sucrose solution (1 %, w/v): two bottles of sucrose solution were placed in each cage for 24 h and then one bottle of sucrose solution was replaced with water for 24 h. After the adaptation, the mice were deprived of water and food for 24 h. The SPT was conducted at 11:30 a.m. on the 57th day on which mice were housed in individual cages and had free access to two bottles containing sucrose solution and water, respectively. After 24 h (11:30 a.m. on the 58th day), the volumes of the consumed sucrose solution and water were recorded. Sucrose preference was calculated using the formula as described below: Sucrose preference = Sucrose consumption/(Water and Sucrose consumption) × 100 %.
Forced swimming test
The forced swimming test was performed according to the method described by Porsolt et al. (1977) with some modifications. After 24 h of the SPT, the forced swimming test was conducted at 11:30 a.m. on the 59th day, and drugs were administered 1 h before the forced swimming test. Briefly, mice were individually placed in a glass cylinder (25 × 12 × 25 cm) filled with water at 15 cm high (23 ± 2 °C). All animals were forced to swim for 6 min, and the duration of immobility was recorded during the final 4-min interval of the test. The immobility period was regarded as the time spent by the mouse floating in the water without struggling and making only the necessary movements to keep its head above the water. Each animal was used only once in this test. The test sessions were recorded by a video camera and scored by a blinded observer.
Protein extraction and western blotting
Mice were sacrificed by decapitation after the forced swimming test. Whole brains were rapidly removed and chilled in an ice-cold saline solution. Hippocampus tissues were dissected on a cold surface and were immediately frozen in liquid nitrogen. The tissue samples were stored at −80 °C until assay.
Tissue samples were homogenized in modified RIPA buffer followed by centrifugation at 1000×g for 5 min at 4 °C to remove nuclei and intact cells. The supernatant was then centrifuged at 12,000×g for 20 min at 4 °C and the resulting supernatant was collected. The protein concentration in the final supernatant was determined by a Bradford protein assay using bovine serum albumin as a standard. The proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5 % (w/v) non-fat dried milk in Tris-buffered saline containing 0.1 % Tween 20 (TBST) and were incubated with the following antibodies in TBST containing 5 % BSA: anti-BDNF (Santa Cruz; 1:500), anti-TrkB (Cell Signaling Technology; 1:1000), anti-p-TrkB (Bioworld Technology; 1:1000), anti-ERK (Cell Signaling Technology; 1:1000), anti-p-ERK (Cell Signaling Technology; 1:1000), anti-Akt (Cell Signaling Technology; 1:1000), anti-p-Akt (Cell Signaling Technology; 1:1000), or GAPDH (Boster; 1:2000). After incubation, the membranes were washed with TBST and were then incubated with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary antibody (Boster; 1:2000) in 5 % non-fat dried milk in TBST. After washing, the immunocomplexes were detected using a Tanon 5200 Chemiluminescence Imaging System. The images were subsequently subjected to densitometric analysis. The BDNF antibody was validated by adding BDNF protein to PBS (Fig. 1S).
Brain extraction and immunohistochemistry
Mice were injected with BrdU (50 mg/kg, dissolved in 0.9 % physiological saline twice per day for two successive days). At 24 h after the last BrdU injection, the mice were immediately anesthetized with chloral hydrate (0.35 g/kg) and were then sacrificed by intracardial perfusion with heparinized 0.9 % saline followed by ice-cold 4 % paraformaldehyde. The brains were removed and postfixed with 4 % paraformaldehyde overnight and incubated with 30 % sucrose solution in PBS at 4 °C for 2 days.
Following fixation, 30-μm-thick coronal brain sections were cut using a cryostat and were then mounted on slides. Every fifth section was collected beginning from bregma coordinate 1.70 mm through 2.57 mm according to a mouse brain atlas. The sections were neutralized by incubating them for 10 min in borate buffer. The anti-BrdU antibody was then used as a primary antibody (1:5000) overnight at room temperature. Subsequently, the sections were intermittently rinsed with PBS and incubated with a biotinylated anti-rat IgG secondary antibody (1:200) followed by an ABC complex (1:100) for 1 h at room temperature. BrdU-incorporated cells were visualized with 0.02% 3,3′-diaminobenzidine tetrahydrochloride and 0.01 % hydrogen peroxide in 0.05 M PBS for approximately 3 min. The number of BrdU-positive cells in the dentate gyrus was counted. The data were obtained from four mice (six sections in total per mouse) per group.
