Abstract
Rationale
The dopaminergic system has been proposed to mediate alcohol-induced locomotor activity, yet the mechanisms underlying this behavioral response remain poorly understood.
Objectives
This study was conducted to investigate the role of dopamine D2-like receptors in mediating alcohol-induced behavioral responses.
Methods
In experiment 1, we examined the effects of high concentrations (0, 2.5, 5, 10 μM) of haloperidol on motor responses. In experiment 2, we examined the effects of low concentrations (0, 0.625, 1.25, 2.5 μM) of haloperidol on anxiety-like behavioral responses using the novel tank test. In experiment 3, we examined the effect of pre-treating zebrafish with different concentrations of haloperidol (0, 0.625, 2.5 μM) and subsequently exposing them to 0 or 1 % alcohol.
Results
In experiment 1, haloperidol induced an inverted U-shaped concentration-dependent increase in locomotor activity. In experiment 2, haloperidol (2.5 μM) reduced the absolute turn angle and freezing behavior in a new environment. In experiment 3, acute alcohol exposure significantly increased locomotor activity and decreased anxiety-like behavioral responses. Pre-treating zebrafish with the lower dose of haloperidol (0.625 μM) abolished the alcohol-induced locomotor activity, without altering anxiety-like behavioral responses. However, pre-treating with the higher dose of haloperidol (2.5 μM) abolished both alcohol-induced increase of locomotor activity and reduction of anxiety-like behavioral responses.
Conclusion
The results suggest alcohol-induced locomotor hyperactivity in zebrafish is mediated via activation of dopamine D2-like receptors, whereas anxiety-like behavioral responses may only be altered by a high haloperidol concentration, at which dose the drug may affect receptors other than D2-R.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol (ethanol, ethyl alcohol, EtOH) is one of the world’s most commonly consumed drugs despite its potential for abuse. Binge drinking is an ever-increasing problem in the world including in the USA with the lifetime prevalence rate for alcohol addiction estimated to be as high as 17 % (Haberstick et al. 2014). Despite the widespread consumption of alcohol and potentially negative health consequences associated with the development of alcohol addiction, current pharmacological treatment options are limited at best (Franck and Jayaram-Lindstrom 2013). Individual variation in how people respond to alcohol’s stimulant effects has been identified as a risk factor for the development of alcohol addiction (Holdstock et al. 2000). Since alcohol-induced locomotor activity in animals parallels the biphasic stimulant and sedative effects observed in humans (Phillips and Shen 1996), investigating the mechanisms underlying alcohol’s stimulant effect may aid in the development of compounds to treat alcohol addiction. Despite concerted efforts using multiple vertebrate models to study alcohol-induced locomotor activity, the mechanisms underlying this seemingly simple behavioral response have remained elusive (Hendler et al. 2013).
The zebrafish has rapidly become a popular animal model for studying the effects of alcohol on the vertebrate brain and behavior due to a number of practical advantages of this species. First, alcohol-induced locomotor activity is easily quantifiable with simple video tracking software (Gerlai et al. 2000; Wong et al. 2010). Second, numerous behavioral parameters have been validated for the quantification of alcohol-induced responses (Egan et al. 2009; Tran and Gerlai 2013). Finally, the method of alcohol administration is simple, controlled, and non-invasive (Dlugos and Rabin 2003; Mathur et al. 2011; Tran et al. 2015a). Since alcohol is water soluble, it can be directly mixed with the tank water at the desired concentration and the immersed zebrafish can take up the compound through its skin and gills in a non-invasive manner compared to stressful injections commonly utilized with rodents.
Zebrafish have been used to investigate the mechanisms mediating alcohol’s locomotor stimulant and anxiety-altering effects in a range of studies (Puttonen et al. 2013; Nowicki et al. 2015; Guo et al. 2015). Anxiety-like behavioral responses of zebrafish can be measured using the novel tank test (Levin et al. 2007; Egan et al. 2009). When zebrafish are placed in a novel environment, they exhibit a number of anxiety-like behavioral responses including increased bottom dwelling and freezing behavior which can be modified by anxiety-altering compounds (Bencan et al. 2009; Ibrahim et al. 2014; Gebauer et al. 2011). At low to moderate doses, acute alcohol exposure has been shown to increase locomotor activity and reduce anxiety-like behavioral responses in zebrafish (Egan et al. 2009; Gerlai et al. 2000). At higher doses, exposure to alcohol has been shown to induce locomotor sedation and to increase anxiety-like behavioral responses (Rosemberg et al. 2012; Tran et al. 2015a) similarly to how it acts in humans. Acute alcohol exposure has also been shown to alter whole-brain levels of the catecholamine neurotransmitter dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in adult (Gerlai et al. 2009; Tran et al. 2015a) and larval zebrafish (Puttonen et al. 2013; Guo et al. 2015). Tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, has recently been examined as a potential target for alcohol’s effects. Acute alcohol exposure has been found to increase both tyrosine hydroxylase gene expression (Puttonen et al. 2013) and enzymatic activity (Chatterjee et al. 2014) in the zebrafish brain. Inhibition of phosphorylated tyrosine hydroxylase (active form) has been shown to attenuate alcohol-induced increases in locomotor activity and dopamine levels in zebrafish (Nowicki et al. 2015). Together, these results suggest a crucial role for dopamine regulating alcohol-induced behavioral responses and, in particular, alcohol-induced locomotor activity. However, it is still unknown how dopamine regulates alcohol-induced behavioral responses and which receptors are activated by this neurotransmitter.
