Abstract
Rationale
Impaired N-methyl-d-aspartate (NMDA) receptor signalling underlies several psychiatric disorders that express high levels of impulsivity. Although synergistic interactions exist between NMDA receptors and metabotropic glutamate receptor 5 (mGluR5), the significance of this interaction for impulsivity is unknown.
Objective
This study aims to investigate the effects of negative and positive allosteric mGluR5 modulation (NAM/PAM) on trait impulsivity and impulsivity evoked by NMDA receptor antagonism in rats.
Methods
Motor and choice impulsivity were assessed using the five-choice serial reaction time task (5-CSRTT) and delayed-discounting task (DDT), respectively. The effects of RO4917523 and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) (NAMs) and ADX47273 (PAM) were investigated in non-impulsive rats and in trait high- and low-impulsive rats. The effects of these compounds on impulsivity induced by NMDA receptor antagonism (MK801) in the 5-CSRTT were also investigated.
Results
RO4917523 (0.1–1 mg/kg) decreased premature responding and increased omissions but had no effect on locomotor activity up to 0.1 mg/kg. MTEP significantly increased omissions, decreased accuracy and slowed responding but had no effect on premature responding. ADX47273 decreased premature responding at doses that had no effect on locomotor activity. MK801 increased premature responding and impaired attentional accuracy; these deficits were dose dependently rescued by ADX47273 pre-treatment. Allosteric modulation of mGluR5 had no significant effect on choice impulsivity, nor did it modulate general task performance.
Conclusions
These findings demonstrate that mGluR5 allosteric modulation selectively dissociates motor and choice impulsivity. We further show that mGluR5 PAMs may have therapeutic utility in selectively targeting specific aspects of impulsivity and executive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impulsivity can be defined as a tendency to act prematurely without foresight (Dalley et al. 2011) and is a multi-faceted behavioural construct spanning several domains from impaired response inhibition to an intolerance of delayed rewards (Cardinal et al. 2004; Evenden 1999; Moeller et al. 2001a). High levels of impulsivity are symptomatic of several major neuropsychiatric disorders, including schizophrenia (Kaladjian et al. 2011; Moeller et al. 2001a), attention deficit/hyperactivity disorder (Aron and Poldrack 2005; Crunelle et al. 2013) and addiction (de Wit 2009; Ersche et al. 2010; Hester and Garavan 2004; Lee et al. 2009; Moeller et al. 2001b). Pre-clinically, individual differences in trait impulsivity predict psychostimulant self-administration (Dalley et al. 2007; Diergaarde et al. 2008), heightened propensity for relapse (Economidou et al. 2009) and the subsequent development of compulsive cocaine self-administration (Belin et al. 2008).
Whilst much research highlights the significant involvement of the dopaminergic, serotonergic and noradrenergic systems in impulsivity (Dalley and Roiser 2012; Pattij and Vanderschuren 2008; Winstanley et al. 2006), there have been fewer studies on the role of glutamate in this area of research. Glutamate is the principal, most abundant excitatory neurotransmitter within the mammalian central nervous system and exerts its effects by activating ionotropic (iGluR) and metabotropic (mGluR) glutamate receptors (Conn and Pin 1997; Schoepp 2001). Based on sequence homology, signal transduction and electrophysiological properties, iGluRs and mGluRs have each been classified into three distinct sub-groups; N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate iGluRs and group I (mGluR1,5), II (mGluR2,3) and III (mGluR4,6,7,8) mGluRs (Conn and Pin 1997; Nakanishi and Masu 1994; Nakanishi 1992).
Dysfunctional glutamatergic signalling has been associated with a number of neuropsychiatric disorders where impulse control deficits are prominent. For example, NMDA receptor hypofunction is postulated to contribute to the pathophysiology of schizophrenia (Deakin et al. 1989; Goff and Coyle 2001; Konradi and Heckers 2003; Lindsley et al. 2006). Thus, dysregulation of cortical glutamatergic signalling by pharmacological blockade of NMDA receptors induces schizophrenia-like symptoms in healthy volunteers and exacerbates positive and negative symptoms in schizophrenic patients (Adler et al. 1999; Krystal et al. 1994; Lahti et al. 2001; Luby 1959). In experimental animals, systemic and local administration of the NMDA receptor antagonists MK801, phencyclidine (PCP) and 3-(2-carboxypiperazine-4-yl)propyl-1-phosphoric acid (CPP) also have the common effect of increasing impulsivity in rats and mice (Agnoli and Carli 2012; Carli et al. 2004; Fletcher et al. 2011; Greco et al. 2005; Higgins et al. 2003; Paine et al. 2007).
The ubiquitous expression and global role of iGluRs in mediating fast, excitatory synaptic transmission limits their use as therapeutic targets for selectively improving impulse control deficits in humans. However, recent evidence suggests that targeting mGluRs may provide a more appropriate, subtle modulation of glutamatergic transmission. Furthermore, the heterogeneous distribution of the eight, diverse subtypes (mGluR1–8) offers an opportunity to selectively modulate glutamatergic transmission in an anatomically and functionally distinct manner (Conn and Pin 1997; Nakanishi 1992; Schoepp and Conn 2002).
In the present study, we investigated the effects of allosteric mGluR5 modulation on two distinct forms of impulsivity. We firstly investigated the effects of negative and positive allosteric mGluR5 modulation on the performance of rats on the five-choice serial reaction time task (5-CSRTT), a widely used operant task to assess sustained visual attention and impulsivity in rodents (Robbins 2002). This research was based on the rationale that mGluR5s, which are excitatory receptors and expressed widely in limbic-cortico-striatal circuitry (Romano et al. 1995; Shigemoto et al. 1993), interact synergistically with NMDA receptors (Awad et al. 2000; Campbell et al. 2004; Doherty et al. 1997; Henry et al. 2002; Homayoun and Moghaddam 2006; Kinney et al. 2003; Pisani et al. 2001). Functional coupling of NMDA and mGluR5 suggests that mGluR5s may be ideally placed to modulate impulsive behaviour sensitive to NMDA receptor transmission. To test this hypothesis, we investigated the effects of the negative allosteric modulators (NAMs), RO4917523 and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP), and the positive allosteric modulator (PAM), ADX47273 (de Paulis et al. 2006), on impulsivity evoked by the NMDA receptor antagonist MK801 and compared these effects with those in rats expressing trait-like impulsivity. We next investigated the specificity of allosteric mGluR5 modulation by evaluating the effects of these compounds on a delay-discounting task (DDT) to assess impulsive choice for immediate, small-magnitude rewards versus larger, but delayed rewards (Ainslie 1975; Evenden 1999).
Material and Methods
Subjects
Male Lister-hooded rats, weighing 200–250 g at the start of training, were obtained from Charles River (UK and Germany) and assessed for performance on the 5-CSRTT and DDT. A separate group of rats weighing 250–300 g was purchased from Charles River (Germany) and used for the assessment of locomotor activity. All rats were housed in groups of four, under a 12-h light/dark cycle with food and water initially available ad libitum. All rats were permitted at least 5 days acclimatisation before training on the 5-CSRTT and DDT commenced. Food restriction was initiated when body weights were at least 300 g. Body weight was then maintained at 80–85 % of free-feeding weight. All training and testing commenced between 0700 and 1200 hours, 5–6 days a week. All experimental procedures were carried out in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986. Studies carried out in Germany were authorised by the Local Animal Care and Use Committee in accordance with local animal care guidelines, AAALAC regulations and the USDA Animal Welfare Act.
Locomotor activity
Locomotor activity was assessed using eight Tru-Scan arena chambers (Coulbourn Instruments, Lehigh Valley, PA, USA), each with two sensing rings (Tru Scan Photo Beam sensor ring—Coulbourn Instruments, Lehigh Valley, PA, USA), detecting activity in three orthogonal planes. Mean distance travelled (cm), total rearing events and rearing time (s) were recorded.
Five-choice serial reaction time task
Twelve five-choice operant chambers (UK) and 32 five-choice operant chambers (Germany) (Med Associates Inc, St. Albans, VT, USA) enclosed in sound-attenuating, fan-ventilated cubicles were used, as described previously (Bari et al. 2008; Carli et al. 1983). Briefly, each chamber consisted of five evenly spaced apertures (2.5 × 2.5 × 4 cm) containing an LED light, set into a curved wall at the rear of the chamber. A centrally located food magazine was located on the opposite wall of the chamber, into which 45-mg reward pellets could be delivered (Sandown Scientific, UK). Infrared beams located at the entrance of each aperture and the food magazine allowed detection of nose pokes. Task parameters and data collection were controlled by ‘Whisker’ software (UK) (Cardinal and Aitken 2010) and Med Associates Inc. software (St. Albans, VT, USA) (Germany).
