Abstract
Rationale
The zebrafish has become an increasingly popular animal model for investigating ethanol’s actions in the brain and its effects on behavior. Acute exposure to ethanol in zebrafish has been shown to induce a dose-dependent increase of locomotor activity, to reduce fear- and anxiety-related behavioral responses, and to increase the levels of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC).
Objectives
The objective of the present study was to investigate the role of dopamine D1 receptors (D1-R) in ethanol-induced locomotor activity in zebrafish.
Methods
Zebrafish were pre-treated with SCH-23390 (0 or 1 mg/L bath concentration), a D1-R antagonist, and subsequently exposed to ethanol (0, 0.25, 0.5, 1.0 % v/v). To explore potential underlying mechanisms, we quantified levels of dopamine, DOPAC, serotonin, and 5-HIAA from whole-brain tissue using high-precision liquid chromatography.
Results
We found pre-treatment with the D1-R antagonist to attenuate locomotor activity independent of ethanol concentration. Furthermore, unlike ethanol, D1-R antagonism did not alter behavioral responses associated with fear and anxiety. Pre-treatment with SCH-23390 decreased levels of dopamine and DOPAC, but this effect was also independent of ethanol concentration. The D1-R antagonist also reduced serotonin and 5-hydroxyindole acetic acid (5-HIAA) levels.
Conclusion
These results suggest a multifaceted and at least partially independent role of dopamine D1 receptors in ethanol-induced locomotor activity and anxiety-related responses as well as in the functioning of the dopaminergic and serotoninergic neurotransmitter systems in zebrafish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A robust and quantifiable behavioral response induced by a non-sedative dose of ethanol in zebrafish is the time- and dose-dependent increase of locomotor activity often measured as the total distance the fish traveled (Echevarria et al. 2011; Rosemberg et al. 2012; Sterling et al. 2015; Tran and Gerlai 2013). Previous studies have found that acute exposure to 1 % v/v ethanol increased locomotor activity with a peak at approximately 20–30 min after the start of exposure (Rosemberg et al. 2012; Tran and Gerlai 2013). Acute exposure to ethanol has been shown to increase the levels of the monoamine neurotransmitter dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in the zebrafish brain (Chatterjee et al. 2014; Gerlai et al. 2009a, b), which was subsequently correlated with increased locomotor activity (Tran et al. 2015a). The increase in both dopamine and DOPAC has also been correlated with increased activity of tyrosine hydroxylase, the rate-limiting enzyme responsible for the synthesis of dopamine, but not monoamine oxidase, the enzyme responsible for the breakdown of dopamine into DOPAC (Chatterjee et al. 2014). Chatterjee et al. (2014) therefore concluded that the increase in dopaminergic activity induced by ethanol is likely due to elevation of synthesis and not to the breakdown of dopamine. However, the time-dependent simultaneous increase in both dopamine and DOPAC following ethanol exposure that was initially reported (Chatterjee and Gerlai 2009) suggests that 0an increasing amount of dopamine is being both synthesized and released into the synaptic cleft and subsequently broken down into DOPAC upon reuptake. In zebrafish, four different dopamine receptor subtypes (D1, D2, D3, and D4) have been identified (Li et al. 2007; Boehmler et al. 2004; 2007). Among the different types of dopaminergic receptors in the brain, the one that is most predominately expressed is the excitatory D1 receptor (D1-R) subtype (Fremeau et al. 1991). D1-R has been shown to mediate locomotor activity in mammals including rodents (Tran et al. 2005; Centonze et al. 2003). Therefore, it is possible that the increase in locomotor activity observed in zebrafish following acute ethanol exposure is due in part to activation of the dopamine D1 receptors.
Previous studies have shown that dopamine D1-like and D2-like receptors contribute to locomotor activity in zebrafish, such that antagonizing these receptor subtypes leads to changes in locomotor activity (Irons et al. 2013; Tran et al. 2015b;). Specifically, D1 receptor blockade by SCH-23990 (a D1 receptor antagonist) has been shown to induce a dose-dependent decrease in locomotor activity in adult zebrafish (Tran et al. 2015b) as well as in larvae (Irons et al. 2013). Activation of D1 receptors by SK-38393 (a D1 receptor agonist) has also been shown to increase locomotor activity in zebrafish larvae (Irons et al. 2013). These studies utilized drugs designed for mammalian dopaminergic receptors. The efficacy of these drugs demonstrated in zebrafish suggests that the zebrafish homologues of these mammalian receptors are structurally and functionally conserved across these highly different vertebrate species.
Together, these studies allow the development of a working hypothesis for how ethanol may increase locomotor activity in zebrafish. For example, the ethanol-induced increase of locomotor activity may be partially mediated by elevated synthesis and release of dopamine, which subsequently activates dopamine D1 receptors. To test this hypothesis, we investigated whether pre-treatment with a dopamine D1 receptor antagonist (SCH-23390) could attenuate the locomotor stimulant effects of ethanol in zebrafish. To examine changes within the dopaminergic system, we quantified the levels of dopamine and its metabolite DOPAC following D1 receptor blockade and ethanol exposure. In addition, due to reported effects of ethanol (Chatterjee et al. 2014; Puttonen et al. 2013) and SCH-23390 on the zebrafish serotonergic system (Scerbina et al. 2012), we also quantified the levels of serotonin and its metabolite 5-hydroxyindoleacetic acid (5-HIAA).
