Abstract
Rationale
Cannabinoid CB1 receptors are implicated in various forms of learning and memory, including acquisition and reinstatement of cocaine-associated memory. However, roles of CB1 receptors in consolidation and extinction processes of cocaine-associated memory and the brain areas potentially involved remain unknown.
Objective
This study examined the effect of rimonabant, a CB1 receptor antagonist, administered systemically or directly into the medial prefrontal cortex (mPFC) on memory consolidation and extinction of cocaine-induced conditioned place preference (CPP).
Materials and methods
Male C57BL/6J mice were trained to acquire cocaine-induced CPP. Rimonabant (0.1–3 mg/kg, i.p. or 1.5 μg bilaterally in the mPFC) or vehicle was administered either immediately after each CPP training (consolidation) or forced extinction (extinction) trial. Cocaine-induced CPP was tested after training, extinction, or cocaine priming.
Results
Systemic or intra-mPFC administration of rimonabant impaired consolidation of CPP induced by a high dose (20 or 40 mg/kg) of cocaine but facilitated that induced by a low dose (2.5, 5, or 10 mg/kg). Moreover, systemic or intra-mPFC administration of rimonabant enhanced extinction of CPP memory induced by a high-dose (20 mg/kg) cocaine.
Conclusion
Our results suggest that antagonism of CB1 receptors in the mPFC bidirectionally modulates consolidation but facilitates extinction of cocaine-induced CPP memory. Therefore, CB1 receptor blockade with the concomitant extinction behavioral procedure may hint important therapeutic intervention strategies for the heavy cocaine addicts in a clinical setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Addiction to the psychostimulant cocaine remains a serious public health problem and currently no effective pharmacotherapies are available. The greatest challenge for treating cocaine addiction is the persistence of psychological craving for cocaine even after prolonged abstinence. Drug-associated cue/context memory may contribute to such motivational changes. Indeed, research suggests that drug memory activated by drug-associated environmental cues or drug re-exposure plays an important role in priming subsequent drug-seeking behavior (Kuo et al. 2007). Therefore, drug-associated memory affects motivational behaviors in drug addiction, which are apparent in two popular animal behavioral paradigms for cocaine-associated memory: conditioned place preference (CPP) and self-administration.
Altered motivation/emotion and learning/memory processes associated with addiction may involve long-lasting neuroplasticity. The cannabinoid CB1 receptors are widely distributed in the brain areas implicated in motivation and memory, such as the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), amygdala and hippocampus (De Vries and Schoffelmeer 2005; Katona et al. 2001; Wilson and Nicoll 2002). Although CB1 receptors have been shown to modulate the primary rewarding effects of several drugs of abuse including cannabinoids, nicotine, alcohol, and opioids, however, they are not involved in that of cocaine (Maldonado et al. 2006). For instance, the CB1 receptor antagonist rimonabant, also known as SR141716A or SR1, does not affect cocaine self-administration in rats (Caille et al. 2007; Caille and Parsons 2006; Filip et al. 2006), mice (Fattore et al. 2007), and squirrel monkeys (Tanda et al. 2000). Moreover, CB1 knockout mice express normal cocaine-induced CPP and self-administration (Cossu et al. 2001; Houchi et al. 2005; Lesscher et al. 2005; Martin et al. 2000). These findings suggest that CB1 receptors do not modulate the primary motivational/rewarding effect of cocaine.
However, CB1 receptors seem to play an important role in cocaine-associated memory, thereby mediating persistence of cocaine addiction. For example, CB1 receptors are implicated in the reinstatement of cocaine self-administration. The cannabinoid receptor agonist HU210 induces, while rimonabant reduces, relapse elicited by cocaine-associated cues or cocaine re-exposure (De Vries and Schoffelmeer 2005; De Vries et al. 2001). Systemic or intra-accumbens (NAc) infusion of another CB1 antagonist AM251 not only impairs cocaine-induced reinstatement of cocaine-seeking behavior, but also inhibits the cocaine-induced release of glutamate but not dopamine in the NAc (Xi et al. 2006). Moreover, rimonabant blocks acquisition, but not expression, of cocaine-induced CPP in rats (Chaperon et al. 1998) and impairs acquisition and reinstatement of cocaine-induced CPP in mice as well (Yu et al. 2011).
However, it is important to point out that all of the above studies adopt a pretraining paradigm, which drugs are injected immediately before the training or testing trial. Because in this pretraining regimen the drug affects the motivation as well as memory processes associated with cocaine addiction, it is hard to discern the pure effect of the CB1 antagonists on cocaine-associated memory. Furthermore, it has little clinical significance to use a pretraining regimen to examine the effect of the CB1 antagonists on acquisition of cocaine-induced CPP memory as most cocaine addicts are treated afterwards. In order to clearly elucidate the role of CB1 receptors in cocaine-associated memory, we tested our hypothesis that systemic blockade of CB1 receptors after training profoundly affected the consolidation and extinction of cocaine-induced CPP. Since the net effect of a CB1 receptor antagonist has recently been shown to vary with the initial level of activity in the network (Piet et al. 2011), we also examined whether rimonabant exerted different effects on memory consolidation of CPP induced by various doses of cocaine.
Several brain areas such as the mPFC, basolateral amygdala, and hippocampus send glutamatergic efferents to the NAc, and all of these projections are implicated in drug relapse behavior (Kalivas et al. 2005; Shaham et al. 2003; Wiskerke et al. 2008). Moreover, inactivation of the mPFC attenuates drug-, cue-, and stress-induced reinstatement of cocaine-seeking (Shaham et al. 2003). Therefore, the mPFC may serve as a final common pathway to mediate drug relapse behavior (Neisewander et al. 2000; See 2002). Because we found that systemic blockade of CB1 receptors affected consolidation and extinction of cocaine-induced CPP as predicted above, we next tested if inactivation of the CB1 receptors in the mPFC also affected the same cocaine-associated memory processes.
Materials and methods
Animals
Eight- to 12-week-old male C57BL/6J mice initially weighing 20–26 g were used as subjects in the current study. They were housed in groups (four to five mice per plastic cage) under controlled lighting conditions (lights on from 0700 to 1900 h) in a temperature (22 ± 1 °C) and humidity (70 %)-controlled colony room. Food (Purina Mouse Chow, Richmond, IN, USA) and water were available ad libitum. All procedures used in this study were approved by the Institutional Animal Care Committee of the National Cheng Kung University College of Medicine and conform to the Guidelines of the National Institutes of Health on the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised in 1996.