Statistical analyses
All data are expressed as the mean ± SD. The data were analyzed using a two-way or one-way ANOVA followed by a Tukey post hoc test. A value of P <0.05 was considered to be statistically significant for analysis.
Results
The effects of gypenosides on sucrose preference in mice
As shown in the Fig. 2a, CUMS induced a significant decrease in the sucrose preference [F(1,18) = 8.222, P = 0.010]. Two-way ANOVA only showed a significant stress effect [F(1,90) = 5.258, P = 0.024]. The post hoc test showed that the 4-week treatment with fluoxetine (20 mg/kg) and gypenosides (at either 50 or 100 mg/kg) significantly increased the sucrose preference (P = 0.039, P = 0.041, and P = 0.024, respectively). Additionally, neither fluoxetine nor gypenosides showed a significant alteration on sucrose preference in control groups.
The effects of gypenosides on the immobility time of mice during the forced swimming test
As shown in Fig. 2b, CUMS induced a significant increase in the immobility time [F(1,18) = 10.296, P = 0.005]. Only two-way ANOVA showed that the interaction [F(4,90) = 2.663, P = 0.038] was significant. The post hoc test revealed that the 4-week treatment with fluoxetine (20 mg/kg) and gypenosides (50 and 100 mg/kg) significantly decreased the immobility time in the CUMS groups (P = 0.004, P = 0.020, and P = 0.003, respectively). Additionally, neither fluoxetine nor gypenosides significantly altered the immobility time in the control groups.
The effects of gypenosides on the body weight of mice
As shown in Fig. 3, one-way ANOVA revealed that CUMS did not significantly alter the body weight of mice [F(1,18) = 3.826, P = 0.066 > 0.05]. Further, the stress effect [F(1,90) = 3.320, P = 0.072 > 0.05], the treatment effect [F(4,90) = 1.642, P = 0.171 > 0.05], and stress × treatment interaction [F(4,90) = 0.717, P = 0.582 > 0.05] were not significant according to two-way ANOVA.
The effects of gypenosides on the hippocampal BDNF expression in mice
As shown in Fig. 4, one-way ANOVA demonstrated that CUMS induced a significant decrease in BDNF expression in the hippocampus [F(1,8) = 7.797, P = 0.023]. Only the two-way ANOVA indicated that the interaction [F(4,40) = 2.634, P = 0.048] was significant. The post hoc test revealed that the 4-week treatment with fluoxetine (20 mg/kg) and gypenosides (100 mg/kg) significantly increased the hippocampal BDNF expression in the CUMS groups (P = 0.039 and P = 0.026, respectively). Additionally, neither fluoxetine nor gypenosides significantly altered the BDNF expression in the control groups.
The effects of gypenosides on the hippocampal neuronal proliferation in mice
As shown in Fig. 5, one-way ANOVA revealed that CUMS induced a significant decrease in hippocampal neuronal proliferation [F(1,6) = 19.951, P = 0.004]. Two-way ANOVA showed that only the stress effect [F(1,30) = 6.337, P = 0.017] was significant. The post hoc test indicated that the 4-week treatment with fluoxetine (20 mg/kg) and gypenosides (100 mg/kg) significantly increased the hippocampal neuronal proliferation in the CUMS groups (P = 0.018 and P = 0.033, respectively). Additionally, neither fluoxetine nor gypenosides significantly altered hippocampal neuronal proliferation in the control groups.
