Abstract
Rationale
Calcium-permeable (GluA2 subunit-free) AMPA receptors (CP-AMPAR) play prominent roles in fear extinction; however, no blockers of these receptors were studied in tests relevant to extinction learning so far.
Methods
The CP-AMPAR antagonist IEM-1460 was administered once before extinction trainings, which were started either 1 or 28 days after fear conditioning (FC). We used a mild extinction protocol that durably decreased but did not abolish conditioned fear. The messenger RNA (mRNA) expression of GluA1 and GluA2 subunits were investigated at both time points in the ventromedial prefrontal cortex (vmPFC) and amygdala.
Results
IEM-1460 transiently facilitated extinction 1 day after conditioning, but learned fear spontaneously recovered 4 weeks later. When the extinction protocol was applied 28 days after training, IEM-1460 enhanced extinction memory, moreover abolished conditioned fear for at least a month. The expression of GluA1 and GluA2 mRNAs was increased at both time points in the vmPFC. In the basolateral and central amygdala, the GluA1/GluA2 mRNA ratio increased, suggesting a shift towards the preponderance of GluA1 over GluA2 expression.
Conclusions
AMPAR blockade lastingly enhanced the extinction of remote but not recent fear memories. Time-dependent changes in AMPA receptor subunit mRNA expression may explain the differential effects of CP-AMPAR blockade on recent and remote conditioned fear, further supporting the notion that the mechanisms maintaining learned fear change over time. Our findings suggest clinical implications for CP-AMPAR blockers, particularly for acquired anxieties (e.g., post-traumatic stress disorder) which have a slow onset and are durable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor in learned fear is demonstrated by several lines of evidence. This receptor—in conjunction with N-methyl-d-aspartate (NMDA)-mediated neurotransmission—has important roles in long-term potentiation (LTP), a form of neural plasticity that substantially contributes to fear learning (Fortin et al. 2010; Kim et al. 2007; Mahanty and Sah 1998; McKernan and Shinnick-Gallagher 1997); its upregulation by the AMPA enhancer PEPA facilitates the extinction of contextual conditioned fear (Yamada et al. 2009; Zushida et al. 2007), and finally, interventions that affect AMPA receptor trafficking influence both fear memory and extinction (Dalton et al. 2008; Migues et al. 2010). It is believed that calcium-permeable AMPA receptors (CP-AMPAR, those lacking the GluA2 subunit) have outstanding roles in both the neural functions underlying and the behaviors reflecting fear (Clem and Huganir 2010; Fortin et al. 2010; Mahanty and Sah 1998; Shepherd 2012). Such findings were primarily obtained in studies targeting the hippocampus and amygdala (particularly its lateral and basolateral nuclei) and by employing various paradigms including contextual and cue-induced conditioned fear as well as fear-potentiated startle, suggesting these are key regions of AMPA receptor involvement in fear and extinction learning (Clem and Huganir 2010; Humeau et al. 2007; Kim et al. 2007; Mahanty and Sah 1998; McKernan and Shinnick-Gallagher 1997; Migues et al. 2010; Ota et al. 2010; Yamada et al. 2009; Zushida et al. 2007). Several studies suggested, however, that AMPA receptors located in the medial prefrontal cortex also have an important role (Popova et al. 2014; Zushida et al. 2007). The ventral part of this area (vmPFC)—i.e., the ventral aspect of the prelimbic cortex and the infralimbic cortex—is especially important for the extinction of fear memories (Lisboa et al. 2010; Morgan and LeDoux 1995), probably due to its distinct neuroarchitectonic features, connectivities, and behavioral functions (Heidbreder and Groenewegen 2003).
Albeit the involvement of AMPA receptors in fear and extinction learning appears established, findings are not entirely congruent regarding their roles. For instance, fear expression was impaired in AMPA receptor knockout mice (Dachtler et al. 2011; Feyder et al. 2007; Humeau et al. 2007; Mead et al. 2006), while systemic pharmacologic interventions affected fear extinction without decreasing fear expression (Dalton et al. 2008; Zushida et al. 2007). In contrast, inhibiting the AMPA receptors in the basolateral complex of the amygdala blocked fear expression but had no effect on extinction (Zimmerman and Maren 2010). The enhancement of AMPA activity by different methodologies, e.g., by the AMPA enhancer PEPA and by the inhibition of endocytosis, facilitated and inhibited, respectively, the extinction of fear (Dalton et al. 2008; Kim et al. 2007; Yamada et al. 2009; Zushida et al. 2007). Furthermore, extinction-related roles were revealed for both calcium-permeable and calcium-impermeable AMPA receptors, while their relative importance remains unclear (Clem and Huganir 2010; Fortin et al. 2010; Mahanty and Sah 1998; Shepherd 2012).
Importantly for the present study, the involvement of AMPA receptors in recent and remote fear memories received little attention so far, although mechanisms underlying conditioned fear undergo substantial changes over time (Frankland et al. 2004; Graff et al. 2014; Mikics et al. 2008). Particularly, it was shown that brain activation patterns elicited by exposure to fear-associated contexts substantially change over time (Frankland et al. 2004; Mikics et al. 2008), and neural plasticity phenomena accompanying recent and remote memories are different (Graff et al. 2014).
