Abstract
Rationale
Drug-associated environmental stimuli elicit craving in humans and drug-seeking in animals.
Objectives
We tested the hypothesis that Pavlovian-conditioned alcohol-seeking is mediated by dopamine, using rats from two vendors.
Methods
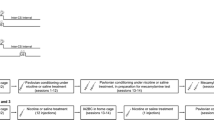
Male, Long–Evans rats (220–240 g) from Charles River (St-Constant, QC, Canada) and Harlan Laboratories (Indianapolis, IN, USA) received 21 sessions of intermittent, 24-h access to ethanol (15 %, v/v) and water in the home-cage. Subsequently, rats were trained to discriminate between one conditioned stimulus (CS+) that was paired with ethanol (0.2 ml per CS+) and a second stimulus (CS−) that was not. Entries into a fluid port where ethanol was delivered were recorded. Next, rats were exposed to a different context where cues and ethanol were withheld. At test, responding to the CS+ and CS− without ethanol was assessed in the second, non-alcohol context. Injections (1 ml/kg; s.c.) of the dopamine D1-receptor antagonist SCH 23390 (0, 3.33, and 10 μg/kg) or dopamine D2-receptor antagonist eticlopride (0, 5, and 10 μg/kg) were administered before test.
Results
Home-cage alcohol consumption was higher in Harlan rats than Charles River rats. At test, saline-treated rats responded more to the alcohol-predictive CS+ than the CS−. While SCH 23390 attenuated CS+ responding in rats from both vendors, eticlopride reduced CS+ responding in Harlan rats only. Subsequently, SCH 23390 but not eticlopride attenuated CS+ responding when the CS+ was again paired with ethanol.
Conclusions
These results indicate important differences in alcohol consumption in Long–Evans rats from different suppliers, and highlight a novel role for dopamine in Pavlovian-conditioned alcohol-seeking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sensory properties of alcohol routinely precede alcohol consumption, and as a consequence of this predictive, temporal relation, such stimuli can elicit conditioned subjective and physiological responses that may facilitate drinking (Litt et al. 2000; McCusker and Brown 1989; Rohsenow et al. 1994). To study the neurobiological mechanisms that mediate Pavlovian-conditioned alcohol-seeking, we developed a procedure in which rats are trained to behaviorally discriminate between one conditioned stimulus (CS+) that is paired with alcohol and a second conditioned stimulus (CS−) that is not. Entries into a fluid port where alcohol is delivered for oral consumption are measured during each CS presentation. Across training, port-entries triggered by the CS+ increase, whereas responding to the CS− stabilizes at a lower level, indicating that rats learn the predictive association between the CS+ and alcohol (Chaudhri et al. 2008a, 2009).
Using this task, the present experiments tested the hypothesis that the expression of alcohol-seeking behavior triggered by a Pavlovian cue is mediated by dopamine. Research conducted using instrumental conditioning procedures in which rats are trained to perform an operant response that is reinforced by the delivery of alcohol (Katner and Weiss 1999; Nie and Janak 2003; Chaudhri et al. 2009) has demonstrated that blocking dopamine neurotransmission at dopamine D1- or D2-like receptors reduces the reinstatement of alcohol-seeking induced by alcohol-predictive discriminative cues (Liu and Weiss 2002). Based on this finding and on evidence that dopamine is involved in cue-induced reinstatement of instrumental drug-seeking (Berglind et al. 2006; Bossert et al. 2007; Ciccocioppo et al. 2001; Liu and Weiss 2002; Liu et al. 2010), we predicted that blocking dopamine D1- or D2-receptors would also dose-dependently attenuate Pavlovian-conditioned alcohol-seeking.
In addition to the sensory stimuli that accompany drug use, environmental contexts in which drug consumption occurs can also induce craving (Conklin et al. 2008, 2010), suggesting that contextual stimuli acquire the capacity to predict drug availability. In support of this hypothesis, research conducted in animal models has shown that placement into a drug-associated context can reinstate drug-seeking behavior that was extinguished in a different environmental context by withholding drug delivery (Chaudhri et al. 2008a; Crombag and Shaham 2002; Fuchs et al. 2004; Hamlin et al. 2006; Wing and Schoaib 2008; Zironi et al. 2006). Contexts can also modulate the level of conditioned responding elicited by cues that are paired with drug delivery. For example, cue-induced reinstatement of alcohol-seeking in mice is greater in a context associated with alcohol consumption, when compared to a context where alcohol was never consumed (Tsiang and Janak 2006). Similarly, reinstatement following extinction can be triggered in rats by presenting them with a small drop of alcohol in an alcohol-associated context, but not in a context where lever-pressing never produced alcohol (Chaudhri et al. 2008b). This effect may be mediated by dopamine, as dopamine release occurs when rats are placed into operant conditioning chambers associated with prior alcohol self-administration (Katner and Weiss 1999). Furthermore, cue- and context-induced reinstatement of drug-seeking have been localized to distinct subregions of the ventral striatum (Bossert et al. 2007; Chaudhri et al. 2009; Ito et al. 2000), suggesting that separate, yet potentially overlapping neural circuits, may mediate these two effects. Consequently, in the present research we sought to minimize the impact of contextual cues associated with alcohol consumption on responding elicited by a Pavlovian-conditioned alcohol-predictive CS+ by conducting tests in a context where subjects had never consumed alcohol.
Lastly, we assessed the role of dopamine in Pavlovian-conditioned alcohol-seeking in male, Long–Evans rats that were obtained from two different breeders, Charles River (St Constant, QC, Canada) and Harlan Laboratories (Indianapolis, IN, USA). This comparison was motivated by a recent report that identified differences in alcohol consumption and alcohol-mediated behaviors in rats of the same strain that were obtained from different suppliers (Palm et al. 2011). In order to determine if choice of supplier could have implications for future research, we systematically compared home-cage alcohol consumption as well as the impact of dopamine D1- and D2-receptor antagonists on the expression of Pavlovian-conditioned alcohol-seeking in Long–Evans rats from these two vendors.