The 5-CSRTT training protocol has been described previously (Bari et al. 2008; Carli et al. 1983). Each training session consisted of 100, self-paced, trials and lasted no longer than 30 min. At later training stages, 100 trials were normally completed within 20 min. Training sessions started with the illumination of the house and magazine lights, and by the delivery of a 45-mg reward pellet (Sandown Scientific, UK). Collection of the reward initiated the first trial. A single trial consisted of an inter-trial interval (ITI), followed by the pseudo-random illumination of one of the five apertures for a fixed duration [stimulus duration (SD)]. Following stimulus detection, a nose poke to the corresponding aperture, within a fixed time interval [limited hold (LH)], was required for reward delivery. Premature responses made during the ITI, incorrect responses and responses made outside the LH (an omission) resulted in a timeout (TO) period, during which time no food was delivered, and the house light was extinguished for 5 s.
Premature responding was calculated as a percentage of completed trials (correct + incorrect + omissions). A premature response was deemed an incomplete trial and re-set the current trial. Percentage accuracy was defined as the number of correct responses divided by the sum of correct and incorrect responses. Perseveration was calculated as the number of additional responses made in the same aperture, following a correct response. Omissions were calculated in terms of the percentage of completed trials.
Animals were deemed to be trained when they completed ≥50 correct trials with ≥70 % accuracy and ≤20 % omissions (SD, 0.7 s; ITI, 5 s; and LH, 5 s). At this stage, perseverative responses (additional responses made to the same aperture following a correct response) resulted in a 5-s TO and loss of food reward.
Impulsivity screening
Screening for impulsivity consisted of three or four ‘challenge’ training sessions where the ITI was extended to 7 s to increase the occurrence of premature responses (Dalley et al. 2007). Each challenge session was separated by four baseline training sessions, where task parameters were restored to the training configuration (ITI, 5 s). The mean percentage of premature responses made by each rat across the challenge sessions was calculated. Rats were excluded from the study if they exhibited poor or unstable performance or failed to complete 100 trials on three of the challenge sessions. All rats were ranked, based on the mean per cent premature responses, from highly to low impulsive. The upper and lower 15th centiles of premature responders were termed high-impulsive (HI) and low-impulsive (LI) rats, respectively. The remaining rats were categorised as mid-impulsive (MI) and were used for studies involving MK801. The MK801 studies were restricted to MI rats only. This was to avoid ceiling effects on premature responding that might occur in HI rats. Furthermore, the behavioural effects of MK801 are known to be highly variable; restricting the selection of animals to the middle of the behavioural distribution was designed to generate a more homogenous cohort of animals.
Delay discounting
Thirty-two operant chambers, enclosed in sound-attenuating, fan-ventilated cubicles (Med Associates Inc, St. Albans, VT, USA) were used, as described previously (Mar and Robbins 2007; Winstanley et al. 2003). Briefly, each chamber consisted of two retractable levers located on either side of a centrally located food magazine into which 45-mg reward pellets (Sandown Scientific, UK) could be delivered. A stimulus light was located above each lever, and an infrared beam at the entrance of the food magazine detected reward collection. Task parameters and data collection were controlled by Med Associates Inc. software (St. Albans, VT, USA).
Pre-training
Rats were habituated to the operant chambers for 2 days and trained under a fixed ratio-1 schedule of reinforcement (FR1). During these sessions, both levers were extended, the lever lights were illuminated and a press on either lever resulted in the delivery of a reward pellet. Rats were required to reach a criterion of 60 lever presses (30 presses on each lever) within 60 min. A simple version of the delay-discounting task was then implemented; trials were initiated every 40 s with the illumination of the house and magazine light. Rats were required to make a nose-poke response in the food magazine within 10 s of trial initiation in order to trigger the presentation of a lever and corresponding lever light. Responding on the lever within 10 s resulted in the retraction of the lever and the lever light being extinguished, the illumination of the food magazine and the delivery of a single reward pellet. Both levers were presented an equal number of times in each session. Rats were required to reach a criterion of at least 60 successfully completed trials in 60 min.
Delay-discounting task
Each training session consisted of 6 blocks of 10 trials (60 trials in total) with each trial lasting exactly 72 s. Each block began with four forced choice trials whereby the left and right lever were each presented twice in a random order. Throughout the task, responding on the right lever resulted in the immediate delivery of a single reward pellet. Responses on the left lever resulted in the delayed delivery of three reward pellets, with increasing delay across blocks from 0 s (block 1), 2 s (block 2), 4 s (block 3), 8 s (block 4), 16 s (block 5) and 32 s (block 6).
Following the completion of four forced trials, six free choice trials were introduced. As in the pre-training protocol, each trial was initiated by the illumination of the house and magazine light. Rats were required to make a nose-poke response in the food magazine within 10 s to trigger the presentation of both levers and lever lights. A failure to respond on either lever within 10 s (an omission) resulted in the retraction of both levers with all lights extinguished and an inter-trial interval (ITI) initiated before the next trial. Responding on one of the levers within 10 s resulted in the retraction of both levers with all lights extinguished. Reward delivery was preceded by the illumination of the magazine light either immediately or after the chosen delay. The length of the ITI was dependent on the choice of the immediate or delayed lever, and followed reward delivery to ensure each trial was exactly 72 s in duration.
Experiment 1: effects of RO4917523, MTEP and ADX47273 on locomotor activity
Three separate groups of rats were assessed for locomotor activity. All rats were habituated in an annex to the testing room, prior to testing. RO4917523, MTEP or ADX47273 administration occurred during the habituation period. Rats received 0.06, 0.1 or 1 mg/kg RO4917523, p.o, 2 h before testing; 3, 10 or 30 mg/kg MTEP, i.p, 15 min before testing; or 60, 80 or 100 mg/kg ADX47273, p.o, 1.5 h before testing. Rats were then placed into the locomotor activity chambers to freely explore in total darkness for 1 h.
Experiment 2: effects of RO4917523, MTEP and ADX47273 on 5-CSRTT performance
Rats that had not undergone impulsivity screening (non-selected rats), but showed stable performance on the 5-CSRTT, were used to assess the effects of (i) RO4917523 (0.03, 0.06, 0.1, 0.3 and 1 mg/kg; p.o), administered 2 h prior to behavioural assessment; (ii) MTEP (1, 3, 10 and 30 mg/kg MTEP; i.p), administered 15 min prior to testing; and (iii) ADX47273 (40, 60, 80 and 100 mg/kg ADX47273; p.o), administered 1.5 h prior to testing.
Experiment 3: effects of RO4917523, MTEP and ADX47273 on 5-CSRTT performance in HI and LI rats
Twelve HI and 12 LI rats were used to assess the effects of 0.03, 0.1 and 0.3 mg/kg RO4917523 and 40, 60, 80 and 100 mg/kg ADX47273 on baseline 5-CSRTT performance. One LI rat was excluded from the ADX47273 study due to a decline in baseline performance. The same rats were also used to assess the effects of ADX47273 on 5-CSRTT performance under a 7-s ITI. Increasing the ITI increases the occurrence of a premature response and thus increases baseline premature responding. In a separate cohort, nine HI and 11 LI trained rats were used to assess the effects of 1, 3, 10 and 30 mg/kg MTEP.
Experiment 4: effects of RO4917523, MTEP and ADX47273 pre-treatment on MK801-modulated 5-CSRTT performance
Since the behavioural response to MK801 administration, as measured on the 5-CSRTT, was variable between different cohorts of rats, two MK801 dose–response studies were carried out (Online Resource 1, Fig. S1 and Fig. S2). This ensured that the dose of MK801 was tailored to each cohort of rats. Based on the results from these studies, two different doses of MK801, 0.03 and 0.06 mg/kg, were used in these studies. In addition, the experimental design was altered from a within-subject’s design to a between-subject’s design to eliminate the possibility of varying effects of repeated MK801 administration. Specifically, in preliminary studies, we found that repeated injections of MK801 led to a reduced behavioural response. Therefore, a between-subject’s design was adopted so that each rat received a single dose of MK801 in the study.
Sixteen MI rats were used in a within subject’s design to assess the effects of 0.06 mg/kg MK801 and its vehicle (0.9 % NaCl, sterile saline) on 5-CSRTT performance following 0.03, 0.1, 0.3 and 1 mg/kg RO4917523 or 1, 3, 10 and 30 mg/kg MTEP pre-treatment. RO4917523 was administered p.o, 2 h prior to testing. MTEP was administered i.p, 15 min prior to testing. MK801 was administered s.c, 10 min before testing.
