Abstract
Rationale
There is presently no approved single treatment for dual alcohol and nicotine dependencies.
Objective
This pilot study investigated baclofen effects in alcoholic smokers.
Methods
This was a preliminary double-blind placebo-controlled randomized clinical study with 30 alcoholic smokers randomized to baclofen at 80 mg/day or placebo. A subgroup (n = 18) participated in an alcohol cue-reactivity experiment.
Results
Baclofen, compared with placebo, significantly decreased the percent days of abstinence from alcohol-tobacco co-use (p = 0.004). Alcohol dependence severity moderated baclofen effects, with the higher severity group having the greater baclofen response (p < 0.001). Although the percent days of alcohol-tobacco co-use declined in both groups, this decline was greater after placebo than baclofen (p < 0.001). Secondary analyses on alcohol or tobacco use alone suggested that the increase in percent days of co-abstinence was driven by the medication differences on heavy drinking days and on percent days smoking. In the cue-reactivity substudy, baclofen slightly decreased alcohol urge (p = 0.058) and significantly reduced salivation (p = 0.001), but these effects were not related to cue type.
Conclusions
This study provides preliminary evidence suggesting a possible role of baclofen in the treatment of alcoholic smokers. However, the mixed results and the small sample require larger confirmatory studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol and nicotine use frequently co-occur (Schlaepfer et al. 2008), and approximately half of the alcoholic population smokes (Grant et al. 2004; Romberger and Grant 2004; Sobell et al. 1990). Spontaneous smoking cessation is infrequent among alcoholic individuals (Falk et al. 2006; Hintz and Mann 2007) and up to 75 % of alcoholic smokers would require treatment for both dependencies (Grant et al. 2004). However, there is presently no approved solitary treatment that addresses both addictions concurrently. Efforts have been made to identify possible treatments that could help patients with alcohol and nicotine co-dependence, e.g., varenicline (Jimenez-Ruiz et al. 2009; Litten et al. 2013), naltrexone (Fucito et al. 2012), topiramate (Baltieri et al. 2009; Johnson et al. 2005), and combined nicotine patch and gum (Cooney et al. 2009).
The GABAB receptor agonist baclofen (BACL) may be an effective pharmacotherapy for alcoholism, as well as for smoking. In animals, BACL diminishes: acquisition of alcohol-drinking behavior (Colombo et al. 2000, 2002; Daoust et al. 1987), the increase in alcohol intake after abstinence (Colombo et al. 2003a, 2006), alcohol self-administration (Anstrom et al. 2003; Besheer et al. 2004; Janak and Michael Gill 2003; Liang et al. 2006; Maccioni et al. 2005; Walker and Koob 2007), alcohol-related motivation (Colombo et al. 2003b; Maccioni et al. 2008b), and cue-induced reinstatement of alcohol-seeking behavior (Maccioni et al. 2008a). In alcohol-dependent individuals, two open-label pilot studies (Addolorato et al. 2000; Flannery et al. 2004) and a small 4-week double-blind placebo (PLA)-controlled randomized clinical trial (DBPCRCT) (Addolorato et al. 2002a) found BACL at 30 mg/day to reduce alcohol drinking. Furthermore, a 12-week DBPCRCT demonstrated that BACL at 30 mg/day was effective in promoting alcohol abstinence in severely ill alcoholic cirrhotic patients (Addolorato et al. 2007), including those with hepatitis C infection (Leggio et al. 2012). Another 12-week DBPCRCT with BACL at 30 mg/day, however, failed to find similar results in alcohol-dependent patients (Garbutt et al. 2010). Differences in alcohol dependence severity are highlighted as a possible explanation of the inconsistencies across these trials (Leggio et al. 2010).
Initial studies also indicate that BACL may be an effective treatment for smoking. Two studies in nondependent rats trained to self-administer nicotine under limited access conditions, found reduced intravenous nicotine self-administration, after BACL microinfusions into the pedunculopontine tegmental nucleus (Corrigall et al. 2001), and the ventral tegmental area (Corrigall et al. 2000), suggesting that BACL reduced the reinforcement effects of nicotine. BACL administered intraperitoneally antagonized intravenous nicotine self-administration and nicotine-rewarding effects in rats trained to chronically self-administer nicotine under a continuous reinforcement schedule (Fattore et al. 2002; Markou et al. 2004; Paterson et al. 2004), and these BACL effects were dose-dependent (Fattore et al. 2009; Markou et al. 2004; Paterson et al. 2004); furthermore, BACL inhibited nicotine stimulant effects (Lobina et al. 2011). A 9-week DBPCRCT in heavy smokers who were contemplating to quit smoking indicated that BACL at 80 mg/day significantly reduced cigarettes smoked per day (CPD) (Franklin et al. 2009).
These results suggest that BACL could represent a unique pharmacotherapy to treat alcoholic smokers. However, this aspect has never been formally investigated. The primary aim of this preliminary study was to investigate the dual effects of BACL on alcohol consumption and smoking concurrently in alcoholic smokers, examining the hypothesis that BACL would significantly reduce alcohol and cigarette co-use. Secondary aims were to investigate the effects of BACL on reduction of or abstinence from either alcohol or tobacco. We also explored whether BACL effects were moderated by alcohol dependence severity, nicotine dependence severity or treatment goals. Finally, in a substudy, we explored the effects of BACL on alcohol cue-elicited craving for alcohol and smoking.