Methods
Subjects
Male, Long–Evans rats (220–240 g on arrival) were obtained from Charles River (St Constant, QC, Canada) and Harlan Laboratories (Indianapolis, IN, USA). Subjects arrived on the same day and were individually housed in polycarbonate shoebox cages containing beta-chip bedding in a colony room that was maintained at constant temperature (21 °C) and on a 12-h light/dark cycle (lights on at 0700 hours; procedures conducted during the light phase). Following arrival, rats received 15 days to acclimate to the colony room, during which time they were regularly weighed and handled. Access to rat chow (Charles River Rodent Animal Diet, St-Hubert, Canada) and water was unrestricted throughout the experiment, unless otherwise indicated. All procedures followed the guidelines of the Canadian Council on Animal Care and were approved by the Concordia University Animal Research Ethics Committee.
Apparatus
Behavioral training and testing was conducted using equipment and software obtained from Med-Associates Inc. (St. Albans, VT, USA). Equipment consisted of twelve operant conditioning chambers (ENV-009A) each contained within a ventilated, sound-attenuating melamine cubicle (53.6 × 68.2 × 62.8 cm; built in-house). Each chamber comprised a stainless steel bar floor, paneled aluminum sidewalls, and a clear, Plexiglas rear wall, ceiling and front door. The right wall featured a central port, which contained a circular fluid receptacle (ENV-200R3AM). Fluid delivery into the receptacle occurred through a 20-ml syringe attached to a pump (PHM-100, 3.33 rpm) that was located outside the cubicle. The upper left wall of the operant conditioning chamber featured a clicker stimulus (ENV-135 M, 76–80 dB, 2 Hz) and white noise stimulus generator (ENV-225SM, 80–85 dB), as well as a white house-light (ENV-215 M). Entries into the fluid port were measured by interruptions of infrared beam across its entrance and recorded to a computer using Med PC-IV software, which also controlled fluid delivery and stimulus presentations.
Drugs
Ethanol (15 %, v/v) was prepared by diluting 95 % ethanol in tap water. SCH 23390 hydrochloride (R (+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2, 3, 4, 5-tetrahydro-1H-3-benzazepine hydrochloride; Sigma Aldrich; catalog # D054) was dissolved in 0.9 % sodium chloride, as was eticlopride hydrochloride (FLB 131, S-(−)-3-Chloro-5-ethyl-N-[(1-ethyl-2- pyrrolidinyl) methyl]-6-hydroxy-2-methoxybenzamide hydrochloride; Sigma Aldrich; catalog # E101). Drug doses were selected based on previous reports that demonstrate behavioral effectiveness using similar behavioral procedures (Bossert et al. 2007; Hamlin et al. 2007; Liu et al. 2010; Liu and Weiss 2002). Lemon oil (SAFC Supply Solutions, St-Louis, USA) or benzaldehyde (used as almond odor; ACP Chemicals Inc., Montreal, Canada) were mixed in tap water to obtain 10 % solutions (v/v).
Alcohol consumption in the home-cage
Two weeks after arrival, ethanol and water were made available via separate bottles that were placed onto the home-cage in 24-h sessions during which rats could consume either solution (procedures adapted from Simms et al. 2008; Wise 1973). These sessions occurred on Monday, Wednesday, and Friday of each week, for a total of 21 sessions. On Tuesday, Thursday, Saturday and Sunday, the ethanol-containing bottle was replaced with an identical bottle containing water. Rats were weighed before each ethanol/water choice session, and ethanol and water bottles were weighed before and after each 24-h session. Detailed procedures for this phase can be found in Supplementary material.
Pavlovian discrimination training
Following 21 sessions of alcohol consumption in the home-cage, rats underwent 17 daily, 1-h Pavlovian discrimination training (PDT) sessions (5–6 days per week). In each session, the house light was illuminated 5-min after rats were placed into the conditioning chamber to indicate the beginning of the session. Rats received 16 random presentations each of two 10-s auditory stimuli (clicker or white noise) delivered according to a variable-time 67-s schedule. One stimulus (CS+) was consistently paired with the delivery of 15 % ethanol (0.2 ml per CS+; delivered over the last 6-s of the CS+; total of 3.2 ml per session) into the fluid port. The alternate stimulus (CS−) was presented without ethanol. Ports were checked following each session to ensure that the ethanol had been consumed.
PDT occurred in one of two contexts created by adding visual, olfactory and tactile stimuli to the operant conditioning chambers. Context 1 consisted of black walls, a smooth Plexiglas floor, and a lemon odor applied to the waste pan under the chamber floor. Context 2 consisted of clear, Plexiglas walls, a perforated stainless steel floor, and an almond odor. Before starting PDT, rats were exposed to each context in 20-min sessions where no ethanol or auditory stimuli were presented. Subsequently, rats from each supplier were separated into 4 counterbalanced groups based on ethanol intake averaged across sessions 19–21 of home-cage ethanol exposure. Groups corresponded to the possible combinations of Context (1 or 2) and CS+ (Click, Noise), which remained constant for each subject for the duration of PDT.
Exposure to a non-alcohol context
Upon completion of PDT, rats were exposed to a different environmental context in 5, daily 1-h sessions. Chambers that had been configured as Context 1 for PDT were re-configured as Context 2, and vice versa. During each session neither the CS+ nor the CS− were presented. In addition, although the syringe pump continued to be activated on the same schedule as during PDT, no ethanol was delivered. The purpose of this phase was to acclimate rats to a second environment where ethanol was never available or consumed, and to extinguish spontaneous entries into the fluid port, which was a feature of both the PDT and non-alcohol contexts.
Test
At 24-h after the last session, responding to the CS+ and CS− in the absence of ethanol was tested in the non-alcohol context. The CS+ and CS− were presented as during PDT. The syringe pump continued to be activated during the CS+, but no ethanol was delivered.
Experiment 1. Effect of SCH 23390 on Pavlovian-conditioned alcohol-seeking
This experiment tested the effect of blocking dopamine D1-like receptors on the expression of Pavlovian-conditioned alcohol-seeking in a non-alcohol context. Fifteen minutes before the test rats received a subcutaneous injection of SCH 23390 (0, 3.33, and 10 μg/kg; 1 ml/kg). Each rat was tested at each dose using a within-subjects, repeated-measures design. Tests were separated by three additional sessions of PDT and four additional sessions of exposure to the non-alcohol context. Dose order was determined by a Latin-Square design.