Fifty-five MI rats were tested using a between-subject’s design to investigate the effects of 0.03 mg/kg MK801 and its vehicle (0.9 % NaCl, sterile saline) on 5-CSRTT performance following 80 and 100 mg/kg ADX47273 pre-treatment. Baseline performance was counter-balanced between vehicle and MK801-treated rats. MK801 was administered s.c., 10 min prior to testing.
Experiment 5: effects of RO4917523, MTEP and ADX47273 on delay discounting
A separate group of rats that showed a stable performance in the delayed-discounting task were used to assess the effects of (i) RO4917523 (0.03, 0.1 and 0.3 mg/kg; p.o; n = 24), administered 2 h prior to behavioural assessment; (ii) MTEP (1, 3, 10 and 30 mg/kg MTEP; i.p; n = 18), administered 15 min prior to testing; and (iii) ADX47273 (40, 60, 80 and 100 mg/kg ADX47273; p.o; n = 20), administered 1.5 h prior to testing.
Drugs
Drugs were administered according to a randomised Latin square design, unless otherwise stated. RO4917523 was synthesised by Boehringer Ingelheim Pharma GmBH & Co. KG and dissolved in 10 % Tween80 (0.1 %) (v/v) and 90 % Natrosol (0.5 %) and administered p.o. The selected dose range and pre-treatment time were based on in-house pharmacokinetic (PK) data (Online Resource, Table S1). MTEP was synthesised by Boehringer Ingelheim Pharma GmBH & Co. KG, dissolved in Tween80 (10 %) (v/v) and administered i.p. The selected dose range and pre-treatment time were based on published literature (Gass et al. 2008; Varty et al. 2005). ADX47273 was synthesised by Boehringer Ingelheim Pharma GmBH & Co. KG, dissolved in 10 % Tween80 (0.1 %) (v/v) and 90 % Natrosol (0.5 %). The selected dose range and pre-treatment time were based on in-house PK data (Online Resource, Table S1). Each compound was characterised using in vitro fluorometric imaging to confirm their allosteric properties and to validate their use as tool compounds in assessing impulsivity in rats (Online Resource, Fig. S4). We confirmed that both RO4917523 and MTEP negatively modulate mGluR5, achieving an IC50 of 4.53 and 19.3 nM, respectively (Fig. S4a). Furthermore, we confirmed positive allosteric modulation of mGluR5 with ADX47273, achieving a maximal glutamate EC50 shift factor of 21.2 at 3 μM. (Fig. S4b). MK801 was purchased from Sigma Aldrich (UK and Germany), dissolved in sterile saline and adjusted to pH 7 with 1 M NaOH. The selected dose range and pre-treatment time were based on published literature (Fletcher et al. 2011).
Data analysis
Data were analysed using SPSS (version 21) and GraphPad Prism 6. Locomotor activity data are expressed as the distance travelled, rearing frequency and rearing time over a test period of 1 h. Data were analysed using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc analysis where indicated by a significant main effect of dose. Behavioural data on the 5-CSRTT were analysed using repeated measures ANOVA, unless otherwise stated. In studies where baseline and evoked impulsivity was not assessed (non-selected rats), drug dose served as a within-subject’s factor. In studies involving HI and LI rats, group served as a between-subject’s factor and drug dose served as a within-subject’s factor. A within-subject’s design was used for the RO4917523 and MTEP studies in MI rats. Dunnett’s and Bonferroni post hoc analyses with paired Student’s t test were used where indicated by significant main effects and interactions. A between-subject’s design was used for the ADX47273 study in MI rats with Bonferroni post hoc analysis for multiple comparisons. Delayed-discounting data were analysed by repeated measures ANOVA with delay and drug dose as within-subject factors. Violation of the requirement of homogeneity of variance, assessed by Mauchley’s sphericity test and confirmed by chi-squared analysis was corrected using the Geisser–Greenhouse epsilon to adjust the degrees of freedom. Statistical significance was set at p < 0.05.
Results
Experiment 1: effects of RO4917523, MTEP and ADX47273 on locomotor activity
Table 1 summarises the effects of RO4917523, MTEP and ADX47273 on locomotor activity.
RO4917523 produced a significant effect on total rearing activity (F 3,28 = 10.5, p < 0.001), rearing time (F 3,28 = 13.4, p < 0.001) and distance travelled (F 3,28 = 7.26, p < 0.001). Although rearing activity and distance travelled were unaffected by lower doses of RO4917523 (0.06 and 0.1 mg/kg), a dose of 1 mg/kg significantly decreased these variables (p < 0.001 and p < 0.05 respectively). The time spent rearing was significantly reduced by all doses (0.06 mg/kg, p < 0.01; 0.1 mg/kg, p < 0.05; 1 mg/kg, p < 0.001). MTEP treatment also had a significant decremental effect on rearing activity (F 3,26 = 10.1, p < 0.001), rearing time (F 3,26 = 23.6, p < 0.001) and distance travelled (F 3,26 = 8.51, p < 0.001). These reductions reached significance at every dose tested (rearing activity: 3 and 10 mg/kg, p < 0.01; 30 mg/kg, p < 0.001; distance travelled: 3 and 10 mg/kg, p < 0.05; 30 mg/kg, p < 0.001; rearing time: 3, 10 and 30 mg/kg, p < 0.001). Although ADX47273 significantly decreased rearing activity (F 3,20 = 5.2, p < 0.01) at 60 mg/kg (p < 0.05), neither distance travelled nor rearing time were affected by this dose. Furthermore, locomotor activity was unaffected by ADX47273 at 80 mg/kg, whereas rearing activity, distance travelled (F 3,20 = 4.4, p < 0.05) and rearing time (F 3,20 = 3.3, p < 0.05) were reduced following 100 mg/kg ADX47273 administration (p < 0.01, p < 0.01 and p < 0.05, respectively).
Experiment 2: effects of RO4917523, MTEP and ADX47273 on 5-CSRTT performance
As shown in Fig. 1a, RO4917523 significantly decreased premature responding on the 5-CSRTT (F 3,27 = 8.4, p < 0.001, X 2 = 34, GG ε = 0.55) at 0.1 mg/kg (p < 0.05) and 0.3 and 1 mg/kg (p < 0.01). However, at these same doses, RO4917523 also significantly increased omissions (F 2,22 = 43.3, p < 0.001, X 2 = 43, GG ε = 0.56) (0.1 mg/kg, p < 0.05; 0.3 and 1 mg/kg, p < 0.001) compared with vehicle treatment (Fig. 1b). Although the accuracy of responding was unaffected by RO4917523 (Fig. 1c), the speed of responding (Fig.1d) was significantly decreased (F 2,18 = 11.43, p < 0.001, X 2 = 25, GG ε = 0.44) following the administration of 0.3 and 1 mg/kg (p < 0.01 and p < 0.05 respectively) compared with the vehicle treated group. However, latencies to collect reward were unaffected by RO4917523.
Effect of RO4917523 on 5-CSRTT performance: a per cent premature responses, b per cent omissions, c per cent accuracy and d correct response latency (s). Bars represent means ± SEM (n = 11). Repeated measures one-way ANOVA, Dunnett’s post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle control
A summary of the effects of MTEP on 5-CSRTT performance is shown in Table 2. MTEP had no significant effect on premature responding nor did it affect attentional accuracy. However, omissions were significantly increased (F 4,40 = 10.1, p < 0.001) at 3 mg/kg (p < 0.01) and higher doses (10 mg/kg, p < 0.05; 30 mg/kg, p < 0.001) together with slower latencies to respond (F 4,40 = 3.9, p < 0.01) at all doses tested compared with the vehicle treated group (1 and 3 mg/kg, p < 0.01; 10 and 30 mg/kg, p < 0.05).
ADX47273 selectively decreased premature responding (F 4,44 = 3.1, p < 0.05), reaching significance at 80 (p < 0.05) and 100 (p < 0.01) mg/kg (Fig. 2a). The observed maximal decrease in premature responding was achieved by 100 mg/kg, reducing premature responding from a mean of 14.2 ± 2.9 % under vehicle conditions to a mean of 8.4 ± 2.2 %. ADX47273 had no significant effect on other behavioural variables (Fig. 2b–d).