Methods
Animals and housing
Male and female zebrafish (8–9 months old) of the AB strain were housed in 37-L tanks prior to testing (n = 20 per tank). Animals were kept on a 13-h light-dark cycle with lights turning on at 08:00 h and off at 21:00 h. Detailed information on housing and rearing conditions are described elsewhere (Tran and Gerlai 2013).
Experimental design and testing procedure
We employed a 2 × 4 between-subject experimental design with pre-treatment (two levels—D1 antagonist or system water) and ethanol (four levels—0, 0.25, 0.5, 1.0 % v/v) as the between-subject factors with 30 fish per group. Individual zebrafish were netted from their home tanks (37-L tanks) and were pre-treated with either 1 mg/L of R(+)-SCH-23390 hydrochloride (Sigma-Aldrich, Oakville, ON, Canada) or system water (reverse osmosis water supplemented with 100 mg/L instant ocean sea salt) for 30 min in a 1.5-L tank. The dose and duration of exposure for SCH-23390 were chosen based upon a previous study (Tran et al. 2015b). Following the 30-min pre-treatment, zebrafish were subsequently challenged with 0, 0.25, 0.50, or 1.00% v/v ethanol in a 1.5-L tank for 30 min.
Video cameras were placed in front of the exposure tanks, and video recordings were taken during the 30-min acute ethanol challenge. The testing tanks were 1.5-L transparent plexi glass tanks with a white background flanking the back and sides to obscure external stimuli. Following the acute ethanol exposure, zebrafish were euthanized by decapitation and their heads stored at −80 °C. Brains were dissected and subsequently prepared for HPLC described below.
Behavioral quantification
Videos were replayed and swim path parameters were quantified using EthoVision XT 8.5, an automated video tracking software. The three behavioral parameters quantified were total distance traveled (cm), a measure of locomotor activity; variance of distance to bottom (cm2), a measure of vertical exploration; and absolute angular velocity (deg/s), a measure of erratic movement.
Quantification of neurochemicals using high-precision liquid chromatography
The levels of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin, and 5-hydroxyindoleacetic acid (5-HIAA) from zebrafish brain tissue were quantified by high-precision liquid chromatography (HPLC) using a previously established method (Chatterjee and Gerlai 2009; Gerlai et al. 2009a, b). Since we did not expect the activity of dopaminergic, or serotonergic, neurons of different brain areas to respond in an opposite manner, and since the zebrafish brain is relatively small, we quantified the levels of these neurochemicals from whole brain samples. Whole brains were dissected and sonicated in 10 μL of artificial cerebral spinal fluid containing 25 μM ascorbic acid. One microliter of the homogenate was used to determine protein concentration using BioRad protein assay reagent (BioRad, Hercules, CA, USA). One microliter of 0.5 N perchloric acid was added to the remaining homogenate and centrifuged at 10,000 rpm for 20 min. The supernatant was collected and analyzed with HPLC using a BAS 461 MICROBORE-HPLC system with electrochemical detection (Bio-analytical Systems Inc, West Lafayette, IN, USA) with a Uniget C18 reverse phase microbore column as the stationary phase (BASi, Cat. No. 8949). The mobile phase consisted of a buffer (14.5 mM sodium phosphate buffer (pH 3.4) containing 30 mM sodium citrate, 27 μM EDTA, 10 mM diethylamine hydrochloride, 2.2 mM sodium octyl sulfate), acetonitrile, and tetrahydrofuran at a ratio of 95:4:1. Dopamine hydrochloride, 3,4-dihydroxyphenylacetic acid, serotonin hydrochloride, and 5-hydroxyindolacetic acid (Sigma-Aldrich, Oakville, ON, Canada) were used as standards to identify and quantify peaks on the chromatographs.
Statistical analysis
Individual trials during which zebrafish exhibited low mobility (defined as movement of less than 0.5 cm/s over a 1-s interval) for a period of time longer than 2 standard deviations from the mean were removed as outliers. A total of 9 out of 246 video trials (i.e., less than 4 % of the trials) were removed from behavioral analysis. First, a two-way repeated measures ANOVA was performed, with the “antagonist” (SCH-23390) pre-treatment (two levels) and “ethanol concentration” (four levels) as the between-subject factors and time (30 levels) as the repeated measures factor, to examine the temporal effects of D1 receptor antagonist pre-treatment during ethanol exposure. Since Tukey post hoc multiple comparison is not appropriate for repeated measures designs, a subsequent two-way analysis of variance (ANOVA) was employed, with the “antagonist” (SCH-23390) pre-treatment (two levels) and “ethanol concentration” (four levels) as the between-subject factors, to analyze the average of behavioral responses in the last 10 min of exposure. We chose to analyze behavior during the last 10 min of the recording session because of the known time-dependent stimulatory effects of ethanol, and also because by this period, zebrafish were expected to have been able to habituate to the novel environment (Tran and Gerlai 2013; Nowicki et al. 2014). Because ANOVA is known to be underpowered to detect the significance of interaction between main factors (Wahlsten 1990), irrespective of the presence or absence of significant interaction terms, we conducted planned independent two-tailed t tests with Bonferroni correction to compare the D1-R-antagonist-treated and vehicle-treated fish exposed to the same ethanol dose.