Based on the findings briefly reviewed above, we hypothesized that the blockade of CP-AMPA receptors affects the efficacy of extinction training in conjunction with conditioning-induced changes in AMPA receptor subunit composition. We also hypothesized that effects on recent and remote memories would be different. This assumption was based on the differential neural underpinnings of recent and remote fear as shown above. We studied the effects of IEM-1460, an adamantane-based CP-AMPAR blocker. Although adamantanes are known to block both NMDA and CP-AMPA receptors (Antonov and Johnson 1996; Magazanik et al. 1997), the ID50 values of IEM-1460 are 310 and 3.1 μM for NMDA and CP-AMPA receptor blockade, respectively, which confer a marked target specificity to the compound at low doses (Magazanik et al. 2003). Not surprisingly, IEM-1460 was used earlier to study the brain distribution of CP-AMPARs (Buldakova et al. 1999; Samoilova et al. 1999; Schlesinger et al. 2005). The compound was not investigated in tests of conditioned fear so far, although its effects on LTP were studied (Fortin et al. 2010). To study both recent and remote fear memories, rats were investigated 1 and 28 days after fear conditioning. A second study investigated the effects of fear learning on the expression of GluA1 and GluA2 AMPA subunits in the vmPFC, basolateral and central amygdala (BLA and CeA, respectively), and their correlation with the expression of conditioned fear. This experiment was considered relevant with respect to the main goal of the study because the relative expression of GluR1 and GluR2 AMPA receptor subunit messenger RNAs (mRNAs) provide information on the abundance of CP-AMPAR in the rat central nervous system; moreover, the level of CP-AMPAR expression can be manipulated by interfering with the expression of mRNAs of subunits (Geiger et al. 1995; Jonas et al. 1994; Studniarczyk et al. 2013).
Method
Animals
Subjects were male Wistar rats (Charles River, Hungary) weighing approximately 300 g at the start of experiments. Food and water were available ad libitum while temperature and relative humidity were kept at 22 ± 2 °C and 60 ± 10 %, respectively. Rats were maintained in a reversed light cycle of 12 h with lights off at 1000 hours. Acclimatization to the day–night schedule lasted more than 2 weeks. Rats were isolated 3 days before fear conditioning and were housed individually thereafter in Tecniplast 1291H Eurostandard Type III H cages (425 × 266 × 185 mm).
Experimental design
Experiment 1 aimed at establishing an extinction protocol, which decreased the expression of conditioned fear without abolishing it. This approach allowed the detection of both facilitatory and inhibitory effects on extinction learning, which seemed appropriate when a compound with unknown effects was studied. One or 28 days after fear conditioning, rats were submitted to a conditioned fear test and submitted thereafter to extinction training. Two protocols were studied. In the case of the 7 × 1 protocol, rats were daily exposed to the conditioning cage for 5 min on seven consecutive days without the delivery of shocks (Fig. 1a). In the case of the 3 × 5 protocol, rats were introduced into the conditioning cage five times a day on three consecutive days at 1-h intervals without the delivery of shocks; trials were performed in the first hours of the dark (active) phase of the day (Fig. 1b). The conditioned fear test was considered the first extinction trial in both cases.
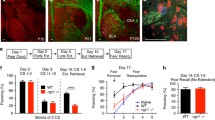
Comparison of different extinction training protocols. a 7 × 1 protocol started 1 or 28 days after fear conditioning. b 3 × 5 protocol started 1 or 28 day after fear conditioning. The precise experimental design is shown on graphs. The first day of the 3 × 5 protocol was chosen for testing the effects of IEM-1460, as after this day, conditioned fear was decreased but not abolished. This protocol allowed for the detection of both enhanced and inhibited extinction learning. FC fear conditioning, CFT conditioned fear test. Data are expressed as mean ± SEM. Asterisk indicates significant difference from session 1; number sign indicates significant difference from session 2 (p < 0.05 at least in all cases)
In Experiment 2, we studied the effects of the CP-AMPAR antagonist IEM-1460 on extinction in two separate experiments. In Experiment 2a, rats were injected intraperitoneally with IEM-1460 (Tocris Bioscience, Hungary) 1 day after fear conditioning and were submitted to extinction training on the same day 1 h later. Experiment 2b was performed in a similar manner, but treatment was administered and training was started 28 days after fear learning. Based on the findings of Experiment 1, an intermediate, 1 × 5 extinction protocol was employed, where five extinction trials were administered on one and the same day at 1-h intervals (Fig. 2a, d; also see below). IEM-1460 doses (0/vehicle, 1, or 3 mg/kg) were selected based on earlier studies (Gmiro et al. 2008; Kobylecki et al. 2010). The efficacy of extinction was investigated the next day, when rats were once again exposed to the fear conditioning chamber for 5 min. The spontaneous recovery of learned fear was investigated in tests performed 4 weeks after extinction training. Noteworthy, the antagonist was administered just once before the first test, and rats were left undisturbed between testing sessions. Behavior was video recorded in each test.