Experiment 3: effects of RO4917523, MTEP and ADX47273 on 5-CSRTT performance in HI and LI rats
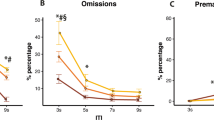
The effect of RO4917523 on 5-CSRTT performance in HI and LI rats is shown in Fig. 3. ANOVA revealed a significant decrease in premature responding (main effect of dose, F 2,47 = 33.9, p < 0.001, X 2 = 19, GG ε = 0.71) (Fig. 3a). This effect depended on impulsivity sub-group (dose × group; F 2,47 = 6.0, p < 0.01, X 2 = 19, GG ε = 0.71). Thus, whilst there was an overall significant difference in premature responding between HI and LI rats (main effect of group, F 1,22 = 15.9, p < 0.01), and specifically following vehicle and 0.03 mg/kg RO4917523 treatment (p < 0.001), the contrast in premature responding between HI and LI rats was abolished at higher doses, with premature responding also being significantly reduced compared with the relative vehicle-treated controls (HI: 0.1 and 0.3 mg/kg p < 0.001; LI: 0.1 and 0.3 mg/kg p < 0.01). Although accuracy was unaffected (Fig. 3c), a significant increase in omissions was observed following the administration of RO4917523 (main effect of dose, F 2,37 = 82.6, p < 0.001, X 2 = 43, GG ε = 0.57); this effect reached significance in HI and LI rats at 0.1 and 0.3 mg/kg compared with vehicle-treated rats (p < 0.001) (Fig. 3b). Omissions were also consistently significantly higher in LI rats compared with HI rats (main effect of group; F 1,22 = 8.0, p < 0.05). The latency to respond correctly was significantly increased (main effect of dose, F 1,31 = 20.0, p < 0.001, X 2 = 42, GG ε = 0.48) following 0.1 and 0.3 mg/kg RO4917523 treatment compared with vehicle (p < 0.001) and was consistently higher in LI compared with HI rats (main effect of group; F 1,22 = 6.6, p < 0.05) (Fig. 3d). However, magazine latencies were unaffected.
Effect of RO4917523 on 5-CSRTT performance in HI (n = 12) and LI (n = 12) rats: a per cent premature responses, b per cent omissions, c per cent accuracy and d correct response latency (s). Data are means ± SEM. Repeated measures ANOVA, Dunnett’s post hoc test. ### p < 0.001 HI versus LI; ***p < 0.001 (HI) or ++ p < 0.01 (LI) or ¥¥¥ p < 0.001 (HI and LI combined) versus relative vehicle control
As summarised in Table 3, MTEP appeared to produce biphasic effects on premature responding on the 5-CSRTT; an increase and decrease in premature responding was observed at lower and higher doses, respectively (main effect of dose, F 2,33 = 4.9, p < 0.05, X 2 = 37, GG ε = 0.47). Post hoc analysis revealed, however, that there was no significant difference between vehicle and any dose of MTEP. All doses of MTEP significantly reduced accuracy of responding in HI and LI rats compared with vehicle [(main effect of dose, F 2,29 = 3.7, p < 0.05, X 2 = 61, GG ε = 0.40) (1 mg/kg, p < 0.01; 3 mg/kg, p < 0.001; 10 and 30 mg/kg, p < 0.05)]. Similarly, 3 and 30 mg/kg MTEP increased omissions [(main effect of dose, F 2,41 = 14.7, p < 0.001, X 2 = 53, GG ε = 0.57) (3 mg/kg, p < 0.01; 30 mg/kg, p < 0.001)]. MTEP also significantly decreased the speed of responding compared with the vehicle control group [(main effect of dose, F 2,42 = 5.4, p < 0.01, X 2 = 32, GG ε = 0.58) (1 mg/kg, p < 0.05; 3 mg/kg, p < 0.01; 30 mg/kg, p < 0.001)].
The effects of ADX47273 on 5-CSRTT performance in HI and LI rats are shown in Fig. 4a–d. ADX47273 significantly decreased premature responding in HI and LI rats (main effect of dose, F 3,59 = 5.3, p < 0.01, X 2 = 20, GG ε = 0.70) at all doses tested (p < 0.05) and had no significant effect on omissions (Fig. 4b) or accuracy (Fig. 4c). Despite correct response latencies being consistently slower in LI rats compared with HI rats (main effect of group, F 1,21 = 10.7, p < 0.01), ADX47273 significantly increased the latency to respond correctly in both HI and LI rats (main effect of dose, F 4,84 = 5.1, p < 0.01) (Fig. 4d) at the three highest doses tested (60 mg/kg, p < 0.01; 80 mg/kg, p < 0.001; and 100 mg/kg, p < 0.01). Extending the ITI to 7 s robustly increased premature responding in vehicle-treated HI and LI rats compared with the performance under a 5-s ITI (main effect of ITI; F 1,21 = 32.1, p < 0.001) (Fig. 4e). ADX47273 decreased premature responding in both HI and LI rats (main effect of dose, F 2,33 = 12.6, p < 0.001, X 2 = 35, GG ε = 0.58) under a 7-s ITI, reaching significance at 60, 80, and 100 mg/kg (p < 0.01, p < 0.001 and p < 0.01, respectively) (Fig. 4f).
Effect of ADX47273 on 5-CSRTT performance in HI (n = 12) and LI (n = 11) rats: a per cent premature responses, b per cent omissions, c per cent accuracy and d correct response latency (s) under a 5-s ITI. e Comparison of per cent premature responses in HI and LI rats at 5-s ITI versus 7-s ITI, f per cent premature responses under a 7-s ITI. Data represent mean ± SEM. Repeated measures ANOVA, Dunnett’s post hoc test. # p < 0.05 HI versus LI; ¥ p < 0.05, ¥¥ p < 0.01, ¥¥¥ p < 0.001 (HI and LI combined) versus vehicle control
Experiment 4: interactive effects of MK801 on RO4917523, MTEP and ADX47273 pre-treatment in MI rats
Based on preliminary studies, doses of 0.03 and 0.06 mg/kg were selected for the two cohorts of rats used for the MK801 studies (Supplementary Fig. 2 and Fig. 3). Both doses increased premature responding without substantially affecting other task parameters.
The interaction of RO4917523 pre-treatment on the effects of MK801 is shown in Fig. 5. As expected, MK801 increased premature responding in MI rats (main effect of drug, F 1,7 = 32.5, p < 0.01; Fig. 5a). In the absence of RO4917523, MK801 increased premature responding from 4.6 ± 1.3 to 43 ± 14.2 % (Fig. 5a). RO4917523 pre-treatment significantly modulated premature responding (main effect of dose, F 4,28 = 3.3, p < 0.05); this effect depended on dose and the presence and absence of MK801 (dose × group interaction, F 4,28 = 2.9, p < 0.05). Higher doses of RO4917523 (0.3 and 1 mg/kg) decreased premature responding. Post hoc analysis revealed significant differences in premature responding between vehicle- and MK801-treated rats following vehicle (p < 0.01), 0.03 and 0.1 mg/kg (p < 0.05) RO4917523, a contrast that was abolished following 0.3 mg/kg RO4917523 pre-treatment. In addition, premature responding following 1 mg/kg RO4917523 pre-treatment was significantly reduced when compared with the maximal response to MK801 and RO4917523 at 0.1 mg/kg (0.1 mg/kg vs 1 mg/kg, p < 0.05). RO4917523 pre-treatment also significantly increased omissions (main effect of dose, F 4,28 = 34.0, p < 0.001), latencies to respond correctly (main effect of dose, F 4,28 = 3.6, p < 0.05) and impaired attentional accuracy (main effect of dose, F 4,28 = 6.4, p < 0.01) at the same doses that significantly decreased premature responding. These effects were more pronounced in vehicle-treated animals, as shown by significant dose × group interactions for omissions (F 4,28 = 10, p < 0.001), correct latencies (F 4,28 = 6.8, p < 0.01) and accuracy (F 4,28 = 4, p < 0.05). Furthermore, there was a significant difference in omissions and attentional accuracy between vehicle- and MK801-treated rats at the highest dose of RO4917523 tested (p < 0.01 and p < 0.05, respectively).
Effect of MK801 (0.06 mg/kg) on 5-CSRTT performance in MI rats following RO4917523 pretreatment (n = 8): a per cent premature responses, b per cent omissions, c per cent accuracy and d correct response latency (s). Bars represent means ± SEM. Repeated measures ANOVA, paired student’s t test. # p < 0.05, ## p < 0.01 vehicle versus MK801; Bonferroni post hoc test. + p < 0.05 versus 0.1 mg/kg; *p < 0.05, **p < 0.01, ***p < 0.001 versus relative vehicle control. Premature responding data were transformed [SQRT (% premature + 1)] to satisfy the requirement of homogeneity of variance
A summary of the effects of 0.06 mg/kg MK801 after MTEP administration is shown in Table 4. MK801 significantly increased premature responding (main effect of drug, F 1,7 = 19.6, p < 0.01); in the absence of MTEP, MK801 increased premature responding from 4.25 ± 0.77 % to 49 ± 15.43 %. MTEP had no significant effect on this response but significantly increased omissions (main effect of dose, F 2,11 = 16.8, p < 0.001, X 2 = 19, GG ε = 0.4) in both MK801- and vehicle-treated rats, at all doses tested (1 mg/kg, p < 0.05; 3 mg/kg, p < 0.01; 10 and 30 mg/kg, p < 0.001). In addition, the latency to respond correctly was significantly decreased in MK801-treated rats versus control animals (main effect of drug, F 1,6 = 16.9, p < 0.01). MTEP also increased response latencies in both treatment groups (main effect of dose, F 4,24 = 13.8, p < 0.001). This effect was apparent at doses of 3 mg/kg (p < 0.05), 10 mg/kg (p < 0.01) and 30 mg/kg (p < 0.05). At the same doses, MTEP decreased attentional accuracy (main effect of dose, F 4,28 = 7.8, p < 0.001; 3 and 10 mg/kg, p < 0.001; 30 mg/kg, p < 0.01).
