Abstract
Rationale
The apolipoprotein E (apoE) genotype influences cognitive performance in humans depending on age and sex. While the detrimental role of the apoE4 isoform on spatial learning and memory has been well-established in humans and rodents, less is known on its impact on the executive functions.
Objectives
We aimed to evaluate the effect of apoE isoforms (apoE2, apoE3, apoE4) on visuospatial attention and inhibitory control performance in female transgenic mice, and to determine the neurochemical and neuropharmacological basis of this potential relationship.
Methods
Female mice carrying apoE2, apoE3, and apoE4 were trained in the five-choice serial reaction time task (5-CSRTT). Upon a stable performance, we manipulated the inter-trial interval and the stimulus duration to elicit impulsive responding and engage attention respectively. We further performed a pharmacological challenge by administering cholinergic and GABAergic agents. Finally, we analyzed the levels of brain amino acids and monoamines by using reversed phase high-performance liquid chromatography (HPLC).
Results
ApoE4 mice showed a deficient inhibitory control as revealed by increased perseveration and premature responding. When attention was challenged, apoE4 mice also showed a higher drop in accuracy. The adverse effect of scopolamine on the task was attenuated in apoE4 mice compared to apoE2 and apoE3. Furthermore, apoE4 mice showed less dopamine in the frontal cortex than apoE2 mice.
Conclusions
We confirmed that the apoE genotype influences attention and inhibitory control in female transgenic mice. The influence of apoE isoforms in the brain neuromodulatory system may explain the cognitive and behavioral differences attributable to the genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apolipoprotein E (apoE), the main apolipoprotein in the brain, contributes to the synaptic development, integrity, and neural plasticity in the central nervous system (CNS), where it is locally synthesized primarily by astrocytes (Hauser et al. 2011).
ApoE in humans is present in three allelic variants (ε2, ε3, ε4) which modulate cognitive functions throughout the life span (Davies et al. 2015). Among them, the ɛ4 allele is a well-established risk factor for Alzheimer's disease (AD) while apoE3 (the most frequent isoform) is regarded as the “neutral form” and apoE2 as neuroprotective against AD (Arendt 2001; Raber et al. 2004; Reitz and Mayeux 2009). However, apoE seems to modulate the cognitive function in the absence of the disease. Particularly, apoE4 has been associated with impaired attention, as well as deficits in verbal and spatial memory in healthy subjects (Berteau-Pavy et al. 2007; De Blasi et al. 2009; Greenwood et al. 2005; Kukolja et al. 2010; Marchant et al. 2010). Interestingly, several lines of evidence supported an apoE4–sex interaction in humans. In fact, apoE4 women carriers have shown more pronounced AD-like changes in neuroimaging, neuropathological, and neuropsychological measures than men (Beydoun et al. 2013; Ungar et al. 2014).
At the preclinical level, initial studies on apoE knockout mice readily suggested an implication of apoE in learning and memory (Champagne et al. 2002; Raber et al. 1998). Subsequently, transgenic lines expressing human apoE isoforms under the control of neuron-specific enolase (NSE) or the glial fibrillary acidic protein (GFAP) promoter revealed impaired spatial learning and increased anxiety in apoE4 mice relative to apoE3 and wild-type controls (Hartman et al. 2001; van Meer et al. 2007). Then, the human apoE targeted replacement (TR) mouse model was created to emulate the human condition since it allows the expression of the apoE protein in the same pattern and level as non-demented humans (Sullivan et al. 1997). Consistently with earlier studies, apoE4-TR mice showed alterations in spatial learning tasks as well as decreased locomotor activity and increased anxiety relative to apoE3 (Reverte et al. 2012; Reverte et al. 2014; Siegel et al. 2012). Notably, preclinical studies reported a decreased learning performance in female apoE4 mice relative to the male counterparts, similarly to that reported in humans (Grootendorst et al. 2005; Reverte et al. 2012; van Meer et al. 2007).
While spatial learning and memory have been extensively studied in apoE transgenic mice, other executive functions such as visuospatial attention and inhibitory control have not been systematically investigated. The aim of the present study was to characterize the differences in attention and inhibitory control between the three major isoforms for the apoE found in humans (apoE2, apoE3, apoE4). We first assessed impulsivity in the context of general attentional abilities by using the five-choice serial reaction time task (5-CSRTT) (Robbins 2002) in female apoE transgenic mice (apoE2, apoE3, and apoE4). Subsequently, we investigated the neuropharmacological basis of these effects. Based on recent evidence supporting abnormal neuronal maturation caused by the dysfunction of GABAergic interneurons in the hippocampus (Li et al. 2009) and a deficient cholinergic system (Yun et al. 2005) in apoE4 mice, we assessed the effects of a GABAergic agonist (alprazolam), a GABAergic antagonist (picrotoxin), and a cholinergic antagonist (scopolamine) in female apoE-TR mice pretrained in the 5-CSRTT. Finally, on a separate cohort of female apoE-TR mice, we further determined the levels of brain amino acids, monoamines, and their metabolites in the frontal cortex, striatum, hippocampus, and thalamus.
Material and methods
Subjects
The human apoE targeted replacement (TR) mice are generated by replacing the murine apoE gene with one of the three apoE human alleles in the C57BL/6 N mice (Sullivan et al. 1997). Adult homozygous (ε2, ε3, and ε4) apoE-TR female mice were obtained from Taconic (N = 35, Taconic Europe, Lille Skensved, Denmark). A wild-type group was not included because our goal was to determine differences in impulsivity and attentional control between the three apoE genotypes so to recapitulate the human spectrum. It is also worth noticing that several studies confirmed a very similar phenotype between apoE3 and the wild-type (WT) or an intermediate phenotype between apoE3 and apoE4 in the WT; please refer to (Bour et al. 2008; Grootendorst et al. 2005; Li et al. 2009; Levi et al. 2003). Subjects were housed in pairs in a room at controlled temperature (22 ± 2 °C) and humidity (50 ± 10 %) and under a 12-h light/dark automatic cycle (light ON at 08:00–20:00). Mice were fed with standard rodent chow (Panlab, Barcelona, Spain). During the behavioral training, mice were food-restricted to achieve the 80–85 % of their free feeding weight, while water was available ad libitum. Nine animals were removed from the experiments because of poor health or poor performance (apoE2 = 2, apoE3 = 4, apoE4 = 2). Another group of adult female apoE transgenic mice (N = 21, Taconic) was housed in groups of two to four per cage with food and water available ad libitum until killing for neurochemical analyses. Experimental procedures complied with the Animal Care and Use Committee of the Universitat Rovira i Virgili (Tarragona, Spain), the Spanish Royal Decree 53/2013 on the protection of experimental animals, and the European Communities Council Directive (86/609/EEC).