Results
Locomotor activity
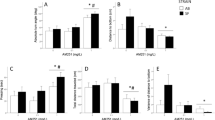
Figure 1a shows the time course of locomotor activity for fish pre-treated with system water or the D1 receptor antagonist and subsequently exposed to different concentrations of ethanol. Upon exposure to the tank, the total distance fish traveled rapidly increased (F(29, 6496) = 19.949, p < 0.001). Exposure to ethanol increased total distance traveled in a time (F(87, 6496) = 6.824, p < 0.001) and dose dependent manner (F(3, 224) = 8.733, p > 0.001). Pre-treatment with the D1 receptor antagonist decreased locomotor activity (F(1, 224) = 17.738, p < 0.001), but there was no significant antagonist × ethanol, antagonist × time, or antagonist × ethanol × time interaction (p > 0.05). Figure 1b shows that acute ethanol exposure increased total distance traveled in a dose-dependent manner in the last 10 min of exposure (F(3, 224) = 14.360, p < 0.001). Pre-treatment with the D1 receptor antagonist had a significant inhibitory effect on locomotor activity (F(1, 224) = 15.167, p < 0.001), but the ethanol concentration × antagonist interaction was not found significant by ANOVA (p > 0.05). To confirm the lack of a significant interaction, we conducted further analysis of these results. Particularly, we were interested in whether the D1-R antagonist had the same effect across the different ethanol dose groups, our null hypothesis. To answer this question, we performed planned independent two-tailed t tests (to compare D1-R antagonist treated and non-treated fish) with Bonferroni correction for multiple comparisons (one t test for each of the four ethanol concentrations). We found control and D1-R-antagonist-treated fish to not significantly differ at any ethanol dose (p > 0.05), which, together with the same ethanol dose response curves found for both the control and the D1-R-treated fish, confirms the absence of ethanol × antagonist interaction.
Mean ± SEM is shown for total distance traveled in 1-min intervals (a) and in the last 10 min (b) of a 30-min acute ethanol exposure session. Ethanol concentrations (0.00, 0.25, 0.50, 1.00 % v/v) are indicated by the legend. Note that zebrafish were exposed to the D1-R antagonist (SCH-23390 at 1 mg/L bath concentration) or to freshwater (0 mg/L bath concentration) for 30 min prior to ethanol exposure (n = 28–30 for each of the eight groups). For the results of statistical analyses, see the “Results” section
Anxiety-like behavioral measures
Figure 2a shows the time course of absolute angular velocity (a measure of erratic movement) for fish pre-treated with system water or the D1 receptor antagonist and subsequently exposed to different concentrations of ethanol. Upon being placed in the tank, zebrafish exhibited a time-dependent decrease of absolute angular velocity (F(29, 6496) = 22.289, p < 0.001). Acute exposure to ethanol decreased absolute angular velocity in a time- (F(87, 9496) = 3.700, p < 0.001) and dose-dependent manner (F(3, 224) = 4.243, p = 0.006). Overall, zebrafish that were pre-treated with the antagonist exhibited higher absolute angular velocity (F(1, 224) = 15.178, p < 0.001), but there was no significant antagonist × time, antagonist × ethanol, or antagonist × ethanol × time interaction (p > 0.05). Figure 2b shows that acute ethanol exposure decreased average absolute angular velocity in a concentration-dependent manner in the last 10 min of exposure (F(3, 224) = 9.513, p < 0.001). Zebrafish pre-treated with the D1 receptor antagonist exhibited an overall increase in absolute angular velocity (F(1, 224) = 12.094, p = 0.001). There was no significant ethanol concentration × antagonist interaction found by ANOVA (p > 0.05). Pre-treatment with the D1-R antagonist did not significantly alter the effect of ethanol at any dose on absolute angular velocity compared to freshwater pre-treated zebrafish exposed to the corresponding dose of ethanol (p > 0.05).
Mean ± SEM is shown for absolute angular velocity (speed and amount of turning) in 1-min intervals (a) and as an average of the last 10 min (b) of a 30-min acute ethanol exposure session. Ethanol concentrations (0.00, 0.25, 0.50, 1.00 % v/v) are indicated by the legend. Note that all zebrafish were exposed to either system water or 1 mg/L of SCH-23390 (the D1-R antagonist) for 30 min before ethanol exposure (n = 28–30 per group). For details of statistical findings, see the “Results” section
Figure 3a shows the time course of variance of distance to bottom (a measure of vertical exploration) for fish pre-treated with system water or the D1 receptor antagonist and subsequently exposed to different concentrations of ethanol. Upon being placed in the tank, zebrafish exhibited a time-dependent increase of variance of distance to bottom (F(29, 6496) = 13.310, p < 0.001). Acute exposure to ethanol decreased variance of distance to bottom in a time- (F(87, 6496) = 2.905, p < 0.001) and dose-dependent manner (F(3, 224) = 19.147, p < 0.001). There was no significant effect of antagonist pre-treatment, antagonist × time, or antagonist × ethanol interaction (p > 0.05). However, there was a significant ethanol × antagonist × time interaction. Figure 3b shows that acute ethanol exposure decreased average variance of distance to bottom in an inverted U-shape dose-dependent manner in the last 10 min of exposure (F(3, 224) = 21.139, p < 0.001). There was no significant main effect of the D1 receptor antagonist (p > 0.05), and the ethanol concentration × antagonist interaction was also found non-significant by ANOVA (p > 0.05). Pre-treatment with the D1-R antagonist did not significantly alter the effect of ethanol at any dose on variance of distance to bottom compared to freshwater pre-treated zebrafish exposed to the corresponding dose of ethanol (p > 0.05).