The effects of the calcium-permeable AMPA receptor antagonist IEM-1460 on fear extinction. Immobility was expressed as percentage of values obtained in CFT in order to control for differences in behavioral baselines. For values expressed as %test time, see Table 2. a, d Experimental designs; b, e fear retrieval (conditioned fear test/first extinction trial); c, f behavior during extinction and in trials that tested extinction memory. FC fear conditioning, CFT conditioned fear test; gray horizontal lines with red horizontal columns, immobility in the first CFT of rats submitted to FC (mean ± SEM, respectively); gray horizontal lines with blue horizontal columns, the level of immobility in the first CFT of rats not submitted to FC (mean ± SEM, respectively); number sign, significant difference from non-conditioned controls (effect of fear conditioning); asterisk, significant effect of extinction training (difference from trial 1); plus sign, significant difference from vehicle-treated conditioned rats (p < 0.05 at least in all cases)
Experiment 3 investigated the impact of fear conditioning on GluA1 and GluA2 mRNA levels in the vmPFC, BLA, and CeA. Brain targets were selected based on earlier studies demonstrating that AMPA receptors in these areas are involved in the control of fear learning and extinction (Clem and Huganir 2010; Popova et al. 2014; Walker and Davis 2008; Zushida et al. 2007). On the first day of the study, rats were submitted to fear conditioning and were re-exposed to the fear conditioning chamber 1 or 28 days later for 5 min. Behavior was video recorded. Rats were decapitated immediately after behavioral testing, and their brains were rapidly removed and processed as described below. Trunk blood was collected in ice-cold EDTA-containing plastic tubes for corticosterone measurements. Note that significant changes in mRNA expression profiles including the genes for AMPA receptor subunits require considerably more time than 5 min, i.e., the duration of the fear conditioning test (Llorente et al. 2015). As such, mRNA expression data reflected the condition which characterized rats when extinction training started (i.e., reflected the delayed effects of fear conditioning rather than those of the acute fear memory reactivation).
Behavior
Fear conditioning
Electric shocks of 3 mA were administered via the grid floor of a Plexiglas cage (30 × 30 × 30 cm) between 1100 hours and 1300 hours (the early hours of the dark period), in a separate, quiet room under regular laboratory illumination (∼400 lx). Two shock trains were administered per min for 5 min, each shock train being 1 s long and consisted of 0.01 s shocks separated by 0.02 s long breaks. Control rats were placed into a similar box for 5 min, but shocks were not delivered. The box was cleaned between shock sessions with soap and tap water. A highly similar procedure was employed in earlier studies (Haller et al. 2011).
Conditioned fear testing and extinction training
Rats were re-introduced into the fear conditioning chamber for 5 min either 1 or 28 days after fear conditioning. Rats did not receive shocks this time. The test took place between 1100 hours and 1300 hours (the early hours of the dark period). This test also constituted the first trial of extinction training that included four additional 5-min-long exposures to the fear conditioning chamber. Extinction trials were performed at 1-h intervals on the same day.
Recent and remote extinction memory
The memory was tested 1 day and 4 weeks after the extinction training by re-exposing rats to the conditioning cage for 5 min both times. Rats were maintained under normal laboratory conditions in the meantime, i.e., were not exposed to additional stressors.
Behavioral variables
Behavior was video recorded and scored from the tapes by an experimenter blind to treatment conditions by means of the H77 event-recording software (Institute of Experimental Medicine, Hungary). The same experimenter scored all tapes; intra-rater reliability was over 90 %. In Experiments 1 and 2, we scored immobility, the main indicator of fear in this test. In Experiment 3, we scored the following behaviors: immobility (complete immobility, no movements of the snout), exploration (searching, sniffing movements directed towards the walls, grid or the air), grooming (“washing” the head with forepaws or scratching the body with hind legs), resting (no locomotion, small postural changes allowed), and escape jumps (rapid jumps toward the wall with the intention of escaping from the box). This broad range of behaviors was scored to allow for evaluating the interaction between gene expression and behavioral profiles.
Real-time quantitative reverse transcription PCR
Brains were collected under RNAase free conditions, were cooled on dry ice, and were dissected into 1-mm-thick coronal slices on a stainless steel brain mould. Tissue blocks containing the vmPFC, BLA, and CeA were identified according to the atlas of Paxinos and Watson (2007) based on clearly visible landmarks (e.g., forceps minor and piriform cortex for the vmPFC; the internal and external capsule as well as the optical tract, and the commissure of the stria terminalis for amygdala regions) (Fig. 3d). In addition to these landmarks, slight color patterns that circumscribed particular brain regions also guided punching. The vmPFC, as well as the BLA and CeA, was punched out bilaterally from the 1–mm-thick slices positioned around Bregma 3.20 and −1.80, respectively. Because of the large rostrocaudal extension of the BLA, another segment of this area was punched out from the next slice that was located at around Bregma −2.80. The micro-dissected areas were quickly moved to the RNA-stabilizing reagent RNAlater (Qiagen, Valencia, CA, USA) on dry ice. Total RNA was isolated by using Qiazol lysis reagent (Qiagen) and RNeasy Lipid Tissue Mini Kit (Qiagen) by means of QIAcube equipment (Qiagen). cDNA was synthesized from 140 ng total RNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Foster City, CA, USA). Taqman gene expression assays and PCR reagents from Roche (Roche Hungary, Budaors, Hungary) were used to quantify messenger RNA (mRNA). GluA1 (Gria1, Rn00709588_m1) and GluA2 (Gria2, Rn00568514_m1) mRNA levels were measured by quantitative RT-PCR by using an ABI StepOnePlus PCR System (Applied Biosystems, Foster City, CA). GAPDH (Rn01775763_g1) was used as reference (housekeeping) gene. The following thermocycling conditions were used: 95 °C for 10 min, 95 °C for 15 s, and 60 °C for 60 s. Forty cycles were run. All samples were prepared in duplicate. PCR amplification of GAPDH was performed for each sample to normalize mRNA levels of the target genes. Relative quantity of mRNAs was referred to corresponding samples of control Wistar rats based on the 2−ΔΔCT method.