Drug treatment
Cocaine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in sterile saline. The CB1 receptor antagonists rimonabant (also known as SR141716A or SR1) and AM281 were purchased from APIChem Technology (Hangzhou, China) and Tocris Bioscience (Bristol, UK), respectively. Drugs were dissolved in the ethanol/cremophor/saline (1:1:18; v/v/v) vehicle for intraperitoneal (i.p.) injection in a volume of 10 ml/kg. For the bilateral intra-mPFC infusion, rimonabant was dissolved in 100 % DMSO. Several labs (Gehani et al. 2007; Hough et al. 2009) have previously used DMSO as a diluent for intracerebroventricular (i.c.v.) or intracerebral (i.c.) studies without any adverse effects. All drugs were freshly prepared before use.
Stereotaxic surgery, guide cannula implantation, and histology
In order to examine the role of the medial prefrontal CB1 receptors in memory consolidation and extinction of cocaine-induced CPP, stereotaxic surgery and bilateral 26-gauge guide cannula implantation (Coordinates: A.P., +1.6 mm; M.L., ±0.5 mm; D.V., −2.3 mm) (Paxinos and Watson 2005) was executed under sodium pentobarbital anesthesia (40 mg/kg) 1 week before the beginning of the experiment. Bregma and the skull surface served as the stereotaxic zero point. Clearance through the guide cannula was maintained with dummy cannulas. The infusion cannula, a 33-gauge dental needle, was inserted into the guide cannula and was lowered 1.0 mm below the guide cannula. A 0.5 μl volume of rimonabant (1.5 μg/μl per side) was infused bilaterally with a Hamilton 10 μl microsyringe driven by a microdialysis pump (CMA 400/Microdialysis, Solna, Sweden) at a rate of 0.1 μl/min. After injection, the infusion cannulas were left for an additional 5 min before withdrawal to avoid reflux of the infused drug solution.
At the conclusion of each experiment, all mice were euthanized with pentobarbital overdose. The mice underwent the guide cannula implantation were deeply anesthetized and perfused with phosphate-buffered saline (PBS) and 4 % paraformaldehyde. Brains were then postfixed with 4 % paraformaldehyde overnight, cryoprotected in 30 % sucrose/PBS and frozenly cut into 40 μm coronal sections with a Cryostat (Leica, CM1850, Wetzlar, Germany) and Nissl stained with cresyl violet. A light microscope (Olympus Microscope Cooperation, Tokyo, Japan) was used to visualize the location of the intracerebral injection sites, which were then verified according to a mouse brain atlas (Paxinos and Watson 2005). Mice with misplaced cannula location were excluded from data analysis.
Cocaine-induced CPP training, test, retest, and priming test
Pretest, cocaine CPP trainings, test, retest, and the cocaine priming test were all conducted in commercial chambers designed for mouse (MedAssociates Inc., Georgia, VT, USA) as described in the previous reports (Fan et al. 2010; Lin et al. 2011). In brief, all mice were examined for their unconditioned preference in the chambers for a 15-min pretest before the trainings. In the pretest, only mice spending shorter than 40 % of time in any one compartment, including the middle one, were included in this study. In the test, mice spending longer than 20 % of the time in the middle compartment were excluded from further experiments because their cocaine-induced CPP could be confounded by a substantial preference toward the neutral, middle compartment. For the cocaine CPP trainings, mice received an i.p. saline injection between 0900 and 1100 h and were immediately confined in their unconditioned preferred compartment for 30 min. Approximately 8 h later, mice received a cocaine hydrochloride injection and were immediately confined in their unconditioned non-preferred compartment for 30 min. These procedures were repeated for three consecutive days. Approximately 20 h (around 1300 h) after the last training trial, mice free of cocaine were placed in the center with guillotine doors open for a 15-min preference test. Durations (in seconds) for mice exploring each of the three compartments were recorded. Cocaine-induced CPP score was represented as “time spent difference” by subtracting the time spent in the saline-paired compartment from the time spent in the cocaine-paired compartment.
As illustrated in Fig. 1a, in order to investigate the role of the CB1 receptors in memory consolidation of cocaine-induced CPP, mice were given rimonabant (SR1, i.p., 3 mg/kg or 1.5 μg bilaterally in the mPFC) or AM281 (i.p., 3 mg/kg) after each saline- or cocaine-training trial during the 3-day CPP trainings. On Day 5, mice were placed in the center with guillotine doors open and a 15-min test was performed (Fig. 1a). In addition, the role of the CB1 receptors in memory extinction of cocaine-induced CPP was tested (Fig. 1b). After the pretest and 3-day cocaine CPP trainings, a preference test was performed to make sure all mice had reliably acquired cocaine-induced CPP on Day 5. Mice then received a 2-day forced extinction procedure. Mice received a saline injection (i.p.) between 0900 and 1100 h and were immediately confined to their unconditioned preferred compartment for 30 min. Approximately 8 h later, mice again received the same saline injection and were immediately confined to their unconditioned non-preferred compartment for 30 min. The no extinction groups did not undergo the forced extinction procedure and instead received morning and afternoon saline injections in their home cages. Rimonabant (SR1, i.p., 3 mg/kg or 1.5 μg bilaterally in the mPFC) or AM281 (i.p., 3 mg/kg) were given immediately after each 30-min forced extinction trial or 30 min after each saline injection in the home cage for the no extinction groups. Mice then performed an additional preference retest on Day 8. On Day 14, a cocaine hydrochloride (i.p., 5 mg/kg) was given before the cocaine priming test (Fig. 1b).
The experimental procedures used to examine the effect of the CB1 receptor antagonists on memory consolidation and extinction of cocaine-induced CPP. a Mice underwent a 15-min pretest for their unconditioned preference first. Rimonabant/AM281 or vehicle injections were given immediately after each saline- or cocaine-training trial during the 3-day cocaine CPP trainings. On Day 5, these mice performed a 15-min cocaine-free preference test to examine the effect of the CB1 receptor antagonists on memory consolidation of cocaine-induced CPP. b Mice underwent the 15-min pretest and 3-day cocaine CPP trainings. After assuring reliable establishment of cocaine CPP on Day 5, mice were divided into different groups undergoing forced extinction or no extinction procedures on Days 6 and 7. Rimonabant/AM281 or vehicle was administered immediately after each forced extinction trial, or 30 min after each saline injection in their home cages for the no extinction group. Retest and the cocaine priming test were performed on Days 8 and 14, respectively
Finally, to rule out the possibility that rimonabant per se may induce CPP or conditioned place aversion (CPA), a separate group of mice underwent a 5-day CPP conditioning procedure similar to that of the pretest, cocaine CPP trainings, and test described above. However, morning vehicle (i.p., ethanol/cremophor/saline, 1:1:18; v/v/v) and afternoon rimonabant (i.p., 3 mg/kg) were injected during the 3-day rimonabant-CPP trainings instead. Rimonabant-induced CPP or CPA was represented as “time spent difference” by subtracting the time spent in preferred/vehicle conditioning compartment from the time spent in non-preferred/rimonabant conditioning compartment.