Methods
Design and setting
This was a pilot between-subject DBPCRCT (Clinical Trial Registration no. ISRCTN62137064) conducted at the Brown University Center for Alcohol and Addiction Studies (CAAS) and Roger Williams Medical Center (RWMC), Providence, RI. Both Brown and RWMC Institutional Review Boards approved the study.
Participants
Participants were alcohol-dependent heavy-drinking and heavy-smoking individuals. To be eligible, participants: (a) had to be between 18 and 75 years old (inclusive); (b) had to have DSM-IV diagnoses of both alcohol and nicotine co-dependency, with heavy use of alcohol (men ≥5 standard drink units (SDUs), and women ≥4 SDUs, a day on average) and cigarettes (≥10 CPD on average) during the last 90 days before screening; (c) had to be interested in receiving treatment for both drinking and smoking (either reducing or stopping both substances; or reducing one substance and stopping the other). Exclusion criteria included: (a) current (i.e., past year) DSM-IV diagnosis of dependence on any psychoactive substance other than alcohol and nicotine; (b) lifetime DSM-IV diagnosis of schizophrenia, bipolar disorder, or other psychosis; (c) past year diagnosis of major depression, anxiety disorders, and eating disorders; (d) risk of suicide (e.g., active plan or recent attempt in last year); (e) positive urine drug screen at baseline for any illegal substance other than marijuana; (f) significant alcohol withdrawal symptoms, as assessed by a Clinical Institute Withdrawal Assessment for Alcohol revised (CIWA-Ar) score >10; (g) history of hospitalization for alcohol intoxication delirium, alcohol withdrawal delirium or seizure; (h) participation in any research study for alcoholism and/or smoking treatment within 3 months prior to signing the consent document; (i) pharmacological treatment with naltrexone, acamprosate, topiramate, disulfiram, nicotine replacement, bupropion, and varenicline within 1 month prior to randomization; (j) current use of psychotropic medications or medications that interfere with the metabolism of BACL, history of allergy to BACL or medical contraindications to take BACL; (k) severe medical diseases, such as cancer, cirrhosis, chronic kidney failure, and chronic neurological disorders; and (l) females who were of child bearing potential and not practicing effective birth control.
Study overview
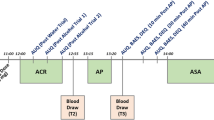
The study consisted of four phases: telephone pre-screening, in-person screening, 12-week treatment, and 4-week follow-up. Potential participants, recruited via advertisements in public transportation and mass media, referrals from other clinics, and by word of mouth, were phone screened. Those meeting initial screening criteria came for an in-person screen in which they provided written informed consent. Screening procedures (week 00 visit) included psychological assessments, blood/urine lab work (e.g., liver and kidney function tests, CBC, urine drug test, and urine pregnancy test), electrocardiogram, medical history, and physical exam. Breath alcohol concentrations (Alco-Sensor IV; Intoximeters, Inc.) were measured; vital signs were taken; alcohol consumption and smoking data were collected using the Timeline Follow-back (TLFB) (Lewis-Esquerre et al. 2005; Sobell et al. 1988); and participants filled out craving assessments. Cravings were measured using the Alcohol Urge Questionnaire (AUQ) (Bohn et al. 1995), the Obsessive Compulsive Drinking Scale (OCDS) (Anton et al. 1995), the Questionnaire on Smoking Urges-Brief (QSU-B) (Cox et al. 2001), and a smoking Visual Analogue Scale (S-VAS) on which scores range from 1 to 10 (Bertholet et al. 2012). Additionally, alcohol and nicotine dependence severity were assessed by the Alcohol Dependence Scale (ADS), on which scores range from 0 to 47 (Skinner and Allen 1982) and the Fagerström Test for Nicotine Dependence (FTND), on which scores range from 0 to 10 (Fagerstrom and Schneider 1989). Readiness to consider smoking cessation was assessed by the smoking contemplation ladder, on which scores range from 0 to 10 (Biener and Abrams 1991).
At week 01 visit, eligible participants were randomized to BACL or PLA using a 3-urn variable procedure (Stout et al. 1994), i.e., gender, baseline heavy drinking days (HDDs), and baseline CPD. BACL at 80 mg/day (20 mg, q.i.d.), or PLA was placed into blister packs as opaque capsules containing drug and 25 mg riboflavin (as a compliance measure; Del Boca et al. 1996). Medication adherence was assessed via pill count, self-report and verified by riboflavin check.
The BACL dose was 80 mg/day. While most prior alcohol studies have targeted 30 mg/day (Addolorato et al. 2000, 2002a, 2007; Garbutt et al. 2010; Leggio et al. 2013), a recent secondary analysis provided preliminary evidence suggesting a possible dose-response BACL effect (e.g., 60 vs. 30 mg/day) in alcoholic patients (Addolorato et al. 2011). Furthermore, Franklin et al. (2009) showed that BACL at 80 mg/day was effective in reducing CPD in smokers. Considering that a three-group design was not feasible for this small-size study, and that this study targeted a population of alcoholic smokers, we opted for a BACL dose of 80 mg/day.