Mean ± SEM are shown for variance of distance to bottom (vertical exploration) in 1-min intervals (a) and as an average for the last 10 min (b) of a 30-min acute ethanol exposure session. Ethanol concentrations (0.00, 0.25, 0.50, 1.00 % v/v) are indicated by the legend. Note that all zebrafish were exposed to either system water or 1 mg/L of SCH-23390 (the D1-R antagonist) for 30 min before ethanol exposure (n = 28–30 per group). For details of statistical findings, see the “Results” section
Dopaminergic neurotransmitter system (HPLC analysis)
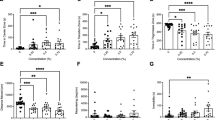
Figure 4a shows that acute ethanol exposure significantly increased the levels of dopamine in the zebrafish brain in a concentration-dependent manner (F(3, 71) = 17.812, p < 0.001), an almost perfect linear dose response. Pre-treatment with the D1 receptor antagonist significantly decreased the levels of dopamine in the brain (F(1, 71) = 110.139, p < 0.001), but there was no significant ethanol concentration × antagonist interaction found by ANOVA (p > 0.05). Subsequent planned t tests also confirmed the lack of interaction and showed that pre-treatment with the D1-R antagonist significantly reduced the levels of dopamine in the zebrafish brain as compared to zebrafish without D1-R antagonist pre-treatment, a difference that was found significant for each ethanol concentration employed (p < 0.001).
The pattern of results observed for the levels of DOPAC, a metabolite of dopamine indicative of release and breakdown of this neurotransmitter, was similar to what we found for dopamine (Fig 4b). We found acute ethanol exposure to significantly increase the levels of DOPAC in the zebrafish brain in a linear ethanol concentration-dependent manner (F(3, 71) = 8.150, p < 0.001). Pre-treatment with the D1 receptor antagonist significantly decreased the levels of DOPAC in the brain (F(1, 71) = 56.372, p < 0.001). However, there was no significant ethanol concentration × antagonist interaction revealed by ANOVA (p > 0.05). Pre-treatment with the D1-R antagonist significantly reduced the levels of DOPAC in the zebrafish brain as compared to zebrafish without D1-R antagonist pre-treatment, a difference that was found significant for each ethanol concentration employed (p < 0.028), again confirming the lack of ethanol concentration × antagonist interaction.
Serotoninergic neurotransmitter system (HPLC analysis)
The pattern of results observed for the levels of serotonin and its metabolite 5-HIAA appeared slightly different from the one obtained for dopamine and DOPAC in that, for the former two neurochemicals, the ethanol-concentration-dependent change was non-linear: The highest concentration of ethanol appeared to induce a disproportionately larger increase (Fig. 5a, b). ANOVA revealed a significant ethanol concentration effect (F(3, 71) = 14.985, p < 0.001) as well as a significant antagonist effect (F(1, 71) = 56.282, p < 0.001) on serotonin levels (Fig 5a). Although the ethanol concentration × antagonist interaction term did not reach significance (F(3, 71) = 2.591, p = 0.059), subsequent t test comparisons did demonstrate that the D1-R antagonist was more effective at higher ethanol concentrations. Pre-treatment with the D1-R antagonist was found not to significantly alter serotonin levels in fish that were not exposed to ethanol (p > 0.05), but the antagonist was found to significantly reduce the levels of serotonin in zebrafish that received acute ethanol at concentrations 0.25 % (t = 3.337, df = 18, p = 0.016) 0.50 % (t = 4.736, df = 17, p < 0.001), and 1.00 % (t = 4.343, df = 18, p < 0.001), respectively.
The results we obtained for the serotonin metabolite 5-HIAA (Fig 5b) were similar to those obtained for serotonin. ANOVA revealed a significant ethanol concentration effect (F(3, 71) = 14.365, p < 0.001) as well as a significant antagonist effect (F(1, 71) = 62.982, p < 0.001) and also detected a significant ethanol concentration × antagonist interaction (F(3, 71) = 4.156, p < 0.01). Planned t tests could not confirm this interaction (they found the D1-R antagonist induced reduction of 5-HIAA levels significant (p < 0.05) for all ethanol concentration groups). Nevertheless, Tukey’s honestly significant distance tests revealed a significant difference between the 0.00 and the 1.00 % ethanol exposed fish without D1-R antagonist pre-treatment (Fig. 5b, first set of four bars), an ethanol effect that was abolished by D1-R antagonist pre-treatment (p > 0.05) (Fig 5b, second set of four bars).
Discussion
Acute exposure to ethanol in zebrafish has been shown to affect a large number of different molecular targets including neurotransmitter systems (Puttonen et al. 2013; Tran et al. 2015a), enzymes (Rico et al. 2007; Tran et al. 2015c), and signal transduction pathways (Peng et al. 2009; Wangle et al. 2011). However, recent studies have provided evidence for a strong relationship between the ethanol-induced increase of locomotor activity observed in zebrafish and the activity of the dopaminergic system (Puttonen et al. 2013; Tran et al. 2015a). In the current study, we focused our attention on dopamine D1 receptors, the most abundantly expressed dopamine receptors in the zebrafish brain (Li et al. 2007), which have a stimulatory effect on locomotor activity when activated (Irons et al. 2013) and an inhibitory effect when blocked (Tran et al. 2015b; Irons et al. 2013). We hypothesized that ethanol’s locomotor stimulant effect may have been, at least partially, mediated by activation of dopamine D1 receptors.