Locomotor activity
We tested if six consecutive rimonabant injections (SR1, i.p., 3 mg/kg or 1.5 μg bilaterally in the mPFC) administered twice per day at 8 h intervals for 3 days would affect mouse activity levels. Approximately 20 h after the 6th injection of rimonabant, the locomotor activity was monitored for 15 min as previously described (Ho et al. 2009; Lin et al. 2011).
Statistical analysis
All results were indicated as the mean ± standard error of mean (SEM). Paired t tests were employed to examine reliable acquisition of cocaine-induced CPP or rimonabant-induced CPP/CPA. Two-tailed Student’s t test was used for two-group comparisons. One-way ANOVA was used for multiple-group comparisons, followed by the Bonferroni post hoc test. Two-way ANOVAs were used to examine the effects of drug (rimonabant/AM281 vs. vehicle) and extinction (extinction vs. no extinction) on cocaine-induced CPP scores. A three-way (drug × cocaine × time) ANOVA with the times of testing (time) as a repeated measure variable was used to examine the overall effect of rimonabant on memory consolidation of CPP induced by various doses of cocaine, followed by the Student’s t test for the effect of rimonabant at each dose of cocaine used. The levels of statistical significance were set at p < 0.05.
Results
Systemic or intra-mPFC administration of rimonabant exerts bidirectional effect on consolidation of CPP memory induced by different doses of cocaine
For the effect of systemic CB1 receptor blockade by rimonabant on memory consolidation, a three-way (drug × cocaine × time) ANOVA with the times of testing (time) as a repeated measure variable revealed a significant three-way (drug × cocaine × time) interaction effect, suggesting that systemic injected rimonabant affected cocaine-induced CPP differentially according to time and cocaine dosage used [F(4, 131) = 4.466, p < 0.005] (Fig. 2). In order to examine which group displayed a significant cocaine CPP score in the test compared to that of in the pretest (its own baseline), a post hoc paired t test was performed. For the vehicle-treated groups, almost all low doses of cocaine induced significant CPP scores in the test when compared to the pretest [paired t 13 = 4.11, p < 0.01, n = 14 for 5 mg/kg; paired t 11 = 3.299, p < 0.01, n = 12 for 10 mg/kg], except for the lowest-dose (2.5 mg/kg) cocaine-induced CPP, which had a trend toward statistic significance [paired t 12 = 1.894, p = 0.083, n = 13]. On the other hand, the rimonabant-treated groups showed significant CPP scores induced by all low doses of cocaine [paired t 12 = 3.232, p < 0.05, n = 13 for 2.5 mg/kg; paired t 13 = 5.912, p < 0.001, n = 14 for 5 mg/kg; paired t 11 = 7.683, p < 0.001, n = 12 for 10 mg/kg]. These results suggest that rimonabant enhanced the consolidation of low-dose cocaine-induced CPP. A post hoc Student’s t test further revealed that there was no difference in cocaine-induced CPP scores between the rimonabant and vehicle groups for each cocaine dosage used in the pretest (all p > 0.05). However, mice treated with systemic rimonabant immediately after each CPP training for 3 days showed significant increase of cocaine-induced CPP when induced by low doses of cocaine (2.5, 5, 10 mg/kg) [t 24 = 2.345, p < 0.05, n = 26 for 2.5 mg/kg; t 26 = 2.479, p < 0.05, n = 28 for 5 mg/kg; t 22 = 2.069, p < 0.05, n = 24 for 10 mg/kg] (Fig. 2a–c). Conversely, the rimonabant group displayed significant decrease of cocaine-induced CPP when induced by high doses of cocaine (20 or 40 mg/kg) [t 30 = 3.036, p < 0.005, n = 32 for 20 mg/kg; t 29 = 3.056, p < 0.005, n = 31 for 40 mg/kg] (Fig. 2d, e). This finding indicates that systemic rimonabant exerts bidirectional effects on memory consolidation of cocaine-induced CPP. That is, systemic blockade of CB1 receptors facilitates consolidation of CPP memory induced by a low dose (2.5, 5, or 10 mg/kg) of cocaine but impairs that by a high dose (20 or 40 mg/kg).
Systemic administration of the CB1 antagonist rimonabant immediately following each saline- or cocaine-training trial exerted bidirectional effects on consolidation of low- vs. high-dose cocaine-induced CPP memory. Mice were injected with various doses of cocaine, a 2.5 mg/kg, b 5 mg/kg, c 10 mg/kg, d 20 mg/kg, or e 40 mg/kg, respectively. *Significantly higher than the vehicle group of the same dosage of cocaine used in the test. #Significantly lower than the vehicle group of the same dosage of cocaine used in the test. Cocaine-induced CPP score was represented as “time spent difference” by subtracting the time spent in the saline-paired compartment from the time spent in the cocaine-paired compartment
For the effect of the medial prefrontal CB1 receptor blockade by rimonabant on memory consolidation, a three-way (drug × cocaine × time) repeated-measure ANOVA revealed a significant three-way interaction effect, suggesting that intra-mPFC infusion of rimonabant affected their cocaine-induced CPP differentially, depending on time and cocaine dosage used [F(1, 64) = 15.238, p < 0.001] (Fig. 3). A post hoc examination showed that the CPP scores were not different between the rimonabant and vehicle groups for 10 or 40 mg/kg cocaine in the pretest (all p > 0.05). However, mice treated with bilateral intra-mPFC infusion of rimonabant showed an increased cocaine CPP induced by 10 mg/kg cocaine [t 31 = 2.116, p < 0.05, n = 33] (Fig. 3a), but had a decreased CPP induced by 40 mg/kg cocaine [t 33 = 4.591, p < 0.001, n = 35] (Fig. 3b). An example of the correct localization of cannulas in the mPFC was shown in Fig. 3c, and mice with wrong injection sites were excluded from data analysis. This result, similar to that of systemic CB1 receptor antagonism, indicates that the medial prefrontal CB1 receptor blockade exerts bidirectional effects on memory consolidation of cocaine-induced CPP.