The effects of ADX47273 pre-treatment on the response of rats to MK801 are shown in Fig. 6. In this experiment, a lower dose of 0.03 mg/kg MK801 was employed, which robustly and selectively increased premature responding (main effect, F 3,42 = 10.5, p < 0.001) from 3.6 ± 0.8 % to 27.5 ± 5.7 % in the absence of ADX47273 (p < 0.001). Both doses of ADX57273 (80 and 100 mg/kg, p < 0.01) significantly attenuated this response such that premature responses in saline control animals were no longer different to those in animals treated with combined MK801 and ADX47273 (Fig. 6a). MK801 also significantly decreased accuracy from 76.24 ± 1.9 to 65.05 ± 2.36 % (main effect, F 3,42 = 5.3, p < 0.01), a deficit that was rescued by pre-treatment with ADX47273 (80 mg/kg, p < 0.05; 100 mg/kg, p < 0.01) (Fig. 6c). Omissions (Fig. 6b) and correct latency (Fig. 6d) were unaffected by either MK801 alone or in the presence of ADX47273.
Effect of MK801 (0.03 mg/kg) on 5-CSRTT performance in MI rats following ADX47273 pretreatment (n = 11–12 per group): a per cent premature responses, b per cent omissions, c per cent accuracy and d correct response latency (s). Bars represent means ± SEM. One-way ANOVA, Bonferroni post hoc test. *p < 0.05, ***p < 0.001 versus saline; + p < 0.05, ++p < 0.01 versus vehicle-MK801
Experiment 5: effects of RO4917523, MTEP and ADX47273 on delay-discounting
The effects of RO4917523, MTEP and ADX47273 pre-treatment on delay-discounting performance are shown in Fig. 7. In all cases, as the delay to the larger reward increased, the choice for that reward decreased (RO4917523; main effect of delay, F 2,115 = 121.9, p < 0.001, X 2 = 93, GG ε = 0.49; MTEP, main effect of delay, F 2,37 = 106.5, p < 0.001, X 2 = 90, GG ε = 0.44; ADX47273; main effect of delay, F 2,37 = 158.2, p < 0.001, X 2 = 123, GG ε = 0.39). However, pre-treatment with RO4917523, MTEP or ADX47273 had no effect on choice behaviour, nor did it affect general task performance, as shown by a lack of effect on omissions.
Discussion
This study investigated the role of mGluR5 in modulating impulsive behaviour on two distinct tasks assessing ‘waiting’ impulsivity using the NAMs RO4917523 and MTEP, and the PAM ADX47273. Our findings indicate a prominent role of mGluR5 in suppressing responses made pre-potent by their association with reward rather than in determining response preference for immediate and delayed rewards (i.e. on temporal discounting). This distinction is theoretically important as both forms of impulsivity appear to depend on subtly different substrates of the nucleus accumbens (Dalley et al. 2011) and have been referred to collectively as exemplary of ‘waiting impulsivity’ in contradistinction to another form of ‘stopping’ impulsivity mediated by the dorsal striatum rather than the nucleus accumbens (Dalley et al. 2011, for a review).
In agreement with a previous study (Semenova and Markou 2007), negative mGluR5 allosteric modulation by RO4917523 and MTEP impaired 5-CSRTT performance. Aside from decreasing premature responding, RO4917523 administration was accompanied by an increase in omissions and response latencies and by a reduction in indices of motor function, namely in rearing and distanced moved, similar to the effects of MTEP. Thus, the effect of RO4917523 to diminish impulsivity was most likely the result of non-specific motoric and/or sedative effects. Indeed, EEG studies in rats demonstrate that mGluR5 NAMs affect distinct phases of the sleep cycle by enhancing deep sleep whilst suppressing REM sleep (Harvey et al. 2013) (Ahnaou et al. 2015). It is noteworthy that RO4921523 (0.1 mg/kg) produced a significant decrease in premature responding and increase in omissions but had no effect on response latencies, rearing behaviour or ambulation. Thus, although attentional accuracy was unaffected by RO4917523, and to some extent MTEP, the concurrent increase observed in omissions suggests that mGluR5 may play some role in attentional processing (Semenova and Markou 2007). Our findings also reveal a novel interaction between NMDA receptor antagonism and positive allosteric modulation of mGluR5 in diminishing impulsive behaviour. Thus, consistent with a cognitive enhancing influence, ADX47273 not only decreased premature responding in all groups tested, but it also attenuated the increase in impulsivity and reversed the attentional impairment produced by the acute systemic administration of MK801.
Although RO4917523 and MTEP exerted similar effects on many task parameters, the inconsistent effects on premature responding are unclear. Both compounds show high selectivity towards mGluR5, reducing the risk of off-target drug–receptor interactions (Anderson et al. 2003; Jaeschke et al. 2015). However, MTEP does exhibit a much lower affinity for mGluR5 compared with RO4917523 (Busse et al. 2004; Jaeschke et al. 2015). Despite compensatory increases in the doses of MTEP administered in the present study, MTEP failed to have any effect on impulsivity and produced a less pronounced increase in omissions and response latencies compared with RO4917523. Since MTEP had no effect on premature responding even at a presumed 100 % mGluR5 receptor occupancy (Busse et al. 2004), these findings confirm that MTEP has a lower potency to modulate mGluR5 compared with RO4917523.
Synergistic interactions between NMDA receptors and mGluR5 have been reported both electrophysiologically and behaviourally (Awad et al. 2000; Campbell et al. 2004; Henry et al. 2002; Homayoun and Moghaddam 2006; Homayoun et al. 2004; Kinney et al. 2003; Lecourtier et al. 2007; Pisani et al. 2001; Rosenbrock et al. 2010). Potentiation of NMDA-induced intracellular calcium mobilisation by mGluR5 activation has been hypothesised to underlie this interaction (Rosenbrock et al. 2010). Based on these studies, we hypothesised that negative allosteric modulators of mGluR5 would potentiate the behavioural deficits induced by NMDA receptor antagonism. Supporting this hypothesis, MPEP (an mGluR5 antagonist) has been shown to potentiate various behaviours and behavioural deficits produced by NMDA receptor antagonism, including hyper-locomotion, impaired pre-pulse inhibition and deficits in mnemonic function (Campbell et al. 2004; Henry et al. 2002; Homayoun et al. 2004; Kinney et al. 2003). However, mGluR5 modulation has also been reported to differentially modulate cognitive and motor function in rats (Gastambide et al. 2013).
In the present study, MTEP pre-treatment failed to have any effect on 5-CSRTT performance following MK801 administration. This apparent discrepancy may reflect the differing pharmacological profiles of MPEP and negative mGluR5 allosteric modulators. Despite MPEP’s high affinity for mGluR5 (Porter et al. 2005), this compound has low in vivo potency (Nordquist et al. 2007). Furthermore, non-specific off-target effects may underlie the behavioural discrepancies observed in the present study. Whereas both MTEP and RO4917523 show high selectivity due to their allosteric properties, (Anderson et al. 2003; Cosford et al. 2003; Jaeschke et al. 2015; Lea and Faden 2006), MPEP also affects the noradrenaline transporter (Heidbreder et al. 2003), NMDA receptors and monoamine oxidase-A (Lea and Faden 2006), which may contribute to the modulation of impulsive behaviour (Carli et al. 2004; Dalley and Roiser 2012; Pattij and Vanderschuren 2008; Winstanley et al. 2006). Alternatively, the lack of effect of MTEP on behavioural changes evoked by MK801 may have been due to a ceiling effect on premature responses. Here, it is interesting to note that low doses of RO4917523 (0.03–0.01 mg/kg, Fig. 5a), which had no effect on omissions, response latencies or two locomotor activity parameters, appeared to potentiate the effect of MK801 on premature responding. However, this effect was highly variable between animals.
