Abstract
Rationale
Neuronal α4β2* nicotinic acetylcholine receptors mediate cognition, pain, and the discriminative and reinforcing effects of nicotine. In addition to traditional orthosteric agonists, α4β2* positive allosteric modulators (PAMs) have recently been identified. With increased subtype selectivity relative to agonists, PAMs administered alone or in combination with low-dose α4β2* agonists may be used as powerful tools for increasing our understanding of α4β2* pharmacology.
Objectives
The present experiments tested the nicotine discriminative-stimulus effects of the α4β2* PAM NS9283 (A-969933) in the presence and absence of low-dose nicotine or nicotinic subtype-selective agonist.
Methods
Rats were trained to discriminate 0.4 mg/kg nicotine from saline in a two-lever drug discrimination paradigm. In subsequent generalization tests, rats were administered nicotine, the α4β2*-preferring agonist ABT-594, and NS9283, alone or in two-drug combinations.
Results
Nicotine and ABT-594 showed dose-dependent nicotine generalization. NS9283 alone resulted in a non-significant increase in nicotine-appropriate lever selection. Combination of non-effective doses of nicotine or ABT-594 with escalating doses of NS9283 resulted in a complete conversion to 100 % nicotine-appropriate choice in the case of nicotine combination and incomplete, though significant, generalization for ABT-594.
Conclusions
The α4β2* PAM NS9283 alone did not produce nicotine-like discriminative effects, but did demonstrate dose-related increases in nicotine lever choice when combined with a non-effective dose of nicotine or the α4β2* agonist ABT-594. This finding provides confirmation of the positive allosteric modulating effect of NS9283 in a functional in vivo paradigm. NS9283 is a potentially valuable tool for studying the role of α4β2* receptors in various nicotinic acetylcholine receptor-related functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuronal nicotinic acetylcholine receptors (nAChRs) are members of a family of pentameric acetylcholine-gated cation channels that are assembled from various combinations of nine alpha subunits (α2–α10) and three beta subunits (β2–β4). For reviews of the morphology and pharmacology of the nAChR subtypes, see Albuquerque et al. (2009), Romanelli et al. (2007), and Dani and Bertrand (2007). These receptors are involved in modulating nicotinic-based signal transmission in the central nervous system, and the α4β2* nAChR subtype is known to participate in attention (Hahn et al. 2003; Grottick et al. 2003; Mohler et al. 2010), cognition (Levin et al. 2006; Radek et al. 2010), motor integration (Quik et al. 2009), pain (Bannon et al. 1998; Ji et al. 2007; Damaj et al. 2007), and reward (Tuesta et al. 2011), among other basic CNS functions.
Nicotine produces a robust discriminative stimulus which has been studied extensively in numerous mammalian species in a variety of drug discrimination paradigms (e.g., Stolerman et al. 1989; Smith and Stolerman 2009). The nicotine stimulus has generally been examined using a two-lever operant drug discrimination procedure. In these studies the animal learns to associate the interoceptive cues of nicotine with selective responding on one of two levers which deliver reward differentially based upon a previous injection of nicotine or vehicle. Recent research has supported the hypothesis that the nicotine interoceptive stimulus is mediated predominantly by the α4β2* nAChR subtype (Picciotto et al. 1998; Zaniewska et al. 2006). Preferential antagonists of the α4β2* nAChR subtype can block the nicotine discriminative stimulus (Shoaib et al. 2000; Stolerman et al. 1999). Knockout mice lacking the β2 subunit failed to acquire the nicotine versus saline discrimination, while acquisition of a morphine stimulus discrimination task remained intact (Shoaib et al. 2002). In contrast, selective agonists for α3β4 (WO 03/062224) and α7 (WO 01/60821A1) nicotinic receptors do not produce the nicotine stimulus (Smith et al. 2007), and antagonists of the α7 subtype fail to block the nicotine cue (Brioni et al. 1996; Gommans et al. 2000; van Haaren et al. 1999). Moreover, α7 knockout mice were still able to acquire discriminative control of responding by nicotine (Stolerman et al. 2004), lending further support to the absence of contribution from α7 receptor subtypes to the discriminative-stimulus effects of nicotine.