Bilateral intra-mPFC rimonabant infusion immediately following each saline- or cocaine-training trial exerted bidirectional effects on consolidation of low- vs. high-dose cocaine-induced CPP memory. Mice were injected with a 10 mg/kg, b 40 mg/kg cocaine, respectively. c Representative photograph of correct cannula location in the mPFC on cresyl violet-stained section. *Significantly higher than the vehicle group of 10 mg/kg cocaine used in the test; #significantly lower than the vehicle group of 40 mg/kg cocaine used in the test
Systemic or intra-mPFC administration of rimonabant facilitates extinction of a high-dose cocaine-induced CPP memory
A two-tailed t test revealed that all mice reliably acquired cocaine-induced CPP [paired t 53 = 10.509, p < 0.001, n = 54]. They were then divided into four groups that underwent the following treatment respectively: rimonabant only (3 mg/kg) (No Extinction-SR1), extinction only (Extinction-Veh), rimonabant with extinction (Extinction-SR1), or control treatment (No Extinction-Veh). A three-way (drug × extinction × time) ANOVA with the times of testing (test vs. retest) as a repeated measure variable revealed significant drug main effect and three-way interaction effect (both p < 0.05), suggesting rimonabant-treated groups had different CPP scores across time (e.g., caused extinction in the retest). A post hoc two-way (drug × extinction) ANOVA revealed that these four groups of mice exhibited comparable cocaine CPP scores before the above treatment in the test [drug, extinction and interaction effect, all p > 0.05]. However, after the treatment, both the rimonabant only and rimonabant with extinction groups displayed significant decrease of cocaine CPP in the retest [drug main effect: F(1, 50) = 9.088, p < 0.005] and in the cocaine priming test [drug main effect: F(1, 50) = 4.979, p < 0.05] (Fig. 4a). Furthermore, the CPP score of the rimonabant with extinction group was significant lower than all other three groups in the retest [interaction effect: F(1, 50) = 5.223, p < 0.05]. This finding indicates that systemic blockade of CB1 receptors by rimonabant facilitates the extinction of a high-dose cocaine-induced CPP memory.
Systemic administration of the CB1 antagonist rimonabant immediately following each extinction behavioral trial facilitated extinction of a high-dose cocaine-induced CPP memory. a Both the No Extinction-SR1 and Extinction-SR1 groups showed substantial decrease of the cocaine CPP scores in the retest and the cocaine priming test. Additionally, the Extinction-SR1 group showed lower retention time than all other three groups in the retest. b Both the Extinction-1 mg/kg SR1 and Extinction-3 mg/kg SR1 groups showed decrease of the CPP scores in the retest, but only the Extinction-3 mg/kg SR1 group displayed a diminished CPP score in the cocaine priming test. *Significantly higher than values in the pretest; #significantly lower than the No Extinction-Veh group in the retest or in the cocaine priming test for a or than the Extinction-Veh group in the retest or in the cocaine priming test for b; @significantly lower than all other groups in the retest for a
A cohort of mice acquired reliable cocaine CPP following the 3-day cocaine CPP trainings [paired t 63 = 9.157, p < 0.001, n = 64] (Fig. 1b). They were then divided into five groups treated with various doses of rimonabant (0.1, 0.3, 1, or 3 mg/kg) or the same volume of vehicle immediately after each forced extinction behavioral confinement (Fig. 1b). A two-way (drug dosage × time) ANOVA with the times of testing (test vs. retest) as a repeated measure variable revealed a significant two-way interaction effect, indicating these groups displayed changes of CPP scores differentially across time. A post hoc one-way (drug dosage) ANOVA revealed that these five groups exhibited comparable cocaine-induced CPP scores in the test [F(4, 59) = 0.084, p = 0.987], indicating that they all acquired reliable cocaine CPP before the above treatments (Fig. 4b). A one-way ANOVA further showed that there were significant differences among these five groups in the retest [F(4, 59) = 4.174, p < 0.005] and in the cocaine priming test [F(4, 59) = 3.285, p < 0.05]. The post hoc analyses showed that only mice injected with 1 or 3 mg/kg rimonabant had significantly lower cocaine CPP scores compared to that of vehicle (p < 0.05) in the retest. On the other hand, only mice treated with 3 mg/kg rimonabant had a lower cocaine CPP score in the cocaine priming test (p < 0.01) (Fig. 4b). This finding indicates that rimonabant exerts a dose-dependent facilitating effect on extinction of a high-dose cocaine-induced CPP memory.
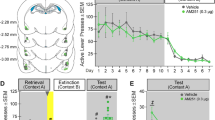
After all mice exhibited reliable cocaine CPP [paired t 34 = 10.111, p < 0.001, n = 35], they were injected with either rimonabant (1.5 μg bilaterally in the mPFC) or vehicle immediately after each extinction behavioral trial as depicted in Fig. 1b. A Student’s t test revealed that these two groups of mice exhibited comparable cocaine-induced CPP scores in the test before the treatment [t 33 = 0.199, p = 0.843] (Fig. 5a). However, after the treatment, the rimonabant group (Extinction-SR1) showed significantly lower cocaine CPP scores than the control group (Extinction-Veh) in the retest [t 33 = 5.141, p < 0.001] and in the cocaine priming test [t 33 = 2.349, p < 0.05] (Fig. 5a). An example of the correct localization of cannulas in the mPFC was shown in Fig. 5b, and mice with wrong injection sites were excluded from data analysis. This finding indicates that inactivation of the medial prefrontal CB1 receptors facilitates memory extinction of a high-dose cocaine-induced CPP.
Bilateral intra-mPFC rimonabant infusion immediately following each extinction behavioral trial facilitated extinction of a high-dose cocaine-induced CPP memory. a The Extinction-SR1 group displayed substantial decrease of the cocaine CPP scores in the retest and in the cocaine priming test. b Representative photograph of correct cannula location in the mPFC on cresyl violet-stained section. *Significantly lower than the Extinction-Veh group in the retest or in the cocaine priming test
Systemic injection of AM281 also exerts bidirectional effects on consolidation of low- vs. high-dose cocaine-induced CPP and facilitates extinction of a high-dose cocaine-induced CPP
For the consolidation of cocaine-induced CPP, a three-way (drug × cocaine × time) repeated-measure ANOVA revealed a significant three-way interaction effect, suggesting that systemic injection of AM281 (3 mg/kg) affected their cocaine-induced CPP differentially, depending on time and cocaine dosage used [F(1, 67) = 8.012, p < 0.01] (Fig. 6a, b). A post hoc examination showed that the CPP scores were not different between the AM281 and vehicle groups for 10 or 40 mg/kg cocaine in the pretest (all p > 0.05). However, AM281-treated mice showed an increased cocaine CPP induced by 10 mg/kg cocaine [t 36 = 2.211, p < 0.05, n = 38] (Fig. 6a), but had a decreased CPP induced by 40 mg/kg cocaine [t 31 = 3.266, p < 0.01, n = 33] (Fig. 6b). This result, similar to that of rimonabant, indicates that CB1 receptor blockade by AM281 exerts bidirectional effects on memory consolidation of cocaine-induced CPP.
