Abstract
This study investigated the effectiveness of ketamine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, in alleviating the enhanced anxiety and fear response in both a mouse model of PTSD induced by inescapable electric foot shocks and a rat model of PTSD induced by a time-dependent sensitization (TDS) procedure. First, we evaluated the effect of ketamine on behavioral deficits in a mouse model of PTSD that consisted of foot shocks followed by three situational reminders. Our results showed that the aversive procedure induced several behavioral deficiencies, such as increased freezing behavior and anxiety, as well as reduced time spent in an aversive-like context, which were reversed by repeated treatment with ketamine. The effect of ketamine on behavioral changes after exposure to TDS was also investigated, and the levels of brain-derived neurotrophic factor (BDNF) in the hippocampus were measured. The results revealed that after TDS, the rats showed a significant increase in contextual freezing and a decrease in the percentage of time spent in and numbers of entries into open arms in the elevated plus maze test. As a positive control drug, sertraline (Ser, 15 mg/kg, i.g.), a selective serotonin reuptake inhibitor (SSRI) ameliorated these behavioral deficits. These behavioral effects were mimicked by chronic ketamine treatment. Furthermore, ketamine normalized the decreased BDNF level in the hippocampus in post-TDS rats. Taken together, these results suggest that ketamine exerts a therapeutic effect on PTSD that might be at least partially mediated by an influence on BDNF signaling in the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating anxiety disorder that is characterized by intrusive reexperiences of traumatic events, avoidance of situations and stimuli that could serve as reminders of these events, and feeling jumpy or easily startled. To date, selective serotonin reuptake inhibitors (SSRIs) have been established as first-line pharmacotherapeutic agents for treating acute and chronic PTSD, based on several well-controlled studies. And, sertraline (Zoloft) and paroxetine (Paxil) are the only FDA-approved medications for PTSD. Although the efficacy of SSRIs has proven to be very high in many sufferers, there are adverse effects (including cognitive dysfunction, weight gain, sexual dysfunction, sedation, dependence, and withdrawal) (Haddad 1998; Nelson and Philbrick 2012; Sheeler et al. 2012) that limit the utility of SSRIs and indicate a major unmet medical need to explore more promising treatments for PTSD.
The noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist ketamine has recently attracted attention for its rapid-onset antidepressant effects (Domino 2010; Hashimoto 2011; Machado-Vieira et al. 2009; Zarate et al. 2010). Ketamine decreased the immobility time in the forced swim test in rats (Engin et al. 2009; Garcia et al. 2008a, b; Li et al. 2010; Yilmaz et al. 2002) and mice (da Silva et al. 2010; Maeng et al. 2008; Rosa et al. 2003) and in the tail suspension test in mice (da Silva et al. 2010; Kos et al. 2006). Ketamine also conveys both anxiolytic activity in humans (Krystal et al. 1994) and fast-acting antidepressant responses in patients suffering from major depressive disorders (Berman et al. 2000; Zarate et al. 2010). Recently, many studies have reported that NMDA receptor activation is also associated with the formation of spontaneous intrusive memories and that high NMDA receptor activity may serve as risk factors for developing PTSD. McGhee et al. (McGhee et al. 2008) reported that patients at a military medical center who received ketamine had significantly lower rates of PTSD than those who did not. These preclinical and clinical findings support the idea that novel pharmacological tools targeting NMDA receptors might be promising candidates for new anti-PTSD drugs.
Given all of the abovementioned factors, it is reasonable to predict that ketamine is effective in the treatment of PTSD. However, studies investigating the anti-PTSD effects of ketamine in animal models are scarce. Thus, the current study was designed to assess the effects of ketamine in alleviating the enhanced anxiety and fear response in both a mouse model of PTSD induced by inescapable electric foot shocks and a rat model of PTSD induced by a time-dependent sensitization (TDS) procedure. Sertraline (Ser, a SSRI) was administered as the positive control.