The effects of gypenosides on the number of BrdU positive cells in mice (n = 4). a Control-vehicle group; b Control-fluoxetine group; c Control-gypenosides (25 mg/kg) group; d Control-gypenosides (50 mg/kg) group; e Control-gypenosides (100 mg/kg) group; f CUMS-vehicle group; g CUMS-fluoxetine group; h CUMS-gypenosides (25 mg/kg) group; i CUMS-gypenosides (50 mg/kg) group; j CUMS-gypenosides (100 mg/kg) group. ## P < 0.01 versus the Control-vehicle group. * P < 0.05 versus the CUMS-vehicle group
The effects of K252a pretreatment on the antidepressant-like effects of gypenosides
To further confirm whether BDNF signaling was necessary for the antidepressant-like effects of gypenosides, we evaluated the effect of K252a pretreatment.
As shown in Fig. 6a, one-way ANOVA displayed that CUMS induced a significant decrease in sucrose preference [F(1,18) = 6.962, P = 0.017]. Two-way ANOVA showed that the treatment effects [F(1,36) = 4.509, P = 0.041] and pretreatment effects [F(1,36) = 5.805, P = 0.021] were significant. The post hoc test revealed that the 4-week treatment with gypenosides (100 mg/kg) increased the sucrose preference in the CUMS groups (P = 0.051). In contrast, this antidepressant-like effect was completely blocked by pretreatment with the K252a antagonist (P = 0.033).
As shown in Fig. 6b, one-way ANOVA revealed that CUMS induced a significant increase in the immobility time [F(1,18) = 12.155, P = 0.003]. Two-way ANOVA showed that only the treatment effect [F(1,36) = 4.203, P = 0.048] was significant. The post hoc test revealed that the 4-week treatment with gypenosides (100 mg/kg) significantly decreased the immobility time in the CUMS groups (P = 0.038). In contrast, this antidepressant-like effect was completely blocked by pretreatment with the K252a antagonist (P = 0.048).
The effects of K252a pretreatment on the BDNF-TrkB signaling pathway induced by gypenosides
As shown in Fig. 7, one-way ANOVA revealed that CUMS induced a significant decrease in the ratio of p-TrkB/TrkB [F(1,8) = 16.663, P = 0.004]. Two-way ANOVA showed a significant pretreatment effect [F(1,16) = 13.310, P = 0.002] and interaction [F(1,16) = 11.112, P = 0.004]. The post hoc test demonstrated that the 4-week treatment with gypenosides (100 mg/kg) significantly increased the ratio of p-TrkB/TrkB in the CUMS groups (P = 0.049). In contrast, this effect was completely blocked by pretreatment with the K252a antagonist (P = 0.001).
As shown in Fig. 8a, one-way ANOVA indicated that CUMS induced a significant decrease in the ratio of p-ERK/ERK [F(1,8) = 13.355, P = 0.006]. Two-way ANOVA showed a significant interaction [F(1,16) = 9.875, P = 0.006]. The post hoc test revealed that the 4-week treatment with gypenosides (100 mg/kg) significantly increased the ratio of p-ERK/ERK in the CUMS groups (P = 0.011). In contrast, this effect was completely blocked by pretreatment with the K252a antagonist (P = 0.026).
As shown in Fig. 8b, one-way ANOVA indicated that CUMS induced a significant decrease in the ratio of p-Akt/Akt [F(1,8) = 9.099, P = 0.017]. Two-way ANOVA showed a significant interaction [F(1,16) = 9.007, P = 0.009]. The post hoc test revealed that the 4-week treatment with gypenosides (100 mg/kg) significantly increased the ratio of p-Akt/AKT in the CUMS groups (P = 0.018). In contrast, this effect was completely blocked by pretreatment with the K252a antagonist (P = 0.031).
As shown in Fig. 9, one-way ANOVA revealed that CUMS induced a significant decrease in hippocampal neuronal proliferation [F(1,6) = 9.253, P = 0.023]. Two-way ANOVA showed the significant effects of treatment [F(1,12) = 11.180, P = 0.006] and pretreatment [F(1,12) = 6.452, P = 0.026]. The post hoc test indicated that the 4-week treatment with gypenosides (100 mg/kg) significantly increased hippocampal neuronal proliferation in the CUMS groups (P = 0.013). In contrast, this effect was completely blocked by pretreatment with the K252a antagonist (P = 0.034).