Our current results do not support this hypothesis, at least not for the swim path parameters quantified. We found that acute ethanol treatment increased locomotor activity (total distance swum) of zebrafish in a time-dependent manner especially in the highest concentration (1 % ethanol) group. Although we found pre-treatment with SCH-23390, a selective D1 receptor antagonist, to apparently attenuate locomotor activity, this effect was independent of the changes induced by ethanol. We therefore conclude that the concentration- and time-dependent ethanol-induced changes in locomotor activity were mediated by neurobiological mechanisms other than D1-R-associated processes.
The result suggesting D1-R-independent ethanol-induced hyperactivity in the zebrafish is noteworthy considering the mixed evidence regarding dopamine D1 receptors in rodent studies. For example, exposure to SCH-23390 has been shown to reduce the locomotor activating effects of ethanol in mice (Shen et al. 1995; Le et al. 1997) and pre-weanling rats (Arias et al. 2010). However, others have failed to replicate these findings (Koechling et al. 1990). Interestingly, Koechling and Amit (1993) demonstrated that SCH-23390 could block ethanol-induced locomotor activation only if mice were first habituated to the testing procedure, suggesting an interaction between ethanol, stress, and dopamine D1 receptors. Unlike dopamine D1 receptors, there is more evidence implicating dopamine D2 receptors in mediating alcohol-induced locomotor activity. In mice, exposure to a D2 receptor antagonist has been shown to block alcohol-induced locomotor activation (Koechling et al. 1990; Shen et al. 1995). Whether D2 or other dopamine receptors underlie ethanol’s effects in zebrafish, will be ascertained in future studies which will characterize psychopharmacological properties of a number of mammalian drugs in zebrafish. Given the high amino acid sequence homologies found between zebrafish and mammalian proteins and given that compounds developed for mammalian systems are often found efficacious in the zebrafish too (Kalueff et al., 2014), such future physchopharmacological analyses are likely to be successful. Nevertheless, given that detailed absorption, distribution, metabolism, and excretion (ADME) data are largely absent for such drugs for the zebrafish, such analyses will require substantial pilot work.
Despite the unclear role of dopamine D1 receptors, it is notable that stimulatory doses of ethanol have been shown to increase the release of dopamine in the nucleus accumbens in mice and rats (Yim et al. 2000; Yim and Gonzales 2000; Yavich and Tiihonen 2000). Similar to mammalian studies, in the current study, we found an ethanol-concentration-dependent increase of the levels of dopamine and DOPAC in the brain of zebrafish following a 30-min acute exposure to this substance. The increased level of dopamine is consistent with previous reports demonstrating increased tyrosine hydroxylase activity induced by acute ethanol exposure (Chatterjee et al. 2014) and increased expression level of mRNA for tyrosine hydroxylase as early as 10 min after ethanol exposure (Puttonen et al. 2013). The elevated level of DOPAC suggests enhanced release of dopamine and subsequent breakdown upon reuptake following acute ethanol exposure. It is important to note that although the D1-R antagonist did reduce the amount of dopamine and DOPAC, the ethanol-concentration-dependent increases in the levels of these neurochemicals were independent of this effect. We therefore conclude that although general locomotor activity may be mediated by D1-R-related mechanisms, the effect of ethanol on the overall activity of the dopaminergic system is not modified by antagonism of D1-R, the most abundant dopamine receptor in the zebrafish brain. It is also notable that while the total distance swum showed a stepwise ethanol dose response curve, dopamine and DOPAC responses demonstrated an almost perfect linear dose response relationship. Based upon these results, we argue that non-D1-R dopaminergic mechanisms may explain ethanol’s locomotor stimulant effects, and other non-dopaminergic mechanisms may also contribute to the observed behavioral changes.
Currently, it is unclear whether the increase in dopaminergic activity observed 30 min after the start of ethanol exposure is a direct or indirect effect of acute ethanol exposure. For example, depletion of dopamine from vesicular stores following release and subsequent breakdown could induce positive feedback leading to increased dopamine synthesis (Puttonen et al. 2013). However, ethanol could also act on different molecular targets (e.g., kinases) and induce tyrosine hydroxylase phosphorylation to increase the total levels of dopamine and DOPAC (Dunkley et al. 2004; Fujisawa and Okuno 2005). Similar pathways have been identified in zebrafish. For example, the locomotor stimulant effect of ethanol in zebrafish larvae has been shown to be dependent on activation of adenylyl cyclase and extracellular-signal-regulated kinase (ERK) phosphorylation (Peng et al. 2009).
Exposure to the D1 receptor antagonist (SCH-23390) decreased levels of dopamine and DOPAC in the zebrafish brain, a finding in line with previous studies (Scerbina et al. 2012). Several potential mechanisms may be responsible for the observed decrease in dopaminergic neurochemicals. For example, competitive antagonism of D1 receptors could elevate the concentration of dopamine in the synaptic cleft leading to increased reuptake and also leakage to extrasynaptic areas. Increased availability of extra-synaptic dopamine would activate dopaminergic autoreceptors on the pre-synaptic neuron leading to inhibition of dopamine synthesis (Tissari and Lillgals 1993). Furthermore, increased reuptake of dopamine would lead to end product catecholamine inhibition of tyrosine hydroxylase via negative feedback inhibition in the pre-synaptic neuron (Dunkley et al. 2004; Fujisawa and Okuno 2005). However, post-synaptic mechanisms downstream of dopamine D1 receptors could also be responsible for the decrease of levels of dopaminergic neurochemicals (Dunkley et al. 2004). Whether the decrease in dopaminergic activity induced by D1-R antagonism is the result of mechanisms upstream and/or downstream of dopamine D1 receptors will be ascertained by future studies.