5-CSRTT
Apparatus
Mice were trained in operant chambers (24 × 20 × 15 cm) placed inside ventilated sound-attenuating cubicles (Med Associates Inc., St. Albans, VT, USA). Each chamber consisted of a curved wall containing nine round apertures equipped with infrared detectors and bright yellow led (1.7 W) at the rear. Four of the nine apertures were blocked with a metal plate, thus allowing five functioning apertures equally spaced 2.5 cm apart. A magazine was located centrally in the opposite wall, equipped with an infrared detector and connected to a liquid dipper delivering 0.01 ml of grape juice (grape juice and 15.13 % sugar, López Morenas, SL, Spain). The chambers were controlled by a PC using a Fader Control interface and Med Pc software (Med Associates Inc., St. Albans, VT, USA).
Habituation to the reinforcer (grape juice) and to the 5-CSRTT apparatus
Prior to training, the preference for the grape juice was tested in a two-bottle choice procedure (Bachmanov et al. 2001). One bottle containing water and one bottle containing grape juice were placed in the home cage. The position of the bottles was counterbalanced across mice. The water and grape juice intakes were recorded after 24 h.
Mice were also habituated to the 5-CSRTT chambers with a 20-min session in which the magazine light remained illuminated and each nose-poke in the magazine triggered the liquid dipper (available for 3 s).
5-CSRTT training
The behavioral training was carried out during the light phase. The training consisted of a 20-min daily session for 5 days a week over a period of 20 weeks. All sessions in the 5-CSRTT were conducted with the houselight of the apparatus extinguished (Humby et al. 2005).
Pretraining and training procedures were adapted from previous studies (Moreno et al. 2010; Oliver et al. 2009; Robbins 2002) (Supplementary Table S1). During the pretraining 0 stage, the five apertures remained illuminated throughout the session and a drop of grape juice was placed in each aperture to elicit exploration. A nose-poke in one of the apertures triggered the liquid dipper delivering the grape juice in the magazine, which was available until collection. Mice were trained at this stage until they performed five nose-pokes in 20 min. In pretraining 1 stage, three random apertures remained illuminated throughout the session. A response into an illuminated aperture triggered the liquid dipper delivering the grape juice in the magazine, which was available until collection. Mice were trained at this stage until they performed 20 correct responses in 20 min.
During training stages, mice learned to detect the location of a brief visual stimulus (cue light) presented in one of the five apertures in a pseudo-random order. During the acquisition of the task, the stimulus duration (SD) was progressively reduced from 30 to 1 s in ten stages. Each session consisted of 20-min or 70 discrete trials. Each trial started with the mouse nose-poking into the illuminated magazine. After an inter-trial interval (ITI) of 5 s, the stimulus was presented.
A correct response was recorded upon successful detection of the spatial location of the visual stimulus, and it was rewarded with 0.01 ml of grape juice. A failure to respond within a limited hold period of 5 s was recorded as an omission and was signaled by a 5-s time-out period during which the houselight was illuminated. Similar feedback was given on trials when mice responded in an adjacent aperture (an incorrect response), or prior to the onset of the light stimulus (a premature response). Furthermore, an additional response to an aperture occurring after a correct response but before the reward collection was recorded as a perseverative response.
Mice were trained until they showed for 5 consecutive days a stable performance: correct trials >50 %, accuracy > 80 %, and omissions < 25 %.
Behavioral challenge
The behavioral testing spanned over a period of 8 consecutive weeks and started upon stable baseline response (Robbins 2002; Sanchez-Roige et al. 2012). A total of 27 female mice were tested (apoE2 = 9, apoE3 = 9, apoE4 = 9). The mean age at the beginning of the challenge was 7.9 ± 1.6 months.
Impulsivity and attentional performance were assessed once a week, typically on Wednesday. Monday, Tuesday, Thursday, and Friday mice were trained with standard baseline parameters. The challenge to elicit impulsive responding consisted in increasing the ITI from 5 s (baseline) to 7 s (weeks 1 and 2) and 10 s (weeks 3 and 4), respectively. The attentional performance was assessed by reducing the stimulus duration from 1 s (baseline) to 0.8 s (weeks 5 and 6) and 0.5 s (weeks 7 and 8), respectively (Fig. 1).
Experimental design of the behavioral and pharmacological challenges in the 5-CSRTT. Upon training completion, once the animals showed a stable performance in the task, the inter-trial interval (ITI) was increased (7–10 s) and the stimulus duration (SD) was decreased (0.8–0.5 s) to challenge impulsivity and attention, respectively. Each parameter was manipulated once a week during 8 weeks: first and second weeks, ITI = 7 s; third and fourth weeks, ITI = 10 s; fifth and sixth weeks, SD = 0.8 s; and seventh and eighth weeks, SD = 0.5 s. After the behavioral challenge, mice were habituated to saline injections for 1 week. During the pharmacological challenge, alprazolam (APZ, 0.06 and 0.12 mg/kg), picrotoxin (PTX, 0.25 and 0.5 mg/kg), and scopolamine (SCP, 0.8 and 1.6 mg/kg) were injected twice a week before the testing session. The order of drug administration was assigned to each mouse using a Latin square design
Pharmacological challenge
All drugs were injected intraperitoneally (i.p.) according to a Latin square design. During the testing weeks, 0.9 % saline was injected i.p. on Tuesdays and Thursdays (baseline condition), while on Wednesdays and Fridays, a given drug/dose was administered 30 min (alprazolam, scopolamine) or 10 min (picrotoxin) before the session (Fig. 1). Mice were subjected to standard sessions of the 5-CSRTT with the same parameters used for the assessment of baseline responding. Mice received infusions of 0.9 % saline, the GABAergic agonist alprazolam (0.06 and 0.12 mg/kg), the GABAergic antagonist picrotoxin (0.25 and 0.5 mg/kg), and the cholinergic antagonist scopolamine (0.8 and 1.6 mg/kg). The dose selection was based on previous studies (Kulkarni and Sharma 1993; Sanchez-Roige et al. 2012; Siegel et al. 2010). Mice were habituated to the i.p. injection (0.9 % saline) daily 20 min before the training session over a period of 1 week. We further performed a pilot study to ensure that the selected doses of picrotoxin did not induce convulsion and the doses of alprazolam did not induce high sedation in mice of any genotype (data not shown).
Neurochemical analyses
A group of naïve female mice (apoE2 = 5, apoE3 = 7, apoE4 = 9; age 7 ± 2 months) were used for this study. Mice were killed by rapid decapitation and the brains were quickly removed and dissected. The frontal cortex, striatum, thalamus, and hippocampus were frozen in liquid nitrogen and stored at −80 °C before processing. Brain region samples were weighed and homogenized in 0.4 N perchloric acid with 0.1 % metabisulfite, 0.01 % EDTA, and 1 mg/ml cysteine. The homogenates were centrifuged at 15,000 rpm for 20 min at 4 °C, and supernatants were collected, filtered (Millipore filters 0.45 micron), and stored at −80 °C until biochemical analyses. The levels of glutamate (Glu), gamma-aminobutyric acid (GABA), norepinephrine (NE), dopamine (DA), serotonin (5-HT), and the metabolites dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxy-3-indolacetic acid (5-HIAA) were measured using reversed phase high-performance liquid chromatography (HPLC).