Among vertebrates, two major families of dopaminergic receptors have been identified including excitatory dopamine D1-like and inhibitory D2-like receptors (Beaulieu and Gainetdinov 2011). In zebrafish, D1-like and D2-like receptors as well as their isoforms have been identified with a high amino acid sequence homology compared to mammalian homologues (Li et al. 2007; Boehmler et al. 2004, 2007). A recent study revealed that alcohol’s locomotor stimulant and anxiety-altering effects were independent of dopamine D1-like receptor activation in zebrafish (Tran et al. 2015b). However, it is unknown whether alcohol-induced behavioral responses are dependent on dopamine D2-like receptor activation. In the current study, we first characterize the behavioral effects of exposure to haloperidol alone (a dopamine D2-like receptor antagonist) following habituation, and subsequently in a new environment, since a behavioral dose-response for this drug has not been established with adult zebrafish of the AB strain. We subsequently determine whether pre-treatment with haloperidol attenuates alcohol-induced locomotor activity and anxiolysis.
Methods and materials
Zebrafish housing and husbandry
Three hundred thirty-four experimentally naïve adult zebrafish (8–9 months old) of the AB strain were used for this study with 70 zebrafish tested in experiment 1, 84 zebrafish tested in experiment 2, and 180 zebrafish tested in experiment 3. AB zebrafish were bred at the University of Toronto Mississauga Vivarium (Mississauga, Ontario, Canada) and raised in 37-L Plexiglas tanks. The progenitors of this population originated from the ZFIN Center (Eugene, OR, USA) and have undergone many generations of breeding. Although not fully inbred, the AB strain is genetically well characterized and is commonly utilized in alcohol-related research due to a high percentage of their loci being in a homozygous form resulting in reduced genetic variance in this strain (Guo et al. 2015; Gerlai et al. 2009). The AB strain was also selected based on the known time and dose-dependent effects of alcohol on behavioral responses previously reported (Tran and Gerlai 2013). Zebrafish were kept on a 14:10-h light/dark cycle with lights turning on at 8 a.m. and off at 10 p.m. Details regarding zebrafish husbandry and housing are described elsewhere (Tran et al. 2015a).
Experiment 1: effects of haloperidol on motor responses
Since the dopaminergic system is known to play roles in motor function and since a dose-response analysis for adult AB zebrafish has not been conducted, in experiment 1, we examined the potential motor-altering effects of high doses of haloperidol (Sigma-Aldrich, Cat# H1512), a dopamine D2-like receptor antagonist. The goal of experiment 1 was to identify doses of haloperidol that can be used on zebrafish of the AB strain without altering baseline motor responses. Zebrafish were exposed to different concentrations of haloperidol (0, 2.5, 5, 10 μM) in home tank water for 90 min in a 1.5-L trapezoidal tank (n = 16–18 per group). The dose and duration of exposure to haloperidol were based on the known time-dependent effects demonstrated in zebrafish larvae (Irons et al. 2013) and the doses commonly administered to adult zebrafish (Magno et al. 2015; Seibt et al. 2010, 2011; Savio et al. 2012). Furthermore, our preliminary data revealed an upper concentration limit with a 90-min exposure to 20 μM haloperidol inducing death in zebrafish of the AB strain. Since the solubility of haloperidol is very low in water, we used dimethyl sulfoxide (DMSO) as a vehicle to dissolve the drug. The final concentration of DMSO in all testing tanks was 0.2 % v/v, which was previously shown not to alter baseline behavioral responses in AB zebrafish (Tran et al. 2015c). Following the preparation of drug exposure tanks, individual zebrafish were netted from their 37-L housing tanks and were individually placed in the 1.5-L trapezoidal drug exposure tank for 90 min. Video cameras were placed in front of individual testing tanks, and the 90-min exposure session was recorded.
Experiment 2: effects of haloperidol on anxiety-like behavioral responses
The purpose of experiment 2 was twofold: (1) to characterize the effects of low doses of haloperidol on anxiety-like behavioral responses using the novel tank test and (2) to identify doses of haloperidol that do not alter behavioral responses in a new environment. Zebrafish were netted from their home tank and individually placed in a 1.5-L trapezoidal tank containing low doses of haloperidol (0, 0.625, 1.25, 2.5 μM) for 30 min (n = 21 per group). Zebrafish were subsequently netted from their haloperidol exposure tank and placed in another 1.5-L trapezoidal tank containing the same concentration of haloperidol for another 30 min. The netting process has been shown to induce a stress response in zebrafish (Tran et al. 2014), which may alter drug-induced behavioral responses in a new environment. For example, when zebrafish are treated with pharmacological compounds in one tank and subsequently netted and placed in a new environment, a number of context-dependent behavioral responses are observed (Quadros et al. 2016; Tran et al. 2016). Therefore, behavioral responses were recorded in the new environment of the second haloperidol exposure tank. Similar to the first experiment, the concentration of DMSO in all the tanks was 0.2 % v/v.