Consistent with the study by Franklin et al. 2009, BACL was titrated up during the first 2 weeks until the targeted dose of 80 mg/day was reached, then administered through a 10-week period at the targeted dose, after which it was tapered and discontinued over 1 week. Patients came back to our facility seven times during the treatment phase, i.e., every week during the first month and then every other week. Participants were also seen at Week 16 for a brief follow-up. At Weeks 5, 7, 9, and 11, a phone call was made to check participants’ well-being. At each visit, measurements of breath alcohol concentration, vital signs, alcohol and smoking TLFB, and craving were taken. At Weeks 4, 8, and 12, blood/urine lab work was repeated to check patients’ safety. Compensation to cover travel expenses and time was provided at each visit.
Medical management
At each visit, patients received a medical management (MM) session, after which study medication for the following study period was dispensed. The MM approach described in the COMBINE (Anton et al. 2006; COMBINE Study Research Group 2003) was used but modified in order to focus the sessions on alcohol- and smoking-related (and their co-use) problems. The sessions provided personalized education regarding alcohol and smoking, helped participants to develop and implement a plan to reduce/stop alcohol and smoking, motivated participants for medication adherence, assessed adverse events, and evaluated concomitant medication use.
Participants recruited for this study were looking for treatment for both alcoholism and smoking, but they could vary in their treatment goals (i.e., reducing or quitting both substances or quitting one and reducing the other). Treatment goals were self-reported at baseline and were further elaborated during the trial with the MM therapist. Although this may have introduced variability across patients, this approach reduces the risk of high dropouts (as it is often observed in studies with a rigid “quit date” pre-set) and is closer to “real-world” settings where health care providers may use a pharmacotherapy to treat alcoholic smokers, and discuss and revise with each patient week-by-week reduction/abstinence goals.
Cue-reactivity experiment
A subgroup of 18 participants (nine BACL and nine PLA) from the main RCT participated in an alcohol cue-reactivity substudy conducted at Brown CAAS.Footnote 1 All procedures were performed on the same day during Week 6, after patients had already reached the targeted BACL dose (80 mg/day). The cue-reactivity procedure was performed as previously described (Monti et al. 2000, 2001). Moreover, consistent with Cooney et al. (2003), both urges to drink and smoke, after exposure to alcohol cues were assessed. Upon arrival, participants provided a urine sample for a drug screen, and carbon monoxide (CO) levels were assessed. Participants had to have a breath alcohol concentration = 0.00 and a CIWA-Ar score ≤10 to participate. Participants were not allowed to smoke for the next 5 h and were under continuous observation, with periodic CO monitoring to assure compliance with abstinence. At the end of this 5-h period, participants first underwent a 3-min relaxation period to collect baseline urge and physiological arousal levels. Then, a staff member entered the room with a tray covered by an inverted pitcher, containing a commercially labeled bottle of water and a glass. The pitcher was removed, the bottle was opened, and the glass was filled. Then the staff member left the room, and the audiotape instructed the participant to sniff the glass of water when s/he heard high tones and stop sniffing when s/he heard low tone tones. Next, participants underwent two 3-min alcohol trials identical to the water trial except the bottle of water was replaced with the commercially labeled participant’s preferred beverage. Cues were always presented in the same order because previous studies reported carry-over effects on urge ratings when alcohol cues are presented first (Monti et al. 1987, 1999; Rohsenow et al. 2000). Self-report ratings were obtained immediately after the baseline period and after each trial. Alcohol and smoking urges were measured by the AUQ (Bohn et al. 1995) and QSU-B (Cox et al. 2001), respectively. The Alcohol Attention Scale (AAS) (Rohsenow et al. 2000) was used to assess attention to the sight/smell of alcohol cues. During each trial, subjects placed three cotton rolls in their mouths to collect saliva (White 1977). Rolls were put in sealed, small-sized plastic bags to prevent loss of saliva, and the weight difference indicated the amount of saliva. Heart rate and mean arterial pressure were assessed continuously using a Welch Allyn monitor. These values were averaged over each 3-min trial.
Statistical analysis
Group comparisons on demographic, drinking, and smoking history measures were conducted using independent-samples t tests for continuous variables and chi-square tests for categorical variables. For the main trial, percent days of abstinence from alcohol-tobacco co-use and percent days of alcohol-tobacco co-use were the two primary dependent measures. Co-use was defined as the consumption on any given day of both any amount of alcohol and any number of cigarettes. These two outcomes do not add up to 100 % as they do not include days when patients used only one substance. The skewness and kurtosis of the outcome variables indicated that the normal distribution was approximated (skewness and kurtosis between −2 and +2).
Most analyses were conducted using mixed model analysis with time (week at full BACL dose, i.e., 8 time points) nested under subjects. If the outcome measure was not a difference score (e.g., percent days co-use), a baseline covariate was included in the analyses. As the two groups were not balanced on race, this variable was added as a covariate to all multivariate analyses. In addition to the main analyses with race added as a covariate, this pair of analyses (effects for percent days co-abstinence and percent days co-use) were each re-conducted three more times, with different covariates added in (separate analyses were conducted for each additional covariate due to the constrained sample size), i.e., adherence, gender, and smoking contemplation ladder score.