Brain-derived neurotrophic factor (BDNF) is one of several endogenous proteins that play critical roles in the survival, maintenance, and growth of the brain and peripheral neurons (Lewin and Barde 1996). A growing body of evidence suggests that BDNF might mediate the pathophysiology of mood disorders, including PTSD. A recent study has demonstrated significantly lower plasma BDNF levels in PTSD patients compared to healthy control subjects (Felmingham et al. 2013; Matsuoka et al. 2013). In addition, sub-chronic ketamine administration has been shown to alter the expression of mRNAs for BDNF in the rat brain (Broekman et al. 2007; Duman and Monteggia 2006; Lommatzsch et al. 2005). We thus hypothesized that alterations in BDNF levels might be involved in the anti-PTSD effect induced by ketamine. BDNF protein levels were measured using Western blotting in the hippocampus of rats treated with ketamine.
Materials and methods
Animals
Both the male ICR mice (18 ± 2 g) and the male Sprague–Dawley rats (180 ± 20 g) were purchased from Beijing SPF Vital Laboratory Animal Technology Company (Beijing, China). Animals were maintained under nonreversed 12-h light/12-h dark cycle conditions (lights on from 7 a.m. to 7 p.m.) at constant room temperature (23 ± 1 °C) and relative humidity (45 %). Experiments were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). The experimental procedures were approved by the institutional committee on animal care and use, and all efforts were made to minimize animal suffering and reduce the number of animals used for the experiments.
Drugs and treatments
The sertraline (Ser) was purchased from Sigma (St. Louis, MO, USA). The ketamine used in the present study was purchased from Fort Dodge Animal Health (Fort Dodge, IA, USA) as injectable solution (concentration 0.1 g/ml).
Long-term behavioral effects of ketamine after electric foot shocks procedures
The experimental procedure was carried out as described previously (Qiu et al. 2013; Zhang et al. 2012). For the training session, a plexiglass chamber (20 × 10 × 10 cm) with a stainless-steel grid floor (9-mm interval) was used. Electric foot shocks were delivered through the grid floor by an isolated shock generator (Med Associates Inc., USA). Each mouse was placed in the chamber, and after a 5-min adaptation period, a total of 15 intermittent, inescapable foot shocks (intensity 0.8 mA, interval 10 s, and duration 10 s) were delivered for 5 min. Control animals were placed in the same chamber for 10 min, without electric foot shocks. Starting the first day (day 1) after the foot shock procedure, Ser (15 mg/kg, i.g.) or ketamine (0.625, 1.25, and 2.5 mg/kg, intraperitoneal (i.p.)) was given once a day at 8:00–9:00 a.m. The drug doses and the administration time were selected according to our previous studies. Each animal was successively tested in the contextual freezing measurement test, the open field test, and the staircase test.
Contextual freezing measurement
The test was performed as described previously, with minor modifications (Zhang et al. 2012). All animals were exposed to the reminder situation, i.e., the same chamber where the foot shocks had been delivered, but with no further foot shocks, for 5 min on days 3, 8, and 15. Freezing behavior, defined as an absence of all movement (except for respiration), was scored during the situational reminder during a 3-min period on day 15. The total cumulative freezing time (total seconds spent freezing during each assessment period) was measured and analyzed automatically using computer software (Med associates Inc Video Freeze SOF-843., USA).
The open field
To evaluate whether the reversion of contextual freezing by ketamine depends on affecting locomotor activity, we assessed the number of line crossings and rears in mice. This test was performed as described previously, with minor modifications (Zhang et al. 2012). On day 16 after foot shocks, mice were placed in the corner of a plastic box (36 × 29 × 23 cm) with a base divided into equal sectors, for a 5-min acclimation period, and the number of crossings (with all four paws placed into a new square) and rears (with both front paws raised from the floor) were recorded in the next 5 min.
The staircase test
The staircase was made from polyvinylchloride and consisted of five identical steps (2.5 cm high, 10 cm wide, and 7.5 cm deep). The height of the walls was constant (12.5 cm above the stairs) along the entire length of the staircase. On day 18 after the foot shocks, the mouse was placed on the floor of the box with its back toward the staircase. Each mouse was individually placed onto the staircase. During a 3-min period, the number of rearings and the number of steps climbed were recorded. A step was considered climbed only if the mouse placed all four paws on the stair. The number of steps descended was not counted. At the end of 3 min, the mouse was removed, and the staircase was cleaned with an alcohol sponge to eliminate any residual odors. The treatments were randomized, and the observer was blind to the grouping. All studies were carried out between 8:00 and 11:00 a.m.