Monoamine measurements
Levels of norepinephrine (NE), dopamine (DA), serotonin (5-HT), and their metabolites dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxy-3-indolacetic acid (5-HIAA) were measured by reversed phase HPLC with amperometric detection (+0.7 V). The mobile phase, containing 0.1 M KH2PO4, 0.1 mM Na2-EDTA, and 2.1 mM 1-octane sulfonic acid, plus 15 % methanol, adjusted to pH 2.65 with 85 % H3PO4, was delivered at 1 ml/min flow rate. Monoamines were separated on a 3-μm particle size column C18 (10 cm × 0.46 cm).Tissue contents of the monoamines are given as picomoles per milligram of tissue. As indices of DA and 5-HT turnover, DOPAC/DA, HVA/DA, and 5-HIAA/5-HT ratios were calculated.
Amino acid measurements
Levels of glutamate and GABA were measured by reversed phase HPLC with fluorescence detection using excitation and emission wavelengths of 360 and 450 nm, respectively. The mobile phase consisted of two components (solution A, containing 0.05 M Na2HPO4, 28 % MeOH, adjusted to pH 5.65 with 85 % H3PO4; and solution B, MeOH/H2O 8:2 ratio) and was delivered at 0.8 ml/min. Glutamate and GABA were separated in a 5-μm particle size C18 column (10 cm × 0.4 cm). The samples were precolumn derivatized with OPA reagent and injected after a 2.5-min reaction time. A gradient was established from 100 % solution A to 100 % solution B. After washing out late eluting peaks, the mobile phase returned to initial conditions. The total gradient programmed time was 20 min.
Statistical analyses
Data were analyzed with the SPSS Statistics 17.0 software. One-way ANOVA (genotype) was used to analyze the number of sessions required at each stage of the training. Repeated-measure ANOVA (genotype) was used to analyze the performance in the 5-CSRTT during baseline, ITI, SD, and pharmacological manipulations. For the behavioral and pharmacological challenges, two measures of each variable taken in two different sessions (5-, 7-, and 10-s ITI; 1-, 0.8-, and 0.5-s SD; vehicle and each drug dose) were used as within-subjects factor and the genotype as the between-subjects factor. A post hoc Tukey test was used to follow-up significant main effects and interactions. Amino acid and monoamine levels in each brain region were analyzed by one-way ANOVA (genotype). The homogeneity of the variance was determined by the Levene’s test. Statistical significance was set at p < 0.05.
The variables considered in the analysis of the performance in the 5-CSRTT were as follows: trials completed (correct responses + incorrect responses + omissions), % accuracy (correct responses / (correct + incorrect responses) × 100), % of omissions (omissions / trials completed × 100), % of premature responses (premature responses / trials completed x 100), perseverative responses (number of responses made after a correct response and before the collection of the reward), correct latency (latency to made a correct response after the onset of the stimulus), and reward latency (latency to collect the reward after a correct response).
Results
Habituation to the reinforcer and 5-CSRTT acquisition phase
In the two-bottle choice procedure, mice of each genotype strongly preferred grape juice over water (p < 0.05, data not shown). Importantly, we did not observe differences between genotypes in water or grape juice total intake (genotype p > 0.1, data not shown). Notably, no differences between genotypes were observed on the total number of sessions required to acquire the task (p > 0.1). However, we observed an effect of the genotype at stage 5 [F(2,34) = 8.920, p < 0.01]. A post hoc analysis revealed that apoE3 mice were significantly slower at this stage relative to apoE2 and apoE4 mice (p < 0.01; Table 1).
5-CSRTT baseline performance
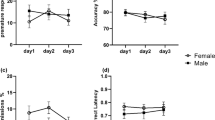
No differences between genotypes were observed in any of the behavioral variables measured, with the exception of perseverative responses [main effect of the genotype, F(2,26) = 3.542, p < 0.05]. A post hoc analysis revealed that perseverative responses were significantly higher in apoE4 than in apoE3 mice (Fig. 2 and Supplementary Table S2). The number of trials completed during baseline is provided in Supplementary Table S3. A main effect of the genotype was observed [F(2,26) = 4.099, p < 0.05]; however, the post hoc analyses failed to show significant differences between groups.
Baseline performance of apoE-TR female mice in the 5-CSRTT. a Percentage of accuracy, b percentage of omissions, c percentage of premature responding, and d number of perseverative responses, during baseline sessions. Data is expressed as mean ± S.E.M. The asterisk indicates differences between apoE4 and apoE3 at p < 0.05
Behavioral challenge
Behavioral attributes of the three genotypes during the challenge sessions on the 5-CSRTT are depicted in Figs. 3 and 4.
Inter-trial interval (ITI) challenge in the 5-CSRTT in apoE-TR female mice. a Percentage of accuracy, b percentage of omission, c percentage of premature responding, and d number of perseverative responses, concurrent with inter-trial interval increments. Data is expressed as mean ± S.E.M. The asterisk indicates differences between genotypes at p < 0.05
Stimulus duration (SD) challenge in the 5-CSRTT in apoE-TR female mice. a Percentage of accuracy, b percentage of omission, c percentage of premature responding, and d number of perseverative responses, concurrent with stimulus duration decrements. Data is expressed as mean ± S.E.M. The asterisk indicates differences between genotypes at p < 0.05
ITI
A significant increase in premature responding [F(2,26) = 26.218, p < 0.001] (Fig. 3c), perseverative responding [F(2,26) = 4.260, p < 0.05] (Fig. 3d), and omissions [F(2,26) = 4.211, p < 0.05] (Fig. 3b) was observed when the ITI was lengthened from 5 to 7 or 10 s. We also observed a main effect of the genotype on both premature [F(2,26) = 3.716, p < 0.05] and perseverative responding [F(2,26) = 3.625, p < 0.05]. A post hoc analysis revealed that apoE4 mice showed higher premature and perseverative responding relative to apoE2 and apoE3 mice (Fig. 3c, d).
SD
A significant decrease in accuracy [F(2,26) = 23.357, p < 0.001] and an increase of omissions [F(2,26) = 14.451, p < 0.001] were observed when the SD was decreased from 1 to 0.8 or 0.5 s (Fig. 4a, b). Furthermore, both response latency and collection latency were reduced [F(2,26) = 5.454, p < 0.05; F(2,26) = 4.349, p < 0.05, respectively] (data not shown). We also observed a main effect of the genotype on accuracy [F(2,26) = 4.089, p < 0.05] (Fig. 4a) and perseverative responses [F(2,26) = 3.833, p < 0.05] (Fig. 4d). A post hoc analysis revealed that apoE4 mice showed a steeper drop in accuracy (p < 0.05) and increased number of perseverative responses (p < 0.05) relative to apoE2 and apoE3 genotypes. An interaction session × genotype was also found in omissions [F(4,26) = 2.941, p < 0.05]. A post hoc analysis revealed a significant increase in apoE3 relative to apoE4 mice when the SD was 0.8 s (Fig. 4b). The maintenance of vigilance in the short SD session (0.5 s) was analyzed during ten-trial bins. A general effect of the trial period was observed on omissions, showing that mice performed more omissions by the end of the session [F(5,25) = 6.113, p < 0.01]. However, no effect of trial period or trial period × genotype interaction was observed in accuracy, which suggest that the deficit observed in apoE4 was present throughout the session (data not shown).