Moderation of medication effects on percent days abstinence from co-use and percent days co-use were examined by adding the relevant moderator term (ADS and FTND score) to the models. Treatment goals were entered as moderators of the medication effect, and a smoking goal × drinking goal interaction was also added. To further clarify the results on percent days co-abstinence and co-use, we also tested for medication effects on five supplemental exploratory outcomes, i.e., percent days smoking, percent days drinking, CPD, drinks per day, and percent HDD. Analyses tested medication effects, while controlling for race and the baseline value of the DV. Furthermore, we examined whether BACL “decouples” alcohol and tobacco use by examining correlations between CPD and drinks per day for the two groups (BACL and PLA).
In the cue-reactivity substudy, changes from pre-stimulus baseline in drinking urge, smoking urge, mean arterial pressure, heart rate, and salivation were examined using mixed model analyses with the predictors medication condition and stimulus condition (water vs. alcohol). As the groups were balanced on race, this variable was not included as a covariate. Baseline covariates paralleling the respective outcome measures were not included in these analyses due to collinearity issues. Moderation of medication effects on cue reactivity by alcohol or nicotine dependence severity was examined by adding the relevant moderator variable (ADS and FTND score) to the models.
Results
Sample description
Of 237 telephone pre-screenings, 45 signed the consent form and were screened in-person; 15 individuals were ineligible, while 30 individuals were eligible and randomized; 24 completed the study (Supplemental Fig. 1). Demographic, alcohol and smoking characteristics of the 30 patients are reported in Table 1. The groups differed on race but not other measures including contemplation ladder score, smoking goals, or drinking goals. In the overall group, 79 % wanted to quit smoking and 48 % wanted to quit drinking. Sixty percent of the BACL group and 36 % of the PLA group identified abstinence as their alcohol treatment goal. Average contemplation ladder scores were 5.3 ± 1.8 for BACL and 5.3 ± 1.0 for PLA. Eighty percent of the BACL group and 79 % of the PLA group identified abstinence as their smoking treatment goal. Medication compliance was 96.4 % in the BACL group and 90.2 % in the PLA group (t(28) = 0.93, p = 0.36).
Primary aims
Drinking and smoking co-use
BACL significantly reduced the % days of abstinence from alcohol-tobacco co-use (BACL, 12.1 ± 2.0 vs. PLA, 3.5 ± 2.2 (M ± SE); F(1,197.6) = 8.27, p = 0.004; Fig. 1a). Although the percent days of alcohol-tobacco co-use declined in both groups, this decline was greater after PLA than BACL (BACL, 44.7 ± 3.1 vs. PLA, 28.8 ± 3.3; F(1, 204.2) = 12.39, p < 0.001) (Fig. 2). The baseline value of the DV, and race covariates, were significant predictors in the latter but not the former analyses.
The BACL effect for percent days co-abstinence persisted when adherence, or gender or the smoking contemplation ladder score was added as a covariate. Likewise, the PLA effect on percent days co-use persisted in the three parallel analyses. Medication compliance did not impact the main outcomes.
Secondary aims
Secondary outcomes
Analyses of the single use of each substance did not reveal an effect of BACL vs. PLA on duration of abstinence from alcohol or tobacco. Post-hoc analyses demonstrated that BACL was slightly more effective in promoting smoking cessation for 2 weeks or more, i.e., 20 % in the BACL group, but 0 % of the PLA group, stopped smoking for ≥2 weeks (X 2(1, N = 30) = 3.33, p = 0.07).
Additional exploratory outcomes on either drinking or smoking were analyzed. BACL significantly reduced percent days smoking (BACL, 85.1 ± 2.3 and PLA, 96.3 ± 2.4; F(1, 188.5) = 11.69, p = 0.001; Fig. 1b) and percent HDD (BACL, 14.1 ± 2.8 and PLA, 39.8 ± 2.6; F(1, 199.4) = 45.3, p < 0.001). PLA significantly reduced percent days drinking (BACL, 48.1 ± 3.0 and PLA, 29.4 ± 3.1; F(1, 204.7) = 18.42, p < 0.001; Fig. 1c) and drinks per day (BACL, 3.68 ± 0.31 and PLA, 2.14 ± 0.33; F(1, 164) = 11.18, p = 0.001). There was no difference between the two groups on CPD (BACL, 12.0 ± 0.7 and PLA, 10.6 ± 0.7, n.s.). Finally, we also analyzed possible BACL effects on de-coupling of tobacco and alcohol use. Correlations between CPD and drinks per day in the BACL and PLA groups indicated that there was a decoupling during Week 9 in the trial. An examination of percent days smoking and percent days drinking was not possible, as for several time points percent days smoking was a constant (100 %) in the PLA group.
Alcohol dependence severity, nicotine dependence severity and treatment goals were analyzed as possible moderators (Electronic supplementary material). Adverse events were also analyzed (Electroni supplementary material).