Long-term behavioral effects of ketamine after TDS procedures
The TDS procedure was performed as described previously (Zhang et al. 2012). Briefly, after a 1-week acclimatization period, the rats were restrained for 2 h, and then each rat was immobilized inside a disposable, clear polyethylene rodent-restraint cone (day 1). The large end of the cone was closed with tape. The bag size was adjusted according to the size of the animal to achieve complete immobilization. A hole in the small end of the cone allowed the rats to breathe freely. After that, rats were individually placed in a clear acrylic cylinder (24-cm diameter, 50-cm height), filled with water (24 °C) to 2/3 of its height, and forced to swim for 20 min. Following a 15-min recuperation period, rats were exposed to diethyl ether until they lost consciousness. The rats were allowed to recover for a week and were then subjected to a brief restress on day 7 (20-min swim stress).
Starting on the first day after the TDS procedure (day 2, rats were exposed to the restraint, forced swim, and exposure to ether), ketamine (0.625, 1.25, and 2.5 mg/kg, i.p.) was given once per day at 8:00–9:00 a.m. To minimize effects of multiple testing, tests were ordered from least to most stressful, and cagemates were tested simultaneously or on consecutive days. The originality of our protocol lies in the fact that the same cohort of animal was tested in different behavioral paradigms, according to the procedure described previously (Zhang et al. 2012).
The locomotor activity test
Locomotor activity was assessed using an open-field test 13 days after TDS. Rats were placed in the corner of a plastic box (76 × 76 × 46 cm), with the base divided into approximately 18 sectors. Rats were allowed to habituate to the environment for 5 min, and the number of crossings (crossing the sector with four paws) and rears (raising the forepaws) were recorded simultaneous for 5 min.
Contextual freezing measurement
The contextual fear paradigm was conducted after the end of the 14-day drug treatments. Each rat was exposed to the conditioning context (180 s, in the conditioning chamber [60 × 21 × 30 cm] without any stimulation). Immediately after that, a foot shock (0.8 mA, 4 s) was given through a stainless steel grid floor (Med Associates Inc. USA). Twenty-four hours after the initial foot shock (day 15 after TDS), the rat was placed in the conditioning chamber where it had previously been foot shocked, and the contextual fear response was then evaluated by measuring the duration of freezing behavior in a 5-min interval. “Freezing” behavior was defined as a total absence of body or head movement except for that associated with breathing, which was rated automatically by computer software (Med associates Inc Video Freeze SOF-843., USA).
The EPM test
This paradigm has proven valid for detecting responses to external stressful stimuli (Liebsch et al. 1998). The apparatus consisted of four branching arms (50 × 10 cm) with two arms open and the other two closed with dark walls (14 cm high). The arms were connected by a center platform (10 × 10 cm), and the maze was 50 cm above the ground. Eighteen days after TDS (day 18), individual rats were placed in the central platform, facing the closed arms. For the purpose of analysis, open-arm activity was quantified as the time spent in the open arms relative to time in the open arms plus time in the closed arms and the total number of entries into open arms relative to the number of in the open arms plus the number of in the closed arms. Rats were scored as entering an open or closed arm only when all four paws passed over the dividing line. The maze was cleaned with a 5 % ethanol/water solution after each test to remove any confounding olfactory cues and dried thoroughly between sessions.
Effects of ketamine on the BDNF level in post-TDS rats
Rats used in the TDS test were sacrificed by decapitation, and the hippocampus was isolated for Western blotting detection. The protein concentration was determined by a BCA assay. Then, 20 μg of total protein was separated by 12 % SDS-PAGE and transferred to PDVF membranes (Millipore, Billerica, MA, USA) by electroblotting. Following blocking in 1 % BSA for 2 h, the protein membrane was incubated in the primary antibody, rabbit anti-BDNF (Sigma Company, 1:1000), at 4 °C overnight and then in the secondary antibody, HRP-conjugated goat anti-rabbit IgG (1:5,000), for 1 h. Bound antibodies were detected using an enhanced chemiluminescence detection reagent (Applygen Company, Beijing, China). Band intensities were quantified using the ImageQuant software package (GE Healthcare).
Statistical analysis
All data were expressed as the mean ± SEM. Student’s t test compares the difference between two groups (i.e., control nonshocked mice vs. shocked mice, shocked mice vs. shocked + Ser treatment mice; and non-TDS rats vs. post-TDS rats, post-TDS rats vs. TDS + Ser treatment rats), with Bonferroni corrections performed where necessary to control for Type I error. Long-term behavioral effects of ketamine after electric foot shocks in mice or TDS in rats were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s t test. For all tests, differences of P < 0.05 were considered significant.