While the field has made significant advances in defining the nAChR subunits involved in nicotine discrimination, definitive research into the role of α4β2* receptors has been challenging due to the lack of highly specific tools. Many agonists possess α3β4 activity, and the prototypical antagonist DHβE is not wholly selective. Mice lacking the β2 subunit have been generated, but many behavioral studies are preferentially conducted in rats, and antisense methods have not been applied to the α4β2* receptor. An alternative method for studying nicotinic receptor activity is to use positive allosteric modulators (PAMs). PAMs bind at locations that are distinct from known orthosteric agonist-binding sites yet increase response to orthosteric agonists by lowering the energy barrier between the resting and active states of the ligand-gated pharmacophore (Bertrand and Gopalakrishnan 2007). Although several PAMs have been reported that are active at α7 or both α7 and α4β2* subtypes (Hurst et al. 2005; Faghih et al. 2008; Kim et al. 2007; Malysz et al. 2009b; Lilienfeld 2002; Schilström et al. 2007), NS9283 (also known as A-969933) is the only compound selective for α4β2* versus α7 (Malysz et al. 2009a) that has been tested in vivo (Lee et al. 2011; Timmermann et al. 2012). NS9283 does not displace the binding of orthosteric ligands including [3H]cytosine at rat α4β2** (cortex), [3H]A-585539 at rat α7* (cortex), or [3H]epibatidine at human α3* receptors (Malysz et al. 2009a). In addition, NS9283 does not directly evoke Ca2+ responses in HEK-293 cells expressing hα4β2* nAChRs but is able to potentiate submaximum evoked responses by nicotine or ABT-594 (EC50 ~ 0.4 μM). The use of subtype-selective nicotinic PAMs represents a plausible alternative approach to disease treatment either given alone or with full or partial nicotinic orthosteric agonists (Maelicke and Albuquerque 1996, 2000; Faghih et al. 2009; Changeux 2010; Lee et al. 2011). Due to the proposed role of α4β2-containing nAChRs in reward, the present studies examined the nicotine stimulus properties of the α4β2 PAM NS9283 in the absence or presence of non-effective doses of either nicotine or the potent α4β2*-preferring agonist ABT-594 (Donnelly-Roberts et al. 1998).
Materials and methods
Drugs
(−)-Nicotine hydrogen tartrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). NS9283 (3-(3-(pyridine-3-yl)-1,2,4-oxadiazol-5-yl)benzonitrile) and ABT-594 (5-[(2R)-2-azetidinylmethoxy]-2-chloropyridine, monohydrochloride) were synthesized at Abbott Laboratories. Nicotine and ABT-594 were dissolved in physiological saline. NS9283 was dissolved in 34 % HBC (2-hydroxypropyl-β-cyclodextrin)/sterile water. Nicotine was administered subcutaneously 10 min before session start. ABT-594 and NS9283 were administered intraperitoneally 20 min before the start of sessions. Doses of nicotine, NS9283, and ABT-594 are expressed as the base.
Apparatus
Training and testing were conducted in eight two-lever rodent chambers and sound-attenuating cubicles obtained from Coulbourn Instruments (Whitehall, PA, USA; internal dimensions 30 cm wide by 25 cm deep by 30 cm high). Two retractable levers were located on either side of a combination pellet/water dispenser that was mounted in the center of a wall adjacent to the Plexiglas door. A dim house light was mounted on the top center of the wall above the dispenser. Control of all chamber stimuli as well as recording of lever presses was accomplished with Graphic State 3.03 software (also from Coulbourn Instruments).
Animals
Male Sprague–Dawley (CD-IGS) rats were obtained from Charles River Labs (Portage, MI, USA) at an arrival weight between 280 and 299 g. Colony lighting was maintained on a 12-h light/dark cycle with lights on at 0600 hours. Temperature and humidity were controlled. Following 1–2 weeks of quarantine and acclimation, rats were singly housed and body weights gradually reduced to 85–90 % of free-feeding weight by maintaining on a food-restricted diet (approximately 15 g per day per rat). At this point rats received a 15-min hopper training session in which a single 45-mg food pellet was delivered to the food magazine on a variable-interval 60-s (VI60) schedule. Levers were retracted and unavailable on this session. Lever-press training began on the following day.
Lever-press training
Rats were first exposed to a VI60 autoshaping procedure on two or more consecutive sessions, as needed. Levers were inserted into the chamber for 10 s and then retracted during VI60 intertrial interval periods. Delivery of a 45-mg food pellet (AIN-76A Rodent Tablet, Test Diet, Richmond, IN, USA) coincided with retraction of the levers to begin the next ITI. A lever press while the levers were extended resulted in the delivery of a food pellet. Conventional lever-press training sessions began following autoshaping. The levers were active throughout these sessions, and each press of either lever produced a pellet. As the training continued, the response requirement was progressively advanced until rats responded reliably on a 10-response fixed-ratio (FR10) schedule in sessions lasting 30 min. Once FR10 responding was established, drug discrimination training commenced. Levers were wiped with a water/Alconox solution before each session.