Craving
BACL, as compared with PLA, did not significantly reduce alcohol craving, assessed by either the AUQ or the OCDS. By contrast, BACL significantly reduced cigarette craving. Specifically, after controlling for baseline scores and race, BACL significantly reduced the QSU-B score (BACL, 23.7 ± 1.5 and PLA, 28.9 ± 1.5; F(1, 180.4) = 5.98, p = 0.02) and slightly reduced the S-VAS score (BACL, 5.04 ± 0.24 and PLA, 5.70 ± 0.25; F(1, 170.0) = 3.60, p = 0.059).Footnote 2
Cue-reactivity experiment
Demographics and baseline characteristics of these 18 participants are in Table 1. The two groups did not differ on any variable. BACL slightly decreased alcohol urge (p = 0.058) and significantly reduced salivation (F(1, 34.6) = 14.38, p = 0.001; Fig. 3), but these effects were not related to cue type (Table 2). No significant differences between BACL and PLA were observed for AAS, mean arterial pressure or heart rate (data not shown).
Discussion
This is the first study to test the dual effects of BACL on concurrent alcohol and tobacco use in alcoholic smokers. Therefore, this study provides novel, albeit very preliminary, information on the possible use of BACL in alcoholism-smoking comorbidity. However, the mixed findings obtained in this study require caution and need for replication in larger trials.
BACL, compared with PLA, was significantly more effective in increasing days of abstinence from alcohol-tobacco co-use in treatment-seeking alcoholic smokers. If confirmed by future larger studies, this finding is important because this population shows an increased risk of tobacco-related mortality and morbidity and smoking-related illnesses that are the leading cause of death among alcoholic patients (Hurt et al. 1996).
Similarly to other treatment studies in the addiction field (e.g., Anton et al. 2006; Garbutt et al. 2010), there was a robust treatment effect in this study. Consistent with previous findings (Anton et al. 2006; Weiss et al. 2008), here the MM itself may have partially contributed to our findings. Conversely, the secondary analyses on either alcohol or tobacco use alone provide alternative and/or additional possible explanations of the different BACL and PLA effects observed in this study. In fact, examination of these outcomes found that the increase in days of co-use appears to be driven by increased percent days drinking in the BACL vs. PLA group. By contrast, the increase in percent days of co-abstinence was driven by the BACL effects on HDD and percent days smoking.
These data appear mixed and as such complex to interpret. However, one may speculate that, in this specific population, BACL effects were selective to specific drinking and smoking patterns. In fact, BACL reduced heavy drinking, an effect that was probably independent from smoking. This suggests that BACL helped to cut drinking below harmful (heavy) levels in patients who were still smoking. This effect might be due to the ability of BACL to alter the biphasic effects of alcohol, as previously reported (Leggio et al. 2013). Notably, compared with alcoholic nonsmokers, alcoholic smokers have reduced sensitivity to the intoxicating effects of alcohol (Funk et al. 2006) and BACL may have altered alcohol sensitivity, which in turn resulted in reduction in heavy drinking. Conversely, BACL’s effect in increasing percent co-abstinent days from both alcohol and cigarettes was driven by the medication difference on percent days smoking. This second effect may be consistent with preclinical research showing that BACL reduced nicotine-reinforced behavior, which resulted in the ability of abstaining from smoking. This, in turn, may have helped patients to abstain from alcohol, thus resulting in the overall ability to abstain from both smoking and drinking concurrently. In summary, BACL’s effects on alcohol sensitivity and nicotine reinforcement may have been responsible, respectively, for the ability of BACL to reduce heavy drinking and increase alcohol/smoking co-abstinence in this population of alcoholic smokers. Although this is merely speculative at this stage, on the other hand, this interpretation would also support an effect of BACL not mediated by possible specific effects on alcohol craving. Consistent with the latter point, this study included a preliminary laboratory experiment which showed BACL effects on alcohol urge (trend) and salivation (an objective physiological index of cue-reactivity), but these effects were not specific for alcohol cues. Considering Drummond’s model which separated withdrawal-related and cue-related cravings (Drummond 2000), and the previous literature showing BACL beneficial effects on alcohol withdrawal (Addolorato et al. 2002b, 2006; Colombo et al. 2000; Knapp et al. 2007; Lyon et al. 2011), we speculate that BACL promotes alcohol abstinence via an effect on tonic, as opposed to phasic craving, and not on cue-elicited craving. This hypothesis is consistent with the fact that BACL was not effective in the co-use of the two substances (a scenario where cues and alcohol-precipitated smoking may play an important role), while the termination of use would affect the withdrawal from both substances and would prolong the abstinence from both, thus explaining the medication effect on alcohol-tobacco abstinent days. Preclinical experiments have indicated, however, that BACL attenuates cue-induced reinstatement of alcohol-seeking when injected specifically in the basolateral amygdala (as a mixture with muscimol) (Chaudhri et al. 2013) or ventral tegmental area (Murschall and Hauber 2006), as well as when injected peripherally in alcohol-preferring rats (Maccioni et al. 2008a). Conversely, Duke et al. (2014) recently reported that, in baboons, BACL decreased alcohol-seeking during extinction effects but the effects were not specific to alcohol, suggesting that these findings may be related to a more general suppression of consummatory and conditioned behaviors. Future fully-powered human studies are needed to draw definitive conclusions on the role of BACL in cue-elicited cravings in alcoholic smokers.