Experiment 3: effects of haloperidol and alcohol exposure
The purpose of experiment 3 was to determine whether alcohol’s locomotor stimulant and anxiolytic effects were mediated via dopamine D2-like receptor activation. To accomplish this, zebrafish were netted from their 37-L home tanks and individually pre-treated with 0, 0.625, or 2.5 μM haloperidol in home tank water for 30 min in a 1.5-L trapezoidal tank. Pre-treated zebrafish were subsequently exposed to 0 or 1 % v/v alcohol in home tank water for 30 min in an identical 1.5-L trapezoidal tank. Thus, we employed a 3 × 2 experimental design with haloperidol pre-treatment (0, 0.625, or 2.5 μM) and alcohol exposure (0 or 1 % v/v) as the between-subjects factor (n = 29–31 per group). Based on the results of experiment 2, we chose to use 0.625 and 2.5 μM as the concentrations of haloperidol for experiment 3. To allow continuous antagonism of dopamine D2-like receptors during alcohol exposure, zebrafish that were pre-treated with 0.625 or 2.5 μM haloperidol were also exposed to the same concentration of haloperidol during the subsequent exposure to 0 or 1 % v/v alcohol. The dose and duration of alcohol exposure correspond to the peak effect of alcohol on locomotor activity and observed reduction in anxiety-like behavioral responses in the AB strain (Tran and Gerlai 2013), and the dose of haloperidol employed is the maximum concentration that, when the drug is administered alone, we have found not to induce behavioral alterations. Similar to experiment 1, the final concentration of DMSO in all the exposure tanks was 0.2 % v/v. Video cameras were set up in front of the alcohol exposure tanks, and the 30-min exposure session was recorded.
Behavioral quantification
Videos were replayed and behavioral responses were quantified using EthoVision XT 8.5 (Noldus Information Technology, Wageningen, The Netherlands), an automated video tracking software. In experiment 1, behavioral responses in the last 10 min of the 90-min exposure to haloperidol were quantified due to the known slow-acting effect of haloperidol exposure demonstrated in zebrafish larvae. For example, Irons et al. (2013) reported haloperidol’s peak effect in zebrafish to occur at 200 min post-exposure. In experiment 2, behavioral responses in the last 10 min of the second haloperidol exposure tank (new environment) were quantified to examine haloperidol’s context-dependent behavioral effects. In experiment 3, behavioral responses in the last 10 min of the 30-min alcohol exposure were quantified since alcohol’s locomotor stimulant and anxiolytic effects have been shown to be at their peak during this time interval (Tran and Gerlai 2013). Furthermore, brain alcohol concentrations approach equilibrium at approximately 30 min post-exposure in zebrafish (Tran et al. 2015a). Although we did not conduct habituation trials prior to treating zebrafish with haloperidol, we quantified behavioral responses in the last 10 min of each experiment, a period of time for which behavioral responses have been shown to habituate in zebrafish (Nowicki et al. 2014). The behavioral responses quantified by EthoVision XT 8.5 include total distance traveled (a measure of locomotor activity), the absolute turn angle (a measure of erratic movement), distance to bottom (a measure of bottom dwelling), freezing duration (a measure of immobility associated with anxiety), the number of freezing bouts (a measure of anxiety), and variance of distance to bottom (a measure of vertical exploration).
Statistical analysis
The data were analyzed using IBM SPSS Statistics 21 for Windows. In experiments 1 and 2, the effect of haloperidol exposure was analyzed using a one-way analysis of variance (ANOVA) with “haloperidol” as the between-subjects factor. In the case of a significant main effect, the different groups were compared using Tukey’s honest significant difference (HSD) multiple-comparison tests with significance reported at p ≤ 0.05. In experiment 3, the effect of haloperidol pre-treatment on alcohol exposure was analyzed using a two-way ANOVA with “haloperidol” and “alcohol” as the between-subjects factors. In the case of a significant haloperidol × alcohol interaction, Tukey HSD tests were used to compare all six groups with significance reported at p ≤ 0.05.
Results
Experiment 1: effect of dopamine D2-like receptor antagonism on motor responses
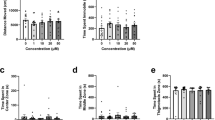
In the first experiment, we quantified behavioral responses to haloperidol following habituation to the test environment. Figure 1a shows that exposure to haloperidol increased the total distance zebrafish swam in an inverted U-shape dose-dependent manner (F(3, 65) = 5.133, p = 0.003). Tukey post hoc HSD confirmed that zebrafish exposed to 5 μM haloperidol swam a significantly larger distance compared to controls (p = 0.002). Zebrafish exposed to 5 μM haloperidol also appeared to swim a larger distance compared to fish exposed to 2.5 μM haloperidol, but this effect did not reach significance (p = 0.054). However, unlike total distance traveled, the exposure to haloperidol did not significantly (p > 0.05) alter the absolute turn angle (erratic movement; Fig. 1b), distance to bottom (bottom dwelling; Fig. 1c), variance of distance to bottom (vertical exploration; Fig. 1d), time spent freezing (immobility; Fig. 1e), and frequency of freezing (Fig. 1f).
Mean ± S.E.M. a Total distance traveled, b absolute turn angle, c distance to bottom, d variance of distance to bottom, e freezing duration, and f number of freezing bouts of zebrafish in the last 10 min of a 90-min exposure to different concentrations of haloperidol (0, 2.5, 5, 10 μM) are shown. Letters that differ from each other represent significance at p ≤ 0.05
Experiment 2: effect of haloperidol on behavioral responses in a new environment
In the second experiment, we quantified behavioral responses to haloperidol in a new environment. Haloperidol treatment did not significantly (p > 0.05) alter total distance traveled (Fig. 2a), distance to bottom (Fig. 2c), or variance of distance to bottom (Fig. 2d) of zebrafish in a new environment. However, haloperidol increased the absolute turn angle (F(3, 80) = 2.916, p = 0.039; Fig. 2b), decreased the time spent freezing (F(3, 80) = 2.872, p = 0.042; Fig. 2e), and decreased the number of freezing bouts (F(3, 80) = 4.150, p = 0.09; Fig. 2f) in a new environment. Tukey post hoc HSD confirmed that zebrafish exposed to the highest dose of haloperidol (2.5 μM) exhibited a lower absolute turn angle and froze less often and for a shorter duration of time compared to controls (p ≤ 0.046).