The effects of pretreatment with K252a on gypenosides-induced BrdU-positive cell number in CUMS (n = 4). a Control-vehicle group; b CUMS-vehicle group; c CUMS-K252a group; d CUMS-gypenosides group; e CUMS-K252a-gypenosides group. # P < 0.05 versus the Control-vehicle group. * P < 0.05 versus the CUMS-vehicle group. &P < 0.05 versus the CUMS-gypenosides group
Discussion
Our present study provided evidence to support the hypothesis that gypenosides have antidepressant-like effects in mice exposed to CUMS. Compared with the Control-vehicle mice, the reduction in sucrose preference and the increase in the immobility time in the CUMS-vehicle group indicated that we successfully created a depression-like animal model. Further, we showed that there was not a significant alteration in body weights when comparing between each animal group. Notably, chronic treatment with gypenosides (at either 50 or 100 mg/kg) for 4 weeks significantly reversed the reduction of sucrose preference and the elevation of immobility time in CUMS mice, which indicate that gypenosides have antidepressant-like effects.
It is widely accepted that BDNF plays a crucial role in antidepressant treatment. BDNF, which maintains the survival of neurons and synaptic plasticity (Dwivedi 2009), has effects on the central nervous system and the peripheral nervous system (Acheson et al. 1995). Although some studies have failed to detect evidence for reduced BDNF levels in depression (Munkholm et al. 2016; Su et al. 2016), accumulating evidence has indicated that BDNF expression may be decreased in the frontal cortex, hippocampus, and serum of depressed suicide victims or patients (Schmidt and Duman 2007; Martinotti et al. 2016). Moreover, depressed patients have shown an increased BDNF concentration in serum after antidepressant treatment. Further, an improvement in depressive symptoms has also been correlated with BDNF levels (Zhao et al. 2015). Therefore, the reduction of BDNF expression in depressed patients suggests that depression is related to BDNF regulation (Dwivedi 2009). Animal studies have shown that many chronic stressors, such as chronic mild stress or social defeat stress, have also been found to down-regulate BDNF expression (Haenisch et al. 2009; Zhang et al. 2010). However, treatment with antidepressant drugs has been shown to reverse the reduction of either BDNF expression or activity that is induced by stress in mice (Lee and Kim 2010). Consistent with previous reports, our current study also revealed that the levels of BDNF expression and neuronal proliferation are lower in the hippocampus of CUMS mice (Li et al. 2011; Mao et al. 2014; Yi et al. 2014a), but chronic gypenoside treatment significantly reversed this reduction. Therefore, these data suggest that up-regulation of BDNF signaling might be involved in the antidepressant-like effects of gypenosides.
To directly confirm this hypothesis, we used K252a, which is an inhibitor of the BDNF receptor TrkB, to block the BDNF signaling pathway. K252a is widely used as a nonspecific protein kinase inhibitor of Trk, especially TrkB, to evaluate the role of BDNF-TrkB signaling (Nye et al. 1992). Previous studies have found that the antidepressant-like effects of BDNF were completely abolished by K252a pretreatment in a depression-like rat model (Shirayama et al. 2002). When other antidepressant agents, such as lamotrigine, piperine, and oleanolic acid, have been used together with K252a pretreatment, antidepressant-like effects have not been observed in behavioral tests, such as the sucrose preference test, forced swimming test, and novelty suppressed feeding test (Li et al. 2011; Mao et al. 2014; Yi et al. 2014a). Similarly, we found that K252a abolished the behavioral antidepressant-like effects of gypenosides in the sucrose preference test and forced swimming test in CUMS mice, which confirms that activation of BDNF signaling is necessary for the antidepressant-like effects of gypenosides.
Our results also showed that CUMS decreased p-TrkB levels and that gypenosides restored this reduction. In contrast, K252a inhibited gypenoside-induced TrkB phosphorylation in the mouse hippocampus. This result demonstrated that gypenosides activated the TrkB receptor and BDNF-TrkB signaling.