Systemic administration of the CB1 antagonist AM281 exerted bidirectional effects on consolidation of low- vs. high-dose cocaine CPP memory and facilitated extinction of a high-dose cocaine CPP memory. Mice were injected with a 10 mg/kg, b 40 mg/kg cocaine, respectively. c Both the No Extinction-AM281 and Extinction-AM281 groups displayed substantial decrease of the cocaine CPP scores in the retest and in the cocaine priming test. Additionally, the Extinction-AM281 group showed lower retention time than all other three groups in the retest. *Significantly higher than the vehicle group of 10 mg/kg cocaine in the test in a or the values in the pretest in c; #significantly lower than the vehicle group of 40 mg/kg cocaine in the test in b or the No Extinction-Veh group in the retest or in the cocaine priming test in c; @significantly lower than all other groups in the retest in c
For the extinction of cocaine-induced CPP, all mice first reliably acquired cocaine-induced CPP following the 3-day CPP trainings [paired t 55 = 11.086, p < 0.001, n = 56] (Fig. 6c). They were then divided into four treatment groups as illustrated in Fig. 4a, except rimonabant was replaced by AM281 (3 mg/kg) in this experiment. A three-way (drug × extinction × time) ANOVA with the times of testing (time) as a repeated measure variable revealed a significant three-way interaction effect, suggesting AM281-treated groups had different CPP scores across time (e.g., caused extinction in the retest). A post hoc two-way (drug × extinction) ANOVA revealed that these four groups of mice exhibited comparable cocaine-induced CPP scores in the test [drug, extinction and interaction effect, all p > 0.05], indicating that they all acquired reliable cocaine CPP before the treatment. After the treatment, both the AM281 only and AM281 with extinction group displayed significantly decreased cocaine CPP in the retest [drug main effect: F(1, 52) = 10.256, p < 0.005] and in the cocaine priming test [drug main effect: F(1, 52) = 9.151, p < 0.005] (Fig. 6c). Furthermore, the CPP score of the AM281 with extinction group was significant lower than all other three groups in retest [interaction effect: F(1, 52) = 4.449, p < 0.05] (Fig. 6c). This finding indicates that systemic blockade of CB1 receptors by AM281 also facilitates the extinction of a high-dose cocaine-induced CPP memory.
Systemic injection of rimonabant does not induce CPP or CPA
Our finding indicates that rimonabant induces neither CPP nor CPA in these mice [paired t 10 = 0.288, p = 0.779, n =11] (Fig. 7a).
Rimonabant did not induce CPP/CPA or affect locomotor activity. a Mice treated with systemic rimonabant during the 3-day rimonabant-CPP trainings did not display any CPP or CPA. b 6 consecutive systemic injections of rimonabant did not affect locomotor activity at approximately 20 h after the last injection. c 6 consecutive bilateral intra-mPFC infusions of rimonabant did not affect locomotor activity at approximately 20 h after the last infusion. Rimonabant-induced CPP or CPA was represented as “time spent difference” by subtracting the time spent in preferred/vehicle conditioning compartment from the time spent in non-preferred/rimonabant conditioning compartment. IR break counts stand for summation of the vertical infrared beam break and the horizontal distance traveled in the chamber
Systemic or intra-mPFC injection of rimonabant does not affect mouse locomotor activity
Another two groups of mice were used for monitoring their locomotor activity approximately 20 h after the 6th injection of rimonabant. We found that 6 consecutive rimonabant administration, either systemically or directly into the bilateral mPFC, did not alter mouse locomotor activity [t 22 = 1.48, p = 0.153, n = 24; t 21 = 0.883, p = 0.387, n = 23] (Fig. 7b, c), suggesting that the above effects of rimonabant on memory consolidation and extinction of cocaine-induced CPP were not caused by changed levels of locomotor activity.
Discussion
The present study examined whether the cannabinoid CB1 receptors, especially those in the mPFC, modulate consolidation and extinction of cocaine-associated memory in mice. Intriguingly, we found that systemic or intra-mPFC administration of the CB1 receptor antagonist rimonabant or systemic injection of another CB1 receptor antagonist AM281 exerted bidirectional effects on consolidation of cocaine-induced CPP memory. That is, rimonabant (and AM281) impaired memory consolidation of CPP induced by a high-dose cocaine but facilitated that induced by a low dose. Moreover, systemic or intra-mPFC administration of rimonabant or systemic injection of AM281 facilitated extinction of a high-dose cocaine-induced CPP memory. These findings, taken together, suggest that the CB1 receptors in the mPFC modulate consolidation and extinction of cocaine-induced CPP memory.
The most intriguing finding in the present study is the bidirectional effects of rimonabant (and AM281) on consolidation of cocaine-associated memory, depending on the cocaine dosage used to acquire cocaine-induced CPP memory. To the best of our knowledge, this is the first report stating that a single dose (3 mg/kg) of rimonabant exerts bidirectional effects on memory consolidation of CPP induced by various doses of cocaine. The reason why we chose 3 mg/kg rimonabant is because this is the only consistently effective dose in the CPP tasks induced by several drugs. For example, when rimonabant doses ranging from 0.1 to 3 mg/kg were used, only the dose of 3 mg/kg effectively disrupted the acquisition of food-induced CPP (Chaperon et al. 1998), the retrieval, reconsolidation and reinstatement of methamphetamine CPP (Yu et al. 2009, 2011), the reinstatement of cocaine CPP (Yu et al. 2011), as well as the retrieval of nicotine CPP (Forget et al. 2005). However, none of the previous studies have tried to examine the effects of rimonabant on CPP induced by various doses of the above drugs of abuse. Interestingly, our finding resembles the bidirectional effect of the post training administration of epinephrine, norepinephrine, adrenocorticotropic hormone (ACTH), or amygdala stimulation on memory consolidation of a one-trial inhibitory avoidance task (Gold et al. 1975; Gold and van Buskirk 1976a, b; Gold and van Buskirk 1975). For example, epinephrine dose-dependently, in an inverted-U manner, facilitates a moderate memory induced by a low intensity of footshock. However, the same facilitating dose of epinephrine (e.g., 0.1 mg/kg) impairs memory induced by a high intensity of footshock (Gold and van Buskirk 1975). In general, avoidance latencies vary with the intensity and duration of the footshock, with a more intense shock induces longer memory retention latencies. The dose-dependent and bidirectional effects of epinephrine (as well as other aforementioned treatments) on memory consolidation can be explained by the Yerkes-Dodson law, which states an inverted-U curve between the arousal level and memory performance.