Experiment 2a. Effect of eticlopride on Pavlovian-conditioned alcohol-seeking
Using different rats from Experiment 1, we tested the effect of blocking dopamine D2-like receptors on the expression of Pavlovian-conditioned alcohol-seeking in a non-alcohol context. Procedures were identical to those described for Experiment 1, except before the test rats received a subcutaneous injection of eticlopride (0, 5, and 10 μg/kg; 1 ml/kg).
Experiment 2b. Effect of SCH 23390 or eticlopride on Pavlovian discrimination training
This study determined the effect of blocking dopamine D1- or D2-like receptors on responding elicited by the CS+ and CS− during PDT sessions where the CS+ was paired with ethanol delivery. Rats utilized in Experiment 2a underwent 2 sessions of PDT re-training in the original training context. Before session 3 of PDT they received a subcutaneous injection of saline (1 ml/kg), SCH 23390 (10 μg/kg; 1 ml/kg) or eticlopride (10 μg/kg; 1 ml/kg). Each rat was tested in each treatment condition, according to a within-subjects design. Tests were separated by two sessions of PDT retraining and drug order was determined by a Latin-Square design.
Statistical analyses
Dependent measures during home-cage ethanol consumption included body weight (g), ethanol and water consumption (ml), ethanol-intake expressed as grams of ethanol consumed per kilogram of bodyweight (g/kg), and ethanol preference (ethanol consumption in ml as a percentage of total fluid intake). Data were analyzed using repeated measures analysis of variance (ANOVA) across the within-subjects factor of Session (1–21) and the between-subjects factor of Vendor (Harlan, Charles River). Polynomial contrasts were used to investigate the relationship between dependent variables as a function of session. All subjects were included in these analyses (Experiment 1, Charles River, n = 18; Harlan, n = 19: Experiment 2, Charles River, n = 18; Harlan, n = 21). However, following the home-cage alcohol consumption phase, 1 Charles River rat from Experiment 1 and 3 Harlan rats from Experiment 2 with low ethanol intakes (g/kg <0.5 averaged across the last 3 sessions) did not continue to behavioral training due to space and time constraints.
During PDT and test, port-entries during 10-s intervals before each CS were subtracted from port-entries during the corresponding CS to obtain normalized CS responses. In addition, total port-entries per session were recorded in each phase. Data from 6 rats from Experiment 1 (1 Charles River and 5 Harlan) and 7 rats from Experiment 2a (2 Charles River and 5 Harlan) were excluded upon completion of each study. Subjects were excluded if the average number of CS+ responses made across the last 5 sessions of PDT was <10, and if they also failed to consume all the ethanol delivered during each session. As a result, final sample sizes for Experiment 1 were 16 Charles River rats and 14 Harlan rats, and for Experiment 2a were 16 Charles River rats and 13 Harlan rats.
For Experiments 1 and 2a, normalized CS data from PDT were analyzed using repeated-measures ANOVA with Session (1–17) and CS (CS+, CS−) as within-subjects variables and Vendor (Charles River, Harlan) as a between-subjects variable. Test data were analyzed using ANOVA with Dose (0, 3.33, and 10 μg/kg of SCH 23390; 0, 5, and 10 μg/kg of eticlopride) and CS (CS+, CS−) as within-subjects variables, and Vendor (Charles River, Harlan) as a between-subjects variable. Total port-entries were analyzed across Session and Vendor during PDT and exposure to the non-alcohol context, and across Dose and Vendor at test.
Experiment 2b (Charles River n = 18; Harlan n = 7) was conducted utilizing rats from Experiment 2a. Two Charles River rats and 3 Harlan rats that were dropped from Experiment 2a were included in Experiment 2b because they showed robust re-acquisition of PDT and consumed all the ethanol delivered during the latter study. The full complement of Harlan rats from Experiment 2a could not be tested because 9 subjects vocalized and struggled considerably when injected at test 3. To determine if this reaction to the injection might have influenced the results of test 3 from Experiment 2a, we compared the behavior of these 9 subjects to rats that did not have a reaction and found no statistically significant main effects or interactions. Test data were analyzed using repeated-measures ANOVA with Treatment (saline, 10 μg/kg SCH 23390, 10 μg/kg eticlopride) and CS (CS+, CS−) as within-subjects variables and Vendor (Charles River, Harlan) as a between-subjects variable. All analyses were conducted using SPSS v 19.
Results
In both experiments significant differences in weight gain and oral ethanol consumption emerged in rats from Harlan and Charles River (Fig. 1). Rats from both vendors gained weight (Fig. 1a, b) across sessions [Session: Exp. 1, F(20,700) = 1065.10, p < 0.001; Exp. 2, F(20,740) = 1747.35, p < 0.001]. However, compared to rats from Harlan, rats from Charles River weighed more overall [Vendor: Exp. 1, F(1,35) = 99.93, p < 0.001; Exp. 2, F(1,37) = 36.36, p < 0.001] and gained more weight per session [Session × Vendor: Exp. 1, F(20,700) = 68.27, p < 0.001; Exp. 2, F(20,740) = 39.20, p < 0.001].
Weight gain and fluid consumption across 21 sessions of ethanol (EtOH) exposure in the home-cage in Charles River (filled symbols) and Harlan (open symbols) rats. In each session, rats received access to both 15 % ethanol and water via two bottles in the home-cage for 24 h. Data represent mean (±SEM) values obtained from each session. Panels on the left depict data from Experiment 1 (Charles River, n = 18; Harlan, n = 19), whereas panels on the right depict data from Experiment 2 (Charles River, n = 18; Harlan, n = 21). Dependent measures shown include body weight in grams (a, b), water and ethanol consumption in milliliters (c, d), ethanol intake in grams of ethanol consumed per kilogram of bodyweight (e, f), and ethanol preference which is calculated as the percentage of total fluid consumption that is accounted for by ethanol (g, h)
Overall, rats from Charles River drank significantly more water (Fig. 1c, d) than Harlan rats [Vendor: Exp. 1, F(1,35) = 50.22, p < 0.001; Exp. 2, F(1,37) = 93.36, p < 0.001]. Water consumption decreased across sessions [Session: Exp. 1, F(20,700) = 11.35, p < 0.001; Exp. 2, F(20,740) = 12.651, p < 0.001], with no Session × Vendor interaction in Experiment 1 but a significant Session × Vendor interaction in Experiment 2 [Session × Vendor, F(20,740) = 4.10, p < 0.001]. Further analyses of Experiment 2 utilizing polynomial contrasts revealed that water consumption decreased linearly for both Charles River [Session, F(1,17) = 27.20, p < 0.001] and Harlan rats [Session, F(1,20) = 13.89, p < 0.01].