Pharmacological challenge
Behavioral attributes of the three genotypes during the pharmacological challenges on the 5-CSRTT are shown in Fig. 5 and Supplementary Figs. S1 and S2.
Effect of scopolamine on the 5-CSRTT performance in apoE-TR female mice. a Percentage of accuracy, b percentage of omissions, c percentage of premature responding, and d number of perseverative responses, after saline and scopolamine injections. Data is expressed as mean ± S.E.M. The asterisk indicates differences between genotypes at p < 0.05
Scopolamine
Scopolamine produced a significant decrease in accuracy, an increase in omissions, and an increase in premature responding [main effect of dose, F(2,23) = 18.686, p < 0.001; F(2,23) = 14.456, p < 0.001; F(2,23) = 10.451, p < 0.001, respectively]. A main effect of the genotype was also observed on accuracy [F(2,22) = 4.370, p < 0.05]. A post hoc analysis revealed differences in accuracy between apoE3 and apoE4 during the scopolamine challenge (p < 0.05; Fig. 5a). An interaction dose × genotype was evident in omissions and premature responding [F(4,23) = 2.837, p < 0.05; F(2,22) = 2.768, p < 0.05, respectively]. Scopolamine-induced omissions were significantly higher in apoE2 relative to apoE4 (p < 0.05), while the increase in premature responding was higher in apoE3 relative to the other genotypes (p < 0.05; Fig. 5b, c). We also observed a main effect of the dose and a dose × genotype interaction in the latency to collect the reward [F(2,23) = 21.891, p < 0.001; F(4,23) = 4.381, p < 0.01, respectively] which increased more in apoE4 than in apoE3 at the high dose of scopolamine (data not shown). The effect of the genotype previously reported in perseverative responses was not observed during the scopolamine challenge (Fig. 5d).
Alprazolam
Alprazolam decreased omissions and increased premature responding [main effect of the dose, F(2,23) = 6.364, p < 0.01; F(2,23) = 5.959, p < 0.01, respectively] (Supplementary Fig. S1b, c). An effect of the genotype on perseverative responding was also observed [F(2,23) = 4.033, p < 0.05]. Post hoc analysis showed that perseverative responding was significantly higher in apoE4 mice relative to the other genotypes (p < 0.05), as observed at baseline and during the behavioral challenge (Supplementary Fig. S1d).
Picrotoxin
Picrotoxin showed an effect on perseverative responding [dose effect, F(2,23) = 5.174, p < 0.05] which was reduced with the low dose. Although perseverative responses were higher in apoE4, we did not observe a significant main effect of the genotype (Supplementary Fig. S2d).
Neurochemical analyses
The amino acid and monoamine baseline levels of female apoE transgenic mice are shown in Tables 2 and 3. ApoE2 mice showed significant higher levels of GABA in the frontal cortex [F(2,18) = 4.819, p < 0.05] and glutamate in the striatum [F(2,17) = 4.119, p < 0.05] relative to apoE3 mice, as well as the highest levels of glutamate in the thalamus [F(2,16) = 9.151, p < 0.01]. However, no differences in the GABA/Glu ratio were observed in any brain region (Table 2).
Genotype differences in DA and DA turnover were observed in several brain regions. Levels of DA in the frontal cortex differed between apoE2 and apoE4, being lower in apoE4 mice [F(2,20) = 3.663, p < 0.05]. In the striatum, the levels of DA were higher in apoE2 mice than in apoE3 [F(2,16) = 6.683, p < 0.001] and the ratio HVA/DA was higher in apoE3 than in mice of other genotypes [F(2,16) = 13.744, p < 0.001]. In the hippocampus, the DOPAC/DA and DOPAC + HVA/DA ratios were higher in apoE2 mice [F(2,19) = 4.848, p < 0.05; F(2,19) = 4.880, p < 0.05]. The levels of NA in the striatum were lower in apoE2 than in apoE3 mice [F(2,17) = 5.875, p < 0.05] (Table 3).
Discussion
In the current study, we first characterized impulsivity in the context of visuospatial attention by using the 5-CSRTT in apoE2, apoE3, and apoE4 transgenic female mice. The main finding was that apoE4 female mice showed a deficit in inhibitory control on the 5-CSRTT as revealed by the increased premature responding during the inter-trial interval challenge. Importantly, we further observed an increased number of perseverative responding under baseline conditions considered a measure of cognitive inflexibility (Dalley et al. 2002). We second investigated the role of a GABAergic agonist (alprazolam), a GABAergic antagonist (picrotoxin), and a cholinergic antagonist (scopolamine) on the 5-CSRTT performance. The second main finding was that scopolamine-induced attentional impairment was significantly less pronounced in apoE4 than in apoE2 and apoE3 female mice. We finally performed a neurochemical analysis of naïve apoE females. We found that apoE4 female mice showed lower levels of dopamine in the frontal cortex relative to apoE2 female mice.
Attention and inhibitory control performance of apoE-TR female mice on the 5-CSRTT
The 5-CSRTT has been extensively used to determine the neural basis of visuospatial attention and inhibitory control prevalently in rats (Robbins 2002). In this study, apoE-TR mice were able to learn the 5-CSRTT, as revealed by a stable performance with minimal differences among genotypes. Notably, no differences in the acquisition of the task between apoE3- and E4-TR mice were observed in a previous study (Siegel et al. 2010). This is consistent with the idea that learning and memory impairments associated to apoE4 are limited to hippocampal-dependent tasks (Acevedo et al. 2010; De Blasi et al. 2009).
ApoE4 mice showed an impaired inhibitory control in the 5-CSRTT as revealed by increased premature and perseverative responding. This is generally considered to reflect a failure of the “executive system” represented by frontal cortical areas exerting a top-down control to limbic and paralimbic areas (Dalley et al. 2011). In rodents, lesions of the ventral hippocampus, prefrontal cortex, and disconnections of the medial prefrontal cortex from the ventral striatum increase impulsivity in the 5-CSRTT (Abela et al. 2013; Dalley et al. 2008). Based on the above, it could be speculated that alterations in the fronto-temporal network associated with the ε4 allele could account for the deficits in inhibitory control. In fact, brain imagining studies reported that human apoE4 carriers show abnormal activity in the fronto-temporal and fronto-parietal systems (Dennis et al. 2010; Filippini et al. 2009; Reiman et al. 2004). Consistently, a recent imaging study in apoE-TR mice showed a volume loss in the cortex and hippocampus associated to age in apoE4 in comparison to wild-type mice (Yin et al. 2011). Furthermore, an abnormal synaptic plasticity in the hippocampus and the amygdala of young apoE4 mice has been reported (Dumanis et al. 2013; Klein et al. 2010; Rodriguez et al. 2013).