Mean ± S.E.M. a Total distance traveled, b absolute turn angle, c distance to bottom, d variance of distance to bottom, e freezing duration, and f number of freezing bouts of zebrafish in the last 10 min of a 30-min exposure to different concentrations of haloperidol (0, 0.625, 1.25, 2.5 μM) in a new environment are shown. Note that zebrafish were first pre-treated with the same concentration of haloperidol for 30 min prior to the new environment. Letters that differ from each other represent significance at p ≤ 0.05
Experiment 3: dopamine D2-like receptor antagonism attenuates alcohol-induced locomotor activity
Figure 3a shows that zebrafish exposed to alcohol exhibited a significant increase in their total distance traveled (F(1, 174) = 18.462, p < 0.001). ANOVA found a significant main effect of haloperidol (F(2, 174) = 5.539, p = 0.005), as well as a haloperidol × alcohol interaction (F(2, 174) = 5.648, p = 0.004). Tukey post hoc HSD confirmed that home tank water-pre-treated zebrafish exposed to alcohol swam a significantly larger distance compared to home tank water-pre-treated controls (p < 0.001). However, alcohol exposure had no significant effect on the locomotor activity of zebrafish pre-treated with haloperidol (0.625 or 2.5 μM), i.e., haloperidol-treated groups did not differ from any groups (p > 0.05).
Mean ± S.E.M. b Absolute turn angle, c distance to bottom, d variance of distance to bottom, e freezing duration, and f number of freezing bouts of zebrafish in the last 10 min of a 30-min exposure to 0 % (white bars) or 1 % (black bars) v/v ethanol are shown. Note that zebrafish were first pre-treated with 0 or 2.5 μM haloperidol for 30 min prior to alcohol exposure. Letters that differ from each other represent significance at p ≤ 0.05
Experiment 3: dopamine D2-like receptor antagonism alters alcohol-induced anxiolysis
The video tracking-quantified absolute turn angle has been shown to correlate well with observation-based quantified values of erratic movement in zebrafish, with an elevated absolute turn angle reflecting increased anxiety (Nowicki et al. 2014; Tran et al. 2015a). Figure 3b shows the effects of haloperidol and alcohol treatment on the absolute turn angle. Although ANOVA found no significant (p > 0.05) main effect of haloperidol or alcohol, it revealed a significant haloperidol × alcohol interaction (F(2, 174) = 12.670, p < 0.001). Tukey post hoc HSD confirmed that home tank water-pre-treated alcohol-exposed (p = 0.02) and haloperidol (2.5 μM)-pre-treated (p = 0.009) fish exhibited significantly lower absolute turn angles compared to home tank water-pre-treated controls. However, alcohol exposure did not significantly alter the absolute turn angle of zebrafish pre-treated with haloperidol (0.625 or 2.5 μM) compared to all other groups (p > 0.05). Notably, zebrafish pre-treated with the highest dose of haloperidol (2.5 μM) exhibited a significant increase in the absolute turn angle when exposed to alcohol (p = 0.029).
Distance to bottom has been utilized as a measure of anxiety as under aversive conditions zebrafish have been found to swim closer to the bottom of the tank (Egan et al. 2009; Bencan et al. 2009). Figure 3c shows the effects of haloperidol and alcohol treatment on distance to bottom. ANOVA revealed no significant (p > 0.05) haloperidol or alcohol main effects, but found the haloperidol × alcohol interaction to be significant (F(2, 174) = 4.518, p = 0.012). Tukey post hoc HSD confirmed that home tank water-pre-treated zebrafish exposed to alcohol swam further away from the bottom of the tank compared to home tank water-pre-treated controls (p = 0.016). Haloperidol treatment (0.625 or 2.5 μM) abolished this alcohol effect. The haloperidol-treated groups did not significantly differ from each other and from any other groups (p > 0.05).
Increased freezing is a sign of anxiety in zebrafish (Blaser and Penalosa 2011). Figure 3e shows the effects of haloperidol and alcohol treatment on freezing. ANOVA found no significant (p > 0.05) main effect of haloperidol or alcohol, but detected a significant haloperidol × alcohol interaction (F(2, 174) = 9.078, p < 0.001). Tukey post hoc confirmed that home tank water-pre-treated zebrafish exposed to alcohol (p = 0.039) or zebrafish pre-treated with 2.5 μM haloperidol (p = 0.045) exhibited a significant reduction in freezing behavior compared to home tank water-pre-treated controls. However, alcohol exposure did not significantly alter freezing behavior in haloperidol (0.625 or 2.5 μM)-pre-treated zebrafish compared to system water-pre-treated controls (p > 0.05). Notably, zebrafish pre-treated with the highest dose of haloperidol (2.5 μM) spent more time freezing when exposed to alcohol (p = 0.046).