Similarly, our result suggests that the modulatory effects of the CB1 receptors on consolidation of cocaine-associated memory depend on the strength of CPP memory. The present and previous studies (Raybuck et al. 2013; Tzeng et al. 2013) depict a graded dose-response curve for CPP induced by various doses of cocaine, with a higher dose of cocaine induces a stronger cocaine-induced CPP (Fig. 2). While the mechanism underlying this bidirectional modulation of network activity by CB1 receptors remain elusive, it is speculated that activity-dependent plasticity of CB1 receptors may be involved (Foldy et al. 2006; Losonczy et al. 2004). A recent research indicates that the net effects of a CB1 receptor antagonist vary with the initial levels of activity in the network. That is, a single dose of AM251 increases network activity when basal burst rates are low, whereas decreases activity in network with higher basal burst rates (Piet et al. 2011). Therefore, it is likely that the bidirectional effect of the CB1 receptor antagonism on consolidation of CPP memory depends on the low vs. high activity level of network including the mPFC induced by the low- vs. high-dose cocaine, respectively. Alternatively, intense network activity, as opposed to the disinhibition of glutamate release by the CB1 receptor antagonism under low network activity, may change the distribution and function of CB1 receptors, thereby altering endocannabinoid signaling (Piet et al. 2011).
Moreover, our findings suggest that systemic injection of rimonabant or AM281 or infusion of rimonabant into the mPFC facilitated the extinction of CPP induced by a high dose (20 mg/kg) of cocaine. It is of importance to emphasize that a total of six injections of rimonabant by themselves did not cause CPP/CPA or affect mouse locomotor activity, we therefore inferred that the CB1 receptor antagonism facilitates extinction of a high-dose cocaine-induced CPP memory. Such findings are opposite to those reported by previous studies that rimonabant disrupts memory extinction of the auditory fear conditioning (Marsicano et al. 2002), Morris water maze (Varvel et al. 2005), conditioned freezing, and passive avoidance tasks (Niyuhire et al. 2007). The discrepancy could be due to difference in the tasks as the impairing effect was mostly demonstrated in aversive learning tasks rather than an appetitive one used in the present studies, consistent with the notion that the modulatory effects of CB1 receptors often varied with experimental paradigms or parameters (Wiskerke et al. 2008). The previous and present findings nonetheless suggest a role of CB1 receptors in extinction of learned responses motivated either appetitively or aversively.
An extinction trial in CPP or any types of learning is essentially a retrieval trial that the cue activates an old memory but in the absence of reward, a process that forges a new memory for the cue. Thus, the facilitated extinction observed in the present study could be due to either enhancement of memory consolidation for the new learning or impediment of reconsolidation of the old memory, because both would yield a reduction of the initially reinforced response in a subsequence test. The present results could not distinguish the two possibilities as rimonabant may facilitate memory consolidation of new learning (extinction, 0 mg/kg; albeit not tested in the current study) or impair reconsolidation of the old memory acquired under a high dose of cocaine (20 mg/kg). It is also likely that the effects that rimonabant exerted on these two processes simultaneously contribute to the present result as both effects yield the same consequence. While exact behavioral mechanisms should be further elucidated by future studies, our results, probably for the first time to the best of our knowledge, hint a possibility that rimonabant could be used together with the extinction behavioral therapy to treat heavy cocaine addicts.
To our surprise, systemic injection of rimonabant neither facilitates nor impairs memory extinction of CPP induced by a low dose (10 mg/kg) of cocaine (data not shown). As discussed above, rimonabant could simultaneously enhance: (1) consolidation of new memory (extinction); (2) reconsolidation of the old memory (cocaine, 10 mg/kg). However, enhancing these two putative underlying processes would yield completely opposite CPP retention scores. That is, if rimonabant enhances the former process, the CPP score should be more negative when compared to the extinction alone group, thereby facilitating the extinction of a low-dose cocaine-induced CPP. In contrast, if rimonabant enhances the latter process, the CPP score should be more positive, thereby impairing the extinction of the same CPP memory.
However, our empirical data indicates that rimonabant neither facilitates nor impairs the extinction of CPP induced by a low dose of cocaine. There are several possibilities for this negative finding. First, it is likely that rimonabant equally enhances the two aforementioned underlying processes thus the net effect is canceled out. Second, it is possible that the former process is dominant and rimonabant facilitates the extinction of CPP induced by a low dose, but hindered by a plausible floor effect to show. That is, our result shows that the same forced extinction procedure seems to induce a relatively low CPP score (approximately −50 s) acquired under a low dose of cocaine, as opposed to that of a highdose (Fig. 4a, the Extinction-Veh Group in the retest: approximately 150 s). Therefore, a floor effect may account for this negative result. Finally, our finding suggests that it is less likely that the latter process is more dominant. If the latter process was indeed more dominant, rimonabant would impair the extinction of CPP induced by a low-dose cocaine. Luckily, rimonabant did not reverse the behavioral extinction effect on a low-dose cocaine-induced CPP memory; otherwise we would need to consider it with caution from both a fundamental and a clinical perspective.
Another significant finding of our study is the role of the medial prefrontal CB1 receptors in both consolidation and extinction of cocaine-induced CPP memory. The cannabinoid CB1 receptors are enriched in the brain areas implicated in motivation and memory, including the mPFC (De Vries and Schoffelmeer 2005; Katona et al. 2001; Wilson and Nicoll 2002). Previous research suggests that CB1 receptors are not involved in the primary motivational/rewarding effects of cocaine, instead they modulate the persistence of cocaine addiction (Maldonado et al. 2006). For instance, AM251 impairs cocaine-induced reinstatement of cocaine-seeking behavior and comparably inhibits cocaine-induced increases of glutamate but not dopamine in the NAc (Xi et al. 2006). While dopamine released from the ventral tegmental pathway projecting to the NAc has been the focus of elucidating the rewarding effects of many abused drugs and the initial development of addiction, the glutamatergic projection from the mPFC to NAc is viewed as the final common pathway for drug relapse behavior (Neisewander et al. 2000; See 2002). The mPFC is well recognized for its role in formation of stimulus-reward associations and executive decision-making processes (Weissenborn et al. 1997).