After binding to its receptor TrkB, BDNF activates its two downstream intracellular signaling cascades, which are ERK and Akt. These two signaling pathways are required for regulation of neuron proliferation and cognitive function (Jiang et al. 2012; Koskimaki et al. 2015). Studies also have shown that the ERK and Akt signaling pathways play a crucial role in the antidepressant-like effects of BDNF (Shirayama et al. 2002; Koskimaki et al. 2015). Chronic stress exposure has been demonstrated to reduce the phosphorylation of p-ERK and p-Akt, whereas chronic antidepressant treatments have reversed this reduction in the brain (Li et al. 2010; Lv et al. 2014; Yi et al. 2014a). These data suggest that the BDNF-related ERK and Akt pathways might be involved in the molecular mechanism of antidepressants. Similarly, our current study found that CUMS decreased p-TrkB/TrkB, p-ERK/ERK, and p-Akt/Akt in the hippocampus, while chronic gypenoside treatment reversed these alterations; however, K252a abolished the action of gypenosides. In concert with the change of p-TrkB/TrkB, p-ERK/ERK, and p-Akt/Akt, CUMS decreased hippocampal neuronal proliferation, and gypenosides reversed the deficits while K252a blocked the gypenoside-dependent improvements. Therefore, these results demonstrate that gypenosides produce antidepressant-like effects and neuronal proliferation via up-regulation of the BDNF-TrkB-ERK/Akt signaling pathway in the hippocampus.
Finally, note that both fluoxetine and gypenosides did not significantly increase the hippocampal BDNF levels nor cell proliferation of naïve animals. These results are not consistent with previous studies (Malberg et al. 2000; Wang et al. 2013; Imoto et al. 2015), which have shown that chronic fluoxetine treatment promotes BDNF expression and/or neuronal proliferation. However, it is widely accepted that BDNF expression depends on numerous experimental factors, such as stressors, drugs, drug dosage, routes of administration, and time windows of BDNF measurement with regard to treatment duration (Tardito et al. 2006). Therefore, we speculated two reasons that might explain this discrepancy. First, the inconsistent studies above sacrificed the animals within 1 day of chronic fluoxetine treatment; however, the animals in our study were sacrificed 3 days after fluoxetine administration. A previous study clearly demonstrated that hippocampal BDNF expression was significantly decreased after 3 weeks of fluoxetine treatment with a 1-week washout period when compared with 3-week fluoxetine treatment without a washout period (Musazzi et al. 2009). Thus, we thought that three washout days after drug treatment might normalize the expression of BDNF. Second, behavioral tests, especially the forced swimming test, were performed before BDNF and cell proliferation measurements were taken in our study; however, in other studies, measurements were performed without a stress-induced paradigm. The Duman group has previously reported that different time points after the last exposure to the stressor affects BDNF expression (Schmidt and Duman 2007). In this way, our present study might not really show the neurotrophic roles of fluoxetine or gypenosides in naïve animals. Considering that sacrifice time and behavioral tests will affect the measurement of BDNF and cell proliferation, further study is needed to explore their roles on naïve animals without any stressors or a washout period.
After binding to G protein–coupled receptors, serotonin and dopamine can modulate gene expression, which includes gene expression regulating neuronal proliferation (Tardito et al. 2006; Musazzi et al. 2009). Therefore, it is well accepted that antidepressants can promote BDNF expression by activating serotonergic and dopaminergic neurotransmission (Imoto et al. 2015). Based on the results from previous studies (Choi et al. 2013; Shin et al. 2014; Zhao et al. 2015) and our present study, we can speculate that gypenosides can increase the levels of serotonin and dopamine, and then these two monoamine neurotransmitters can activate downstream signaling to promote the expression of BDNF. Of course, this speculation needs to be validated in the further study. This is one of the limitations of the present study.