Conflicting results among previous alcoholism RCTs with BACL led to a comparative analysis that indicated that patients in the positive RCT were likely to have a more severe alcohol dependence, compared with those in the negative RCT (Leggio et al. 2010). Consistent with this hypothesis, here we found that BACL was more effective in increasing days of abstinence from alcohol-tobacco co-use in those patients with a greater degree of alcohol dependence. Conversely, BACL was more effective at promoting abstinence from co-use in those with lower nicotine dependence severity. This finding is consistent with the strong predictive effects of nicotine dependence severity on cessation in smoking treatments in general (Vangeli et al. 2011). Furthermore, the present findings are intriguing given that previous studies suggest a stronger pharmacological association between alcohol and smoking among low dependent smokers (Harrison et al. 2009; Peloquin et al. 2013).
In this study, BACL was titrated up to 80 mg/day. This dose administered for 9 weeks reduced CPD (Franklin et al. 2009). By contrast, a lab study with nontreatment seeking smokers found that a single dose of 20 mg BACL negatively impacted cigarette enjoyment but did not reduce smoking (Cousins et al. 2001). This was likely due to two reasons: (1) the dose used (20 vs. 80 mg), as dose-related differences have been observed in animal studies (Fattore et al. 2009; Markou et al. 2004; Paterson et al. 2004); and (2) the need for the drug to be administered for a period of time enough to reach a steady state and show its effects, like in our study and that of Franklin et al. (2009). Therefore, this study does not allow us to draw conclusions on whether the abstinence from alcohol-tobacco co-use is related to the length of time on the medication and/or to the dose chosen here. Therefore, this study leaves open the question if doses <80 mg/day are still clinically effective and/or if doses >80 mg/day are more effective in promoting abstinence from alcohol-tobacco co-use.
From a neurobiological standpoint, the combination of alcohol and nicotine provides additive neurochemical effects, which potentiate reinforcement for both substances. Alcohol and nicotine co-administration results in increased dopamine release in the nucleus accumbens (Tizabi et al. 2002). Alcohol may exert its reinforcing effects through an interaction with nicotinic acetylcholine receptors in the mesolimbic tract, thus providing a basis for the alcoholism-smoking comorbidity (Tizabi et al. 2002). By stimulating GABAB receptors on the cell bodies of ventral tegmental area dopamine neurons and on the terminals of glutamatergic afferent neurons (Bowery et al. 1987), BACL may directly and indirectly inhibit dopamine neurons (Yoshida et al. 1994). Through this mechanism, BACL suppresses alcohol- and drug-stimulated dopamine release and, in turn, dopamine-mediated behaviors. Co-administration of alcohol and nicotine results in increased dopamine release, which may be blocked by BACL, thus resulting in its ability to promote abstinence from both substances. This hypothesis has never been formally tested in animals; therefore, this clinical pilot study might serve as a platform for future reverse translational animal experiments.
These partially conflicting findings among different outcomes might be due to the small sample, which is an important limitation of this study. For example, this does not allow us to conduct additional sub-analyses aimed to identify possible responders vs. non-responders to BACL treatment, as has been done with pharmacological treatments for other disorders (Gueorguieva et al. 2011).
The human laboratory findings also need to be considered preliminary and need to be replicated and expanded. Although future laboratory studies of BACL effects could include an ad lib smoking period to validate self-reported effects on smoking urge, an ad lib smoking period was not included in the current study for ethical reasons, given that these outpatient individuals were seeking treatment from smoking. Participants were also seeking treatment for alcoholism; therefore, an alcohol self-administration experimental session was not included either.
The above limitations notwithstanding, this is the first RCT testing BACL in treatment-seeking alcoholic smokers and suggesting a possible role of BACL in treating alcoholic smokers. However, larger studies are needed to confirm these preliminary findings.
Notes
This substudy was funded when the main RCT was already ongoing, thus not all patients were able to participate in. Specifically, after it was funded, the cue-reactivity sub-study was offered to any ongoing participant at Week 6, and all individuals, among those who were invited, volunteered to take part in the sub-study. A separate consent form was obtained, which stated clearly that their decision to participate or not in the cue-reactivity substudy was completely independent and would not compromise their participation in the main treatment RCT
Other parameters, including anxiety, saliva cotinine (nicotine metabolite) levels (Salimetrics, LLC, State College, PA), carbon monoxide (Smokerlyzer®; Bedfont Scientific Ltd.), and liver tests (GGT, AST, ALT) were not significantly different between the two groups either at baseline or during any other time point in the study (data not shown).