Ethanol consumption (Fig. 1c, d) increased across sessions [Session: Exp. 1, F(20,700) = 12.35, p < 0.001; Exp. 2, F(20,740) = 18.03, p < 0.001], and rats from Harlan drank more ethanol than rats from Charles River [Vendor: Exp. 1, F(1,35) = 5.61, p < 0.05; Exp. 2, F(1,37) = 13.49, p < 0.01]. There was no Session × Vendor interaction in Experiment 1, but this interaction was significant in Experiment 2 [Session × Vendor F(20,740) = 3.20, p < 0.001]. In Experiment 2, ethanol consumption followed a linear trend for Charles River rats [Session, F(1,17) = 27.81, p < 0.001] and a linear [Session, F(1,20) = 22.75, p < 0.001] and quadratic trend [Session, F(1,20) = 16.03, p < 0.01] for Harlan rats.
Ethanol intake expressed as g/kg (Fig. 1e and f) increased across session [Session: Exp. 1, F(20,700) = 6.27, p < 0.001; Exp. 2, F(20,740) = 8.15, p < 0.001] with Harlan rats achieving higher overall ethanol intakes [Vendor: Exp. 1, F(1,35) = 14.14, p < 0.01; Exp. 2, F(1,37) = 24.23, p < 0.001] in both experiments. There was no Session × Vendor interaction in Experiment 1, but a significant Session × Vendor interaction in Experiment 2 [Session × Vendor, F(20,740) = 3.23, p < 0.001]. In Charles River rats, ethanol consumption expressed as g/kg in Experiment 2 followed a linear trend [Session, F(1,17) = 17.84, p < 0.01]; however, in Harlan rats, the trend was revealed to be quadratic [Session, F(1,20) = 12.46, p < 0.01].
Ethanol preference (Fig. 1g, h) increased across session [Session: Exp. 1, F(20,700) = 15.72, p < 0.001; Exp. 2, F(20,740) = 8.15, p < 0.001] and was higher overall in Harlan rats [Vendor: Exp. 1, F(1,35) = 26.01, p < 0.001; Exp. 2, F(1,37) = 24.23, p < 0.001]. There was no Session × Vendor interaction in Experiment 1 but a significant Session × Vendor interaction in Experiment 2 [Session × Vendor, F(20,740) = 3.23, p < 0.001]. Trend analyses on Experiment 2 indicated that ethanol preference followed a linear trend in Charles River rats [Session, F(1,17) = 26.08, p < 0.001] and a linear [Session, F(1,20) = 18.23, p < 0.001] and quadratic trend [Session, F(1,20) = 12.81, p < 0.01] for Harlan rats.
Rats from both vendors learned to discriminate between the CS+ and CS− across PDT sessions in both experiments (Fig. 2a, b). Normalized port-entries increased across session [Session, Exp. 1, F(16,448) = 19.79, p < 0.001; Exp. 2, F(16,432) = 20.78, p < 0.001], with responding to the CS+ stabilizing at a higher level than CS− responding [Session × CS: Exp. 1, F(16,448) = 10.34, p < 0.001; Exp. 2, F(16,432) = 11.57, p < 0.001]. Overall, more responding occurred during the CS+ than the CS− [CS: Exp. 1, F(1,28) = 43.19, p < 0.001; Exp. 2, F(1,27) = 68.40, p < 0.001]. In Experiment 1 (Fig. 2a), there was no difference in the acquisition of PDT as a function of vendor. Nor were there significant Session × Vendor, CS × Vendor, or Session × CS × Vendor interactions. Follow-up paired samples t tests on data collapsed across vendor indicated that with the exception of PDT sessions 1 and 3, CS+ responding was significantly higher than CS− responding at each session (p < 0.05 for each comparison). In Experiment 2 (Fig. 2b), rats from Harlan achieved and maintained a higher level of CS+ responses than rats from Charles River. These results are supported by ANOVA, indicating a near-significant main effect of vendor [Vendor, F(1,27) = 4.13, p = 0.052], a significant Session × Vendor interaction [Session × Vendor, F(16,432) = 2.14, p < 0.01] and a significant 3-way interaction [Session × CS × Vendor, F(16,432) = 2.61, p < 0.01]. There was no significant CS × Vendor interaction. Follow-up t-tests for independent samples verified that rats from Harlan made more CS+ responses than Charles River rats on sessions 13, 14, 16, and 17 (p < 0.05) and session 12 (p < 0.01).
Acquisition of Pavlovian-conditioned alcohol-seeking across 17 sessions of Pavlovian discrimination training (PDT) in Charles River (filled symbols) and Harlan (open symbols) rats. In each PDT session, rat received presentations of a CS+ that was paired with 15 % ethanol (0.2 ml/CS+; 3.2 ml total) and a CS− that was presented without ethanol. Experiment 1 is depicted in panels on the left, and Experiment 2a is shown in panels on the right. Rats in both experiments learned to discriminate between the CS+ (circles) and the CS− (triangles) (a, b). Data represent mean (±SEM) normalized port-entries during each CS, calculated by subtracting responding during 10-s pre-CS intervals from responding during the corresponding 10-s CS. Total port-entries decreased as a function of session in rats from both vendors (c, d). Data represent mean (±SEM) total port-entries from each session
Total port-entries (Fig. 2c and d) decreased across PDT sessions in both experiments [Session: Exp. 1, F(16,448) = 3.87, p < 0.001; Exp. 2, F(16,432) = 4.28, p < 0.001]. In Experiment 1, there was no main effect of Vendor, or Session × Vendor interaction, whereas in Experiment 2 there was a trend for total port-entries to be lower in Harlan rats [Vendor, F(1,27) = 3.88, p = 0.059], and a difference in the number of total port-entries across session as a function of vendor [Session × Vendor, F(16,432) = 2.69, p < 0.01]. Independent samples t tests confirmed that rats from Harlan made fewer total port-entries on sessions 1–3 (p < 0.01) and sessions 14 and 15 (p < 0.05).