In addition to the duration of freezing, we also quantified the number of times zebrafish froze. Figure 3f shows the effects of haloperidol and alcohol treatment on the number of freezing bouts. ANOVA found no significant (p > 0.05) main effect of haloperidol or alcohol, but detected a significant haloperidol × alcohol interaction (F(2, 174) = 10.125, p < 0.001). Tukey post hoc confirmed that home tank water-pre-treated zebrafish exposed to alcohol (p = 0.032) froze less often compared to home tank water-pre-treated controls. However, alcohol exposure did not significant alter the number of freezing bouts in haloperidol (0.625 or 2.5 μM)-pre-treated zebrafish compared to system water-pre-treated controls (p > 0.05). Notably, zebrafish pre-treated with the highest dose of haloperidol (2.5 μM) froze a significantly more number of times when exposed to alcohol (p = 0.019).
Experiment 3: dopamine D2-like receptor antagonism does not alter alcohol-induced motor impairment
Variance of distance to bottom has previously been shown to be sensitive to alcohol’s motor-impairing effects and less sensitive to alcohol’s anxiety-altering effects (Tran et al. 2015a). Figure 3d shows that zebrafish exposed to alcohol exhibited a significant decrease in the variance of distance to bottom (F(1, 174) = 43.975, p < 0.001) and that this decrease was haloperidol independent (haloperidol pre-treatment main effect (p > 0.05); haloperidol × alcohol interaction (p > 0.05)).
Discussion
In the current study, we characterized the dose-dependent effects of haloperidol and demonstrate that alcohol-induced locomotor activity and behavioral responses of AB zebrafish are altered by haloperidol (a dopamine D2-like receptor antagonist). Exposure to haloperidol was first reported to inhibit the locomotor activity of zebrafish larvae of an unspecified genetic background at a high dose of 9 μM following a 2-h acute exposure (Giacomini et al. 2006). However, follow-up studies using outbred adult zebrafish found no significant effect of exposure to 9 μM haloperidol on locomotor activity or anxiety-like behavioral responses following 15 (Seibt et al. 2011; Savio et al. 2012) or 30 min of exposure to the antagonist (Seibt et al. 2010). The inconsistent findings between these studies may reflect differences in the genetic background and/or the age of the tested zebrafish and/or the duration of exposure to haloperidol, prompting the need for a systematic dose-response analysis employed with specific zebrafish strains. A dose-response analysis for haloperidol has been conducted using outbred larval zebrafish revealing an inverted U-shaped dose-dependent increase in locomotor activity peaking at 5.5 μM following a 200-min exposure (Irons et al. 2013). In contrast, a recent dose-response analysis for haloperidol using adult zebrafish of a mixed, genetically undefined and likely highly variable, population (short- and long-fin zebrafish) was conducted, and revealed a non-significant U-shaped dose-dependent increase in locomotor activity following a 5-min exposure session (Magno et al. 2015). These differences may be due to the slow-acting effect of haloperidol and the significant difference in the duration of exposure between the two studies (5 vs. 200 min). Since the genetic background and age of zebrafish have also been shown to alter their behavioral responses to different pharmacological compounds (Liu et al. 2014; Pannia et al. 2014; de Esch et al. 2012), it was unknown whether the quasi-inbred adult zebrafish of the AB strain would respond to haloperidol in a similar manner as outbred larval and adult zebrafish did.
In experiment 1, we used a moderately long exposure time (90 min) due to haloperidol’s slow-acting effect and quantified behavioral responses following habituation to the test environment (in the last 10 min). We found that AB zebrafish exposed to moderately high doses of haloperidol exhibited a biphasic inverted U-shaped dose-dependent increase in locomotor activity with a peak observed at 5 μM similar to the effects previously reported for outbred zebrafish larvae (Irons et al. 2013). Although speculative at this point, the biphasic effect of haloperidol on locomotor activity we and others found in zebrafish may be attributed to the dose-dependent blockade of pre-synaptic dopamine autoreceptors at low concentrations and postsynaptic dopamine receptors at higher concentrations similarly to what has been found in mammals (Frussa-Filho et al. 1997). Another similarity between our zebrafish findings and those obtained with mammals is that haloperidol has been shown to produce akathisia, a subjective desire to move in humans manifesting as increased agitation and restlessness (Arvanitis and Miller 1997), which may lead to increased locomotion in animals too.
In experiment 2, we examined the effect of lower doses of haloperidol on zebrafish behavioral responses in a novel environment. When netted and placed in a novel environment, zebrafish exhibit increased anxiety-like behavioral responses including an elevated absolute turn angle and duration of freezing (Nowicki et al. 2014). We found that zebrafish pre-treated with low doses of haloperidol and subsequently transferred to a new environment containing the same concentration of the antagonist exhibited a concentration-dependent decrease in the absolute turn angle and freezing (frequency and duration). An increased freezing and turn angle have been observed in response to aversive stimuli or contexts, and have been consistently interpreted as signs of anxiety in zebrafish (Blaser and Penalosa 2011; Nowicki et al. 2014). Although a single behavioral test and a limited number of behavioral measures may not allow one to conclusively decide about the behavioral state of the studied subjects, the low-dose haloperidol-induced reduction of freezing and turn angle we observed may be interpreted as signs of reduced anxiety. Notably, we found that exposure to 2.5 μM haloperidol reduced the absolute turn angle and freezing in a context-dependent manner (i.e., only in a new environment). However, haloperidol is a typical antipsychotic and is not considered an anxiolytic. In humans, haloperidol reduces agitation and restricts motor behavior (e.g., hand movements, facial expression, natural arm movements while walking) (Asadollahi et al. 2015). The antipsychotic effects of haloperidol in humans may explain the reduction of the absolute turn angle and freezing behavior (duration and frequency) observed in zebrafish. Our results suggest that low doses of haloperidol reduce motor agitation and restrict normal movements in a new environment, effects that are similar to those previously described in zebrafish (Magno et al. 2015) and humans (Ammar et al. 2015).