High impulsivity is negatively correlated to attentional accuracy (Dalley et al. 2008). Likewise, apoE4 mice displayed a greater drop in accuracy when attention was challenged. Interestingly, this effect was present during the whole session, indicative of a deficit in selective attention rather than difficulty to maintain sustained attention. Similarly, in apoE-TR mice that also overexpress the human amyloid precursor protein (APP), those carrying apoE4 showed poor accuracy in a two-choice operant visual discrimination task (Kornecook et al. 2010). We observed in apoE3 mice a higher rate of omissions than in apoE4 mice when the stimulus duration was decreased. A similar finding was reported by Siegel et al. who reported a higher number of omissions in apoE3 than in apoE4 mice at baseline and after scopolamine injections in the 5-CSRTT (Siegel et al. 2010).
Comparatively, human studies have found a worse execution of apoE4 carriers in neuropsychological tests with a greater attention load (Caselli et al. 2001; Rosen et al. 2002; Wisdom et al. 2011). As far as we know, only the group of Pasuraman used a specific task to compare visuospatial attention in subjects with different apoE genotypes. They observed selective attentional deficits in apoE4 carriers with an additive effect of ε4 allele dosage, and an effect of age. While the attentional deficit was evident in middle-aged and old apoE4 individuals, it was not present in very old individuals without dementia (Greenwood et al. 2000; Greenwood et al. 2005; Negash et al. 2009). Whether the attentional and inhibitory control deficits in apoE4 individuals could be indicators of higher risk for AD is a future venue of investigation.
Effects of scopolamine, alprazolam and picrotoxin on the 5-CSRTT performance in apoE-TR female mice
The basal forebrain cholinergic system is involved in sustained attention (Paolone et al. 2013; Sarter and Paolone 2011), and the muscarinic antagonist scopolamine disrupts accuracy and increases omissions in both rats and mice in the 5-CSRTT (Sanchez-Roige et al. 2012). In the current study, apoE4 showed a lower sensitivity to scopolamine-induced attentional impairment. Specifically, the negative effect of scopolamine on attentional performance was more pronounced in apoE2 and apoE3 than in apoE4 mice.
An interaction between apoE and the cholinergic system has been suggested to underlie the cognitive deficit associated to apoE4 in humans. In fact, several indicators of a cholinergic dysfunction have been reported in apoE4, ranging from decreased neuronal activity in the basal nucleus of Meynert, which is the main source of cholinergic projections to the cortex (Salehi et al. 1998), to decreased hippocampal and cortical choline acetyltransferase activity (Allen et al. 1997; Lai et al. 2006; Poirier et al. 1995; Soininen et al. 1995a), higher levels of acetylcholinesterase (Eggers et al. 2006; Soininen et al. 1995b), and higher levels of muscarinic receptors (Cohen et al. 2003). The presence of the ε4 allele has also shown to modulate the response to cholinergic agents. Young and healthy apoE4 carriers benefit more of the cognitive effects of nicotine (Evans et al. 2013; Marchant et al. 2010), while the prolonged use of anti-cholinergic medications have a worse cognitive effect in non-demented apoE4 carriers (Nebes et al. 2012; Pomara et al. 2008; Pomara et al. 2004). On the other hand, anticholinesterase medications used to improve cognitive function in AD patients seem to be less effective in those carrying apoE4 (Braga et al. 2014; Farlow et al. 1996).
In rodents, the blockade of nicotinic acetylcholine receptors has shown to suppress hippocampal long-term potentiation in wild-type but not in apoE4-TR mice (Yun et al. 2005). Remarkably, decreased levels of choline acetyltransferase have also been reported in apoE4 mice (Buttini et al. 2002) while the exposure to the pesticide chlorpyrifos, a cholinesterase inhibitor, impaired memory in apoE3 mice but not in apoE4 mice (Peris-Sampedro et al. 2015). However, the alleged cholinergic hypo-function related to apoE4 remains controversial since some studies failed to find cholinergic alterations in both human apoE4 carriers (Corey-Bloom et al. 2000; Reid et al. 2001; Svensson et al. 1997; Uusvaara et al. 2009) and apoE4 transgenic mice (Bronfman et al. 2000; Siegel et al. 2010). Overall, our results suggest that the antagonism of muscarinic receptors has a less pronounced effect on visuospatial attention in apoE4 mice compared to apoE2 and apoE3 mice.
Higher anxiety in apoE4 mice has been previously reported (Reverte et al. 2012; Siegel et al. 2012). Here we inquired whether the administration of anxiolytic or anxiogenic drugs would induce a differential effect in apoE-TR mice depending on the genotype. However, we did not observe a genotype effect on the 5-CSRTT performance after alprazolam and picrotoxin administration. Accordingly, the effects of lorazepam on attention and reaction time were similar in human apoE4 carriers and non-carriers (Stonnington et al. 2009). The systemic administration of alprazolam improved attention by decreasing omissions, but slightly increased premature responding. On the other hand, picrotoxin decreased perseveration. Similarly, GABAergic agonists have shown to increase impulsivity in several mouse strains (Oliver et al. 2009). Increasing GABAergic activity in the PFC increases impulsivity, probably because of the disinhibition of downstream areas such as the ventral striatum (Hayes et al. 2014). Coupled with this, the reduction of GABA in the NAc increases impulsivity in low impulsive rats (Caprioli et al. 2014). However, the levels of GABA in the PFC and the striatum did not differ in apoE4 mice compared to the other genotypes.
Neurotransmitters in apoE-TR female mice
Cortico-striatal and cortico-limbic networks involved in attention and inhibitory control are modulated by dopaminergic, serotonergic, and noradrenergic neurons originating in the midbrain (Dalley et al. 2011). We observed lower levels of dopamine in the frontal cortex in apoE4 mice than in apoE2 and apoE3 mice. Consistently, the depletion of dopamine in the medial prefrontal cortex (mPFC) induces impulsive choice in a delay discounting task (Freund et al. 2014), and reduced cortical dopamine levels have been reported in patients with ADHD (Del Campo et al. 2011). Furthermore, reduced mPFC dopamine activity levels also correlate with poor attention outcome (Logue and Gould 2014). Taken these results together, the lower levels of dopamine in the frontal cortex found in apoE4 mice may account for both the decreased accuracy and increased premature responding.
Dopaminergic and noradrenergic alterations in the striatum play a key role in the expression of impulsivity (Caprioli et al. 2013; Caprioli et al. 2015; Dalley et al. 2007; Economidou et al. 2012; Moreno et al. 2013). In the present study though, we did not observe in apoE4 mice deficiencies in the levels of dopamine and norepinephrine in the striatum relative to apoE2 and apoE3, but quite the opposite. The reasons for these discrepancies are unclear and obviously require further investigation. A possible explanation for this discrepancy could derive from the fact that we analyzed the entire striatum (nucleus accumbens, shell and core, and caudate putamen) while in the previous studies the main differences were confined to the nucleus accumbens.