Emotional and hormonal responses to the conditioning chamber 1 and 28 days after conditioning. The timing of testing did not affect these measures, suggesting that pharmacological responsiveness was not affected by differences in emotionality. FC fear conditioning, CORT corticosterone; number sign, significant effect of training
Hormone measurements
Blood was centrifuged at 4 °C. The plasma was separated and kept at −20 °C till analysis. Plasma corticosterone concentrations were measured in serum by radioimmunoassay. The assay was performed in duplicate. Prior to assay, corticosterone was separated from CBG at low pH (200 μl 0.1 mol citric acid; 1 h incubation at room temperature). Antiserum was raised in rabbits against corticosterone-3-carboxymethyloxime bovine serum albumin (prepared at the Institute of Experimental Medicine, Budapest), and 125I-labeled carboxymethyloxime–tyrosine–methyl ester derivative (Isotope Institute Ltd., Budapest, Hungary) was used as tracer. Final dilution of the antibody was 1:40,000. Incubation time was 24 h at 4 °C, and a second antibody (anti-rabbit from goat), for separation 6 % polyethylene glycol solution, was used. A calibration curve was prepared from corticosterone (Calbiochem) and ranged from 0.27 to 40 pmol/tube. The sensitivity of the assay was 1 pmol/ml. Intra-assay coefficient of variation was 7.5 %. All samples were measured in the same assay.
Statistics
Values were expressed as means ± SEM. Statistical analysis was performed by means of the StatSoft 12.0 software (StatSoft, Inc., Tulsa, OK, USA). Data were evaluated by two- or three-factor repeated measures ANOVA as explained below. Post hoc comparisons were made by the Tukey HSD method. The relationship between gene expression profiles and behavior was studied by multiple regression. p ≤ 0.05 was considered significant.
Results
Experiment 1: extinction protocols
The duration of immobility was dramatically reduced by extinction training both 1 day (7 × 1 protocol: F(6,54) = 15.3; p < 0.01; 3 × 5 protocol: F(14,126) = 21.3; p < 0.01) and 28 days after fear conditioning (7 × 1 protocol: F(6,54) = 24.7; p < 0.01; 3 × 5 protocol: F(14,126) = 15.3; p < 0.01) (Fig. 1a, b, respectively). The expression of fear was abolished by the end of trainings, which would have prevented the detection of pharmacologic effects if IEM-1460 facilitated conditioned fear. Therefore, a 1-day protocol was chosen for pharmacologic testing, as conditioned fear was significantly decreased but was not abolished over the first day in either protocol. As 1-day extinction seemed appropriate for the purposes of the present study, we chose to administer five extinction trials on that day, to increase the number of associations between the effect of the agent and extinction trials. Noteworthy, the effects of the 1 × 5 protocol chosen for testing carried over to the next day (Fig. 1b, see trial 6).
Experiment 2: behavioral effects of IEM-1460
In non-conditioned rats, immobility was not affected by treatments at any time point (Table 1). In conditioned rats, the CP-AMPAR antagonist IEM-1460 did not affect the expression of conditioned fear during the first test (1 day after fear conditioning: F(2,25) = 1.86; p = 0.17; 28 days after fear conditioning: F(2,24) = 3.11; p = 0.06) (Fig. 2b, e). However, treatments did affect the efficacy of extinction learning.
When IEM-1460 and extinction training were administered 1 day after conditioning, immobility depended on the interaction between pharmacologic treatment and time (i.e., the succession of trials) (F interaction(6,150) = 2.56; p < 0.01) (Fig. 2c). In controls, extinction was not evident during extinction training; however, immobility was reduced the next day (trial 6), demonstrating that the training was efficacious. Conditioned fear did not recover in this group 4 weeks later (trial 7). Differences from non-conditioned rats remained significant throughout, i.e., the employed extinction protocol decreased but did not abolish conditioned fear. No fear extinction was observed in conditioned rats treated with 1 mg/kg IEM-1460, but the 3 mg/kg dose accelerated extinction. One day after extinction learning, the decrease was still observable, but a spontaneous recovery occurred 4 weeks later (trial 7). Thus, CP-AMPAR blockade accelerated learning during extinction trials but inhibited it on the long run when training and extinction were administered 1 day after conditioning.