Following PDT, rats were exposed to an alternate context in which the cues and alcohol were withheld. Total port-entries decreased across sessions, as depicted in Supplemental Material (Supplementary Fig. 1).
Blocking dopamine D1-like receptors in Experiment 1 significantly reduced Pavlovian-conditioned alcohol-seeking in rats from both vendors. Figure 3 depicts normalized port-entries during the CS+ and CS− at test for rats from Charles River (Fig. 3a; n = 16) and Harlan (Fig. 3b; n = 14) when both cues were presented without ethanol in the non-alcohol context. Overall, rats responded more to the CS+ than the CS− [CS, F(1,28) = 97.45, p < 0.001], and Charles River rats made more port-entries compared to Harlan rats [Vendor, F(1,28) = 5.25, p < 0.05]. SCH 23390 pre-treatment reduced CS+ responding, with no effect on CS− responding [Dose, F(2,56) = 10.60, p < 0.001; Dose × CS, F(2,56) = 10.36, p < 0.001]. There were no Dose × Vendor or Dose × CS × Vendor interactions, suggesting that SCH 23390 reduced CS+ responding in rats from both vendors.
Blocking dopamine D1-receptors reduced the expression of Pavlovian-conditioned alcohol-seeking (Experiment 1). At test rats received presentations of the CS+ and CS− as during PDT. However, no ethanol was delivered and tests were conducted in a context where alcohol had never previously been consumed. Data represent mean (±SEM) normalized port-entries during the CS+ (filled bars) and CS− (open bars) following saline and SCH 23390 (3.33 or 10 μg/kg) pre-treatment in a Charles River (n = 16) and b Harlan (n = 14) rats. * p < 0.03, compared to saline and 3.33 microgram/kilogram dose
Because of the main effect of Vendor, separate analyses were conducted to investigate the impact of SCH 23390 on normalized CS+ responding in Charles River and Harlan rats. There was a main effect of dose in rats from either vendor [Charles River, F(2,30) = 4.65, p < 0.05; Harlan, F(2,26) = 6.91, p < 0.01]. Follow-up t tests for paired samples were conducted that were corrected for multiple comparisons using a modified Bonferroni test (α = 0.03; see Keppel 1991). In Charles River rats, normalized CS+ responding was reduced by 10 μg/kg of SCH 23390, compared to saline [t(15) = 3.08, p = 0.008], and the 3.33 μg/kg dose [t(15) = 2.56, p = 0.022]. There was no difference between saline and 3.33 μg/kg dose. The same pattern of effects was found in Harlan rats. Normalized CS+ responding was reduced by 10 μg/kg of SCH 23390 compared to saline [t(13) = 4.61, p = 0.003] and 3.33 μg/kg [t(13) = 2.90, p = 0.012], with no difference between saline and the 3.33 μg/kg dose.
Trend analyses were conducted to compare the shape of the dose response curves in rats from Charles River and Harlan. These revealed that the attenuation of normalized CS+ responding followed a linear trend in rats from both vendors [Dose; Charles River, F(1,15) = 9.48, p < 0.01; Harlan, F(1,13) = 12.88, p < 0.01].
The impact of SCH 23390 on total port-entries at test is described in Supplemental Material (Supplementary Fig. 2).
Blocking dopamine D2-like receptors in Experiment 2a significantly reduced Pavlovian-conditioned alcohol-seeking in rats from Harlan, but was ineffective in rats from Charles River. Figure 4 depicts normalized port-entries made during the CS+ and CS− at test for Charles River (Fig. 4a; n = 16) and Harlan rats (Fig. 4b; n = 13) when both cues were presented without ethanol in the non-alcohol context. Overall, rats responded more to the CS+ than the CS− [CS, F(1,27) = 117.45, p < 0.001]. Blocking dopamine D2-receptors dose-dependently reduced CS+ responding, with no effect on CS− responding [Dose, F(2,54) = 8.17, p < 0.01; Dose × CS, F(2,54) = 6.08, p < 0.01]. There was no main effect of Vendor or significant Vendor × CS interaction, indicating no difference across vendors in the number of CS responses made at test. Interestingly, the effect of eticlopride on responding at test differed as a function of vendor [Dose × Vendor, F(2,54) = 3.44, p < 0.05], with no Dose × CS × Vendor interaction.
Blocking dopamine D2-receptors reduced the expression of Pavlovian-conditioned alcohol-seeking in Harlan, but not Charles River rats (Experiment 2a). At test rats received presentations of the CS+ and CS− as during PDT. However, no ethanol was delivered and tests were conducted in a context where alcohol had never previously been consumed. Data represent mean (± SEM) normalized port-entries during the CS+ (filled bars) and CS− (open bars) following saline and eticlopride (5 μg/kg or 10 μg/kg) pre-treatment in a Charles River (n = 16) and b Harlan (n = 13) rats. * p < 0.03, compared to saline and 5 microgram/kilogram dose
Separate ANOVA conducted on normalized CS+ responding for rats from Charles River and Harlan revealed a main effect of Dose in Harlan rats only [Harlan, F(2,24) = 8.01, p < 0.01]. In Harlan rats, paired-samples t-tests indicated a significant reduction in normalized CS+ responding following 10 μg/kg when compared to saline [t(12) = 3.63, p = 0.003] and to the 5 μg/kg dose of eticlopride [t(12) = 2.57, p = 0.025]. There was no difference between saline and the 5 μg/kg dose.
Trend analyses conducted to examine the shape of the dose response curve revealed that the attenuation of normalized CS+ responding followed a linear trend in rats from Harlan [Dose, F(1,12) = 13.17, p < 0.01], which was not observed in Charles River rats.
The impact of eticlopride on total port-entries at test is described in Supplemental Material (Supplementary Fig. 3).