Glutamate and GABA are the main excitatory and inhibitory neurotransmitters in the brain. ApoE2 mice showed significant higher levels of glutamate in the striatum relative to apoE3 mice and in the thalamus relative to both apoE3 and apoE4 mice. GABA in the frontal cortex was also higher in apoE2, which would account for a trend toward an increased sensitivity of this genotype to picrotoxin. However, no differences in the ratio of GABA/glutamate were observed in any brain region, which indicates that the balance of brain excitation/inhibition was not compromised in apoE2 female mice. Higher levels of glutamate in the whole brain of apoE2 mice were reported by Dumanis et al. which might be related to the neuroprotective role attributed to the apoE2 isoform (Dumanis et al. 2013).
Concluding remarks
The results from the present study demonstrate that the human apolipoprotein E isoforms impact visuospatial attention and inhibitory control as measured in the 5-CSRTT, as well as the underlying neuromodulatory brain systems. Finally, further studies are needed to determine to what extent these results generalize to male apoE-TR and human population. The current findings have relevance because they provide valuable information on the underlying neural basis of the cognitive dysfunction related to apoE4 before the onset of neurodegenerative patterns.
Abbreviations
- 5-CSRTT:
-
five-choice serial reaction time task
- 5-HIAA:
-
5-Hydroxy-3-indolacetic acid
- AD:
-
Alzheimer's disease
- ANOVA:
-
Analysis of variance
- apoE:
-
Apolipoprotein E
- CNS:
-
Central nervous system
- DOPAC:
-
Dihydroxyphenylacetic acid
- DA:
-
Dopamine
- Glu:
-
Glutamate
- GABA:
-
Gamma-aminobutyric acid
- HPLC:
-
High-performance liquid chromatography
- HVA:
-
Homovanillic acid
- ITI:
-
Inter-trial interval
- LH:
-
Limited hold
- NE:
-
Norepinephrine
- 5-HT:
-
Serotonin
- SD:
-
Stimulus duration
- TR:
-
Targeted replacement mice
- TO:
-
Time-out
References
Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y (2013) Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb Cortex 23:1396–409
Acevedo SF, Piper BJ, Craytor MJ, Benice TS, Raber J (2010) Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatr Res 67:293–9
Allen SJ, MacGowan SH, Tyler S, Wilcock GK, Robertson AG, Holden PH, Smith SK, Dawbarn D (1997) Reduced cholinergic function in normal and Alzheimer’s disease brain is associated with apolipoprotein E4 genotype. Neurosci Lett 239:33–6
Arendt T (2001) Alzheimer’s disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience 102:723–65
Bachmanov AA, Tordoff MG, Beauchamp GK (2001) Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses 26:905–13
Berteau-Pavy F, Park B, Raber J (2007) Effects of sex and APOE epsilon4 on object recognition and spatial navigation in the elderly. Neuroscience 147:6–17
Beydoun MA, Beydoun HA, Kaufman JS, An Y, Resnick SM, O’Brien R, Ferrucci L, Zonderman AB (2013) Apolipoprotein E ε4 allele interacts with sex and cognitive status to influence all-cause and cause-specific mortality in U.S. older adults. J Am Geriatr Soc 61:525–34
Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C (2008) Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res 193(2):174–82
Braga IL, Silva PN, Furuya TK, Santos LC, Pires BC, Mazzotti DR, Bertolucci PH, Cendoroglo MS, Smith MC (2014) Effect of APOE and CHRNA7 Genotypes on the Cognitive Response to Cholinesterase Inhibitor Treatment at Different Stages of Alzheimer’s Disease. Am J Alzheimers Dis Other Demen
Bronfman FC, Tesseur I, Hofker MH, Havekens LM, Van Leuven F (2000) No evidence for cholinergic problems in apolipoprotein E knockout and apolipoprotein E4 transgenic mice. Neuroscience 97:411–8
Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L (2002) Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci 22:10539–48
Caprioli D, Hong YT, Sawiak SJ, Ferrari V, Williamson DJ, Jupp B, Adrian Carpenter T, Aigbirhio FI, Everitt BJ, Robbins TW, Fryer TD, Dalley JW (2013) Baseline-dependent effects of cocaine pre-exposure on impulsivity and D(2/3) receptor availability in the rat striatum: Possible relevance to the attention-deficit hyperactivity syndrome. Neuropsychopharmacology
Caprioli D, Sawiak SJ, Merlo E, Theobald DE, Spoelder M, Jupp B, Voon V, Carpenter TA, Everitt BJ, Robbins TW, Dalley JW (2014) Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol Psychiatry 75(2):115–23
Caprioli D, Jupp B, Hong YT, Sawiak SJ, Ferrari V, Wharton L, Williamson DJ, McNabb C, Berry D, Aigbirhio FI, Robbins TW, Fryer TD, Dalley JW (2015) Dissociable rate-dependent effects of oral methylphenidate on impulsivity and D2/3 receptor availability in the striatum. J Neurosci 35:3747–55
Caselli RJ, Osborne D, Reiman EM, Hentz JG, Barbieri CJ, Saunders AM, Hardy J, Graff-Radford NR, Hall GR, Alexander GE (2001) Preclinical cognitive decline in late middle-aged asymptomatic apolipoprotein E-e4/4 homozygotes: a replication study. J Neurol Sci 189(1–2):93–98
Champagne D, Dupuy JB, Rochford J, Poirier J (2002) Apolipoprotein E knockout mice display procedural deficits in the Morris water maze: analysis of learning strategies in three versions of the task. Neuroscience 114:641–54
Cohen RM, Podruchny TA, Bokde AL, Carson RE, Herscovitch P, Kiesewetter DO, Eckelman WC, Sunderland T (2003) Higher in vivo muscarinic-2 receptor distribution volumes in aging subjects with an apolipoprotein E-epsilon4 allele. Synapse 49:150–6
Corey-Bloom J, Tiraboschi P, Hansen LA, Alford M, Schoos B, Sabbagh MN, Masliah E, Thal LJ (2000) E4 allele dosage does not predict cholinergic activity or synapse loss in Alzheimer’s disease. Neurology 54:403–6
Dalley JW, Theobald DE, Pereira EA, Li PM, Robbins TW (2002) Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berlin) 164(3):329–40
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–70
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–60
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–94