Nicotine discrimination training
An errorless learning procedure was used during the first eight sessions of discrimination training. Rats were injected subcutaneously with vehicle or nicotine (0.4 mg/kg as base) and immediately placed into test chambers with house light off and levers unavailable in order to allow animals to adapt to the train/test environment. Following the 10-min habituation period, the start of the session was marked by insertion of levers and illumination of the house light. Only the lever scheduled to be active in conjunction with the prior injection was extended into the chamber and available for reinforcement on FR10 on errorless training days. An equal number of errorless sessions (four) were given with each treatment/lever combination on a single alternation schedule for the first four sessions, then double alternation for the remaining four sessions (e.g., nicotine–saline–nicotine–saline, nicotine–nicotine–saline–saline, and vice versa). Normal discrimination training began following these errorless sessions.
Both levers were extended throughout training sessions (except during time-out periods and before or after a session). Presses on the correct lever (left lever for half of the rats when previously administered nicotine, right for the other half) were reinforced with delivery of a food pellet on an FR10 schedule. The opposite lever was active during sessions in which saline was previously administered. Injections of nicotine or saline were given daily in a pseudorandom order such that animals never received more than three consecutive daily administrations of either nicotine or saline. Ten presses on the incorrect lever resulted in a 5-s time-out, with house light extinguished and levers retracted. Training sessions ended after 15 min or the successful receipt of 75 reinforcements.
Generalization tests
Rats receiving ABT-594, NS9283, or vehicle were injected 20 min prior to each session. Therefore, rats were placed into test chambers 10 min after injection and 10 min prior to the actual start of sessions. Rats receiving nicotine or vehicle were injected 10 min prior to each session as described above. Therefore rats receiving NS9283 and nicotine were injected both 20 min and 10 min before the session, and placed in the chambers immediately following the nicotine injection. Test sessions were conducted once or twice weekly when subjects met the following criteria during prior training sessions: (1) first FR10 completed on the correct lever and (2) greater than 90 % correct lever choice over the entire session for 9 out of 10 consecutive sessions before the start of testing. Rats were also required to meet a minimum response output criterion of greater than 20 responses per minute on vehicle sessions. Test sessions lasted for 15 min or until 75 reinforcements were obtained. Responses on either lever were reinforced on an FR10. Subsequent test sessions were given only following completion of at least one intervening vehicle and drug training session in which the training criteria were met. Compounds were determined to be nicotine-like if animals made 80 % or more session responses on the nicotine-associated lever during generalization tests and vehicle-like if 20 % or fewer responses were made on the nicotine lever. The primary generalization measure was based on overall session lever selection, but a comparison was made to lever choice before the first reinforcement and noted if these measures were noticeably different. Response rate (lever presses per minute) was calculated for the whole session.
Each drug or combination experiment was conducted with a group of rats (n = 11–12) which received each of the dose combinations in a counterbalanced order over a period of 2–4 weeks, with two or more training sessions preceding each test session. Pretreatment times and routes of administration were chosen on the basis of in-house pharmacokinetic analyses indicating significant levels of compound during the selected time points. In experiment 1, dose–response effects of both nicotine and ABT-594 were evaluated. In experiment 2, the effects of various doses of NS9283 alone were compared with effects in combination with a non-effective, 0.025 mg/kg dose of nicotine. In experiment 3, the same doses of NS9283 used in experiment 2 were combined with a non-effective dose of ABT-594 (0.001 mg/kg).
Statistical analysis
Results are expressed as the percentage of total lever presses made on the nicotine-appropriate lever and rate per minute of responding based upon the sum of lever presses on both levers on test sessions. All studies utilized a within-subjects design, with counterbalanced ordering of treatments. Lever choice measures tend to be quantal in nature and were not assumed to be distributed normally, so non-parametric analyses (Friedman or Kruskal–Wallis ANOVA; Dunn’s comparisons) were used to confirm statistically significant changes from vehicle, in addition to the convention of accepting 80 % or greater nicotine-associated lever choice as full nicotine-like generalization. Two-way repeated measures ANOVA (NS9283 doses with vehicle or with drug) was utilized in experiments 2 and 3. Analysis of response rate data was conducted using one-way repeated measures ANOVA, except for a high dose of nicotine, 0.8 mg/kg, added later and an additional low dose of ABT-594 (0.001 mg/kg, also added after the initial dose–response determination). These additional doses were tested with between-group statistics. Planned comparisons (Dunnett’s multiple comparison test) of response rates were conducted between vehicle and drug treatment sessions following significant ANOVA. GraphPad Prism 4.03 software was used for statistical analyses.