Figure 5 depicts data from Experiment 2b, in which Charles River and Harlan rats received an injection of saline, SCH 23390 or eticlopride before a PDT session in which the CS+ was paired with ethanol. Overall, rats from both vendors responded more to the CS+ than the CS− [CS, F(1,23) = 81.48, p < 0.001], with no CS × Vendor interaction. There were no main effects of Vendor or Treatment, and no Treatment × Vendor interaction. However, a significant Treatment × CS interaction [Treatment × CS, F(2,46) = 3.53, p < 0.05] indicated that blocking dopamine receptors reduced responding to the CS+ but not the CS−. There was no Treatment × CS × Vendor interaction, indicating that pattern was consistent in rats from both vendors. Follow-up ANOVA conducted on normalized CS+ responding collapsed across vendor revealed a main effect of Treatment [Treatment, F(2,48) = 3.85, p < 0.05]. Paired-samples t-tests indicated a significant reduction in CS+ responding following SCH 23390 when compared to saline [t(24) = 2.31, p < 0.05], with no significant difference between saline and eticlopride. There was no main effect of treatment on normalized responding to the CS−.
Blocking dopamine D1 receptors but not D2 receptors reduced Pavlovian-conditioned alcohol-seeking during Pavlovian-discrimination training sessions in which the CS+ was paired with 15 % ethanol. Data represent mean (±SEM) normalized port-entries during the CS+ (filled bars) and the CS− (open bars) following saline, eticlopride (10 μg/kg) and SCH 23390 (10 μg/kg) pre-treatment in Charles River (n = 18) and Harlan (n = 7) rats. * p < 0.05, compared to saline
Discussion
These results highlight important differences in home-cage alcohol consumption and the contribution of dopamine receptor subtypes to Pavlovian-conditioned alcohol-seeking in male, Long–Evans rats from different vendors. Alcohol intake and preference during home-cage alcohol consumption sessions were higher in Harlan rats than Charles River rats. In tests for conditioned behavior, saline-infused rats from both vendors checked the fluid port for alcohol more frequently during the CS+ than the CS−, when both cues were presented without alcohol in a non-alcohol context. By comparison, blocking dopamine D1-like receptors with 10 μg/kg SCH 23390 reduced CS+ responding in Harlan and Charles River rats, and blocking dopamine D2-like receptors with 10 μg/kg eticlopride reduced CS+ responding in rats from Harlan only. Lastly, SCH 23390 but not eticlopride reduced CS+ responding in PDT sessions where the CS+ was paired with alcohol. Thus, both dopamine receptor subtypes are involved in Pavlovian-conditioned alcohol-seeking, with D1-like receptors having a more prominent role in this effect.
Home-cage alcohol consumption was assessed using a procedure that induces robust voluntary ethanol consumption in standard laboratory rats without sucrose-fading (Carnicella et al. 2008; Simms et al. 2008; Wise 1973). Overall, the consumption of 15 % ethanol increased across session in rats from both suppliers. While Harlan rats drank more ethanol than Charles River rats in earlier sessions, there was no difference in the volume of ethanol consumed as a function of vendor by the last session. Marked differences in weight gain as a function of vendor resulted in substantially lower ethanol-intake measured in g/kg in Charles River rats compared to Harlan rats. Nonetheless, this measure also increased across session in rats from both vendors. The rapid increase in ethanol intake across session observed in Charles River rats is similar to the “escalation” in ethanol consumption reported by other researchers using this procedure with Harlan rats (Simms et al. 2008). However, Harlan rats in our studies achieved high ethanol-intakes on the first session and consequently showed a less pronounced escalation in g/kg relative to published data. One explanation for this discrepancy is that we utilized 15 % ethanol whereas others report an escalation in consumption with 20 % ethanol (Simms et al. 2008). Overall, values for ethanol intake (g/kg/24 h) in the present studies are a bit lower than those reported in the literature. For example, Simms and colleagues (2008) found that male, Long–Evans rats from Harlan achieved stable consumption of 20 % ethanol at 5.1 ± 0.6 g/kg, whereas averaged across the last two sessions of the present studies Harlan rats consumed 3.4 ± 0.2 g/kg of 15 % ethanol.
While the volume of ethanol consumed did not differ across vendor by the end of this phase, water consumption remained higher in Charles River rats. Consequently, preference for ethanol was more robust in Harlan rats, although preference increased across session in rats from both suppliers. Interestingly, a large number of Charles River rats and fewer Harlan rats required a sweetened ethanol solution to encourage ethanol consumption (see details in Supplemental material). Together, these vendor differences support the use of Harlan rats in experiments involving voluntary ethanol consumption.
Rats from both suppliers arrived on the same day, were of comparable weights on arrival, and were maintained under identical conditions. Therefore, it is notable that Charles River rats appeared unable to regulate weight gain when given unrestricted access to food. That Charles River rats consumed more water than Harlan rats suggests that they also ate more food, given that water and food consumption typically co-occur (Bolles 1961). Because fluid intake and body weight influence measures of ethanol-intake such as g/kg and preference, future studies should compare blood ethanol concentrations following oral consumption as a function of supplier.
The present findings are consistent with a recent report (Palm et al. 2011) of differences in weight gain and alcohol consumption in male, Wistar rats obtained from five unique suppliers. Interestingly, Wistar rats from Harlan Laboratories (The Netherlands) drank significantly more ethanol than rats from other suppliers, with 80 % of rats exhibiting a mean ethanol intake of 3.0 g/kg/day. Within-strain discrepancies in weight gain and alcohol consumption could result from genetic factors arising from years of breeding within a facility, or be attributable to environmental early life differences, such as maternal separation and weaning age, which can impact alcohol consumption in adulthood (Gustafsson and Nylander 2006; Ploj et al. 2003; Roman et al. 2005). The present data, together with the study by Palm and colleagues (2011), emphasize the value of considering breeder in alcohol research, with rats from Harlan Laboratories being particularly suitable for studies that require high levels of oral ethanol consumption.
Unlike the replicable differences during home-cage alcohol exposure, there were no consistent vendor differences in the acquisition of PDT. Charles River and Harlan rats in both experiments learned to discriminate between the CS+ and CS−. However, in Experiment 1 the levels of CS+ responding were similar in Charles River and Harlan rats, whereas in Experiment 2 Harlan rats responded more vigorously to the CS+. Furthermore, Harlan rats responded more to the CS+ in Experiment 2 than in Experiment 1. Thus, Harlan rats might be more prone to variability in behavioral data when compared to rats from Charles River.