In the third part of our study, we examined whether alcohol-induced behavioral responses were dependent on dopamine D2-like receptor activation. Since dopamine appears to be important for alcohol-induced locomotor activity in zebrafish (Puttonen et al. 2013; Guo et al. 2015; Chatterjee et al. 2014), and since a recent study provided evidence against the role of dopamine D1-like receptors (Tran et al. 2015b), it was hypothesized that dopamine D2-like receptors contributed to the observed behavioral responses. Our results now give strong support for this hypothesis as they demonstrate that pre-treatment with haloperidol (0.625 or 2.5 μM) abolishes alcohol-induced locomotor activity in zebrafish. Our findings are also in line with those that have shown alcohol-induced locomotor activity to be attenuated by haloperidol pre-treatment in mice (Risinger et al. 1992). Notably, neither doses of haloperidol (0.625 and 2.5 μM) altered baseline locomotor activity in the new environment, suggesting the attenuation of alcohol-induced locomotor activity is not due to direct inhibition of locomotor activity. Dopaminergic receptors have been known to play an important role in regulating locomotor activity in zebrafish (Anichtchik et al. 2004; Bretaud et al. 2004). Our results suggest that alcohol increases locomotor activity in zebrafish via a dopamine D2-like receptor-dependent mechanism.
Based on our results, the role of dopamine D2-like receptors in regulating alcohol-induced alterations to freezing and the turn angle in zebrafish is more difficult to ascertain. In experiment 3, our analysis revealed a significant interaction between haloperidol and alcohol. Pre-treatment with the lower dose of haloperidol (0.625 μM) did not alter baseline behavioral responses or alcohol-induced behavioral responses. In contrast, the higher dose of haloperidol (2.5 μM) and alcohol exposure both reduced the absolute turn angle and freezing in zebrafish when these drugs were administered alone. However, when the two drugs were co-administered, the resulting behavioral response was similar to that of controls, revealing an antagonistic interaction between haloperidol and alcohol. It is likely that at the higher dose of haloperidol (2.5 μM), these two drugs act through different pathways in the zebrafish brain to mediate the change in behavioral responses. Although the mechanisms through which haloperidol altered the alcohol-induced turn angle and freezing behavior remain speculative, it is likely that dopamine D2-like receptors can modulate alcohol’s effect in an indirect way. For example, it is unlikely that dopamine displaces haloperidol since the antagonist is present during alcohol exposure and its affinity for dopamine D2-like receptors is higher than that for dopamine itself (Seeman 2002). Since haloperidol’s affinity for antagonizing dopamine D2-like receptors is much greater than that for D1-like receptors (Seeman and Van Tol 1994), synaptic dopamine may activate non-D2-like receptors, which may contribute to the observed antagonistic interaction between haloperidol and alcohol at the behavioral level.
It is also important to note that although haloperidol is classified as a typical antipsychotic with preferential selectivity for dopamine D2-like receptors, at high concentrations, the drug has been shown to exhibit affinity for serotonin 2A (5-HT2A) and alpha 1A adrenergic (α1A) receptors (Kroeze et al. 2003). Zebrafish pharmacology is in its infancy. Whether the orders of magnitude for concentration differences found for haloperidol binding to different target receptors in mammals mimic those typical of zebrafish is not yet known. Nevertheless, if haloperidol’s binding affinity profile is similar between zebrafish and mammals, the doses utilized in the current study are unlikely to have had off-target effects. For example, locomotor sedation commonly associated with blockade of dopamine D2 receptors (Simon et al. 2000) was not observed even at the highest dose of haloperidol in mammals (mice). Furthermore, the doses required to inhibit α1A and 5-HT2A receptors are approximately 3 and 13 times higher than what is required to inhibit dopamine D2 receptors in mammals (Kroeze et al. 2003). Notably, haloperidol has been shown to be more potent (Simon et al. 2000), to exhibit a higher affinity for D2 over D3 receptors (Seeman and Van Tol 1994), and to exhibit greater potency for blocking pre-synaptic autoreceptors (Imazu et al. 1989) compared to the alternative D2-like receptor antagonist, sulpiride. Future studies will be able to compare the effects of different antagonists targeting dopamine D2-like receptors such as sulpiride or amisulpride, which exhibit preferential limbic selectivity in mammals (Schoemaker et al. 1997) to distinguish between striatal and limbic D2 receptors. In addition, it is important to note that with regard to alcohol’s effects we also cannot exclude the role of neurotransmitter systems other than the dopaminergic system. These systems include but are not limited to the GABAergic and adenosinergic systems, which may also contribute to alcohol’s locomotor stimulant and anxiety-altering effects (Phillips and Shen 1996; Nam et al. 2013).
The haloperidol-induced biphasic activation of locomotor activity and its attenuation of alcohol-induced locomotor activity in zebrafish parallel the effects observed in mammals including humans (Enggasser and de Wit 2001). Our findings from experiments 1 and 2 suggest the behavioral pharmacology and functional role of dopamine D2-like receptors are evolutionarily conserved in fish and mammals. In addition, haloperidol was developed to target mammalian D2-like receptors, and the similarities between the phenotypical effects reported in our study for zebrafish and those published on mammals imply that this compound may have a similar mode of action, i.e., target the same D2-like receptors in both zebrafish and mammals. Thus, results of experiment 3 suggest that alcohol-induced locomotor hyperactivity in zebrafish is dependent on activation of dopamine D2-like receptors, whereas alcohol-induced reduction of the absolute turn angle and freezing behavior may only be altered by high haloperidol concentration, at which dose the drug may affect receptors other than D2-R, possibly including receptors of other neurotransmitter systems. In conclusion, our results show both the utility of and the challenges associated with zebrafish, a novel research subject in psychopharmacology.