γ-Aminobutyric acid type A (GABAA) receptors are also known to be important mediators of ethanol’s effects (Davies 2003). In addition, the GABAergic and dopaminergic neurotransmitter systems interact. For example, administration of a GABAA receptor agonist in the ventral tegmental area increases dopamine levels (Kalivas et al. 1990). A number of studies suggest that ethanol may increase dopamine levels in the brain through an indirect manner, one of which could be through the GABAergic system (Cowen and Lawrence 1999), a possibility that will be examined in the future in zebrafish.
Interestingly, although D1 receptor antagonism significantly reduced both locomotor activity and the levels of neurochemicals of the dopaminergic neurotransmitter system tested here, we found almost no effect of this drug on behavioral measures associated with fear and/or anxiety. On the other hand, previous studies have shown that ethanol induces an inverted U-shape dose response with anxiolytic-like behaviors at low doses and anxiogenic-like behaviors at higher doses in zebrafish (Egan et al. 2009; Echevarria et al. 2011; Rosemberg et al. 2012; Mathur and Guo 2011; Tran and Gerlai 2013). The behavioral effects observed at high ethanol doses in our current study are unlikely to be due to sedation since we found increased locomotor activity in response to this dose. Furthermore, in the past, increased whole-body cortisol levels were found in response to this ethanol dose, which suggests elevated anxiety/stress induced (Tran et al. 2015a). Angular velocity (a measure of erratic movement) and the variability of distance to bottom (a measure of vertical exploration) have been argued to be good measures of fear and/or anxiety in zebrafish (Gerlai et al. 2009b; Tran et al. 2015a; Nowicki et al. 2014). In line with previous studies (Rosemberg et al. 2012; Tran et al. 2015a), here we report that zebrafish exposed to ethanol exhibited significant alterations in variance of distance to bottom and absolute angular velocity with both behaviors showing dose-dependent changes in response to ethanol. Notably, the employed D1-R antagonist did not alter the effect of ethanol on these behavioral responses, and the ethanol dose response curves obtained for dopamine and DOPAC (linear) were also different from the inverted U-shaped dose response curves obtained for these two behavioral measures. Considering the lack of D1-R-antagonist-induced changes on anxiety-like behavioral responses, as well as the ethanol dose response curves found for these behavioral measures versus those obtained for the dopaminergic neurochemicals, we argue that the anxiolytic and anxiogenic effects of ethanol are likely to be mediated by non-dopaminergic processes.
Another noteworthy finding of the current study is the significant ethanol-concentration-dependent increase of the levels of serotonin and 5-HIAA in the brain of zebrafish as well as the attenuation or abolishment of this concentration-dependent ethanol effect by pre-treatment with the D1-R antagonist. The increase in serotonergic activity following acute exposure to ethanol is in line with previous zebrafish (Chatterjee et al. 2014; Tran et al. 2015a) as well as rodent studies showing increases in extracellular levels of serotonin in response to this substance (Yan 1999; McBride et al. 1993). Furthermore, in response to acute SCH-23390 exposure, we found a decrease in the activity of the serotonergic system similarly to findings published previously (Scerbina et al. 2012). Notably, although SCH-23390 is a selective D1 receptor antagonist, in vitro, it has been found to exhibit significant affinity for 5-HT1C (Briggs et al. 1991) and 5-HT2C receptors (Millan et al. 2001) acting as an agonist for these serotonin receptors. However, in vivo, the concentrations required to activate these receptors are greater than 10-fold higher than those required to induce a D1-R-mediated response (Bourne 2001). Given that we may expect approximately 1/1000th of the external drug concentration of the water bath to enter the zebrafish brain (Sackerman et al. 2010), SCH-23390 at the concentration employed here is more likely to exclusively act through D1-R receptors, although we cannot completely exclude the possibility of this drug exerting direct effects on serotonergic neurotransmission via 5-HT1C and 5-HT2C receptor activation. Notably, acute exposure to 5-HT1B and 5-HT2A agonists has been shown to reduce brain serotonin synthesis in rats (Hasegawa et al. 2005; 2012). Alternatively, the D1-R antagonist treatment may have modulated serotonergic tone by inhibiting the D1 dopaminergic receptors of the postsynaptic terminal of neurons with presynaptic serotonergic terminals. Furthermore, although uncommon, dopamine receptors have been reported on presynaptic terminals of serotonergic synapses modulating serotonin release in the substantia nigra of the rat (Benkirane et al. 1987), and SCH-23390 could act through such receptors and influence serotoninergic responses in the zebrafish brain as well.