Davies G, Armstrong N, Bis JC, Bressler J, Chouraki V, Giddaluru S, Hofer E, Ibrahim-Verbaas CA, Kirin M, Lahti J, van der Lee SJ, Le Hellard S, Liu T, Marioni RE, Oldmeadow C, Postmus I, Smith AV, Smith JA, Thalamuthu A, Thomson R, Vitart V, Wang J, Yu L, Zgaga L, Zhao W, Boxall R, Harris SE, Hill WD, Liewald DC, Luciano M, Adams H, Ames D, Amin N, Amouyel P, Assareh AA, Au R, Becker JT, Beiser A, Berr C, Bertram L, Boerwinkle E, Buckley BM, Campbell H, Corley J, De Jager PL, Dufouil C, Eriksson JG, Espeseth T, Faul JD, Ford I, Gottesman RF, Griswold ME, Gudnason V, Harris TB, Heiss G, Hofman A, Holliday EG, Huffman J, Kardia SL, Kochan N, Knopman DS, Kwok JB, Lambert JC, Lee T, Li G, Li SC, Loitfelder M, Lopez OL, Lundervold AJ, Lundqvist A, Mather KA, Mirza SS, Nyberg L, Oostra BA, Palotie A, Papenberg G, Pattie A, Petrovic K, Polasek O, Psaty BM, Redmond P, Reppermund S, Rotter JI, Schmidt H, Schuur M, Schofield PW, Scott RJ, Steen VM, Stott DJ, van Swieten JC, Taylor KD, Trollor J, Trompet S, Uitterlinden AG, Weinstein G, Widen E, Windham BG, Jukema JW, Wright AF, Wright MJ, Yang Q, Amieva H, Attia JR, Bennett DA, Brodaty H, de Craen AJ, Hayward C, Ikram MA, Lindenberger U, Nilsson LG, Porteous DJ, Räikkönen K, Reinvang I, Rudan I, Sachdev PS, Schmidt R, Schofield PR, Srikanth V, Starr JM, Turner ST, Weir DR, Wilson JF, van Duijn C, Launer L, Fitzpatrick AL, Seshadri S, Mosley TH, Deary IJ, Scotland G (2015) Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53949). Mol Psychiatry 20:183–92
De Blasi S, Montesanto A, Martino C, Dato S, De Rango F, Bruni AC, Mari V, Feraco E, Passarino G (2009) APOE polymorphism affects episodic memory among non demented elderly subjects. Exp Gerontol 44:224–7
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW (2011) The roles of dopamine and norepinephrine in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry 69:e145–57
Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R (2010) Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement 6:303–11
Dumanis SB, DiBattista AM, Miessau M, Moussa CE, Rebeck GW (2013) APOE genotype affects the pre-synaptic compartment of glutamatergic nerve terminals. J Neurochem 124:4–14
Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW (2012) Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology 37:2057–66
Eggers C, Herholz K, Kalbe E, Heiss WD (2006) Cortical acetylcholine esterase activity and ApoE4-genotype in Alzheimer disease. Neurosci Lett 408:46–50
Evans S, Dowell NG, Tabet N, Tofts PS, King SL, Gray M, Rusted JM (2013) Nicotine effects on attentional reorienting in mid-age adults, and interactions with apolipoprotein E status. J Psychopharmacol 27:1007–14
Farlow MR, Lahiri DK, Poirier J, Davignon J, Hui S (1996) Apolipoprotein E genotype and gender influence response to tacrine therapy. Ann N Y Acad Sci 802:101–10
Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009) Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 106:7209–14
Freund N, MacGillivilray HT, Thompson BS, Lukkes JL, Stanis JJ, Brenhouse HC, Andersen SL (2014) Sex-dependent changes in ADHD-like behaviors in juvenile rats following cortical dopamine depletion. Behav Brain Res 270:357–63
Greenwood PM, Sunderland T, Friz JL, Parasuraman R (2000) Genetics and visual attention: selective deficits in healthy adult carriers of the epsilon 4 allele of the apolipoprotein E gene. Proc Natl Acad Sci U S A 97(21):11661–11666
Greenwood PM, Lambert C, Sunderland T, Parasuraman R (2005) Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health’s BIOCARD study. Neuropsychology 19:199–211
Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C (2005) Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res 159:1–14
Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM (2001) Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology. Exp Neurol 170:326–44
Hauser PS, Narayanaswami V, Ryan RO (2011) Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res 50:62–74
Hayes DJ, Jupp B, Sawiak SJ, Merlo E, Caprioli D, Dalley JW (2014) Brain γ-aminobutyric acid: a neglected role in impulsivity. Eur J Neurosci 39(11):1921–32
Humby T, Wilkinson L, Dawson G (2005) Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr Protoc Neurosci Chapter 8: Unit 8.5H
Klein RC, Mace BE, Moore SD, Sullivan PM (2010) Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2. Neuroscience 171:1265–72
Kornecook TJ, McKinney AP, Ferguson MT, Dodart JC (2010) Isoform-specific effects of apolipoprotein E on cognitive performance in targeted-replacement mice overexpressing human APP. Genes Brain Behav 9:182–92
Kukolja J, Thiel CM, Eggermann T, Zerres K, Fink GR (2010) Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience 168:487–97
Kulkarni SK, Sharma K (1993) Alprazolam modifies animal behaviour on elevated plus-maze. Indian J Exp Biol 31:908–11
Lai MK, Tsang SW, Garcia-Alloza M, Minger SL, Nicoll JA, Esiri MM, Wong PT, Chen CP, Ramírez MJ, Francis PT (2006) Selective effects of the APOE epsilon4 allele on presynaptic cholinergic markers in the neocortex of Alzheimer’s disease. Neurobiol Dis 22:555–61
Levi O, Jongen-Relo AL, Feldon J, Roses AD, Michaelson DM (2003) ApoE4 impairs hippocampal plasticity isoform-specifically and blocks the environmental stimulation of synaptogenesis and memory. Neurobiol Dis 3:273–82
Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y (2009) GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 5:634–45
Logue SF, Gould TJ (2014) The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav 123:45–54
Marchant NL, King SL, Tabet N, Rusted JM (2010) Positive effects of cholinergic stimulation favor young APOE epsilon4 carriers. Neuropsychopharmacology 35:1090–6
Moreno M, Cardona D, Gómez MJ, Sánchez-Santed F, Tobeña A, Fernández-Teruel A, Campa L, Suñol C, Escarabajal MD, Torres C, Flores P (2010) Impulsivity characterization in the Roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology 35:1198–208
Moreno M, Economidou D, Mar AC, López-Granero C, Caprioli D, Theobald DE, Fernando A, Newman AH, Robbins TW, Dalley JW (2013) Divergent effects of D(2/3) receptor activation in the nucleus accumbens core and shell on impulsivity and locomotor activity in high and low impulsive rats. Psychopharmacology (Berl)
Nebes RD, Pollock BG, Perera S, Halligan EM, Saxton JA (2012) The greater sensitivity of elderly APOE ε4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. Am J Geriatr Pharmacother 10:185–92
Negash S, Greenwood PM, Sunderland T, Parasuraman R, Geda YE, Knopman DS, Boeve BF, Ivnik RJ, Petersen RC, Smith GE (2009) The influence of apolipoprotein E genotype on visuospatial attention dissipates after age 80. Neuropsychology 23(1):81–9
Oliver YP, Ripley TL, Stephens DN (2009) Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology (Berlin) 204:679–92
Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M (2013) Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci 33:8321–35
Peris-Sampedro F, Basaure P, Reverte I, Cabré M, Domingo JL, Colomina MT (2015) Chronic exposure to chlorpyrifos triggered body weight increase and memory impairment depending on human apoE polymorphisms in a targeted replacement mouse model. Physiol Behav 144:37–45
Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D, Hui S, Bertrand P, Nalbantoglu J, Gilfix BM, Gauthier S (1995) Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A 92:12260–4