Our primary objective was to examine the contribution of dopamine receptors to Pavlovian-conditioned alcohol-seeking in a non-alcohol context. Following PDT rats were placed into a different context where the CS+, CS− and ethanol were withheld. As a consequence, spontaneous entries into the fluid port decreased across session (see Supplementary Fig. 1), suggesting that this context came to signal the absence of ethanol. At test, responding to the CS+ and CS− without ethanol was assessed in the non-alcohol context. Consistent with previous findings (Chaudhri et al. 2009), saline-injected rats from both suppliers responded more to the alcohol-predictive CS+ than the CS−, indicative of Pavlovian-conditioned alcohol-seeking. Blocking dopamine D1-receptors reduced CS+ responding in rats from both vendors, whereas blocking dopamine D2-receptors produced this effect in Harlan rats only. These results demonstrate a novel role for dopamine in alcohol-seeking triggered by discrete, Pavlovian-conditioned, alcohol-predictive cues, which is consistent with evidence that dopamine D1- and D2-receptor subtypes are involved in the reinstatement of instrumental alcohol-seeking induced by discriminative olfactory cues that signal the alcohol availability (Liu and Weiss 2002). Thus, dopamine may mediate the conditioned incentive effects of alcohol-associated olfactory cues (Liu and Weiss 2002), as well as Pavlovian-conditioned approach responses triggered by auditory cues that predict alcohol. Interestingly, in the study by Liu and Weiss (2002) the discriminative olfactory cue was extinguished before test, whereas in the present experiment responding to the CS+ and CS− was not extinguished before test. Thus, dopamine appears to mediate alcohol-seeking triggered by both extinguished and non-extinguished alcohol-predictive cues. In addition, the present study assessed alcohol-seeking driven by the non-extinguished CS+ in a context that had never been paired with alcohol delivery or consumption, confirming a role for dopamine in responding to discrete Pavlovian-conditioned alcohol-cues specifically.
That eticlopride produced different effects in Harlan and Charles River rats suggests that male Long–Evans rats from these suppliers may differ in terms of dopamine D2-receptor expression or availability, and/or dopamine sensitivity. Rats from different strains exhibit differences in dopamine sensitivity (Shoaib et al. 1995), suggesting the possibility of within-strain variations as a function of breeder as well. Alternatively, 10 μg/kg eticlopride might not have been a sufficiently high dose to affect CS+ responding in Charles River rats, a question that should be addressed in future studies.
There are several explanations for why blocking dopamine receptors reduced the expression of Pavlovian-conditioned alcohol-seeking. Dopamine has been implicated in a myriad of cognitive and behavioral processes, including reward (Berridge and Robinson 1998, 2003; Wise and Rompré 1989; Wise 2004) motivation (Di Chiara 2002; Koob et al. 1998; Wise 2004) incentive-salience (Berridge 2012; Berridge and Robinson 1998), and locomotor behavior (Beninger 1983; Hoffman and Beninger 1985). The present findings cannot dissociate between the first three explanations. However, it is noteworthy that eticlopride only reduced CS+ responding when the cue was not paired with alcohol delivery, whereas SCH 23390 reduced CS+ responding in the presence and absence of alcohol. Thus, dopamine D1-receptors may play a more important role in responding to Pavlovian-conditioned cues per se, whereas D2-receptors may be involved in the expression of conditioned drug-seeking in the absence of the drug. Importantly, fluid ports were dry at the end of test session conducted during PDT, suggesting that neither antagonist affected ability or motivation to consume ethanol at test.
While dopamine antagonists can impair locomotor activity, these effects are usually observed at higher doses than those employed here (Hoffman and Beninger 1985). In addition, dopamine D2-receptor antagonists induce motoric impairments at doses of 20 μg/kg or higher (Bardo et al. 1999; Bevins et al. 2001). SCH 23390 doses similar to those in the present experiments have minimal effect on high rates of instrumental responding for sucrose or food (Crombag et al. 2002; Nakajima 1989). Similarly, eticlopride does not cause locomotor deficits within the dose range used in the present studies (Liu and Weiss 2002). Although we observed a dose-dependent decrease in the total number of port-entries at test in rats infused with 10 μg/kg SCH 23390 relative to saline, this measure did not differ relative to total port-entries averaged across the last 2 sessions of exposure to the non-alcohol context (see Supplementary Fig. 2). That there was no difference between these two phases suggests that SCH 23390 did not reduce port-entries to a level below what is typically observed in the absence of cues or ethanol, but likely blocked the specific increase in port-entries attributable to the presence of the alcohol-predictive CS+. There was no impact of eticlopride on total port-entries in rats from either vendor (see Supplementary Fig. 3).
There is support for the hypothesis that distinct neurobiological processes mediate conditioned responding elicited by discrete Pavlovian cues and environmental contexts (Bossert et al. 2007; Chaudhri et al. 2009; Ito et al. 2000, 2002, 2004). For example, SCH 23390 infusions into the nucleus accumbens (NAc) shell, but not the NAc core reduce context-induced reinstatement of heroin-seeking, whereas SCH 23390 infused into the NAc core but not shell attenuates cue-induced reinstatement of heroin-seeking (Bossert et al. 2007). Also, pharmacologically inactivating the NAc core, but not shell reduces CS+ responding in a procedure identical to the present experiments (Chaudhri et al. 2009), suggesting that the NAc core is particularly important for responding to Pavlovian-conditioned alcohol-predictive cues. Our ongoing research will determine if Pavlovian-conditioned alcohol-seeking is reduced by dopamine receptor antagonist administration in the NAc core, but not shell.
In conclusion, alcohol-predictive cues reliably elicit Pavlovian-conditioned alcohol-seeking, even when experienced in a context where alcohol was never previously consumed. Dopamine D1-receptor antagonists reduce Pavlovian-conditioned alcohol-seeking in both the presence and absence of alcohol. D2-receptors antagonists reduce Pavlovian-conditioned alcohol-seeking only in the absence of alcohol, although this effect depends on the vendor that supplied the rats. These findings provide novel evidence of the involvement of dopamine receptors in the expression of Pavlovian-conditioned alcohol-seeking. In addition, they highlight the importance of considering vendor as a source of variability in pre-clinical alcohol research.