Results
Experiment 1
Training to the criterion to discriminate 0.4 mg/kg nicotine from saline required an average of 26 ± 1.1 sessions (n = 36). Drug generalization testing began once all rats in a designated test group achieved criterion performance. Experiment 1 compared the dose–response effects of ABT-594 and nicotine in nicotine-discriminating rats (Fig. 1). Both nicotine (Friedman = 26.02; six groups (excluding 0.8 mg/kg dose tested with different rats); P < 0.0001) and ABT-594 (Friedman = 18.77; five groups; P < 0.0009) fully substituted for the nicotine stimulus at doses of 0.4–0.8 and 0.025–0.05 mg/kg, respectively. The median effective dose (ED50) for generalization with ABT-594 was 0.005 mg/kg (95 % confidence interval (CI) = 0.002 to 0.012), compared to 0.08 mg/kg (95 % CI = 0.034 to 0.19) for nicotine (see Table 1 for all ED50 values). Thus, ABT-594 displayed potency approximately 16 times greater than nicotine based upon their relative ED50 doses. This roughly aligns with the reported α4β2* binding affinities of 0.037 nM for ABT-594 and 1 nM for nicotine (Donnelly-Roberts et al. 1998), a 27-fold difference.
Experiment 1. Discriminative stimulus (top) and response rate (bottom) effects of ABT-594 (open squares) and nicotine (open triangles) in rats trained to discriminate 0.4 mg/kg nicotine from 0.9 % saline. Results are presented as mean ± standard error of the mean on a 15-min test in which responses on both levers are reinforced on FR10. Asterisks indicate differences from appropriate vehicle with significance levels of P < 0.05 or P < 0.01 for one or two asterisks, respectively. Dotted lines at 20 and 80 % nicotine lever choice indicate presumptive maximal and minimal levels, respectively, associated with full saline or nicotine generalization. N = 12 with each compound
The lowest dose of nicotine (0.025 mg/kg) resulted in a significant increase in response rate over vehicle, while the highest dose tested (0.8 mg/kg) significantly reduced responding to 18 % of vehicle levels. The ED50 for response rate reduction by nicotine was 0.68 mg/kg (95 % CI = 0.38 to 1.22), representing an approximate 8.5-fold separation between the discriminative stimulus-producing dose and the response rate-reducing dose for nicotine. ABT-594 at the highest dose (0.05 mg/kg) tested produced a suggestive, but non-significant, reduction in response rate. This high dose of ABT-594 resulted in numerous behavioral effects prior to testing, including mild ataxia and a flattened body posture. Despite these short-term side effects, food responding was not significantly affected. The ED50 for response rate reduction by ABT-594 was estimated to be at a higher dose than actually tested, 0.11 mg/kg (95 % CI = 0.05 to 0.25). Comparison of the ED50 values between discrimination and response rate reduction with ABT-594 revealed a 22-fold difference. Results of these dose–response tests were used to select similar non-effective doses of ABT-594 and nicotine for combination with the α4β2* PAM in experiments 2 and 3. The selected dose of nicotine, 0.025 mg/kg, is 25 times greater than the 0.001 mg/kg dose of ABT-594 used in these experiments, approximating the 16-fold potency difference in ED50 values for the discrimination measure noted in Experiment 1 and the 27-fold difference in reported K i values.
Experiment 2
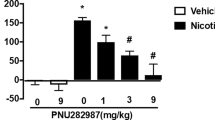
Experiment 2 provided dose–response data for NS9283 alone or in combination with a dose of nicotine (0.025 mg/kg) which by itself only elicited vehicle lever choice. As presented in the top panel of Fig. 2, NS9283 treatment alone did not result in a significant increase in nicotine lever choice above that found with vehicle (the highest level of generalization was 33 ± 14 % at the 7.5 mg/kg dose of NS9283). In contrast to this lack of effect, combination of the same doses of NS9283 with 0.025 mg/kg nicotine resulted in a dose-dependent, full generalization of the 7.5 mg/kg dose of NS9283 plus nicotine for the nicotine-training stimulus, although three rats failed to respond with this combination (ANOVA: F(4,51) = 12.25; P < 0.001). Treatment with doses of 2.5 and 7.5 mg/kg NS9283 plus nicotine resulted in significant increases in nicotine lever selection compared to both the 0.025 mg/kg dose of nicotine/vehicle combination and corresponding doses of NS9283 alone. The dose of NS9283 which, in combination with 0.025 mg/kg nicotine, produced an ED50 response on nicotine discrimination was 0.95 mg/kg (95 % CI = 0.53 to 1.72). The ED50 for response rate reduction by NS9283 in combination with 0.025 mg/kg nicotine was 3.24 mg/kg (95 % CI = 2.03 to 5.18).