When IEM-1460 and extinction training were administered 28 days after fear conditioning, behavior also depended on the interaction between pharmacologic treatment and time (i.e., the successive trials) (F interaction(18,204) = 3.43; p < 0.01). Immobility declined in all groups on the extinction training day and remained low thereafter (Fig. 2f). In controls, the duration of immobility remained low in the following two trials as compared to the first extinction trial but remained larger than immobility observed in non-conditioned rats showing again that the employed protocol decreased but did not abolish conditioned fear. The 1 mg/kg dose accelerated learning and abolished conditioned fear both 1 day (trial 6) and 4 weeks (trial 7) after extinction trials. Immobility was lower than that seen in vehicle-treated, fear-conditioned rats throughout and was not different from that seen in non-conditioned rats. The 3 mg/kg dose did not accelerate learning during extinction trials but abolished conditioned fear both 1 day (trial 6) and 4 weeks later (trial 7). Albeit larger effects were observed with the lower dose, both IEM-1460-treated groups were similar to non-conditioned rats in trials 6 and 7, showing that extinction training abolished conditioned fear in these rats, and the effect lasted at least a month.
Experiment 3: emotional responses and GluA mRNA expression
The behavior of fear-conditioned rats was dominated by immobility in the conditioned fear test irrespective to the time elapsed from conditioning (Fig. 3a). In addition, grooming was virtually eliminated, exploration was markedly reduced, resting was increased, and escape jumps emerged after fear conditioning (Table 3). Fear conditioning increased the corticosterone response to the conditioning cage as compared to non-conditioned rats; this response did not depend on the timing of the test either (F conditioning(1,26) = 19.0; p < 0.01; F timing(1,26) = 0.30; p = 0.60; F interaction(1,26) = 0.01; p = 0.98) (Fig. 3b). These findings show that the differential efficacy of IEM-1460 on recent and remote fear memory was not secondary to time-dependent alterations in behavioral and stress responses.
In the vmPFC, fear conditioning increased mRNA levels (F conditioning(1,19) = 5.16; p < 0.05) independently of the specific GluA subunit (F GluA(1,19) = 0.03; p = 0.87) and of time elapsed from conditioning (F timing(1,19) = 0.20; p = 0.66) (Fig. 4a, b). No interactions were significant (F values were between 0.03 and 0.27; p values were between 0.87 and 0.61). The GluA1/GluA2 ratio was not affected in the vmPFC (Fig. 4c).
The impact of fear conditioning on GluR1 and GluR2 mRNA expression 1 day or 4 weeks after conditioning. Upper panels show data obtained in the vmPFC; the middle and lower rows show those obtained in the amygdala. a, d The localization of brain punches in which gene expression was studied (schematics based on Paxinos and Watson. 2007); b, e Subunit mRNA expression; c, f GluA1/GluA2 ratios; FC fear conditioning, vmPFC ventral medial prefrontal cortex, BLA basolateral amygdala, CeA central amygdala; horizontal lines and gray bars on graphs, mean ± SEM, respectively, of gene expression in non-conditioned controls; number sign, significant difference from non-conditioned rats; asterisk, significant within-group difference between GluA1 and GluA2 expression (p < 0.05 at least in both cases)
In the amygdala, gene expression depended on the interaction between conditioning, the specific GluA subunit, and the time elapsed from conditioning (F interaction(1,46) = 3.91; p = 0.05) (Fig. 4d, e). No main effect of amygdala sub-regions (BLA and CeA) was observed, (F area(1,46) = 0.66; p = 0.41), and no interaction was observed between these and other factors (interaction F values were between 0.01 and 0.67; p values were between 0.99 and 0.41). Pairwise comparisons showed that GluA2 expression was weaker than GluA1 expression 1 day after fear conditioning. As a consequence, the GluA1/GluA2 mRNA ratio was significantly affected by conditioning (F conditioning(1,46) = 5.92; p = 0.01). The two subregions were similar (F area(1,46) = 0.08; p = 0.77) (Fig. 4f). A trend was observed in the interaction between the time elapsed from conditioning and GluA1/GluA2 ratio (F interaction = 3.59; p = 0.06), which prompted pairwise comparisons. These indicated that the ratio was increased 1 day but not 4 weeks after fear conditioning. Taken together, these data show that fear conditioning durably increased the expression of both GluA1 and GluA2 subunit mRNAs in the vmPFC without altering their ratio. In the amygdala, no global changes in expression levels were observed, but the relative expression of the two specific GluA subunits was altered 1 day but not 4 weeks after fear conditioning.