Clinical studies indicate that the basal activity in the mPFC, manifested by changes in blood flow and metabolism, is reduced in addicts during withdrawal. When drug-associated cues are presented, the activities of the PFC and NAc increase dramatically, accompanied by the increased self-reports of “drug craving” (Goldstein and Volkow 2002). Research shows that the dopamine release in the mPFC and the glutamate release in the mPFC-NAc projection are the two prerequisites for the reinstatement of cocaine-seeking behavior in rats (McFarland et al. 2003). Inhibition of dopamine release in the mPFC blocks stress- or drug-induced glutamate release in the NAc (Capriles et al. 2003; McFarland et al. 2004). The reinstatement induced by dopamine release in the mPFC can be blocked by infusion of the glutamate receptor antagonists into the NAc (Park et al. 2002). These findings suggest that the meso-prefrontal dopamine release is a critical antecedent to the activation of the prefrontal glutamatergic projection to the NAc, thereby reinstating drug seeking.
Interestingly, the CB1 receptor activation increases the firing rate and burst firing of the meso-prefrontal dopaminergic neurons (Diana et al. 1998), while rimonabant attenuates the food-induced dopamine increment in the mPFC in those food-restricted rats (Dazzi et al. 2014). By using a post training regimen, our results argue that the CB1 receptor blockade did not decrease motivation or alter sensitivity during cocaine CPP training or its retrieval test, but affected consolidation and extinction processes of cocaine-associated memory. Although further studies are needed to determine the underlying mechanisms, however, it is possible that the modulatory effects of rimonabant on cocaine-associated memory processes observed is mediated by the reduction of dopamine release in the mPFC, thereby inhibiting cocaine-induced increases of glutamate in the NAc.
It is of importance to note that the mPFC is composed of the ventral infralimibic cortex (IL) and the dorsal prelimbic cortex (PL), and previous research suggests that only IL is involved in the consolidation, but not acquisition or expression of extinction of conditioned fear. For example, animals with lesions of IL or infusion of various receptor antagonists, including the CB1 antagonist AM251, into IL acquired fear extinction normally, but had difficultty retrieving extinction the following day (Laurent and Westbrook 2009; Lin et al. 2009; Quirk and Mueller 2008; Quirk et al. 2000). In contrast, PL seems to be involved in the expression of fear extinction, as CS-induced PL activation is negatively correlated with extinction expression during the training session (acquisition) as well as in the test session a day after training (retrieval) (Burgos-Robles et al. 2009). Therefore, it is likely that PL and IL are involved in regulating the balance between fearful and nonfearful memories (Burgos-Robles et al. 2009; Schiller and Johansen 2009). However, it awaits further study to decipher if IL and PL are differently involved in the consolidation and extinction of cocaine-associated memory as our study used a midline infusion and a large infused volume of rimonabant (0.5 μl), which did not allow a distinction between IL and PL on their roles in the two processes.
In conclusion, the present study indicates that the antagonism of CB1 receptors in the mPFC modulates memory consolidation of cocaine-induced CPP bidirectionally, while facilitates its extinction. Consistent with previous research, our results support that CB1 receptor blockade by rimonabant, administered systemically or directly into the mPFC, can act as a useful anti-relapse agent for treating cocaine addiction. More importantly, the bidirectional effects of rimonabant on consolidation of cocaine-associated memory urge the caution when using rimonabant as the therapeutic intervention, depending on the doses of cocaine consumed by the addicts. Finally, the facilitating effect of rimonabant on extinction of cocaine-associated memory provides insight for applying the concomitant behavioral extinction procedure together with rimonabant for treating the heavy cocaine addicts in a clinical setting. From a pharmacological perspective, it would also be of interest to examine if the effects of the CB1 receptor antagonist/inverse agonist rimonabant on the consolidation and extinction of cocaine-induced CPP can be mimicked by the putative neutral CB1 antagonists such as O-2050 (Breivogel and Childers 2000; Pertwee et al. 1996) or AM4113 (Landsman et al. 1997; Mato et al. 2002; Sink et al. 2008). However, since the classification of O-2050 as a neutral CB1 antagonist is problematic (Balster and Prescott 1992; Wiley et al. 1995) and the characterization of AM4113 as an authentic neutral CB1 antagonist awaits further investigation, their effects on cocaine CPP memory are not tested in the current study. Moreover, due to the mood- and anxiety-related side effects of rimonabant in several clinical trials reported by the US Food and Drug Administration (FDA Advisory Committee 2007), further investigation should focus on deciphering whether these side effects are specific to the compound rimonabant per se or to an endocannabinoid-mediated mechanism of action instead. If the former is the case, other CB1 antagonists or inhibitors of the endocannabinoid synthesis and degradation enzymes should be tested for their efficacy as anti-craving drugs. Alternatively, if the side effect is caused by the endocannabinoid system being manipulated, then finding the novel compounds targeting specific downstream effectors of the endocannabinoid system involved in cocaine addiction would prove to be helpful.