Results
Long-term behavioral effects of ketamine after electric foot shocks in mice
There was no significant effect on the number of line crossings and rearings between the control nonshocked mice and the shocked mice. Daily oral administration of either Ser (15 mg/kg) or ketamine also did not significantly affect number of line crossings and rearings. These results indicate that neither foot shocks nor repeated ketamine treatment affected locomotor activity in this animal mode (figures are not shown).
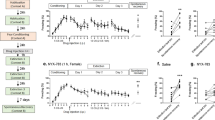
Electric foot shocks caused a significant increase in the contextual freezing response (Student’s t test, P < 0.001) of shocked mice compared to control, nonshocked mice, indicating that the electric foot shock model was successful. As a positive control, Ser (15 mg/kg) also significantly reduced the freezing behavior (Student’s t test, P < 0.01) induced by foot shock. However, since multiple tests were undertaken in this analysis, we used the Bonferroni correction to identify these effects. After this correction, the significant difference remained, which demonstrates the predictive validity of this model. Chronic treatment with ketamine showed a main effect on the contextual freezing response in mice exposed to foot shock (one-way ANOVA, F[3,36] = 3.931, P < 0.05). Further post hoc analysis revealed that ketamine (0.625, 1.25, and 2.5 mg/kg) decreased the contextual freezing response (Dunnett’s test, P < 0.01 compared with foot shock-vehicle group) on day 15 (Fig. 1b). These results indicate that mice exhibit a persistent fear response to the context associated with traumatic events and that repeated treatment with ketamine can alleviate the contextual freezing behavior in the shocked mice.
Long-term behavioral effects of ketamine (KET) after electric foot shocks in mice. a Treatment schedule and order of behavioral tests for the inescapable electric foot shock model in mice. b Exposure to foot shocks significantly increased the contextual freezing response. The freezing behavior was significantly reduced in the groups that were administered either Ser or ketamine (1.25 and 2.5 mg/kg, respectively, i.g.) on day 15. c, d Exposure to foot shocks resulted in an increased number of rearings but failed to significantly affect the number of steps. Ketamine treatment decreased the number of rearings without affecting the climbing behavior in the staircase test. Data are presented as the means ± SEM (n = 8–10). *P < 0.05, ***P < 0.001, compared with the foot shock (−) group; #P < 0.05, ##P < 0.01 compared with the saline-treated foot shock (+) group (ANOVA followed by Dunnett’s t test)
In the staircase test, mice that had been previously exposed to foot shocks exhibited an increased number of rears (Student’s t test, P < 0.05) but failed to demonstrate a significant change in the number of steps. These results indicate that the animals still avoided the aversive-like compartment and that they exhibited a fear response to the context associating with traumatic events. We also found that repeated administrations of ketamine or Ser decreased the number of rearings (ketamine 1.25 and 2.5 mg/kg, one-way ANOVA, F[3,36] = 3.046, P < 0.05; Ser 15 mg/kg, Student’s t test, P < 0.05) without affecting climbing behavior in the staircase test (Fig. 1c), suggesting that ketamine significantly improved the behavioral deficits induced by the aversive procedures.
Long-term behavioral effects of ketamine after exposure to TDS
To examine the possibility that TDS and/or drug treatments influenced baseline locomotor activity, we investigated the level of spontaneous locomotor activity in each group. The results showed that there was no significant difference between the control, non-TDS rats and the post-TDS rats. As a positive control, Ser (15 mg/kg) also did not significantly affect the number of line crossings and rearings. The daily oral administration of ketamine did not show an effect on the number of line crossings and rears (figures are not shown). Compared to vehicle treatment, exposure to TDS significantly increased the contextual freezing response (Student’s t test, P < 0.05). A 14-day chronic coadministration with Ser alleviated the enhanced contextual freezing in rats that experienced TDS (Student’s t test, P < 0.001). A similar effect was also observed with repeated ketamine (0.625 and 2.5 mg/kg) treatment (one-way ANOVA, F[3,36] = 4.743, P < 0.01) (Fig. 2b).