Experiment 2. Percentage nicotine-appropriate lever choice (top) and response rate (bottom) effects of NS9283 alone (filled triangles) or NS9283 plus 0.025 mg/kg nicotine (open triangles) in rats trained to discriminate 0.4 mg/kg nicotine from saline. All results are presented as mean ± SEM for a 15-min test with both levers reinforced on FR10. Asterisks indicate significance levels of P < 0.05 or P < 0.01 for one or two asterisks, respectively, compared to vehicle plus 0.025 mg/kg nicotine. Pound signs indicate significance levels of P < 0.05 or P < 0.01 for one or two signs, respectively, compared to the same dose of NS9283 alone. N = 12 per drug combo, with each rat serving at each dose within each group in a counterbalanced order
Experiment 3
The same doses of the PAM tested in experiment 2 were administered in combination with 0.001 mg/kg ABT-594 in experiment 3. As seen in Fig. 3, there was a partial but highly significant increase in nicotine lever selection when either the 2.5 or 7.5 mg/kg dose of NS9283 was administered in combination with ABT-594 (Friedman = 27.38; five groups; P < 0.001) and compared to vehicle. Two-way ANOVA of lever selection yielded a significant interaction factor (F(4,80) = 3.83; P < 0.01) between main effects of ABT-594 presence or absence (F(1,80) = 1.2; n.s.) and NS9283 doses (F(4,80) = 21.63; P < 0.0001). Generalization of nicotine to these combination doses of ABT-594 and NS9283 only reached 60 %, short of the 80 % threshold for concluding that complete generalization had occurred. The ED50 for nicotine lever selection was 2.91 mg/kg (95 % CI = 1.58 to 5.37) NS9283. The response rate following the 7.5 mg/kg NS9283 dose combination with ABT-594 reduced to 29 % of vehicle control (Friedman = 21.74; five groups; P < 0.0002). The ED50 for response rate reduction by the combination of NS9283 with 0.001 mg/kg ABT-594 was 5.21 mg/kg (95 % CI = 3.36 to 8.08), slightly higher than the ED50 with the nicotine combination.
Experiment 3. Discriminative stimulus (top) and response rate (bottom) effects of NS9283 alone (filled squares) or NS9283 plus ABT-594 (open squares) in rats trained to discriminate 0.4 mg/kg nicotine from 0.9 % saline. Results are presented as mean ± standard error of the mean on a 15-min test in which responses on both levers are reinforced on FR10. Asterisks indicate differences from appropriate vehicle with significance level of P < 0.05 for one asterisk using Dunn’s or Dunnett’s MCT. Pound signs indicate significance levels of P < 0.01 compared to the same dose of NS9283 alone using Bonferroni posttests. Dotted lines at 20 and 80 % nicotine lever choice indicate presumptive maximal and minimal levels, respectively, associated with full saline or nicotine generalization. Plus signs indicate significance levels of P < 0.01 compared to vehicle–saline using Student’s t test. N = 11 per dose in the NS9283 alone tests, and N = 12 per dose in the NS9283 plus ABT-594 tests, with each rat serving at each dose within each group in a counterbalanced order
Discussion
The present experiments demonstrate the ability of a novel α4β2* PAM, NS9283, to markedly increase the nicotine-like discriminative-stimulus effects of non-effective doses of both nicotine and a α4β2*-preferring agonist. In contrast, the α4β2* PAM administered alone resulted predominantly in vehicle lever selection with minimal indication of an increase in nicotine lever selection. This suggests the need for coadministration of at least a very low dose of an orthosteric agonist with this PAM in order to achieve adequate α4β2* receptor activation for the purpose of producing a nicotine-like subjective cue. In contrast, the degree of activation required for cognitive improvement can be met by this PAM without exogenous agonist supplementation (Timmermann et al. 2012). Cognition-engaging activities may result in the release of endogenous acetylcholine that is sufficient to interact with a PAM, while the subjective cue associated with nicotine drug discrimination appears to require exogenous activation of the α4β2* orthosteric site. This is also consistent with the higher dose of nicotine required for drug discrimination (0.4 mg/kg) compared to that necessary for cognitive enhancement (0.05–0.2 mg/kg) (e.g., Allison and Shoaib 2013). It is also possible that the rats used in a drug discrimination paradigm may have altered sensitivity to nicotine based on repeated dosing over a period of months, thereby changing the sensitivity to a PAM. However, this is unlikely for two reasons. First, the well-trained rats in this study displayed very similar nicotine dose–response curves to rats tested in their first nicotine dose–response study (data not shown). Second, cognition-enhancing doses of nicotine in rodents remain effective even after prolonged exposure (e.g., Hahn and Stolerman 2002). This suggests that repeated experience with nicotine is unlikely to be affecting the interaction with NS9283, although this has not been specifically tested.