References
Balster RL, Prescott WR (1992) Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev 16:55–62
Breivogel CS, Childers SR (2000) Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther 295:328–336
Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ (2009) Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29:8474–8482
Caille S, Parsons LH (2006) Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology 31:804–813
Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH (2007) Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci 27:3695–3702
Capriles N, Rodaros D, Sorge RE, Stewart J (2003) A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berlin) 168:66–74
Chaperon F, Soubrie P, Puech AJ, Thiebot MH (1998) Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berlin) 135:324–332
Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W (2001) Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res 118:61–65
Dazzi L, Talani G, Biggio F, Utzeri C, Lallai V, Licheri V, Lutzu S, Mostallino MC, Secci PP, Biggio G, Sanna E (2014) Involvement of the cannabinoid CB1 receptor in modulation of dopamine output in the prefrontal cortex associated with food restriction in rats. PLoS One 9:e92224
De Vries TJ, Schoffelmeer AN (2005) Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci 26:420–426
De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN (2001) A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7:1151–1154
Diana M, Melis M, Gessa GL (1998) Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci 10:2825–2830
Fan HY, Cherng CG, Yang FY, Cheng LY, Tsai CJ, Lin LC, Yu L (2010) Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behav Brain Res 208:522–527
Fattore L, Spano MS, Deiana S, Melis V, Cossu G, Fadda P, Fratta W (2007) An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev 53:1–16
FDA Advisory Committee (2007) FDA briefing document: NDA 21–888, Zimulti (rimonabant) Tablets, 20 mg; Sanofi Aventis. US Food and Drug Administration, Rockville
Filip M, Golda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegalinski E (2006) Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep 58:806–819
Foldy C, Neu A, Jones MV, Soltesz I (2006) Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci 26:1465–1469
Forget B, Hamon M, Thiebot MH (2005) Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology (Berlin) 181:722–734
Gehani NC, Nalwalk JW, Razdan RK, Martin BR, Sun X, Wentland M, Abood ME, Hough LB (2007) Significance of cannabinoid CB1 receptors in improgan antinociception. J Pain Off J Am Pain Soc 8:850–860
Gold PE, van Buskirk RB (1975) Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 13:145–153
Gold PE, van Buskirk R (1976a) Effects of posttrial hormone injections on memory processes. Horm Behav 7:509–517
Gold PE, van Buskirk R (1976b) Enhancement and impairment of memory processes with post-trial injections of adrenocorticotrophic hormone. Behav Biol 16:387–400
Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL (1975) Memory interference and facilitation with posttrial amygdala stimulation: effect on memory varies with footshock level. Brain Res 86:509–513
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Ho MC, Cherng CG, Tsai YP, Chiang CY, Chuang JY, Kao SF, Yu L (2009) Chronic treatment with monoamine oxidase-B inhibitors decreases cocaine reward in mice. Psychopharmacology (Berlin) 205:141–149
Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M (2005) CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology 30:339–349
Hough LB, Svokos K, Nalwalk JW (2009) Non-opioid antinociception produced by brain stem injections of improgan: significance of local, but not cross-regional, cannabinoid mechanisms. Brain Res 1247:62–70
Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650
Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF (2001) Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci 21:9506–9518
Kuo YM, Liang KC, Chen HH, Cherng CG, Lee HT, Lin Y, Huang AM, Liao RM, Yu L (2007) Cocaine-but not methamphetamine-associated memory requires de novo protein synthesis. Neurobiol Learn Mem 87:93–100
Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI (1997) SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol 334:R1–R2
Laurent V, Westbrook RF (2009) Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem 16:520–529
Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA (2005) Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 15:31–37
Lin HC, Mao SC, Su CL, Gean PW (2009) The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex 19:165–175
Lin KY, Cherng CG, Yang FR, Lin LC, Lu RB, Yu L (2011) Memantine abolishes the formation of cocaine-induced conditioned place preference possibly via its IL-6-modulating effect in medial prefrontal cortex. Behav Brain Res 220:126–131
Losonczy A, Biro AA, Nusser Z (2004) Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A 101:1362–1367
Maldonado R, Valverde O, Berrendero F (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O (2000) Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci 12:4038–4046
Mato S, Pazos A, Valdizan EM (2002) Cannabinoid receptor antagonism and inverse agonism in response to SR141716A on cAMP production in human and rat brain. Eur J Pharmacol 443:43–46
McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537
McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–1560
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20:798–805
Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH (2007) The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berlin) 191:223–231
Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC (2002) Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci 22:2916–2925
Paxinos G, Watson C (2005) The mouse brain in stereotaxic coordinates. Elsevier, Amsterdam
Pertwee RG, Fernando SR, Griffin G, Ryan W, Razdan RK, Compton DR, Martin BR (1996) Agonist–antagonist characterization of 6′-cyanohex-2′-yne-delta 8-tetrahydrocannabinol in two isolated tissue preparations. Eur J Pharmacol 315:195–201
Piet R, Garenne A, Farrugia F, Le Masson G, Marsicano G, Chavis P, Manzoni OJ (2011) State-dependent, bidirectional modulation of neural network activity by endocannabinoids. J Neurosci 31:16591–16596
Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72
Quirk GJ, Russo GK, Barron JL, Lebron K (2000) The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20:6225–6231
Raybuck JD, McCleery EJ, Cunningham CL, Wood MA, Lattal KM (2013) The histone deacetylase inhibitor sodium butyrate modulates acquisition and extinction of cocaine-induced conditioned place preference. Pharmacol Biochem Behav 106:109–116
Schiller D, Johansen J (2009) Prelimbic prefrontal neurons drive fear expression: a clue for extinction–reconsolidation interactions. J Neurosci 29:13432–13434
See RE (2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71:517–529
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berlin) 168:3–20
Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD (2008) The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology 33:946–955
Tanda G, Munzar P, Goldberg SR (2000) Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci 3:1073–1074
Tzeng WY, Chuang JY, Lin LC, Cherng CG, Lin KY, Chen LH, Su CC, Yu L (2013) Companions reverse stressor-induced decreases in neurogenesis and cocaine conditioning possibly by restoring BDNF and NGF levels in dentate gyrus. Psychoneuroendocrinology 38:425–437
Varvel SA, Anum EA, Lichtman AH (2005) Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berlin) 179:863–872
Weissenborn R, Robbins TW, Everitt BJ (1997) Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology (Berlin) 134:242–257
Wiley JL, Lowe JA, Balster RL, Martin BR (1995) Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther 275:1–6
Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296:678–682
Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ (2008) The role of CB1 receptors in psychostimulant addiction. Addict Biol 13:225–238
Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL (2006) Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci 26:8531–8536
Yu LL, Wang XY, Zhao M, Liu Y, Li YQ, Li FQ, Wang X, Xue YX, Lu L (2009) Effects of cannabinoid CB1 receptor antagonist rimonabant in consolidation and reconsolidation of methamphetamine reward memory in mice. Psychopharmacology (Berlin) 204:203–211
Yu LL, Zhou SJ, Wang XY, Liu JF, Xue YX, Jiang W, Lu L (2011) Effects of cannabinoid CB(1) receptor antagonist rimonabant on acquisition and reinstatement of psychostimulant reward memory in mice. Behav Brain Res 217:111–116
Acknowledgments
We thank Drs. Keng-Chen Liang and Tsu-Wei Wang for comments and suggestions on the manuscript; Heng-Ai Chang for help with the experiment; Li-Hsien Chen and Wen-Yu Tzeng for technical assistance; and all the members of the Hu lab for their support. This work was supported by ROC National Science Council grant Nos. 100-2410-H-006-085-MY2, 101-2320-B-006-007-, and 102-2410-H-006-016-MY2 to S.S.H.; No. 101-2922-I-006-338 to L.Y.; and by NCKU Aiming for the Top University and Elite Research Center Development Plan (MoE ATU Plan) to S.S.H.. The authors declare no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, S.SJ., Liu, YW. & Yu, L. Medial prefrontal cannabinoid CB1 receptors modulate consolidation and extinction of cocaine-associated memory in mice. Psychopharmacology 232, 1803–1815 (2015). https://doi.org/10.1007/s00213-014-3812-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3812-y