Positive allosteric modulation of mGluR5 with ADX47273 dose-dependently attenuated the disruptive effects of MK801 on response inhibition and visual attention. These findings provide further support for a functional interaction between NMDA receptors and mGluR5, as discussed above, and show that positive mGluR5 allosteric modulation is sufficient to reverse behavioural and cognitive deficits associated with NMDA receptor hypofunction. Similar cognitive enhancing effects of mGluR5 PAMs have been reported in relation to behavioural flexibility, learning and memory, executive control and social cognition (Clifton et al. 2013; Darrah et al. 2008; Fowler et al. 2011; Stefani and Moghaddam 2010; Uslaner et al. 2009). Although we cannot rule out possible off-target effects of ADX47273, this would appear unlikely as the enhancing effects of ADX47273 was blocked by a selective mGluR5 antagonist (Clifton et al 2013). Furthermore, since we dosed ADX47273 orally, plasma levels were comparable to studies using intraperitoneal dosing (Schlumberger et al. 2009).
Importantly, systemic administration of ADX47273 selectively reduced baseline premature responding in all rats tested as well as impulsivity evoked by lengthening the ITI (i.e., the waiting period prior to stimulus onset). Such effects were not accompanied by global impairments in motor activity with doses as high as 80 mg/kg. Furthermore, ADX47273 did not affect the number of omissions or latencies to respond and collect food reward. Our findings are thus consistent with an earlier study showing ADX47273 to selectively reduce impulsivity when the ITI is increased and hyperactivity induced by NMDA receptor antagonism (Liu et al. 2008). However, we now extend these findings by showing that deficits in visual attention and impulse control induced by NMDA receptor antagonism can be restored by positive mGluR5 allosteric modulation.
The neural mechanisms responsible for the observed interactive effects of NMDA receptor antagonism and mGluR5 allosteric modulation on attentional control processes are unclear but may involve modulation of glutamate release in the medial prefrontal cortex (mPFC). Microdialysis studies have shown that NMDA receptor antagonists cause excessive neuronal firing (Jackson et al. 2004; Lecourtier et al. 2007), leading to increased extracellular glutamate efflux in the mPFC of freely moving rats (Adams and Moghaddam 1998; Ceglia et al. 2004; Moghaddam and Adams 1998; Moghaddam et al. 1997). It has been hypothesised that altered glutamatergic tone in the mPFC may underpin changes in impulsivity following administration of an NMDA receptor antagonist (Ceglia et al. 2004; Moghaddam and Adams 1998; Moghaddam et al. 1997; Pozzi et al. 2011). Indeed, an mGluR2/3 agonist has been shown to block both the increase in glutamate efflux in the mPFC and impulsivity resultant from NMDA receptor antagonism (Pozzi et al. 2011).
However, it remains unclear how NMDA receptor antagonists increase neuronal firing and extracellular glutamate release in the PFC, an effect described recently as ‘paradoxical’ (Pozzi et al. 2011). Within the PFC, there is a high density of inhibitory GABAergic interneurons that project onto and inhibit excitatory glutamatergic pyramidal neurons. A prevailing hypothesis is that increased glutamate release in the PFC depends on the inhibition of NMDA receptors predominantly expressed by GABAergic interneurons. Inhibition of GABAergic interneurons disinhibits glutamatergic neurons resulting in increased glutamate release (Moghaddam et al. 1997). Consistent with this hypothesis, intra-PFC administration of PCP and MK801 reduced extracellular levels of GABA in the PFC (Yonezawa et al. 1998). This effect depended on NMDA receptor antagonism as co-perfusion with NMDA reduced the effects of PCP and MK801 on extracellular GABA levels (Yonezawa et al. 1998). Interestingly, MK801 predominantly decreased the firing rate of GABAergic interneurons and, at a delayed rate, increased the firing activity of glutamatergic pyramidal neurons, suggesting that inhibition of GABAergic interneurons precedes the disinhibition of glutamatergic neurons (Homayoun and Moghaddam 2007).
Positive allosteric modulation of mGluR5 by ADX47273 may partly exert its restorative effects on impaired impulse control by indirectly decreasing glutamatergic tone in the mPFC. Thus, it has been demonstrated that 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)-benzamide (CDPPB), an mGluR5 PAM, attenuates MK801-induced neuronal activity and spontaneous burst firing of mPFC neurons in freely moving rats (Lecourtier et al. 2007). The behavioural effects of ADX47273 may be exerted by mGluR5-expressing GABAergic interneurons in the mPFC. Indeed, it has previously been demonstrated that activation of mGluR5 potentiates GABAergic inhibition of glutamatergic pyramidal neurons through excitation of GABAergic interneurons (Chu and Hablitz 1998). This effect may be sufficient to counteract the increase in glutamatergic tone in the mPFC following NMDA receptor blockade by reducing the excitation of glutamatergic pyramidal neurons and glutamate release in this region.
In addition to effects on glutamate and GABA function, NMDA receptor antagonists and mGluR5 modulators may interact at the level of the mesolimbic and mesocortical dopamine systems. For example, the NMDA receptor antagonists PCP and MK801 increase dopamine release in the PFC and nucleus accumbens (Adams and Moghaddam 1998; Homayoun et al. 2004). Since dopamine inputs to the nucleus accumbens are important determinants of impulsivity on this task (Cole and Robbins 1987; Van Gaalen et al. 2006), the effect of ADX47273 in reducing impulsivity may be mediated in part through effects on dopamine transmission in this region. Indeed, it has been shown that systemic administration of ADX47273 decreases dopamine release in the nucleus accumbens but not the dorsal striatum (Liu et al. 2008). However, the dependence of this effect on positive mGluR5 allosteric modulation requires further research as CDPPB was reported to have no effect on dopamine release in either the nucleus accumbens or the mPFC (Lecourtier et al. 2007). Furthermore, MPEP has been reported to potentiate the effects of MK801 on dopamine release in the PFC (Homayoun et al. 2004).
Our findings show that ADX47273 reduced premature responding on the 5-CSRTT but had no effect on the sensitivity of animals for discounting delayed, relatively large magnitude rewards. Similarly, negative mGluR5 allosteric modulation by RO4917523 and MTEP had no effect on this dissociable aspect of ‘waiting’ impulsivity. A recent study provides additional evidence for this distinction; a selective mGluR1 antagonist, EMQMCM, reportedly enhanced motor impulsivity in the differential reinforcement of low rates (DRL) task but attenuated choice impulsivity by increasing tolerance of delayed rewards (Sukhotina et al. 2008). Furthermore, MK801 reportedly improved choice impulsivity by appearing to decrease sensitivity to delayed reinforcement (Yates et al. 2014). This is in stark contrast to the behavioural effect that we observe on motor impulsivity in the present study. Neurally, the orbital frontal cortex (OFC) has consistently been implicated in modulating choice impulsivity. Thus, lesions to the OFC promote a shift in preference for smaller, immediate rewards, over larger delayed rewards in rats (Mar et al. 2011; Mobini et al. 2002; Rudebeck et al. 2006). An involvement of the OFC in choice impulsivity is further supported by the work of Winstanley et al. (2006), who reported increased dopamine release and molecular changes in this region during the choice phase of delayed discounting. In contrast, lesions to the mPFC reportedly had no effect on impulsive choice (Cardinal et al. 2001). However, the mPFC, but not the OFC, plays a prominent role in modulating motor impulsivity (Chudasama et al. 2003; Muir et al. 1996; Murphy et al. 2005, 2012; Pezze et al. 2009). While mGluR5 expression in the PFC has consistently been reported (Gupta et al. 2005; Romano et al. 1995; Shigemoto et al. 1993), evidence of mGluR5 expression in the mPFC or OFC sub-regions of the PFC specifically is limited. Anatomical selectivity of mGluR5 modulation towards the mPFC versus the OFC may be one possible explanation for the dissociable behavioural effects on motor and choice impulsivity observed in the present study; however, more specific sub-regional mGluR5 expression studies are required to confirm this. Collectively, these data suggest that the glutamatergic system in the OFC may be involved in regulating choice behaviour for delayed gratification; however, these effects appear to be mGluR sub-type specific.
In summary, we have demonstrated that only one form of ‘waiting impulsivity’ is subject to modulation by allosteric mGluR5 modulators, perhaps reflecting a distinction between ‘choice’ and ‘motor’ forms. In particular, positive allosteric modulation of mGluR5 was efficacious in decreasing not only pharmacologically evoked state impulsivity but also pre-existing trait impulsivity. In addition, we have further highlighted the prominent functional interactions that exist between mGluR5 and NMDA receptors. This study encourages the further investigation of the role of mGluR5 in impulsive behaviour and introduces the possible utility of mGluR5 PAMs as cognitive enhancers and potential therapeutic treatments for targeting specific aspects of maladaptive impulsivity.