Pomara N, Willoughby LM, Wesnes K, Sidtis JJ (2004) Increased anticholinergic challenge-induced memory impairment associated with the APOE-epsilon4 allele in the elderly: a controlled pilot study. Neuropsychopharmacology 29:403–9
Pomara N, Belzer K, Hernando R, De La Pena C, Sidtis JJ (2008) Increased mental slowing associated with the APOE epsilon4 allele after trihexyphenidyl oral anticholinergic challenge in healthy elderly. Am J Geriatr Psychiatry 16:116–24
Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L (1998) Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A 95:10914–9
Raber J, Huang Y, Ashford JW (2004) ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 25:641–50
Reid RT, Sabbagh MN, Thal LJ (2001) Does apolipoprotein E (Apo-E) genotype influence nicotinic receptor binding in Alzheimer’s disease. J Neural Transm 108:1043–50
Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J (2004) Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A 101:284–9
Reitz C, Mayeux R (2009) Endophenotypes in normal brain morphology and Alzheimer’s disease: a review. Neuroscience 164:174–90
Reverte I, Klein AB, Ratner C, Domingo JL, Colomina MT (2012) Behavioral phenotype and BDNF differences related to apoE isoforms and sex in young transgenic mice. Exp Neurol 237:116–25
Reverte I, Pujol A, Domingo JL, Colomina MT (2014) Thyroid hormones and fear learning but not anxiety are affected in adult apoE transgenic mice exposed postnatally to decabromodiphenyl ether (BDE-209). Physiol Behav 133:81–91
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berlin) 163:362–80
Rodriguez GA, Burns MP, Weeber EJ, Rebeck GW (2013) Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learn Mem 20:256–66
Rosen VM, Bergeson JL, Putnam K, Harwell A, Sunderland T (2002) Working memory and apolipoprotein E: what’s the connection? Neuropsychologia 40(13):226–2233
Salehi A, Dubelaar EJ, Mulder M, Swaab DF (1998) Aggravated decrease in the activity of nucleus basalis neurons in Alzheimer’s disease is apolipoprotein E-type dependent. Proc Natl Acad Sci U S A 95:11445–9
Sanchez-Roige S, Peña-Oliver Y, Stephens DN (2012) Measuring impulsivity in mice: the five-choice serial reaction time task. Psychopharmacology (Berlin) 219:253–70
Sarter M, Paolone G (2011) Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci 125:825–35
Siegel JA, Craytor MJ, Raber J (2010) Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav Pharmacol 21:602–14
Siegel JA, Haley GE, Raber J (2012) Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging 33:345–58
Soininen H, Kosunen O, Helisalmi S, Mannermaa A, Paljärvi L, Talasniemi S, Ryynänen M, Riekkinen P (1995a) A severe loss of choline acetyltransferase in the frontal cortex of Alzheimer patients carrying apolipoprotein epsilon 4 allele. Neurosci Lett 187:79–82
Soininen H, Lehtovirta M, Helisalmi S, Linnaranta K, Heinonen O, Riekkinen P (1995b) Increased acetylcholinesterase activity in the CSF of Alzheimer patients carrying apolipoprotein epsilon4 allele. Neuroreport 6:2518–20
Stonnington CM, Snyder PJ, Hentz JG, Reiman EM, Caselli RJ (2009) Double-blind crossover study of the cognitive effects of lorazepam in healthy apolipoprotein E (APOE)-epsilon4 carriers. J Clin Psychiatry 70:1379–84
Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N (1997) Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 272:17972–80
Svensson AL, Warpman U, Hellström-Lindahl E, Bogdanovic N, Lannfelt L, Nordberg A (1997) Nicotinic receptors, muscarinic receptors and choline acetyltransferase activity in the temporal cortex of Alzheimer patients with differing apolipoprotein E genotypes. Neurosci Lett 232:37–40
Ungar L, Altmann A, Greicius MD (2014) Apolipoprotein E, gender, and Alzheimer’s disease: an overlooked, but potent and promising interaction. Brain Imaging Behav 8:262–73
Uusvaara J, Pitkala KH, Tienari PJ, Kautiainen H, Tilvis RS, Strandberg TE (2009) Association between anticholinergic drugs and apolipoprotein E epsilon4 allele and poorer cognitive function in older cardiovascular patients: a cross-sectional study. J Am Geriatr Soc 57:427–31
van Meer P, Acevedo S, Raber J (2007) Impairments in spatial memory retention of GFAP-apoE4 female mice. Behav Brain Res 176:372–5
Wisdom NM, Callahan JL, Hawkins KA (2011) The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 32:63–74
Yin JX, Turner GH, Lin HJ, Coons SW, Shi J (2011) Deficits in spatial learning and memory is associated with hippocampal volume loss in aged apolipoprotein E4 mice. J Alzheimers Dis 27:89–98
Yun SH, Park KA, Sullivan P, Pasternak JF, Ladu MJ, Trommer BL (2005) Blockade of nicotinic acetylcholine receptors suppresses hippocampal long-term potentiation in wild-type but not ApoE4 targeted replacement mice. J Neurosci Res 82:771–7
Acknowledgments
The authors would like to thank Luis Heredia, PhD; Jennifer Calzado, MPh; Phoebe Soebagyo, MPh; Teresa Neri, MPh; and Raissa Alvear, MPh for their helpful collaboration. This research was supported by PSI2010-21743-C02-01, the Ministry of the Economy and Competitiveness (MINECO, Spain), and PI 13/01252 from the Instituto de Salud Carlos III (ISCIII, Spain), the European Regional Development Fund (ERDF), the Commission for Universities and Research of the Department of Innovation, Universities and Enterprise of the Generalitat de Catalunya (2009 FI-B 00256/2010 and BE-1 0072), and the European Social Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest has influenced the results presented in this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 15.7 kb)
Supplementary Table 2
(DOC 15.8 kb)
Supplementary Table 3
(DOC 14.7 kb)
Online Supplementary Figure 1
Effect of alprazolam on the 5-CSRTT performance in apoE-TR female mice. (A) Percentage of accuracy, (B) Percentage of omissions, (C) Percentage of premature responding and (D) Number of perseverative responses, after saline and alprazolam injections. Data is expressed as mean ± S.E.M. The asterisk indicates differences between genotypes at p < 0.05. (GIF 89 kb)
Online Supplementary Figure 2
Effect of picrotoxin on the 5-CSRTT performance in apoE-TR female mice. (A) Percentage of accuracy, (B) Percentage of omissions, (C) Percentage of premature responding and (D) Number of perseverative responses, after saline and picrotoxin injections. Data is expressed as mean ± S.E.M. (GIF 86.5 kb)
Rights and permissions
About this article
Cite this article
Reverte, I., Peris-Sampedro, F., Basaure, P. et al. Attentional performance, impulsivity, and related neurotransmitter systems in apoE2, apoE3, and apoE4 female transgenic mice. Psychopharmacology 233, 295–308 (2016). https://doi.org/10.1007/s00213-015-4113-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4113-9