References
Bardo MT, Valone JM, Bevins RA (1999) Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berlin) 143:39–46
Beninger RJ (1983) The role of dopamine in locomotor activity and learning. Brain Res Rev 6:173–196
Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE (2006) Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience 137:699–706. doi:10.1016/j.neuroscience.2005.08.064
Berridge KC (2012) From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci 35:1124–1143. doi:10.1111/j.1460-9568.2012.07990.x
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28:309–369
Berridge KC, Robinson TE (2003) Parsing reward. Trends Neurosci 26:507–513. doi:10.1016/S0166-2236(03)00233-9
Bevins RA, Besheer J, Pickett KS (2001) Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav 68:135–145
Bolles RC (1961) The interaction of hunger and thirst in the rat. J Comp Physiol Psychol 54:580
Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin-seeking induced by contextual and discrete cues. J Neurosci 27:12655–12663. doi:10.1523/JNEUROSCI.3926-07.2007
Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D (2008) GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A 105:8114–8119. doi:10.1073/pnas.0711755105
Chaudhri N, Sahuque LL, Janak PH (2008a) Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry 64:203–210. doi:10.1016/j.biopsych.2008.03.007
Chaudhri N, Sahuque LL, Cone JJ, Janak PH (2008b) Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci 28:2288–2298. doi:10.1111/j.1460-9568.2008.06517.x
Chaudhri N, Sahuque LL, Schairer WW, Janak PH (2009) Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology 35:783–791. doi:10.1038/npp.2009.187
Ciccocioppo R, Sanna PP, Weiss F (2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A 98:1976–1981. doi:10.1073/pnas.98.4.1976
Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ (2008) Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol 16:207–214. doi:10.1037/1064-1297.16.3.207
Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP (2010) Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend 111:58–63. doi:10.1016/j.drugalcdep.2010.03.017
Crombag HS, Shaham Y (2002) Renewal of drug-seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116:169–173. doi:10.1037//0735-7044.116.1.169
Crombag HS, Grimm JW, Shaham Y (2002) Effect of dopamine receptor antagonists on renewal of cocaine-seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 27:1006–1015
Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137:75–114
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE (2004) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine-seeking in rats. Neuropsychopharmacology 30:296–309. doi:10.1038/sj.npp.1300579
Gustafsson L, Nylander I (2006) Time-dependent alterations in ethanol intake in male Wistar rats exposed to short and prolonged daily maternal separation in a 4-bottle free-choice paradigm. Alcohol Clin Exp Res 30:2008–2016. doi:10.1111/j.1530-0277.2006.00247.x
Hamlin AS, Blatchford KE, McNally GP (2006) Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience 143:25–38. doi:10.1016/j.neuroscience.2006.07.035
Hamlin AS, Newby J, McNally GP (2007) The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 146:525–536. doi:10.1016/j.neuroscience.2007.01.063
Hoffman DC, Beninger RJ (1985) The D1 dopamine receptor antagonist, SCH 23390 reduces locomotor activity and rearing in rats. Pharmacol Biochem Behav 22:341–342
Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ (2000) Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 20:7489–7495
Ito R, Dalley JW, Robbins TW, Everitt BJ (2002) Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22:6247–6253
Ito R, Robbins TW, Everitt BJ (2004) Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7:389–397. doi:10.1038/nn1217
Katner SN, Weiss F (1999) Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res 23:1751–1760
Keppel G (1991) Design and analysis: a researcher’s handbook. Prentice-Hall, New Jersey
Koob GF, Sanna PP, Bloom FE (1998) Neuroscience of addiction. Neuron 21:461–476
Litt MD, Cooney NL, Morse P (2000) Reactivity to alcohol–related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction 95:889–900
Liu X, Weiss F (2002) Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther 300:882–889
Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF (2010) Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol 21:153–160. doi:10.1097/FBP.0b013e328337be95
McCusker CG, Brown K (1989) Alcohol-predictive cues enhance tolerance to and precipitate “craving” for alcohol in social drinkers. J Stud Alcohol 51:494–499
Nakajima S (1989) Subtypes of dopamine receptors involved in the mechanism of reinforcement. Neurosci Biobehav Rev 13:123–128
Nie H, Janak PH (2003) Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berlin) 168:222–228. doi:10.1007/s00213-003-1468-0
Palm S, Roman E, Nylander I (2011) Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol 45:607–614. doi:10.1016/j.alcohol.2010.11.005
Ploj K, Roman E, Nylander I (2003) Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience 121:787–799. doi:10.1016/S0306-4522(03)00499-8
Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM et al (1994) Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol 62:620–626
Roman E, Gustafsson L, Hyyti P, Nylander I (2005) Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res 29:591–601. doi:10.1097/01.ALC.0000158933.70242.FC
Shoaib M, Spanagel R, Stohr T, Shippenberg TS (1995) Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology (Berlin) 117:240–247
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. doi:10.1111/j.1530-0277.2008.00753.x
Tsiang MT, Janak PH (2006) Alcohol-seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol 38:81–88. doi:10.1016/j.alcohol.2006.05.004
Wing VC, Schoaib M (2008) Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacology (Berlin) 200:357–365. doi:10.1007/s00213-008-1211-y
Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacology (Berlin) 29:203–210
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494. doi:10.1038/nrn1406
Wise RA, Rompré PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225
Zironi I, Burattini C, Aicardi G, Janak PH (2006) Context is a trigger for relapse to alcohol. Behav Brain Res 167:150–155. doi:10.1016/j.bbr.2005.09.007
Acknowledgments
The National Institute of Alcohol Abuse and Alcoholism (RO1 AA14925; Patricia H. Janak, PI) funded this research. NC is the recipient of a Chercheurs-Boursiers award from Fonds de recherche du Québec - Santé, and a member of the FRQS-funded Center for Studies in Behavioral Neurobiology/Groupe de recherche en neurobiologie comportementale (CSBN/GRNC). The authors would like to thank Dr. Uri Shalev for comments on the manuscript and Atyeh Heidari for assistance in running the experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 70 kb)
Rights and permissions
About this article
Cite this article
Sparks, L.M., Sciascia, J.M., Ayorech, Z. et al. Vendor differences in alcohol consumption and the contribution of dopamine receptors to Pavlovian-conditioned alcohol-seeking in Long-Evans rats. Psychopharmacology 231, 753–764 (2014). https://doi.org/10.1007/s00213-013-3292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3292-5