References
Ammar G, Naja WJ, Pelissolo A (2015) Treatment-resistant anxiety disorders: a literature review of drug therapy strategies. Encéphale 41:260–265
Anichtchik OV, Kaslin J, Peitsaro N, Scheinin M, Panula P (2004) Neurochemical and behavioral changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem 88:443–453
Arvanitis LA, Miller BG (1997) Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry 42:233–246
Asadollahi S, Heidari K, Hatamabadi H, Vafaee R, Yunesian S, Azadbakht A, Mirmohseni L (2015) Efficacy and safety of valproic acid versus haloperidol in patients with acute agitation: results of a randomized, double-blind, parallel-group trial. Int Clin Psychopharmacol 30:142–150
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Bencan Z, Sledge D, Levin ED (2009) Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav 94:75–80
Blaser RE, Penalosa YM (2011) Stimuli affecting zebrafish (Danio rerio) behavior in the light/dark preference test. Physiol Behav 104:831–837
Boehmler W, Obrecht-Pflumio S, Canfield V, Thisse C, Thisse B, Levenson R (2004) Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev Dyn 230:481–493
Boehmler W, Carr T, Thisse C, Thisse B, Canfield VA, Levenson R (2007) D4 dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behavior. Genes Brain Behav 6:155–166
Bretaud S, Lee S, Guo S (2004) Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol 26:857–864
Chatterjee D, Shams S, Gerlai R (2014) Chronic and acute alcohol administration induced neurochemical changes in the brain: comparison of distinct zebrafish populations. Amino Acids 46:921–930
de Esch C, van der Linde H, Slieker R, Willemsen R, Wolterbeek A, Woutersen R, de Groot D (2012) Locomotor activity assay in zebrafish larvae: influence of age, strain and ethanol. Neurotoxicol Teratol 34:425–433
Dlugos CA, Rabin RA (2003) Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacol Biochem Behav 74:471–480
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44
Enggasser JL, de Wit H (2001) Haloperidol reduces stimulant and reinforcing effects of ethanol in social drinkers. Alcohol Clin Exp Res 25:1448–1456
Franck J, Jayaram-Lindstrom N (2013) Pharmacotherapy for alcohol dependence: status of current treatments. Curr Opin Neurobiol 23:692–699
Frussa-Filho R, Abilio VC, Bergamo M, Palermo-Neto J (1997) Behavioral subsensitivity induced by long-term administration of a low dose of haloperidol to rats. J Pharm Pharmacol 49:412–415
Gebauer DL, Pagnussat N, Piato AL, Schaefer IC, Bonan CD, Lara DR (2011) Effects of anxiolytics in zebrafish: similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol Biochem Behav 99:480–486
Gerlai R, Lahav M, Guo S, Rosenthal A (2000) Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67:773–782
Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R (2009) Acute and chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes Brain Behav 8:586–599
Giacomini NJ, Rose B, Kobayashi K, Guo S (2006) Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol Teratol 28:245–250
Guo N, Linn J, Peng X, Chen H, Zhang Y, Liu X, Li Q (2015) Influences of acute ethanol exposure on locomotor activities of larvae under different illumination. Alcohol, In Press
Haberstick BC, Young SE, Lessem JM, Zeiger JS, Kewitt KL, Hopfer CJ (2014) Prevalence and correlates of alcohol and cannabis use disorders in the United States: results from the national longitudinal study of adolescent health. Drug Alcohol Depend 136:158–161
Hendler RA, Ramchandani VA, Gilman J, Hommer DW (2013) Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci 13:489–509
Holdstock L, King AC, de Wit H (2000) Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res 24:789–794
Ibrahim M, Mussulini BH, Moro L, de Assis AM, Rosemberg DB, de Oliveira DL, Rocha JB, Schwab RS, Schneider PH, Souza DO, Rico EP (2014) Anxiolytic effects of diphenyl diselenide on adult zebrafish in a novelty paradigm. Prog Neuropsychopharmacol Biol Psychiatry 54:187–194
Imazu Y, Kobayashi K, Shohmori T (1989) Comparative study of sulpiride and haloperidol on dopamine turnover in the rat brain. Neurochem Res 14:459–464
Irons TD, Kelly PE, Hunter DL, MacPhail RC, Padilla S (2013) Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol Biochem Behav 103:792–813
Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL (2003) H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28:519–526
Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90:54–58
Li P, Shah S, Huang L, Carr AL, Gao Y, Thisse C, Thisse B, Li L (2007) Cloning and spatial and temporal expression of the zebrafish dopamine D1 receptor. Dev Dyn 236:1339–1346
Liu X, Guo N, Lin J, Zhang Y, Chen XQ, Li S, He L, Li Q (2014) Strain-dependent differential behavioral responses of zebrafish larvae to acute MK-801 treatment. Pharmacol Biochem Behav 127:82–89
Magno LD, Fontes A, Goncalves BM, Gouveia A Jr (2015) Pharmacological study of the light/dark preference test in zebrafish (Danio rerio): waterborne administration. Pharmacol Biochem Behav 135:169–176