Treatment schedules and order of behavioral tests for the PTSD model of TDS a. The post-TDS rats showed a significant increase in contextual freezing b and a decreased percentage of both time spent in and entries into open arms in the elevated plus maze test c. Repeated administration of ketamine (KET) ameliorated these behavioral deficits. Daily administrations of either Ser or KET were begun on the first day after the TDS procedure. Data are presented as the means ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001, compared with TDS (−) group; ##P < 0.01, ###P < 0.001 compared with the saline-treated TDS (+) group
As shown in Fig. 2c, Student’s t test revealed that TDS-exposed animals showed significant reductions in the percentage of time spent in open arms (Student’s t test, P < 0.05) and in percent number of entries into open arms (Student’s t test, P < 0.05) in the elevated plus maze (EPM) test. One-way ANOVA analyses revealed that the chronic coadministration of ketamine (0.1 and 0.3 mg/kg) significantly increased the percentage of time spent in the open arms (F[3,36] = 4.416, P < 0.05) and the number of entries into the open arms (F[3,36] = 16.93, P < 0.05), as did the repeated administrations of Ser (15 mg/kg, Student’s t test, P < 0.05).
Effects of ketamine on the BDNF level in post-TDS rats
The levels of BDNF in the hippocampus of post-TDS rats were measured at the end of the EPM test. As shown in Fig. 3, statistical analysis revealed that the TDS procedure significantly reduced the level of BDNF in the hippocampus (Student’s t test, P < 0.05). This effect was clearly reversed by the chronic administration of ketamine treatment at 1.25 and 2.5 mg/kg (one-way ANOVA, F[3,36] = 10.71, P < 0.05).
Effects of ketamine (KET) on the level of BDNF in the hippocampus of post-TDS rats. The upper panels are quantification of BDNF. Values shown are means ± SEM of four rats per group; the immunoblotting data are expressed as percentages of corresponding optical density (normalized to β-actin) in the vehicle control samples. **P < 0.01 compared with TDS (−); ###P < 0.001 compared with TDS-exposed group (ANOVA followed by Dunnett’s t test)
Discussion
The present study demonstrated that chronic treatment with ketamine caused significant suppression of enhanced anxiety and contextual fear induced by inescapable electric foot shocks in mice and TDS in rats, which is similar to the effects of the first-line anti-PTSD drug sertraline (SSRI). Furthermore, ketamine increased the level of BDNF in the hippocampus of post-TDS rats.
Our results demonstrated that repeated situational reminders followed by electric foot shocks elicited the acquisition of conditioned fear and that the mice showed an innate aversive freezing behavior. Repeated ketamine treatment significantly increased the time spent in the aversive-like context, indicating that ketamine alleviated the stressed animals’ fear of the context associated with the traumatic event. Moreover, the aversive procedure did not affect the animals’ spontaneous locomotor activity or the number of steps climbed in the staircase test in mice. These results are in accordance with the studies of Pynoos et al. (1996), who showed that foot shocks associated with situational reminders did not affect the motor activity of male mice in an open field test performed 3–6 weeks after the first foot shock. The present study indicates that aversive foot shocks followed by repeated reminders are a reliable long-lasting animal model for PTSD and that ketamine has a therapeutic effect in this animal model within a certain dose range.
TDS models have utilized intense stressors, aversive challenges, and situational reminders of a traumatic stress in an attempt to model long-term effects on behavioral, autonomic, and hormonal responses seen in humans with PTSD (Uys et al. 2003). In this model, a single exposure to stress causes subsequent sensitization during the restress. Rats are exposed to various stressors (restraint stress, forced swim, and ether exposure). Then, the rats are allowed to recover for a week and are then subjected to a brief restress on day 7 (20-min swim stress). There is growing evidence that the TDS model is a proven PTSD animal model that utilizes intense stressors, aversive challenges, and situational reminders of a traumatic stress in an attempt to model the long-term behavioral, autonomic, and hormonal responses observed in humans with PTSD (Khan and Liberzon 2004; Uys et al. 2003). To date, the TDS model has been proven by different laboratories to resemble the clinical condition with accurate face, construct, and predictive validity (Oosthuizen et al. 2005). The data presented here demonstrate that contextual freezing is significantly enhanced in rats exposed to TDS and that the chronic administration of ketamine successfully reverses these adverse effects. EPM, a model that uses rodents’ natural fear of open and elevated places, has been proven valid for detecting responses to external stressful stimuli. It has been found that the TDS procedure induced anxiety-like behavior in the EPM test. The present study also shows that TDS exposure produces representative anxiety-like behavior, as evidenced by the fact that TDS-exposed animals significantly decreased the percentage of time spent in and the number of entries into the open arms, while ketamine reversed these behavioral changes and alleviated anxiety in rats after TDS exposure. We also found that TDS and/or ketamine did not significantly influence spontaneous locomotor activity in rats, which is consistent with earlier reports that ketamine does not affect locomotion in male adult Sprague–Dawley rats, over a large range of doses (e.g., 0–10 mg/kg) (Wilson et al. 2007). These findings suggesting that the behavioral changes observed in this study were not due to a change in basal locomotor activity.