References
Adams B, Moghaddam B (1998) Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18:5545–5554
Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A (1999) Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry 156:1646–1649
Agnoli L, Carli M (2012) Dorsal–striatal 5-HT2A and 5-HT2C receptors control impulsivity and perseverative responding in the 5-choice serial reaction time task. Psychopharmacology 219:633–645. doi:10.1007/s00213-011-2581-0
Ahnaou A, Langlois X, Steckler T, Bartolome-Nebreda JM, Drinkenburg WHIM (2015) Negative versus positive allosteric modulation of metabotropic glutamate receptors (mGluR5): indices for potential pro-cognitive drug properties based on EEG network oscillations and sleep-wake organization in rats. Psychopharmacology 232:1107–1122. doi:10.1007/s00213-014-3746-4
Ainslie G (1975) Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82:463–496
Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford NDP, Varney MA (2003) In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine). Eur J Pharmacol 473:35–40
Aron AR, Poldrack RA (2005) The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1285–1292. doi:10.1016/j.biopsych.2004.10.026
Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ (2000) Activation of metabotropic glutamate receptor 5 Has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci 20:7871–7879
Bari A, Dalley JW, Robbins TW (2008) The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protocols 3:759–767. doi:10.1038/nprot.2008.41
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355
Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND (2004) The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[lsqb](2-methyl-1,3-thiazol-4-yl)ethynyl[rsqb]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29:1971–1979
Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ (2004) The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl) 175:310–318. doi:10.1007/s00213-004-1827-5
Cardinal RN, Aitken MRF (2010) Whisker: a client-server high-performance multimedia research control system. Behav Res Methods 42:1059–1071. doi:10.3758/BRM.42.4.1059
Cardinal RN, Pennicott DR, Lakmali C, Robbins TW, Sugathapala CL, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499–2501
Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ (2004) Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci 1021:33–50. doi:10.1196/annals.1308.004
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Carli M, Baviera M, Invernizzi R, Balducci C (2004) The Serotonin 5-HT2A Receptors Antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology 29. doi:10.1038/sj.npp.1300479
Ceglia I, Carli M, Baviera M, Renoldi G, Calcagno E, Invernizzi RW (2004) The 5-HT2A receptor antagonist M100,907 prevents extracellular glutamate rising in response to NMDA receptor blockade in the mPFC. J Neurochem 91:189–199. doi:10.1111/j.1471-4159.2004.02704.x
Chu Z, Hablitz JJ (1998) Activation of group I mGluRs increases spontaneous IPSC frequency in rat frontal cortex. J Neurophysiol 80:621–627
Chudasama Y, Baunez C, Robbins TW (2003) Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci 23:5477–5485
Clifton NE, Morisot N, Girardon S, Millan MJ, Loiseau F (2013) Enhancement of social novelty discrimination by positive allosteric modulators at metabotropic glutamate 5 receptors: adolescent administration prevents adult-onset deficits induced by neonatal treatment with phencyclidine. Psychopharmacology (Berl) 225:579–594. doi:10.1007/s00213-012-2845-3
Cole BJ, Robbins TW (1987) Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology 91:458–466. doi:10.1007/BF00216011
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. doi:10.1146/annurev.pharmtox.37.1.205
Cosford NDP, Roppe J, Tehrani L, Schweiger EJ, Seiders TJ, Chaudary A, Rao S, Varney MA (2003) [3H]-Methoxymethyl-MTEP and [3H]-Methoxy-PEPy: potent and selective radioligands for the metabotropic glutamate subtype 5 (mGlu5) receptor. Bioorg Med Chem Lett 13:351–354
Crunelle CL, Veltman DJ, van Emmerik-van Oortmerssen K, Booij J, van den Brink W (2013) Impulsivity in adult ADHD patients with and without cocaine dependence. Drug Alcohol Depend 129:18–24. doi:10.1016/j.drugalcdep.2012.09.006
Dalley JW, Roiser JP (2012) Dopamine, serotonin and impulsivity. Neuroscience 215:42–58. doi:10.1016/j.neuroscience.2012.03.065
Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron J-C, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270. doi:10.1126/science.1137073
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top–down cognitive control. Neuron 69:680–694. doi:10.1016/j.neuron.2011.01.020
Darrah JM, Stefani MR, Moghaddam B (2008) Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol 19:225–234. doi:10.1097/FBP.0b013e3282feb0ac
De Paulis T, Hemstapat K, Chen Y, Zhang Y, Saleh S, Alagille D, Baldwin RM, Tamagnan GD, Conn PJ (2006) Substituent effects of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides on positive allosteric modulation of the metabotropic glutamate-5 receptor in Rat cortical astrocytes. J Med Chem 49:3332–3344. doi:10.1021/jm051252j
De Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22–31. doi:10.1111/j.1369-1600.2008.00129.x
Deakin JFW, Slater P, Simpson MDC, Gilchrist AC, Skan WJ, Royston MC, Reynolds GP, Cross AJ (1989) Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J Neurochem 52:1781–1786. doi:10.1111/j.1471-4159.1989.tb07257.x
Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, De Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301–308. doi:10.1016/j.biopsych.2007.07.011
Doherty A, Palmer M, Henley J, Collingridge G, Jane D (1997) (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology 36:265–267. doi:10.1016/S0028-3908(97)00001-4
Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ (2009) High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry 65:851–856. doi:10.1016/j.biopsych.2008.12.008
Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW (2010) Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry 68:770–773. doi:10.1016/j.biopsych.2010.06.015
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146:348–361. doi:10.1007/PL00005481
Fletcher PJ, Rizos Z, Noble K, Higgins GA (2011) Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT2C receptor stimulation and 5-HT2A receptor blockade. Neuropharmacology 61:468–477. doi:10.1016/j.neuropharm.2011.02.025
Fowler SW, Ramsey AK, Walker JM, Serfozo P, Olive MF, Schachtman TR, Simonyi A (2011) Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiol Learn Mem 95:73–79. doi:10.1016/j.nlm.2010.11.009
Gaalen M, Brueggeman R, Bronius PC, Schoffelmeer AM, Vanderschuren LMJ (2006) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology 187:73–85. doi:10.1007/s00213-006-0396-1
Gass JT, Osborne MPH, Watson NL, Brown JL, Olive MF (2008) mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 34:820–833
Gastambide F, Gilmour G, Robbins TW, Tricklebank MD (2013) The mGlu5 positive allosteric modulator LSN2463359 differentially modulates motor, instrumental and cognitive effects of NMDA receptor antagonists in the rat. Neuropharmacology 64:240–247. doi:10.1016/j.neuropharm.2012.07.039
Goff DC, Coyle JT (2001) The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158:1367–1377
Greco B, Invernizzi R, Carli M (2005) Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU2/3 receptor agonist LY379268. Psychopharmacology 179:68–76. doi:10.1007/s00213-004-2127-9
Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH (2005) Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57:123–131. doi:10.1002/syn.20164
Harvey BD, Siok CJ, Kiss T, Volfson D, Grimwood S, Shaffer CL, Hajós M (2013) Neurophysiological signals as potential translatable biomarkers for modulation of metabotropic glutamate 5 receptors. Neuropharmacology 75:19–30. doi:10.1016/j.neuropharm.2013.06.020
Heidbreder CA, Bianchi M, Lacroix LP, Faedo S, Perdona E, Remelli R, Cavanni P, Crespi F (2003) Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse 50:269–276. doi:10.1002/syn.10261
Henry S, Lehmann-Masten V, Gasparini F, Geyer M, Markou A (2002) The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology 43:1199–1209. doi:10.1016/S0028-3908(02)00332-5
Hester R, Garavan H (2004) Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24:11017–11022. doi:10.1523/JNEUROSCI.3321-04.2004
Higgins GA, Enderlin M, Haman M, Haman PI (2003) The 5-HT2A receptor antagonist M100,907 attenuates motor and “impulsive-type” behaviours produced by NMDA receptor antagonism. Psychopharmacology 170:309–319. doi:10.1007/s00213-003-1549-0
Homayoun H, Moghaddam B (2006) Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex 16:93–105. doi:10.1093/cercor/bhi087
Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500
Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B (2004) Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 29:1259–1269
Jackson ME, Homayoun H, Moghaddam B (2004) NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A 101:8467–8472