Mathur P, Berberoglu MA, Guo S (2011) Preference for ethanol in zebrafish following a single exposure. Behav Brain Res 217:128–133
Nam HW, Bruner RC, Choi DS (2013) Adenosine signaling in striatal circuits and alcohol use disorders. Mol Cells 36:195–202
Nowicki M, Tran S, Muraleetharan A, Markovic S, Gerlai R (2014) Serotonin antagonists induce anxiolytic and anxiogenic-like behavior in zebrafish in a receptor subtype dependent manner. Pharmacol Biochem Behav 126:170–180
Nowicki M, Tran S, Chatterjee D, Gerlai R (2015) Inhibition of phosphorylated tyrosine hydroxylase attenuates ethanol-induced hyperactivity in adult zebrafish (Danio rerio). Pharmacol Biochem Behav 138:32–39
Pannia E, Tran S, Rampersad M, Gerlai R (2014) Acute ethanol exposure induces behavioral differences in two zebrafish (Danio rerio) strains: a time course analysis. Behav Brain Res 259:174–185
Phillips TJ, Shen EH (1996) Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol 243:243–282
Puttonen HA, Sundvik M, Rozov S, Chen YC, Panula P (2013) Acute ethanol treatment upregulates Th1, Th2, and Hdc in larval zebrafish in stable networks. Front Neural Circuits 7:1–10
Quadros VA, Silveira A, Giuliani GS, Didonet F, Silveira AS, Nunes ME, Silva TO, Loro VL, Rosemberg DB (2016) Strain- and context-dependent behavioral responses of acute alarm substance exposure in zebrafish. Behav Process 122:1–11
Risinger FO, Dickinson SD, Cunningham CL (1992) Haloperidol reduces ethanol-induced motor activity stimulation but not conditioned place preference. Psychopharmacology (Berlin) 107:453–456
Rosemberg DB, Braga MM, Rico EP, Loss CM, Cordova SD, Mussulini BH, Blaser RE, Leite CE, Campos MM, Dias RD, Calcagnotto ME, de Oliveira DL, Souza DO (2012) Behavioral effects of taurine pretreatment in zebrafish acute exposed to ethanol. Neuropharmacology 63:613–623
Savio LE, Vuaden FC, Piato AL, Bonan CD, Wyse AT (2012) Behavioral changes induced by long-term proline exposure are reversed by antipsychotics in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry 36:258–263
Schoemaker H, Claustre Y, Fage D, Rouquier L, Chergui K, Curet O, Oblin A, Gonon F, Carter C, Benavides J, Scatton B (1997) Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with presynaptic and limbic selectivity. J Pharmacol Exp Ther 280:83–97
Seeman P (2002) Atypical antipsychotics: mechanism of action. Can J Psychiatry 47:27–38
Seeman P, Van Tol HH (1994) Dopamine receptor pharmacology. Trends Pharmacol Sci 15:264–270
Seibt KJ, da Luz Oliveira R, Zimmermann FF, Capiotti KM, Bogo MR, Ghisleni G, Bonan CD (2010) Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio). Behav Brain Res 214:417–422
Seibt KJ, Piato AL, da Luz Oliveira R, Capiotti KM, Vianna MR, Bonan CD (2011) Antipsychotic drugs reverse MK-801-induced cognitive and social interaction deficits in zebrafish (Danio rerio). Behav Brain Res 224:135–139
Simon VM, Parra A, Minarro J, Arenas MC, Vinader-Caerols C, Aguilar MA (2000) Predicting how equipotent doses of chlorpromazine, haloperidol, sulpiride, raclopride and clozapine reduce locomotor activity in mice. Eur Neuropsychopharmacol 10:159–164
Tran S, Gerlai R (2013) Time-course of behavioral changes induced by ethanol in zebrafish (Danio rerio). Behav Brain Res 252:204–213
Tran S, Chatterjee D, Gerlai R (2014) Acute net stressor increases whole-body cortisol levels without altering whole-brain monoamines in zebrafish. Behav Neurosci 128:621–624
Tran S, Chatterjee D, Gerlai R (2015a) An integrative analysis of ethanol tolerance and withdrawal in zebrafish (Danio rerio). Behav Brain Res 276:161–170
Tran S, Nowicki M, Muraleetharan A, Chatterjee D, Gerlai R (2015b) Differential effects of acute administration of SCH-23390, a D1 receptor antagonist, and of ethanol on swimming activity, anxiety-related responses, and neurochemistry of zebrafish. Psychopharmacology 232:3709–3718
Tran S, Nowicki M, Muraleetharan A, Gerlai R (2015c) Differential effects of dopamine D1 and D2/3 receptor antagonism on motor responses. Psychopharmacology 232:795–806
Tran S, Muraleetharan A, Fulcher N, Chatterjee D, Gerlai R (2016) MK-801 increases locomotor activity in a context-dependent manner in zebrafish. Behav Brain Res 296:26–29
Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu N, Cachat J, Kalueff AV (2010) Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav Brain Res 208:450–457
Acknowledgments
The research was supported by an NSERC Discovery Grant (#311637) and a Brain Canada Foundation grant issued to Robert Gerlai and an NSERC Canadian Graduate Scholarship (doctoral level) issued to Steven Tran.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tran, S., Facciol, A. & Gerlai, R. Alcohol-induced behavioral changes in zebrafish: The role of dopamine D2-like receptors. Psychopharmacology 233, 2119–2128 (2016). https://doi.org/10.1007/s00213-016-4264-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4264-3