Over the past decades, many studies have suggested that several neurotrophic factors and related signaling cascades might be involved in the pathophysiology of mood disorders, including depression and PTSD (Broekman et al. 2007; Pivac et al. 2012; Zhang et al. 2013). The hippocampus is a limbic structure that is important in the control of learning and memory and in the regulation of the hypothalamic–pituitary–adrenal (HPA) axis. Moreover, the hippocampus has connections with the amygdala and the prefrontal cortex. The hippocampus, prefrontal cortex, and amygdala have all been implicated in PTSD. It is known that stress decreases the expression of BDNF in limbic structures and that chronic antidepressant treatment reverses the effects of stress (Dell’Osso et al. 2009). Our data demonstrates that the repeated administration of ketamine increased hippocampal BDNF protein levels in post-TDS rats. It should be stated that, despite the role played by amygdala and prefrontal cortex in mediating PTSD behaviors, in this study, we did not evaluate BDNF protein levels in these areas. Therefore, we cannot discard the idea that BDNF protein levels might also be altered in the amygdala and the prefrontal cortex.
Previous studies have demonstrated that acute administration of ketamine (15 mg/kg) causes an increase in BDNF protein levels in the rat hippocampus, while repeated administration (for 14 days) of ketamine (15 mg/kg) does not alter hippocampal BDNF protein levels(Garcia et al. 2008a, b). It should be noted that, in those studies, BDNF levels were assessed in rats that were not subjected to the TDS procedures. Thus, we must consider that these studies used different behavioral procedures, different doses, different routes, and different durations of ketamine administration.
It is important to note that NMDA receptor activation has been suggested to play a role in some of the kindling-like processes that have been associated with the formation of spontaneous intrusive memories (Adamec 1997; Grillon et al. 1996) and that states of high NMDA receptor activity may increase the risk of developing PTSD because they may increase the likelihood for aversive memory encoding (Mehta and Binder 2012; Reul and Nutt 2008). NMDA receptors play an important role in the memory consolidation processes (Lee et al. 2006), and antagonizing NMDA receptors in the hippocampus impairs the consolidation of fear conditioning (Liu et al. 2009; Zimmerman and Maren 2010), suggesting that NMDA receptor antagonists may be useful in the treatment of PTSD. NMDA receptor antagonists also interfere with anxiety-related behavior in rats if they are given shortly after exposure to predator stress (Adamec et al. 1999). In this respect, it is worth noting that in a preliminary retrospective study, McGhee et al. (McGhee et al. 2008) found that in a group of burned service men, those treated with the NMDA receptor antagonist ketamine during hospitalization had a lower incidence of PTSD than did the others. These reports were consistent with our finding that chronic injection of ketamine shortly after exposure to TDS ameliorated behavioral deficits.
PTSD is an illness with high chronicity, comorbidity, and severity, accompanied by different neuroendocrine and neurochemical alterations and different clinical manifestations. In fact, we recently found that NMDA receptor antagonist MK-801, at doses of 0.0125, 0.025, and 0.05 mg/kg, i.p., which did not significantly influence spontaneous locomotor activity, produced no significant behavioral changes in mice after electric foot shocks (figures are not shown). While ketamine, in addition to binding with NMDA receptors, also binds to α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors, GABAA receptors, opioid receptors, cholinergic receptors, substance P receptors, dopamine D-2 receptors, 5-HT2 receptors, and voltage-sensitive Ca2+ and Na1+ channels, at physiologically relevant concentrations (Lang and Borgwardt 2013a). Furthermore, ketamine binding at serotonergic and dopaminergic receptors occurs with an affinity (e.g., 0.5 μM) that is quite comparable to its affinity for NMDA receptors (Kapur and Seeman 2002). Ketamine also interacts with monoamine reuptake transporters in the same way as antidepressant drugs, increasing synaptic monoamine concentrations (Tso et al. 2004; Lang and Borgwardt 2013b). Considering this background, it seems plausible that ketamine may be a strongly multi-target drug which may exert its anti-PTSD-like effect via several receptor pathways. The precise mechanism of action warrants further studies, which now are now being undertaken by our group.