To the best of our knowledge, the present study is the first reported demonstration of a α4β2* PAM enhancing the discriminative-stimulus effects of either nicotine or a selective nAChR subtype agonist. It is interesting to note that full generalization was observed with the combination of NS9283 and a non-effective dose of nicotine, while a non-effective dose of ABT-594 combined with NS9283 failed to reach greater than 60 % nicotine-appropriate lever responding. Previous research has demonstrated that Ca2+ efflux in HEK-293 cells expressing human α4β2* was more robustly affected by the addition of NS9283 to nicotine and ACh than ABT-594 (Malysz et al. 2009a), a result similar to the in vivo effect we observe in drug discrimination. It is possible that a higher dose of ABT-594 in combination with the PAM would yield complete generalization. However, the purpose in choosing the dosage tested was to equate the ABT-594 dose with nicotine and demonstrate the ability of a PAM to enhance the discriminative properties of a dose with no detectable effect of its own. Moreover, despite the higher ED50 for response rate reduction with ABT-594, significantly decreased responding was encountered with the 2.5 mg/kg NS9283 combination with ABT-594 but not nicotine, suggesting that increasing the dose in combination with a PAM might not achieve complete generalization.
Another possible explanation for greater generalization with NS9383 and nicotine could be a secondary contribution from another nAChR subtype, e.g., α6- or β4-containing receptors, that is lacking with ABT-594. The absence of this “enabling” receptor subtype activity with ABT-594 could prevent full generalization from occurring. NS9283 did not potentiate Ca2+ in HEK-293 cells expressing human α3β4 or α4β4 receptors in the presence of ABT-594 (Lee et al. 2011). However, ABT-594 has 30-fold greater potency than nicotine at α3β4 receptors (Donnelly-Roberts et al. 1998), and previous work suggests that α3β4 lack the discriminative-stimulus properties necessary for nicotine generalization (Smith et al. 2007). In contrast, NS9283 was able to potentiate nicotine-evoked responses at human α4β4 receptors (Malysz et al. 2009a). It is possible that α4β4 or other receptors such as α6* (Exley et al. 2011) could be potentiated by NS9283 with nicotine and not ABT-594, and are contributing to the stimulus generalization effects at the lower doses tested in the combination studies. With greater activation of α4β2* receptors from a fully effective dose of ABT-594, this secondary receptor activity becomes unnecessary or redundant. In addition, NS9283 also shows stoichiometry-specific activity. Specifically, NS9283 shows PAM activity for the low-sensitivity (α4)3(β2)2 subform of the α4β2 receptor, but no detectable activity at the high-sensitivity (α4)2(β2)3 receptor (Timmermann et al. 2012). Additional PAM activity was also noted at the (α2)3(β2)2, (α2)3(β4)2, and (α4)3(β4)2 receptor subforms. These specific subforms may also be contributing to mediation of the nicotine discriminative-stimulus effects and explain some of the differences between modulation of nicotine and ABT-594. Further work is necessary to identify whether the effects of NS9283 on other receptor subtypes or even receptor subforms are selectively contributing to nicotine discriminative-stimulus properties.
In conclusion, these studies confirm and extend previous work that demonstrates the key role α4β2* receptors play in generating the discriminative-stimulus properties of nicotine. Positive allosteric modulation of the α4β2* receptor by NS9283 can significantly enhance the discriminative-stimulus properties of a non-effective dose of nicotine and the agonist ABT-594. As a non-effective dose of ABT-594 did not produce full generalization when combined with a PAM, it is possible that other receptor mechanisms may play a role in observed potentiation of nicotine’s discriminative-stimulus effects. However, it is also possible that complete generalization would have occurred following combination of a slightly higher dose of ABT-594 with NS9283. Additional studies with PAMs such as NS9283 may clarify the contributions, if any, of additional receptor subtypes. More generally, the studies further demonstrate that α4β2 PAMs are a useful new tool in the study of nAChR function.