In summary, our study shows that chronic administration with gypenosides produces antidepressant-like effects in CUMS mice and ameliorates CUMS-induced down-regulation of BDNF expression and neuronal proliferation in the hippocampus. Blockade of BDNF signaling abolishes the behavioral improvement of gypenosides in CUMS mice. Additionally, K252a also blocks the up-regulation of TrkB, ERK, and p-Akt as well as neuronal proliferation. The present study strongly suggests that the BDNF-ERK/Akt signaling pathway is required for producing the antidepressant-like effects of gypenosides.
References
Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM (1995) A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374:450–453
Angelucci F, Brene S, Mathe AA (2005) BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry 10:345–352
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95
Chiranthanut N, Teekachunhatean S, Panthong A, Khonsung P, Kanjanapothi D, Lertprasertsuk N (2013) Toxicity evaluation of standardized extract of Gynostemma pentaphyllum Makino. J Ethnopharmacol 149:228–234
Choi HS, Zhao TT, Shin KS, Kim SH, Hwang BY, Lee CK, Lee MK (2013) Anxiolytic effects of herbal ethanol extract from Gynostemma pentaphyllum in mice after exposure to chronic stress. Molecules 18:4342–4356
Duman RS (2004) Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med 5:11–25
Duman RS, Voleti B (2012) Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35:47–56
Dwivedi Y (2009) Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat 5:433–449
Haenisch B, Bilkei-Gorzo A, Caron MG, Bönisch H (2009) Knockout of the norepinephrine transporter and pharmacologically diverse antidepressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J Neurochem 111:403–416
Imoto Y, Kira T, Sukeno M, Nishitani N, Nagayasu K, Nakagawa T, Kaneko S, Kobayashi K, Segi-Nishida E (2015) Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol Brain 8:29
Jiang B, Xiong Z, Yang J, Wang W, Wang Y, Hu ZL, Wang F, Chen JG (2012) Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol 166:1872–1887
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey R (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105
Koskimaki J, Matsui N, Umemori J, Rantamaki T, Castren E (2015) Nimodipine activates TrkB neurotrophin receptors and induces neuroplastic and neuroprotective signaling events in the mouse hippocampus and prefrontal cortex. Cell Mol Neurobiol 35:189–196
Krishnan V, Nestler EJ (2011) Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7:121–147
Lee BH, Kim YK (2010) The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig 7:231–235
Li N, He X, Zhang Y, Qi X, Li H, Zhu X, He S (2011) Brain-derived neurotrophic factor signalling mediates antidepressant effects of lamotrigine. Int J Neuropsychopharmacol 14:1091–1098
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
Li W, Keifer J (2012) Rapid enrichment of presynaptic protein in boutons undergoing classical conditioning is mediated by brain-derived neurotrophic factor. Neuroscience 203:50–58
Lv QQ, Wu WJ, Guo XL, Liu RL, Yang YP, Zhou DS, Zhang JX, Liu JY (2014) Antidepressant activity of astilbin: involvement of monoaminergic neurotransmitters and BDNF signal pathway. Biol Pharm Bull 37:987–995
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110
Mao QQ, Huang Z, Zhong XM, Xian YF, Ip SP (2014) Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav Brain Res 261:140–145
Martinotti G, Pettorruso M, De Berardis D, Varasano PA, Lucidi Pressanti G, De Remigis V, Valchera A, Ricci V, Di Nicola M, Janiri L, Biggio G, Di Giannantonio M (2016) Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. Int J Neuropsychopharmacol. doi: 10.1093/ijnp/pyw003
Mattson MP, Maudsley S, Martin B (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27:589–594
Monteleone P, Serritella C, Martiadis V, Maj M (2008) Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord 10:95–100
Munkholm K, Vinberg M, Kessing LV (2016) Peripheral blood brain-derived neurotrophic factor in bipolar disorder: a comprehensive systematic review and meta-analysis. Mol Psychiatry 21:216–228