References
Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G (2000) Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence. Alcohol Clin Exp Res 24:67–71
Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G (2002a) Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol 37:504–508
Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, Colombo G, Gessa GL, Gasbarrini G (2002b) Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med 112:226–229
Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G (2006) Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med 119(276):e213–e278
Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D'Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G (2007) Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet 370:1915–1922
Addolorato, G., Leggio, L., Ferrulli, A., Cardone, S., Bedogni, G., Caputo, F., Gasbarrini, G., Landolfi, R., Baclofen Study, G., 2011. Dose–response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol and Alcoholism 46, 312–317
Anstrom KK, Cromwell HC, Markowski T, Woodward DJ (2003) Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res 27:900–908
Anton RF, Moak DH, Latham P (1995) The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res 19:92–99
Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, COMBINE Study Research Group (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017
Baltieri DA, Daro FR, Ribeiro PL, Andrade AG (2009) Effects of topiramate or naltrexone on tobacco use among male alcohol-dependent outpatients. Drug Alcohol Depend 105:33–41
Bertholet N, Gaume J, Faouzi M, Gmel G, Daeppen JB (2012) Predictive value of readiness, importance, and confidence in ability to change drinking and smoking. BMC Public Health 12:708
Besheer J, Lepoutre V, Hodge CW (2004) GABA(B) receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology (Berl) 174:358–366
Biener L, Abrams DB (1991) The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol: Official Journal of the Division of Health Psychology, American Psychological Association 10:360–365
Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606
Bowery NG, Hudson AL, Price GW (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20:365–383
Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH (2013) Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci 38:2751–2761
Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL (2000) Ability of baclofen in reducing alcohol intake and withdrawal severity: I—preclinical evidence. Alcohol Clin Exp Res 24:58–66
Colombo G, Serra S, Brunetti G, Atzori G, Pani M, Vacca G, Addolorato G, Froestl W, Carai MA, Gessa GL (2002) The GABA(B) receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol Alcohol 37:499–503
Colombo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL (2003a) Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend 70:105–108
Colombo G, Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL (2003b) Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology (Berl) 167:221–224
Colombo G, Serra S, Vacca G, Carai MA, Gessa GL (2006) Baclofen-induced suppression of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats exposed to different alcohol concentrations. Eur J Pharmacol 550:123–126
COMBINE Study Research Group (2003) Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcohol Clin Exp Res 27:1107–1122
Cooney JL, Cooney NL, Pilkey DT, Kranzler HR, Oncken CA (2003) Effects of nicotine deprivation on urges to drink and smoke in alcoholic smokers. Addiction 98:913–921
Cooney NL, Cooney JL, Perry BL, Carbone M, Cohen EH, Steinberg HR, Pilkey DT, Sevarino K, Oncken CA, Litt MD (2009) Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction 104:1588–1596
Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J (2000) Response of nicotine self-administration in the rat to manipulations of mu-opioid and gamma-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology (Berl) 149:107–114
Corrigall WA, Coen KM, Zhang J, Adamson KL (2001) GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl) 158:190–197
Cousins MS, Stamat HM, de Wit H (2001) Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tobacco Research: Off J Society Research Nicotine Tobacco 3:123–129
Cox LS, Tiffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tobacco Research: Off J Society Research Nicotine Tobacco 3:7–16
Daoust M, Saligaut C, Lhuintre JP, Moore N, Flipo JL, Boismare F (1987) GABA transmission, but not benzodiazepine receptor stimulation, modulates ethanol intake by rats. Alcohol 4:469–472
Del Boca FK, Kranzler HR, Brown J, Korner PF (1996) Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res 20:1412–1417
Drummond DC (2000) What does cue-reactivity have to offer clinical research? Addiction 95(Suppl 2):S129–S144
Duke AN, Kaminski BJ, Weerts EM (2014) Baclofen effects on alcohol seeking, self-administration and extinction of seeking responses in a within-session design in baboons. Addict Biol 19:16–26
Fagerstrom KO, Schneider NG (1989) Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med 12:159–182
Falk DE, Yi HY, Hiller-Sturmhofel S (2006) An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research Health: J National Institute Alcohol Abuse Alcoholism 29:162–171
Fattore L, Cossu G, Martellotta MC, Fratta W (2002) Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol 37:495–498
Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P (2009) Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. Eur Neuropsychopharmacol: J European College Neuropsychopharmacol 19:487–498
Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A (2004) Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res 28:1517–1523
Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O'Brien CP, Childress AR (2009) The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend 103:30–36
Fucito LM, Park A, Gulliver SB, Mattson ME, Gueorguieva RV, O'Malley SS (2012) Cigarette smoking predicts differential benefit from naltrexone for alcohol dependence. Biol Psychiatry 72:832–838
Funk D, Marinelli PW, Le AD (2006) Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Research Health: J National Institute Alcohol Abuse Alcoholism 29:186–192
Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA (2010) Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res 34:1849–1857
Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA (2004) Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 61:1107–1115
Gueorguieva R, Mallinckrodt C, Krystal JH (2011) Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses. Arch Gen Psychiatry 68:1227–1237
Harrison EL, Hinson RE, McKee SA (2009) Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav 34:484–486
Hintz T, Mann K (2007) Long-term behavior in treated alcoholism: Evidence for beneficial carry-over effects of abstinence from smoking on alcohol use and vice versa. Addict Behav 32:3093–3100
Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ 3rd (1996) Mortality following inpatient addictions treatment. Role of Tobacco Use in a Community-Based Cohort. JAMA: J Am Med Assoc 275:1097–1103
Janak PH, Michael Gill T (2003) Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 30:1–7
Jimenez-Ruiz C, Berlin I, Hering T (2009) Varenicline: a novel pharmacotherapy for smoking cessation. Drugs 69:1319–1338
Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA (2005) Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med 165:1600–1605
Knapp DJ, Overstreet DH, Breese GR (2007) Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res 31:582–595
Leggio L, Garbutt JC, Addolorato G (2010) Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurological Disorders Drug Targets 9:33–44
Leggio L, Ferrulli A, Zambon A, Caputo F, Kenna GA, Swift RM, Addolorato G (2012) Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav 37:561–564
Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, Addolorato G, Swift RM, Kenna GA (2013) A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav 103:784–791
Lewis-Esquerre JM, Colby SM, Tevyaw TO, Eaton CA, Kahler CW, Monti PM (2005) Validation of the timeline follow-back in the assessment of adolescent smoking. Drug Alcohol Depend 79:33–43
Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ (2006) The GABA(B) receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology 50:632–639
Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R, N.S.G (2013) A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addiction Medicine 7:277–286
Lobina C, Carai MA, Froestl W, Mugnaini C, Pasquini S, Corelli F, Gessa GL, Colombo G (2011) Activation of the GABA(B) Receptor prevents nicotine-induced locomotor stimulation in mice. Frontiers Psychiatry 2:76
Lyon JE, Khan RA, Gessert CE, Larson PM, Renier CM (2011) Treating alcohol withdrawal with oral baclofen: a randomized, double-blind, placebo-controlled trial. J Hosp Med: Off Publication Soc Hosp Med 6:469–474
Maccioni P, Serra S, Vacca G, Orru A, Pes D, Agabio R, Addolorato G, Carai MA, Gessa GL, Colombo G (2005) Baclofen-induced reduction of alcohol reinforcement in alcohol-preferring rats. Alcohol 36:161–168
Maccioni P, Bienkowski P, Carai MA, Gessa GL, Colombo G (2008a) Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend 95:284–287
Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G (2008b) Specific reduction of alcohol's motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783—comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res 32:1558–1564
Markou A, Paterson NE, Semenova S (2004) Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann N Y Acad Sci 1025:491–503
Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR (1987) Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol 96:122–126
Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB (1999) Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res 23:1386–1394
Monti PM, Rohsenow DJ, Hutchison KE (2000) Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction 95(Suppl 2):S229–S236
Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK (2001) Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res 25:1634–1647
Murschall A, Hauber W (2006) Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learning Memory 13:123–126
Paterson NE, Froestl W, Markou A (2004) The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 172:179–186
Peloquin MP, Hecimovic K, Sardinha J, Stewart SH, Barrett SP (2013) The effect of snus on alcohol-related cigarette administration in dependent and non-dependent smokers. Pharmacol Biochem Behav 114–115:97–102
Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB (2000) Naltrexone's effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol 109:738–742
Romberger DJ, Grant K (2004) Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother = Biomedecine and Pharmacotherapie 58:77–83
Schlaepfer IR, Hoft NR, Ehringer MA (2008) The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev 1:124–134
Skinner HA, Allen BA (1982) Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol 91:199–209
Sobell LC, Sobell MB, Leo GI, Cancilla A (1988) Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict 83:393–402
Sobell LC, Sobell MB, Kozlowski LT, Toneatto T (1990) Alcohol or tobacco research versus alcohol and tobacco research. Br J Addict 85:263–269
Stout RL, Wirtz PW, Carbonari JP, Del Boca FK (1994) Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl 12:70–75
Tizabi Y, Copeland RL Jr, Louis VA, Taylor RE (2002) Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res 26:394–399
Vangeli E, Stapleton J, Smit ES, Borland R, West R (2011) Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction 106:2110–2121
Walker BM, Koob GF (2007) The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 31:11–18
Weiss RD, O'Malley SS, Hosking JD, Locastro JS, Swift R, Group, C.S.R (2008) Do patients with alcohol dependence respond to placebo? Results from the COMBINE Study. J Stud Alcohol Drugs 69:878–884
White KD (1977) Salivation: a review and experimental investigation of major techniques. Psychophysiology 14:203–212
Yoshida M, Yokoo H, Tanaka T, Emoto H, Tanaka M (1994) Opposite changes in the mesolimbic dopamine metabolism in the nerve terminal and cell body sites induced by locally infused baclofen in the rat. Brain Res 636:111–114
Acknowledgments
The pilot treatment randomized clinical trial was supported by a grant from the ABMRF/The Foundation for Alcohol Research (PI: Leggio). The human laboratory cue-reactivity substudy was supported by an NIH grant jointly funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA) (R03AA020169; PI: Leggio). Both grants were awarded to Dr. Leggio, while he was at Brown University. Dr. Leggio’s current work is supported by the NIAAA Division of Intramural Clinical and Biological Research and the NIDA Intramural Research Program.
Conflict of interest
Dr. Swift has received travel and honorarium from D&A Pharma, and consultant fees from CT Laboratories. Dr. Kenna has received consultant fees from CT Laboratories. The other authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1S
Study flow chart (PPTX 81 kb)
Supplemental Fig. 2S
Effects of BACL vs. placebo in participants with high (>14.5) vs. low (<14.5) alcohol dependence scale (ADS) score on percent days abstinent from alcohol-tobacco co-use (PPTX 61 kb)
Supplemental Fig. 3S
Effects of BACL vs. placebo in participants with high (>7.5) vs. low (<7.5) nicotine dependence severity (Fagerström Test for Nicotine Dependence (FTND) score) on percent days abstinent from alcohol-tobacco co-use (PPTX 56 kb)
ESM 4
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Leggio, L., Zywiak, W.H., Edwards, S.M. et al. A preliminary double-blind, placebo-controlled randomized study of baclofen effects in alcoholic smokers. Psychopharmacology 232, 233–243 (2015). https://doi.org/10.1007/s00213-014-3652-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3652-9