Multiple regression analysis
The relationship between gene expression and behavior was investigated by multiple regression. As genetic changes were complex, four models were tested. The first model focused on GluA expression profiles. Immobility was not predicted significantly by the expression of GluA1 mRNA (multiple R = 0.51; F(3,19) = 2.24; p = 0.11) but was significantly predicted by GluA2 mRNA expression (multiple R = 0.58; F(3,19) = 3.29; p < 0.05). GluA2 expression explained 24 % of variation in immobility (adjusted R 2 = 0.24), and the interaction was due to GluA2 changes in the vmPFC (β = 0.55; p < 0.01) but not by similar changes in the BLA (β = −0.20; p = 0.30) or CeA (β = −0.04; p = 0.83). The second model focused on GluA1/GluA2 ratios, which did not predict immobility significantly (multiple R = 0.45; F(3,19) = 1.58; p = 0.22). In the third model, we investigated the impact of those genetic changes that were significant according to ANOVA comparisons. Particularly, the predictive power of vmPFC GluA1 and GluA2 expressions and of amygdala GluA1/GluA2 ratios was studied. These predicted the duration of immobility significantly (multiple R = 0.67; F(4,18) = 4.09; p < 0.05) and explained 36 % of variation in immobility. The interaction was due to the GluA1/GluA2 ratio in the BLA (β = 0.37; p < 0.05); the impact of vmPFC GluA2 expression was observed at trend level only (β = 0.44; p = 0.06), whereas GluA1 changes in the vmPFC and the GluA1/GluA2 ratio in the CeA had no impact on the interaction (β = 0.23; p = 0.34 and β = −0.20; p = 0.33). The fourth analysis was prompted by the results of the first and the third analyses which suggested that vmPFC GluA2 expression is more important for immobility than GluA1 expression. vmPFC GluA2 expression and the GluA1/GluA2 ratio in amygdala subdivisions predicted immobility highly significantly (multiple R = 0.69; F(3,19) = 5.16; p < 0.01), explained 45 % of variation in behavior, and was due to GluA2 expression in the vmPFC and the GluA1/GluA2 ratio in the BLA (β = 0.56; p < 0.01 and β = 0.36; p < 0.05) (Fig. 5a). The impact of GluA1/GluA2 ratio in the CeA was not significant (β = −0.13; p = 0.51). Thus, the results of multiple regression analysis suggest that immobility was associated in the vmPFC with increased expression of the subunit that confers calcium permeability to the AMPA receptor (GluA2); by contrast, it was associated with the relative decrease of this subunit in the BLA (i.e., with the increase of the GluA1/GluA2 expression ratio; Fig. 5b).
The contribution of gene expression profiles to conditioned fear. a Conditioned fear (immobility in the fear conditioning chamber) was best predicted by the prefrontal increase in GluA2 expression and the increase in GluA1/GluA2 ratios in the BLA and CeA. Partial correlations were significant for the vmPFC and BLA (see p values in brackets). b A 3D graph that illustrates the results of multiple regression analysis. vmPFC ventral medial prefrontal cortex, BLA basolateral amygdala, CeA central amygdala
Discussion
Single administration of the CP-AMPAR antagonist IEM-1460 before extinction trials facilitated extinction learning in the case of both recent and remote fear memories. However, this effect was only transient in the case of recent memories; moreover, learned fear spontaneously recovered after 4 weeks in treated rats, a phenomenon not observed in saline-treated controls. By contrast, remote conditioned fear was durably abolished by extinction training performed in the presence of CP-AMPAR blockade. Fear conditioning affected AMPA receptor subunit mRNA expression in a time-dependent manner, which may explain the differential effects of CP-AMPAR blockade on recent and remote conditioned fear. Interestingly, subunit expression explained a substantial share of variation in the expression of contextual conditioned fear and pointed towards the possibility that AMPA receptors with different subunit composition play roles in conditioned fear in the vmPFC and amygdala.
Although not tested directly so far, earlier studies suggest that the inhibition of CP-AMPARs by specific antagonists should facilitate fear extinction (Clem and Huganir 2010; Fortin et al. 2010; Mahanty and Sah 1998). The study by Clem and Huganir (2010) is especially relevant in this respect as it showed that fear extinction is associated with the removal of CP-AMPARs from synapses in the amygdala. Other findings are indirectly supportive of this assumption. For instance, Popova et al. (2014) showed that fear extinction increases GluA2 mRNA expression in all four parts of the vmPFC as well as the lateral, basolateral, and central amygdala. Our study partly supports the inference based on earlier ones, because IEM-1460 facilitated fear extinction 1 day after conditioning. Surprisingly, however, effects reversed later on, as a single treatment with the compound before the first extinction trial induced a spontaneous recovery of conditioned fear 4 weeks after extinction learning, a phenomenon that was not observed in vehicle-treated controls. The effects of the antagonist were stronger when combined with extinction training 28 days after fear conditioning. At this time point, IEM-1460 not only facilitated extinction learning but durably abolished contextual fear.
The genetic study was undertaken to get insights into the mechanisms that underlie the effects of IEM-1460 on fear extinction. We hypothesized that by revealing the AMPA receptor milieu in which the antagonist acted during extinction would provide clues on how and where it could influence glutamatergic neurotransmission. As repeatedly shown, mRNA expression has a considerable inertia, and changes—with the exception of immediate early genes—require 1–2 h to emerge and even more time to peak (Llorente et al. 2015; Ryan et al., 2013). As such, the time elapsed from conditioning (24 h) was fully sufficient to affect mRNA expression, whereas the 5 min of the conditioned fear test, after which rats were immediately sacrificed, was not. Consequently, the subunit mRNA profile observed—which is an indicator of the subunit composition of AMPA receptors (Geiger et al. 1995; Jonas et al. 1994; Studniarczyk et al. 2013)—provides clues on the AMPA receptor background which was influenced by the CP-AMPAR blocker in extinction trials.