Abbreviations
- PAM:
-
Positive allosteric modulator
- nAChR:
-
Nicotinic acetylcholine receptor
- FR10:
-
Fixed ratio 10
- ED50 :
-
Median effective dose
References
Albuquerque EX, Pereira EFR, Alkondon M, Rodgers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120
Allison C, Shoaib M (2013) Nicotine improves performance in an attention set shifting task in rats. Neuropharmacology 64:314–320
Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, Bitner RS, Diaz A, Dickenson AH, Porsolt RD, Williams M, Arneric SP (1998) Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science 279:77–81.
Bertrand D, Gopalakrishnan M (2007) Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 74:1155–1163
Brioni JD, Kim DJB, O-Neill AB (1996) Nicotine cue: lack of effect of the alpha 7 nicotinic receptor antagonist methyllycaconitine. Eur J Pharmacol 301:1–5
Changeux JP (2010) Allosteric receptors: from electric organ to cognition. Annu Rev Pharmacol Toxicol 50:1–38
Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47:699–729
Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, Collins AC, Martin BR (2007) Genetic approaches identify differential roles for α4β2* nicotinic receptors in acute models of antinociception in mice. J Pharmacol Exp Ther 321:1161–1169
Donnelly-Roberts D, Puttfarcken PS, Kuntzweiler TA, Briggs CA, Anderson DJ, Campbell JE, Piattoni-Kaplan M, McKenna DG, Walczak J, Holladay MW, Williams M, Arneric SP (1998) ABT-594 [(R)-5-(2-azetidinylmethoxy)-2-chloropyridine]: a novel, orally effective analgesic acting via neuronal nicotinic acetylcholine receptors: I. In vitro characterization. J Pharmacol Exp Ther 285:777–786
Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux J-P, Maskos U, Cragg SJ, Faure P (2011) Distinct contributions of nicotinic acetylcholine receptor subunit α4 and subunit α6 to the reinforcing effects of nicotine. PNAS 108(18):7577–7582
Faghih R, Gopalakrishnan M, Briggs CA (2008) Allosteric modulators of the α7 nicotine acetylcholine receptor. J Med Chem 51(4):701–712
Faghih R, Gopalakrishnan M, Gronlien JH, Malysz J, Briggs CA, Wetterstrand C, Ween H, Curtis MP, Sarris KA, Gfesser GA et al (2009) Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl_benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the α7 nicotinic acetylcholine receptor. J Med Chem 52:3377–3384
Gommans J, Stolerman IP, Shoaib M (2000) Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology 39:2840–2847
Grottick AJ, Haman M, Wyler R, Higgins GA (2003) Reversal of a vigilance decrement in the aged rat by subtype-selective nicotinic ligands. Neuropsychopharmacology 28:880–887
Hahn B, Sharples CGV, Wonnacott S, Shoaib M, Stolerman IP (2003) Attentional effects of nicotinic agonists in rats. Neuropharmacology 44:1054–1067
Hahn B, Stolerman IP (2002) Nicotine-induced attentional enhancement in rats: Effects of chronic exposure to nicotine. Neuropsychopharmacology 27:712–722
Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP (2005) A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25(17):4396–4405
Ji J, Bunnelle WH, Anderson DJ, Faltynek C, Dyhring T, Ahring PK, Rueter LE, Curzon P et al (2007) A-366833: a novel nicotinonitrile-substituted 3,6-diazabicyclo[3.2.0]-heptane α4β2* nicotinic acetylcholine receptor selective agonist: synthesis, analgesic efficacy and tolerability profile in animal models. Biochem Pharmacol 74:1253–1262
Kim JS, Padnya A, Weltzin M, Edmonds BW, Schulte MK, Glennon RA (2007) Synthesis of desformylflustrabromine and its evaluation as an α4β2* and α7 nACh receptor modulator. Bioorg Med Chem Lett 17(17):4855–4860
Lee C-H, Zhu C, Malyszm H, Campbell T, Shaughnessy T, Honore P, Polakowski J, Gopalakrishnan M (2011) α4β2* neuronal nicotinic receptor positive allosteric modulation: an approach for improving the therapeutic index of α4β2* nAChR agonists in pain. Biochem Pharmacol 82:959–966
Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharm 184:523–539
Lilienfeld S (2002) Galantamine—a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev 8:159–176
Maelicke A, Albuquerque EX (1996) New approach to drug therapy in Alzheimer’s disease. Drug Discov Today 1:53–59
Maelicke A, Albuquerque EX (2000) Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol 393:165–170