In addition to the inhibitory effects of SCH-23390 on the serotonergic system, our results also demonstrated that this D1-R antagonist significantly altered ethanol-induced serotonergic responses, i.e., we found a D1-R antagonist × ethanol concentration interaction. Ethanol-induced increases in extracellular levels of serotonin in mammals have been partially attributed to inhibition of serotonin reuptake (Daws et al. 2006; Boyce-Rustay et al. 2006). Blockade of dopamine D1 receptors in the current study may have interfered with ethanol’s inhibitory actions on serotonin reuptake. For example, exposure to SCH-23390 has been shown to attenuate drug-induced inhibition of serotonin reuptake by locomotor stimulants such as methamphetamine (Haughey et al. 2000). Characterization of the role of the serotoninergic system will require detailed psychopharmacological and ADME studies using existing and perhaps novel serotonin-receptor-specific compounds.
In conclusion, our results reveal dissociation between ethanol’s anxiety modulating and locomotor stimulant effects. They also demonstrate the role of D1-R-mediated and other dopaminergic and serotonergic mechanisms in these processes, results that delineate the complex nature of ethanol’s actions in the brain of zebrafish. These findings raise numerous questions as to the specific mechanisms underlying the dopaminergic and serotoninergic systems and their role in ethanol-induced functional changes in the brain of the zebrafish. They uncover the limitation of our currently existing knowledge but also demonstrate excellent future opportunities for and the utility of the zebrafish in this research area.
References
Arias C, Mlewski EC, Hansen C, Molina JC, Paglini MG, Spear NE (2010) Dopamine receptors modulate ethanol’s locomotor-activating effects in preweanling rats. Dev Psychobiol 52:13–23
Benkirane S, Arbilla S, Langer SZ (1987) A functional response to D1 dopamine receptor stimulation in the central nervous system: inhibition of the release of [3H]-serotonin from the rat substantia nigra. Naunyn Schmiedebergs Arch Pharmacol 335:502–507
Boehmler W, Carr T, Thisse C, Thisse B, Canfield VA, Levenson R (2007) D4 Dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behavior. Genes Brain Behav 6:155–166
Boehmler W, Obrecht-Pflumio S, Canfield V, Thisse C, Thisse B, Levenson R (2004) Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev Dyn 230:481–493
Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A (2006) Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res 30:1957–65
Bourne JA (2001) SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev 7:399–414
Briggs CA, Pollock NJ, Frail DE, Paxson CL, Rakowski RF, Kang CH, Kebabian JW (1991) Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SK 38393. Br J Pharmacol 104:1038–1044
Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P (2003) Distinct roles of D1 and D5 dopamine recepotors in motor activity and striatal synaptic plasticity. J Neurosci 23:8506–8512
Chatterjee D, Gerlai R (2009) High precision liquid chromatography analysis of dopaminergic and serotonergic responses to acute alcohol exposure in zebrafish. Behav Brain Res 200:208–213
Chatterjee D, Shams S, Gerlai R (2014) Chronic and acute alcohol administration induced neurochemical changes in the brain: comparison of distinct zebrafish populations. Amino Acids 46:921–930
Cowen MS, Lawrence MJ (1999) The role of opiod-dopamine interactions in the introduction and maintenance of ethanol consumption. Prog Neuropsychopharmacol Biol Psychiatry 23:1171–1212
Davies M (2003) The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci 28:263–274
Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A (2006) Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci 26:6431–6438
Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW (2004) Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91:1025–1043
Echevarria DJ, Toms CN, Jouandot DJ (2011) Alcohol-induced behavior change in zebrafish models. Rev Neurosci 22:85–93
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebraifsh. Behav Brain Res 205:38–44
Fremeau RT Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG (1991) Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci USA 88:3772–3776
Fujisawa H, Okuno S (2005) Regulatory mechanisms of tyrosine hydroxylase activity. Biochem Biophys Res Commun 338:271–276
Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R (2009a) Acute and chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes Brain Behav 8:586–599
Gerlai R, Fernandes Y, Pereira T (2009b) Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav Brain Res 201:318–324
Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR (2000) The effects of methamphetamine on serotonin transporter activity: role of dopamine and hypothermia. J Neurochem 75:1608–1617
Hasegawa S, Fikre-Merid M, Diksic M (2012) 5-HT2A receptor antagonist M100907 reduces serotonin synthesis: an autoradiographic study. Brain Res Bull 87:44–49
Hasegawa S, Watanabe A, Nishi K, Nguyen KQ, Diksic M (2005) Selective 5-HT1B receptor agonist reduces serotonin synthesis following acute, and not chronic, drug administration: results of an autoradiographic study. Neurochem Int 46:261–272
Irons TD, Kelly PE, Hunter DL, Macphail RC, Padilla S (2013) Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol Biochem Behav 103:792–813
Kalivas PW, Duffy P, Eberhart H (1990) Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther 253:858–866
Kalueff AV, Stewart AM, Gerlai R (2014) Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35:63–75
Koechling UM, Amit Z (1993) Effects of CA antagonist on ethanol-induced excitation in habituated and nonhabituated mice: interaction with stress factors? Pharmacol Biochem Behav 44:791–796