Our findings concerning the amygdala, particularly 1 day after conditioning, suggest that in the extinction study, the CP-AMPAR blocker counteracted a change induced by fear conditioning. In CP-AMPARs, GluA2 is replaced by other subunits, usually by GluA1; as such the relative overexpression of the GluA1 subunit indirectly indicates an increase in CP-AMPARs in this area, the behavioral consequences of which where likely counteracted by IEM-1460. The finding that the GluA1/GluA2 ratio in the basolateral amygdala contributed to fear expression (immobility) lends further support to this assumption.
Subunit expression dynamics was rather different in the vmPFC, where the expression of both GluA1 and GluA2 mRNA was increased, suggesting an increase in the expression of AMPA receptors overall, irrespective to their calcium permeability. The importance of these prefrontal changes for conditioned fear is indicated by both our multiple regression analysis and a study showing that the AMPA receptor enhancer PEPA—which is not selective for subunits—had larger effects on learned fear when administered into the vmPFC than when administered into the amygdala (Zushida et al. 2007). Noteworthy, calcium-impermeable (GluA2-containing) AMPA receptors also have roles relevant to fear learning; e.g., they affect synaptic scaling, another form of neuronal plasticity and also receptor trafficking (Dalton et al. 2008; Gainey et al. 2009; Isaac et al. 2007; Kim et al. 2007). One can hypothesize that the changes induced by fear conditioning in vmPFC function were also affected by IEM-1460, despite the fact that these were not CP-AMPAR specific, e.g. the inhibition of CP-AMPARs may have indirectly enhanced the behavioral impact of glutamatergic neurotransmission mediated by other receptors (e.g., GluA2-containing AMPA or NMDA receptors), and the favorable outcome may have been explained by this indirect effect of the treatment.
It is premature to speculate on the precise role of receptor expression profiles on the differential impact of the antagonist on recent and remote fear memories. Albeit the association or non-association of AMPA receptor overexpression in the prefrontal cortex associated with changes in amygdala subunit ratio (recent and remote memory, respectively) may be a factor, glutamatergic neurotransmission in other brain areas, e.g., the hippocampus certainly, had contributions of their own. Nevertheless, our findings show that the impact of fear conditioning on AMPA receptor subunit composition changes over time and may be a reason why the effects of CP-AMPAR depended on the time elapsed from conditioning.
Although the relationship between AMPA receptor subunit dynamics and the behavioral effects of CP-AMPAR blockade needs further studies, our findings suggest that this compound has clinical implications. Novel treatment strategies for acquired anxiety such as post-traumatic stress disorder recently shifted focus from symptom relief to the disruption of dysfunctional aversive memories either by exposure therapy, pharmacotherapy, or their combination (Cain et al. 2012; Fitzgerald et al. 2014; McNally 2007; Pape and Pare 2010). Extinction training in the laboratory is considered to model human exposure therapy, and compounds that enhance extinction learning can be used to enhance the efficacy of exposure therapy in humans (Cain et al. 2012; Davis 2011; Fitzgerald et al. 2014; Herry et al. 2010). IEM-1460 appears promising in this respect, particularly for patients with chronic symptoms.
References
Antonov SM, Johnson JW (1996) Voltage-dependent interaction of open-channel blocking molecules with gating of NMDA receptors in rat cortical neurons. J Physiol 493(Pt 2):425–445
Buldakova SL, Vorobjev VS, Sharonova IN, Samoilova MV, Magazanik LG (1999) Characterization of AMPA receptor populations in rat brain cells by the use of subunit-specific open channel blocking drug, IEM-1460. Brain Res 846:52–58
Cain CK, Maynard GD, Kehne JH (2012) Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Invest Drugs 21:1323–1350
Clem RL, Huganir RL (2010) Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330:1108–1112
Dachtler J, Fox KD, Good MA (2011) Gender specific requirement of GluR1 receptors in contextual conditioning but not spatial learning. Neurobiol Learn Mem 96:461–467
Dalton GL, Wang YT, Floresco SB, Phillips AG (2008) Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 33:2416–2426
Davis M (2011) NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci 13:463–474
Feyder M, Wiedholz L, Sprengel R, Holmes A (2007) Impaired associative fear learning in mice with complete loss or haploinsufficiency of AMPA GluR1 receptors. Front Behav Neurosci 1:4
Fitzgerald PJ, Seemann JR, Maren S (2014) Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull 105:46–60
Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR (2010) Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci Off J Soc Neurosci 30:11565–11575
Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ (2004) The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304:881–883
Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG (2009) Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci Off J Soc Neurosci 29:6479–6489
Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H (1995) Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15:193–204
Gmiro VE, Serdyuk SE, Efremov OM (2008) Peripheral and central routes of administration of quaternary ammonium compound IEM-1460 are equally potent in reducing the severity of nicotine-induced seizures in mice. Bull Exp Biol Med 146:18–21
Graff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, Rei D, Bero AW, Phan TX, Wagner F, Holson E, Xu J, Sun J, Neve RL, Mach RH, Haggarty SJ, Tsai LH (2014) Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 156:261–276