Musazzi L, Cattaneo A, Tardito D, Barbon A, Gennarelli M, Barlati S, Racagni G, Popoli M (2009) Early raise of BDNF in hippocampus suggests induction of posttranscriptional mechanisms by antidepressants. BMC Neurosci 10:48
Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H (2013) Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience 239:157–172
Nye SH, Squinto SP, Glass DJ, Stitt TN, Hantzopoulos P, Macchi MJ, Lindsay NS, Ip NY, Yancopoulos GD (1992) K-252a and staurosporine selectively block autophosphorylation of neurotrophin receptors and neurotrophin-mediated responses. Mol Biol Cell 3:677–686
Pincus HA, Pettit AR (2001) The societal costs of chronic major depression. J Clin Psychiatry 62(Suppl 6):5–9
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Schmidt HD, Duman RS (2007) The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 18:391–418
Sen S, Duman R, Sanacora G (2008) Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64:527–532
Shin KS, Zhao TT, Choi HS, Hwang BY, Lee CK, Lee MK (2014) Effects of gypenosides on anxiety disorders in MPTP-lesioned mouse model of Parkinson’s disease. Brain Res 1567:57–65
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261
Su CL, Su CW, Hsiao YH, Gean PW (2016) Epigenetic regulation of BDNF in the learned helplessness-induced animal model of depression. J Psychiatr Res 76:101–110
Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M (2006) Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev 58:115–134
Wang P, Niu L, Gao L, Li WX, Jia D, Wang XL, Gao GD (2010a) Neuroprotective effect of gypenosides against oxidative injury in the substantia nigra of a mouse model of Parkinson’s disease. J Int Med Res 38:1084–1092
Wang P, Niu L, Guo XD, Gao L, Li WX, Jia D, Wang XL, Ma LT, Gao GD (2010b) Gypenosides protects dopaminergic neurons in primary culture against MPP(+)-induced oxidative injury. Brain Res Bull 83:266–271
Wang XJ, Sun T, Kong L, Shang ZH, Yang KQ, Zhang QY, Jing FM, Dong L, Xu XF, Liu JX, Xin H, Chen ZY (2014) Gypenosides pre-treatment protects the brain against cerebral ischemia and increases neural stem cells/progenitors in the subventricular zone. Int J Dev Neurosci 33:49–56
Wang Z, Gu J, Wang X, Xie K, Luan Q, Wan N, Zhang Q, Jiang H, Liu D (2013) Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: the HPA axis, BDNF expression and phosphorylation of ERK. Pharmacol Biochem Behav 112:104–110
Yi LT, Li J, Liu BB, Luo L, Liu Q, Geng D (2014a) BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J Psychiatry Neurosci 39:348–359
Yi LT, Liu BB, Li J, Luo L, Liu Q, Geng D, Tang Y, Xia Y, Wu D (2014b) BDNF signaling is necessary for the antidepressant-like effect of naringenin. Prog Neuropsychopharmacol Biol Psychiatry 48:135–141
Zhang GL, Deng JP, Wang BH, Zhao ZW, Li J, Gao L, Liu BL, Xong JR, Guo XD, Yan ZQ, Gao GD (2011) Gypenosides improve cognitive impairment induced by chronic cerebral hypoperfusion in rats by suppressing oxidative stress and astrocytic activation. Behav Pharmacol 22:633–644
Zhang Y, Gu F, Chen J, Dong W (2010) Chronic antidepressant administration alleviates frontal and hippocampal BDNF deficits in CUMS rat. Brain Res 1366:141–148
Zhao TT, Shin KS, Choi HS, Lee MK (2015) Ameliorating effects of gypenosides on chronic stress-induced anxiety disorders in mice. BMC Complement Altern Med 15:323
Acknowledgments
The project was supported by grants from the National Natural Science Foundation of China (no. 81202940; 81303278) and the Science Research Foundation of Ministry of Health and United Fujian Provincial Health and Education Project for Tacking the Key Research (WKJ-FJ-31).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors reported no biomedical financial interests or potential conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 603 kb)
Rights and permissions
About this article
Cite this article
Mu, RH., Fang, XY., Wang, SS. et al. Antidepressant-like effects of standardized gypenosides: involvement of brain-derived neurotrophic factor signaling in hippocampus. Psychopharmacology 233, 3211–3221 (2016). https://doi.org/10.1007/s00213-016-4357-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4357-z