Jaeschke G, Kolczewski S, Spooren W, Vieira E, Bitter-Stoll N, Boissin P, Borroni E, Büttelmann B, Ceccarelli S, Clemann N, David B, Funk C, Guba W, Harrison A, Hartung T, Honer M, Huwyler J, Kuratli M, Niederhauser U, Pähler A, Peters J-U, Petersen A, Prinssen E, Ricci A, Rueher D, Rueher M, Schneider M, Spurr P, Stoll T, Tännler D, Wichmann J, Porter RH, Wettstein JG, Lindemann L (2015) Metabotropic glutamate receptor 5 negative allosteric modulators: discovery of 2-chloro-4-[1-(4-fluorophenyl)-2,5-dimethyl-1H-imidazol-4-ylethynyl]-pyridine (Basimglurant, RO4917523), a promising novel medicine for psychiatric diseases. J Med Chem. doi:10.1021/jm501642c
Kaladjian A, Jeanningros R, Azorin J-M, Anton J-L, Mazzola-Pomietto P (2011) Impulsivity and neural correlates of response inhibition in schizophrenia. Psychol Med 41:291–299. doi:10.1017/S0033291710000796
Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ (2003) Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther 306:116–123. doi:10.1124/jpet.103.048702
Konradi C, Heckers S (2003) Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther 97:153–179. doi:10.1016/S0163-7258(02)00328-5
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Lahti AC, Weiler MA, Michaelidis T, Parwani A, Tamminga CA (2001) Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25:455–467
Lea PM, Faden AI (2006) Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev 12:149–166. doi:10.1111/j.1527-3458.2006.00149.x
Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B (2007) Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry 62:739–746. doi:10.1016/j.biopsych.2006.12.003
Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29:14734–14740. doi:10.1523/JNEUROSCI.3765-09.2009
Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL Jr, Sur C, Kinney GG (2006) Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem 6:771–785
Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL (2008) ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327:827–839
Luby C (1959) STudy of a new schizophrenomimetic drug—sernyl. A.M.A. Arch Neurol Psychiatry 81:363–369. doi:10.1001/archneurpsyc.1959.02340150095011
Mar AC, Robbins TW (2007) Delay discounting and impulsive choice in the rat. Curr Protoc Neurosci Chapter 8, Unit 8.22. doi:10.1002/0471142301.ns0822s39
Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW (2011) Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci 31:6398–6404. doi:10.1523/JNEUROSCI.6620-10.2011
Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2002) Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 160:290–298. doi:10.1007/s00213-001-0983-0
Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001a) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793. doi:10.1176/appi.ajp.158.11.1783
Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J (2001b) The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198
Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Muir JL, Everitt BJ, Robbins TW (1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6:470–481. doi:10.1093/cercor/6.3.470
Murphy E, Dalley J, Robbins T (2005) Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology 179:99–107. doi:10.1007/s00213-004-2068-3
Murphy E, Fernando AP, Urcelay G, Robinson EJ, Mar A, Theobald DH, Dalley J, Robbins T (2012) Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology 219:401–410. doi:10.1007/s00213-011-2572-1
Nakanishi S (1992) Molecular diversity of glutamate receptors and implications for brain function. Science 258:597–603. doi:10.1126/science.1329206
Nakanishi S, Masu M (1994) Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct 23:319–348. doi:10.1146/annurev.bb.23.060194.001535
Nordquist RE, Durkin S, Jaeschke G, Spooren W (2007) Stress-induced hyperthermia: effects of acute and repeated dosing of MPEP. Eur J Pharmacol 568:199–202. doi:10.1016/j.ejphar.2007.04.034
Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA Jr (2007) Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague–Dawley rats. Biol Psychiatry 62:687–693. doi:10.1016/j.biopsych.2006.11.017
Pattij T, Vanderschuren LJMJ (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29:192–199. doi:10.1016/j.tips.2008.01.002
Pezze MA, Dalley JW, Robbins TW (2009) Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D2/3 receptor antagonist sulpiride. Psychopharmacology 202:307–313. doi:10.1007/s00213-008-1384-4
Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P (2001) Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106:579–587. doi:10.1016/S0306-4522(01)00297-4
Porter RHP, Jaeschke G, Spooren W, Ballard TM, Büttelmann B, Kolczewski S, Peters J-U, Prinssen E, Wichmann J, Vieira E, Mühlemann A, Gatti S, Mutel V, Malherbe P (2005) Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther 315:711–721. doi:10.1124/jpet.105.089839
Pozzi L, Baviera M, Sacchetti G, Calcagno E, Balducci C, Invernizzi RW, Carli M (2011) Attention deficit induced by blockade of N-methyl D-aspartate receptors in the prefrontal cortex is associated with enhanced glutamate release and cAMP response element binding protein phosphorylation: role of metabotropic glutamate receptors 2/3. Neuroscience 176:336–348. doi:10.1016/j.neuroscience.2010.11.060
Robbins T (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163:362–380. doi:10.1007/s00213-002-1154-7
Romano C, Sesma MA, McDonald CT, O’malley K, van den Pol AN, Olney JW (1995) Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355:455–469. doi:10.1002/cne.903550310
Rosenbrock H, Kramer G, Hobson S, Koros E, Grundl M, Grauert M, Reymann KG, Schröder UH (2010) Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. Eur J Pharmacol 639:40–46. doi:10.1016/j.ejphar.2010.02.057
Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS (2006) Separate neural pathways process different decision costs. Nat Neurosci 9:1161–1168. doi:10.1038/nn1756
Schlumberger C, Pietraszek M, Gravius A, Klein K-U, Greco S, Morè L, Danysz W (2009) Comparison of the mGlu5 receptor positive allosteric modulator ADX47273 and the mGlu2/3 receptor agonist LY354740 in tests for antipsychotic-like activity. Eur J Pharmacol 623:73–83. doi:10.1016/j.ejphar.2009.09.006
Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299:12–20
Schoepp DD, Conn PJ (2002) Metabotropic glutamate receptors. Pharmacol, Biochem Behav 74:255–256. doi:10.1016/S0091-3057(02)00953-X
Semenova S, Markou A (2007) The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats’ performance in the 5-choice serial reaction time task. Neuropharmacology 52:863–872. doi:10.1016/j.neuropharm.2006.10.003
Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163:53–57. doi:10.1016/0304-3940(93)90227-C
Stefani MR, Moghaddam B (2010) Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol 639:26–32. doi:10.1016/j.ejphar.2010.01.028
Sukhotina IA, Dravolina OA, Novitskaya Y, Zvartau EE, Danysz W, Bespalov AY (2008) Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats. Psychopharmacology (Berl) 196:211–220. doi:10.1007/s00213-007-0953-2
Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JSH, McNaughton CH, Jacobson MA, Hutson PH (2009) Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 57:531–538. doi:10.1016/j.neuropharm.2009.07.022
Varty G, Grilli M, Forlani A, Fredduzzi S, Grzelak M, Guthrie D, Hodgson R, Lu S, Nicolussi E, Pond A, Parker E, Hunter J, Higgins G, Reggiani A, Bertorelli R (2005) The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology 179:207–217. doi:10.1007/s00213-005-2143-4
Winstanley CA, Dalley JW, Theobald DEH, Robbins TW (2003) Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 170:320–331. doi:10.1007/s00213-003-1546-3
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26:379–395. doi:10.1016/j.cpr.2006.01.001
Yates JR, Batten SR, Bardo MT, Beckmann JS (2014) Role of ionotropic glutamate receptors in delay and probability discounting in the rat. Psychopharmacology (Berl). doi:10.1007/s00213-014-3747-3
Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H (1998) Involvement of γ-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol 341:45–56. doi:10.1016/S0014-2999(97)01435-0
Acknowledgements
This research was supported by a Medical Research Council (MRC) grant to JWD (G0701500) and a grant from Boehringer Ingelheim Pharma GmbH & Co. KG. This work was carried out in the Behavioural and Clinical Neuroscience Institute (BCNI) at Cambridge University with joint support from the MRC (G1000183) and Wellcome Trust (093875/Z/10/Z) and at Boehringer Ingelheim Pharma GmbH & Co. KG, Germany. We thank David Theobald, Johannes Freudenreich, Peter Schorn, Alfie Wearn and Benjamin Jaehnke for technical support and Gert Kramer, Dr. Holger Rosenbrock and Dr. Cornelia Dorner-Ciossek for helpful scientific discussions. The authors declare that the experiments performed in this manuscript followed the principles of laboratory animal care and are in compliance with the current laws of the UK and Germany.
Conflict of interest
The authors declare no conflict of interest.
Sarah Isherwood, Anton Pekcec and Janet Nicholson are employees of Boehringer Ingelheim Pharma GmbH & Co. KG
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 777 kb)
Rights and permissions
About this article
Cite this article
Isherwood, S.N., Pekcec, A., Nicholson, J.R. et al. Dissociable effects of mGluR5 allosteric modulation on distinct forms of impulsivity in rats: interaction with NMDA receptor antagonism. Psychopharmacology 232, 3327–3344 (2015). https://doi.org/10.1007/s00213-015-3984-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3984-0