Haller J, Nagy R, Toth M, Pelczer KG, Mikics E (2011) NR2B subunit-specific NMDA antagonist Ro25-6981 inhibits the expression of conditioned fear: a comparison with the NMDA antagonist MK-801 and fluoxetine. Behav Pharmacol 22:113–121
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579
Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A (2010) Neuronal circuits of fear extinction. Eur J Neurosci 31:599–612
Humeau Y, Reisel D, Johnson AW, Borchardt T, Jensen V, Gebhardt C, Bosch V, Gass P, Bannerman DM, Good MA, Hvalby O, Sprengel R, Luthi A (2007) A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. J Neurosci Off J Soc Neurosci 27:10947–10956
Isaac JT, Ashby MC, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871
Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H (1994) Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 12:1281–1289
Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S (2007) Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A 104:20955–20960
Kobylecki C, Cenci MA, Crossman AR, Ravenscroft P (2010) Calcium-permeable AMPA receptors are involved in the induction and expression of l-DOPA-induced dyskinesia in Parkinson’s disease. J Neurochem 114:499–511
Lisboa SF, Stecchini MF, Correa FM, Guimaraes FS, Resstel LB (2010) Different role of the ventral medial prefrontal cortex on modulation of innate and associative learned fear. Neuroscience 171:760–768
Llorente IL, Landucci E, Pellegrini-Giampietro DE, Fernandez-Lopez A (2015) Glutamate receptor and transporter modifications in rat organotypic hippocampal slice cultures exposed to oxygen-glucose deprivation: the contribution of cyclooxygenase-2. Neuroscience 292:118–28
Magazanik LG, Buldakova SL, Samoilova MV, Gmiro VE, Mellor IR, Usherwood PN (1997) Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J Physiol 505(Pt 3):655–663
Magazanik LG, Tikhonov DB, Bol’shakov KV, Gmiro VE, Buldakova SL, Samoilova MV (2003) Studies of the structure of glutamate receptor ion channels and the mechanisms of their blockade by organic cations. Neurosci Behav Physiol 33:237–246
Mahanty NK, Sah P (1998) Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 394:683–687
McKernan MG, Shinnick-Gallagher P (1997) Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390:607–611
McNally RJ (2007) Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev 27:750–759
Mead AN, Morris HV, Dixon CI, Rulten SL, Mayne LV, Zamanillo D, Stephens DN (2006) AMPA receptor GluR2, but not GluR1, subunit deletion impairs emotional response conditioning in mice. Behav Neurosci 120:241–248
Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K (2010) PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci 13:630–634
Mikics E, Toth M, Varju P, Gereben B, Liposits Z, Ashaber M, Halasz J, Barna I, Farkas I, Haller J (2008) Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology 33:1198–1210
Morgan MA, LeDoux JE (1995) Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109:681–688
Ota KT, Monsey MS, Wu MS, Schafe GE (2010) Synaptic plasticity and NO-cGMP-PKG signaling regulate pre- and postsynaptic alterations at rat lateral amygdala synapses following fear conditioning. PLoS One 5:e11236
Pape HC, Pare D (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90:419–463
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic, San Diego
Popova D, Agustsdottir A, Lindholm J, Mazulis U, Akamine Y, Castren E, Karpova NN (2014) Combination of fluoxetine and extinction treatments forms a unique synaptic protein profile that correlates with long-term fear reduction in adult mice. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 24:1162–1174
Samoilova MV, Buldakova SL, Vorobjev VS, Sharonova IN, Magazanik LG (1999) The open channel blocking drug, IEM-1460, reveals functionally distinct alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors in rat brain neurons. Neuroscience 94:261–268
Schlesinger F, Tammena D, Krampfl K, Bufler J (2005) Two mechanisms of action of the adamantane derivative IEM-1460 at human AMPA-type glutamate receptors. Br J Pharmacol 145:656–663
Shepherd JD (2012) Memory, plasticity and sleep—a role for calcium permeable AMPA receptors? Front Mol Neurosci 5:49
Studniarczyk D, Coombs I, Cull-Candy SG, Farrant M (2013) TARP gamma-7 selectively enhances synaptic expression of calcium-permeable AMPARs. Nat Neurosci 16:1266–1274
Walker DL, Davis M (2008) Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 213:29–42
Yamada D, Zushida K, Wada K, Sekiguchi M (2009) Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacol Official Publ Am Coll Neuropsychopharmacol 34:2574–2584
Zimmerman JM, Maren S (2010) NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci 31:1664–1670
Zushida K, Sakurai M, Wada K, Sekiguchi M (2007) Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci Off J Soc Neurosci 27:158–166
Acknowledgments
The authors are grateful to Carmen Sandi for her help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experiments were carried out in accordance with the European Communities Council Directive recommendations for the care and use of laboratory animals (2010/63/EU) and were reviewed and approved by the Animal Welfare Committee of the Institute of Experimental Medicine.
Funding
This study was supported by OTKA grant no. 101645.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Dóra Zelena and Éva Mikics contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zelena, D., Mikics, É., Balázsfi, D. et al. Enduring abolishment of remote but not recent expression of conditioned fear by the blockade of calcium-permeable AMPA receptors before extinction training. Psychopharmacology 233, 2065–2076 (2016). https://doi.org/10.1007/s00213-016-4255-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4255-4