Koechling UM, Smith BR, Amit Z (1990) Differential effects of catecholamine antagonists on ethanol-induced excitation in mice. Psychopharmacology (Berl) 102:234–238
Le AD, Tomkins D, Higgins G, Quan B, Sellers EM (1997) Effects of 5-HT3, D1 and D2 receptor antagonists on ethanol- and cocaine-induced locomotion. Pharmacol Biochem Behav 57:325–332
Li P, Shah S, Huang L, Carr AL, Gao Y, Thisse C, Thisse B, Li L (2007) Cloning and spatial and temporal expression of the zebrafish dopamine D1 receptor. Dev Dyn 236:1339–1346
Mathur P, Guo S (2011) Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav Brain Res 219:234–239
McBride WJ, Murphy JM, Gatto GJ, Levy AD, Yoshimoto K, Lumeng L, Li TK (1993) CNS mechanisms of alcohol administration. Alcohol Alcohol Suppl 2:463–467
Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D (2001) The “selective” dopamine D1 receptor antagonist, SCH23390, is potent and high efficacy agonist at cloned humane serotonin2C receptors. Psychopharmacology (Berl) 156:58–62
Nowicki M, Tran S, Muraleetharan A, Markovic S, Gerlai R (2014) Serotonin antagonist induce anxiolytic and anxiogenic-like behavior in zebrafish in a receptor-subtype dependent manner. Pharmacol Biochem Behav 126:170–180
Peng J, Wagle M, Mueller T, Mathur P, Lockwood BL, Bretaud S, Guo S (2009) Ethanol-modulated camouflage response screen in zebrafish uncovers a novel role for cAMP and extracellular signal-regulated kinase signalling in behavioural sensitivity to ethanol. J Neurosci 29:8408–8418
Puttonen HA, Sundvik M, Rozov S, Chen YC, Panula P (2013) Acute ethanol treatment upregulatesth1, th2, and hdc in larval zebrafish in stable networks. Front Neural Circuits 7:102
Rico EP, Rosemberg DB, Dias RD, Bogo MR, Bonan CD (2007) Ethanol alters acetylcholinesterase activity and gene expression in zebrafish brain. Toxicol Lett 174:25–30
Rosemberg DB, Braga MM, Rico EP, Loss CM, Cordova SD, Mussulini BH, Blaser RE, Leite CE, Campos MM, Dias RD, Calcagnotto ME, de Oliveira DL, Souza DO (2012) Behavioral effects of taurine pretreatment in zebrafish acutely exposed to ethanol. Neuropharmacology 63:613–623
Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A, Benno RH, Gould GG (2010) Zebrafish behaviour in novel environments: effects of acute exposure to anxiolytic compounds and choice of Danio rerio line. Int J Comp Psychol 23:43–61
Scerbina T, Chatterjee D, Gerlai R (2012) Dopamine receptor antagonism disrupts social preference in zebrafish: a strain comparison study. Amino Acids 43:2059–2072
Shen EH, Crabbe JC, Phillips TJ (1995) Dopamine antagonist effects on locomotor activity in naive and ethanol-treated FAST and SLOW selected lines of mice. Psychopharmacology (Berl) 118:28–36
Sterling ME, Karatayev O, Chang GQ, Algava DB, Leibowitz SF (2015) Model of voluntary ethanol intake in zebrafish: effect on behaviour and hypothalamic orexigenic peptides. Behav Brain Res 278C:29–39
Tissari AH, Lillgals MS (1993) Reduction of dopamine synthesis inhibition by dopamine autoreceptor activation in striatal synaptosomes with in vivo reserpine administration. J Neurochem 61:231–238
Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T (2005) Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci U S A 102:2117–2122
Tran S, Chatterjee D, Gerlai R (2015a) An integrative analysis of ethanol tolerance and withdrawal in zebrafish (Daniorerio). Behav Brain Res 276:161–170
Tran S, Gerlai R (2013) Time-course of behavioural changes induced by ethanol in zebrafish (Daniorerio). Behav Brain Res 252:204–213
Tran S, Nowicki M, Chatterjee D, Gerlai R (2015b) Acute and chronic ethanol exposure differentially alters alcohol dehydrogenase and aldehyde dehydrogenase activity in the zebrafish liver. Prog Neuropsychopharmacol Biol Psychiatry 56:221–226
Tran S, Nowicki M, Muraleetharan A, Gerlai R (2015c) Differential effects of dopamine D1 and D2/3 receptor antagonism on motor responses. Psychopharmacology 232:795–806
Wahlsten D (1990) Insensitivity of the analysis of variance to heredity x environment interaction. Behav Brain Sci 13:109–61
Wangle M, Mathur P, Guo S (2011) Corticotropin-releasing factor critical for zebrafish camouflage behavior is regulated by light and sensitive to ethanol. J Neurosci 31:214–224
Yan QS (1999) Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Alcohol 19:1–7
Yavich L, Tiihonen J (2000) Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. Eur J Pharmacol 401:365–373
Yim HJ, Gonzales RA (2000) Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol 22:107–115
Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, Gonzales RA (2000) Dissociation between the time course of ethanol and extracellular concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol Clin Exp Res 24:781–788
Acknowledgments
This study was supported by an NSERC Discovery Grant (#311637) issued to RG and an NSERC CGSD issued to ST. We would like to thank Niveen Fulcher for her assistance with behavioral testing.
Ethical statement
The research reported here was reviewed and approved by the Local Animal Care Committee (LACC) of the University of Toronto Mississauga and is in accordance with the guidelines of the Canadian Council for Animal Care (CCAC).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tran, S., Nowicki, M., Muraleetharan, A. et al. Differential effects of acute administration of SCH-23390, a D1 receptor antagonist, and of ethanol on swimming activity, anxiety-related responses, and neurochemistry of zebrafish. Psychopharmacology 232, 3709–3718 (2015). https://doi.org/10.1007/s00213-015-4030-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4030-y