Conclusion
In summary, our findings indicate that ketamine has a clear anti-PTSD-like effect that might be at least partially mediated by an influence on BDNF signaling in the hippocampus. Future studies are needed to clarify which receptor system is responsible for the anti-PTSD effects of ketamine in animal models. The results of these investigations have theoretical implications for the neural theory of PTSD and clinical implications for the treatment of this mental disorder.
Abbreviations
- ANOVA:
-
Analysis of variance
- BDNF:
-
Brain-derived neurotrophic factor
- NMDA:
-
N-methyl-d-aspartate
- PTSD:
-
Posttraumatic stress disorder
- TDS:
-
Time-dependent sensitization
- SSRIs:
-
Selective serotonin reuptake inhibitors
- EPM:
-
Elevated plus maze
- ST:
-
Staircase test
References
Adamec R (1997) Transmitter systems involved in neural plasticity underlying increased anxiety and defense—implications for understanding anxiety following traumatic stress. Neurosci Biobehav Rev 21:755–765
Adamec RE, Burton P, Shallow T, Budgell J (1999) NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure—implications for anxiety associated with posttraumatic stress disorder. Physiol Behav 65:723–737
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Broekman BF, Olff M, Boer F (2007) The genetic background to PTSD. Neurosci Biobehav Rev 31:348–362
da Silva FC, do Carmo de Oliveira Cito M, da Silva MI, Moura BA, de Aquino Neto MR, Feitosa ML, de Castro Chaves R, Macedo DS, de Vasconcelos SM, de Franca Fonteles MM, de Sousa FC (2010) Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res Bull 83:9–15
Dell'Osso L, Carmassi C, Del Debbio A, Catena Dell'Osso M, Bianchi C, da Pozzo E, Origlia N, Domenici L, Massimetti G, Marazziti D, Piccinni A (2009) Brain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorder. Prog Neuro-psychopharmacol Biol Psychiatry 33:899–902
Domino EF (2010) Taming the ketamine tiger. 1965. Anesthesiology 113:678–684
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127
Engin E, Treit D, Dickson CT (2009) Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience 161:359–369
Felmingham KL, Dobson-Stone C, Schofield PR, Quirk GJ, Bryant RA (2013) The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biol Psychiatry 73:1059–1063
Garcia LS, Comim CM, Valvassori SS, Reus GZ, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008a) Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol 103:502–506
Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008b) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuro-psychopharmacol Biol Psychiatry 32:140–144
Grillon C, Southwick SM, Charney DS (1996) The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry 1:278–297
Haddad P (1998) The SSRI discontinuation syndrome. J Psychopharmacology 12:305–313
Hashimoto K (2011) Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev Neurother 11:33–36
Kapur S, Seeman P (2002) NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry 7:837–844
Khan S, Liberzon I (2004) Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology 172:225–9
Kos T, Popik P, Pietraszek M, Schafer D, Danysz W, Dravolina O, Blokhina E, Galankin T, Bespalov AY (2006) Effect of 5-HT3 receptor antagonist MDL 72222 on behaviors induced by ketamine in rats and mice. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol 16:297–310
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Lang UE, Borgwardt S (2013a) Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem Pharmacol 31:761–777
Lang UE, Borgwardt S (2013b) Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol Biochem 31:761–777
Lee JL, Milton AL, Everitt BJ (2006) Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci: Off J Soc Neurosci 26:10051–10056
Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19:289–317
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
Liebsch G, Montkowski A, Holsboer F, Landgraf R (1998) Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res 94:301–310
Liu JL, Li M, Dang XR, Wang ZH, Rao ZR, Wu SX, Li YQ, Wang W (2009) A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS One 4:e7548
Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC (2005) The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging 26:115–123
Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA Jr (2009) Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther 123:143–150
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352