Malysz J, Dyhring PK, Ahring GM, Olsen D, Peters D, Gronlien JG, Wetterstrand H, Ween M, Haakerud M, Thorin-Hagene K, Andersen E, Anderson DJ, Hu M, Kroeger PE, Lee C-H, Gopalakrishnan M, Timmerman DB (2009a) In vitro pharmacological profile of a novel α4β2* positive allosteric modulator NS9283 (A-969933). Biochem Pharmacol 78:919–920
Malysz J, Gronlien JH, Anderson DJ, Haakerud M, Thorin-Hagene K, Ween H, Wetterstrand C, Briggs CA et al (2009b) In vitro pharmacological characterization of a novel allosteric modulator of α7 neuronal acetylcholine receptor, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744), exhibiting unique pharmacological profile. J Pharmacol Exp Ther 330:257–267
Mohler EG, Franklin SR, Rueter LE, Fox GB, Decker MW, Browman KE (2010) ABT-594 improves performance in the 5-choice serial reaction time task under conditions of increased difficulty, sub-chronic dosing, and in poorly-performing subjects. Pharmacol Biochem Behav 95:146–157
Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Merlo-Pich E, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta-2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA (2009) Multiple roles for nicotine in Parkinson’s disease. Biochem Pharmacol 78:677–685
Radek RJ, Kohlhaas KL, Rueter LE, Mohler EG (2010) Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Current Pharm Design 16:309–322
Romanelli MN, Gratteri P, Guandalini L, Martini E, Bonaccini C, Gualtieri F (2007) Central nicotinic receptors: structure, function, ligands, and therapeutic potential. Chem Med Chem 2:746–767
Schilström B, Ivanov VB, Wiker C, Svensson TH (2007) Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacol 32:43–53
Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux J-P (2002) The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology 42:530–539
Shoaib M, Zubaran C, Stolerman IP (2000) Antagonism of stimulus properties of nicotine by dihydro-beta-erythroidine (DHbetaE) in rats. Psychopharmacology (Berl) 149(2):140–146
Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M (2007) Ligands selective for α4β2* but not α3β4 or α7 nicotinic receptors generalize to the nicotine discriminative stimulus in the rat. Psychopharmacology 190:157–170
Smith JW, Stolerman IP (eds) (2009) Nicotine psychopharmacology. Handbook of psychopharmacology, vol 192. Springer, 295, pp 295–333
Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L (2004) The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology 46:363–371
Stolerman IP, Chandler CJ, Garcha HS, Newton JM (1999) Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharm 129:390–397
Stolerman IP, Rasul F, Shine PJ (1989) Trends in drug discrimination research analysed with a cross-indexed bibliography, 1984–1987. Psychopharmacology 98:1–19
Timmermann DB, Sandager-Nielsen K, Dyhring T, Smith M, Jacobsen AM, Nielsen EO, Grunnet M, Christensen JK, Peters D, Kohlhaas K, Olsen GM, Ahring PK (2012) Augmentation of cognitive function by NS9283, a stoichiometry-dependent positive allosteric modulator of α2- and α4-containing nicotinic acetylcholine receptors. Br J Pharmacol 167:164–182
Tuesta LM, Fowler CD, Kenny PJ (2011) Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol 82:984–995
van Haaren F, Anderson KG, Haworth SC, Kem WR (1999) GTS-21, a mixed nicotinic receptor agonist/antagonist, does not affect the nicotine cue. Pharmacol Biochem Behav 64:439–444
Zaniewska M, McCreary AC, Przegaliński E, Filip M (2006) Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine. Eur J Pharmacol 540:96–106
Acknowledgments
Animals were treated in accordance with guidelines established by the AbbVie Animal Care and Use Committee and National Institutes of Health Guide for The Care and Use of Laboratory Animals in an AAALAC-accredited facility.
This study was sponsored by AbbVie, Inc. AbbVie contributed to the study design, research, interpretation of data, writing, reviewing, and approval of the manuscript. All authors are current or past employees of AbbVie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohler, E.G., Franklin, S.R. & Rueter, L.E. Discriminative-stimulus effects of NS9283, a nicotinic α4β2* positive allosteric modulator, in nicotine-discriminating rats. Psychopharmacology 231, 67–74 (2014). https://doi.org/10.1007/s00213-013-3207-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3207-5