Matsuoka Y, Nishi D, Noguchi H, Kim Y, Hashimoto K (2013) Longitudinal changes in serum brain-derived neurotrophic factor in accident survivors with posttraumatic stress disorder. Neuropsychobiology 68:44–50
McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH (2008) The correlation between ketamine and posttraumatic stress disorder in burned service members. The Journal of Trauma 64:S195–198, Discussion S197-8
Mehta D, Binder EB (2012) Gene x environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology 62:654–662
Nelson EM, Philbrick AM (2012) Avoiding serotonin syndrome: the nature of the interaction between tramadol and selective serotonin reuptake inhibitors. Ann Pharmacother 46:1712–1716
Oosthuizen F, Wegener G, Harvey BH (2005) Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): evidence from an animal model. Neuropsychiatr Dis Treat 1:109–123
Pivac N, Kozaric-Kovacic D, Grubisic-Ilic M, Nedic G, Rakos I, Nikolac M, Blazev M, Muck-Seler D (2012) The association between brain-derived neurotrophic factor Val66Met variants and psychotic symptoms in posttraumatic stress disorder. World J Biol Psychiatry: Off J World Fed Soc Biol Psychiatry 13:306–311
Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I (1996) A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry 39:129–134
Qiu ZK, Zhang LM, Zhao N, Chen HX, Zhang YZ, Liu YQ, Mi TY, Zhou WW, Li Y, Yang RF, Xu JP, Li YF (2013) Repeated administration of AC-5216, a ligand for the 18kDa translocator protein, improves behavioral deficits in a mouse model of post-traumatic stress disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 45:40–46
Reul JM, Nutt DJ (2008) Glutamate and cortisol—a critical confluence in PTSD? J Psychopharmacol 22:469–472
Rosa AO, Lin J, Calixto JB, Santos AR, Rodrigues AL (2003) Involvement of NMDA receptors and L-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav Brain Res 144:87–93
Sheeler RD, Ackerman MJ, Richelson E, Nelson TK, Staab JP, Tangalos EG, Dieser LM, Cunningham JL (2012) Considerations on safety concerns about citalopram prescribing. Mayo Clin Proc Mayo Clin 87:1042–1045
Tso MM, Blatchford KL, Callado LF, McLaughlin DP, Stamford JA (2004) Stereoselective effects of ketamine on dopamine, serotonin and noradrenaline release and uptake in rat brain slices. Neurochem Int 44:1–7
Uys JD, Stein DJ, Daniels WM, Harvey BH (2003) Animal models of anxiety disorders. Curr Psychiatry Rep 5:274–281
Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A (2007) Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol Behav 91:202–207
Yilmaz A, Schulz D, Aksoy A, Canbeyli R (2002) Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol, Biochem Behav 71:341–344
Zarate C Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G (2010) Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry 18:293–303
Zhang LM, Yao JZ, Li Y, Li K, Chen HX, Zhang YZ, Li YF (2012) Anxiolytic effects of flavonoids in animal models of posttraumatic stress disorder. Evid-based Complement Alternat Med: eCAM 2012:623753
Zhang LM, Zhao N, Guo WZ, Jin ZL, Chen HX, Xue R, Zhang YZ, Yang RF, Li YF (2013) Antidepressant-like and anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa). Neuropharmacology. 81:116–125
Zimmerman JM, Maren S (2010) NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci 31:1664–1670
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81001653, 30973516, 81102423, 81102498, 81072624, and 81173036) and the National Key New Drug Creation Program (No. 2012ZX09102101-004, 2012ZX09J12110-02C, and 2012ZX09J12201-004).
Conflicts of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Author contributions
Author Zhang (Li-ming Zhang) performed the research design, data analysis, and manuscript writing. Authors Wen-Wen Zhou, Ya-Jun Ji, and Ying Li performed the behavioral tests and Western blotting. Nan-Zhao, Hong-Xia Chen, and Rui Xue participated in behavioral tests. Authors Zhang (You-Zhi Zhang) and Wang contributed to research design, data analysis, and manuscript revision. Authors Mei and Li contributed to research design and manuscript revision.
Li-Ming Zhang and Wen-Wen Zhou contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 130 kb)
Rights and permissions
About this article
Cite this article
Zhang, LM., Zhou, WW., Ji, YJ. et al. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacology 232, 663–672 (2015). https://doi.org/10.1007/s00213-014-3697-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3697-9