Abstract
Rationale
The rewarding and reinforcing effects of nicotine are produced, in large part, by activation of neuronal α4β2* nicotinic acetylcholine receptors (nAChRs), pentameric protein complexes comprised of different stoichiometries of α4 and β2 subunits. However, little is known about the functional role of distinct subtypes of α4β2* nAChRs in nicotine addiction.
Objectives
NS9283 represents a new class of stoichiometry-selective positive allosteric modulators (PAMs) that selectively bind to α4β2 nAChRs containing three α4 and two β2 subunits (3(α4)2(β2) nAChRs). The present experiments were designed to determine the effects of NS9283 on nicotine self-administration and the reinstatement of nicotine-seeking behavior, an animal model of smoking relapse. Parallel studies of sucrose self-administration and reinstatement were conducted in separate cohorts of rats to determine if the effects of NS9283 generalized to other reinforced behaviors.
Results
Acute and repeated administration of NS9283 dose-dependently reduced nicotine self-administration and reinstatement in male Sprague Dawley rats. These effects were reinforcer specific as no effects of NS9283 on sucrose self-administration and reinstatement were noted. NS9283 also failed to substitute for nicotine in supporting self-administration behavior suggesting that, at the doses tested, NS9283 alone is not reinforcing.
Conclusion
Taken together, these results provide compelling evidence that stoichiometry-selective PAMs of 3(α4)2(β2) nAChRs attenuate nicotine taking and seeking in rats and suggest that targeting 3(α4)2(β2) nAChRs may represent a promising therapeutic strategy for preventing smoking relapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With 40 million American adults (∼16.8% of the population) smoking traditional cigarettes on a regular basis and the prevalence of smoking electronic cigarettes (e-cigarettes) tripling in middle and high school students in the USA, cigarette smoking continues to be a significant public health concern (CDC CfDCaP 2015; CDCP CfDCaP 2015). The primary psychoactive compound found in tobacco and e-cigarettes is nicotine, which functions as a nonselective agonist at nicotinic acetylcholine receptors (nAChRs) (Stolerman and Jarvis 1995). There is a clear evidence that neuronal α4β2* nAChRs play a critical role in nicotine reinforcement (Changeux 2010; Picciotto et al. 1998; Tuesta et al. 2011), which supports the development of novel smoking cessation pharmacotherapies targeting this receptor population. Indeed, varenicline, currently the best-in-class treatment for smoking cessation, was developed as such based on its action as a partial agonist at α4β2* nAChRs (Coe et al. 2005). However, varenicline has a modest efficacy in promoting a long-term abstinence highlighting a need to develop more efficacious smoking cessation pharmacotherapies for nicotine dependence (Lerman et al. 2007).
Recently, we showed that systemic administration of galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator (PAM) of α4β2* nAChRs, attenuates voluntary nicotine taking and seeking in rats (Hopkins et al. 2012). These results suggest that PAMs of α4β2* nAChRs may represent potential smoking cessation medications. PAMs bind nAChRs at allosteric sites that are distinct from the orthosteric binding site for nicotine. Therefore, PAMs have a low intrinsic activity in the absence of acetylcholine or nicotine (Taly et al. 2009; Uteshev 2014). By enhancing receptor activity as well as the probability of nicotine-induced channel opening, PAMs can substantially increase and prolong α4β2* nAChR responses to nicotine from tobacco smoke (Williams et al. 2011). Based on this unique pharmacological mechanism of action, PAMs of α4β2* nAChRs may reduce the amount of nicotine consumed by enhancing the reinforcing effects of lower unit doses of nicotine—analogous to higher nicotine doses. Indeed, an emerging literature supports this hypothesis. Consistent with their mechanism of action, PAMs of α4β2* nAChRs are not reinforcing (Liu 2013) and do not produce nicotine-like discriminative stimulus effects on their own (Mohler et al. 2014). Moreover, PAMs of α4β2* nAChRs enhance the discriminative stimulus effects of nicotine (Mohler et al. 2014) and attenuate nicotine self-administration in rats (Liu 2013). Taken together, these findings suggest that PAMs of α4β2* nAChRs may reduce smoking behaviors in humans.
To our knowledge, the only study examining the effects of a PAM of α4β2* nAChRs on nicotine taking utilized desformylflustrabromine (dFBr) (Liu 2013). While dFBR selectively binds to heteromeric α4β2* nAChRs, it does not distinguish between α4β2* nAChR subtypes consisting of different ratios of α4:β2 subunits (Williams et al. 2011). α4β2* nAChRs assemble into two stoichiometrically and functionally different combinations characterized by the α4:β2 subunit ratio. The 2(α4)3(β2) and 3(α4)2(β2) nAChR subtypes represent channels with a high (EC50 = 1 μM) or low (EC50 = 100 μM) sensitivity, respectively, to acetylcholine (Moroni et al. 2006; Nelson et al. 2003). Nicotine and varenicline bind to and activate both high- and low-sensitivity populations of α4β2 nAChRs, albeit with different potencies and binding affinities (Anderson et al. 2009; Moroni et al. 2006). While the relevance of these findings to the therapeutic effects of nicotine and varenicline is unknown, it raises the intriguing possibility that compounds targeting high- or low-sensitivity α4β2 nAChRs alone may be more efficacious anti-smoking medications. Recently, PAMs that bind selectively to low-sensitivity 3(α4)2(β2) nAChRs have been identified (Grupe et al. 2013a; Timmermann et al. 2012). These compounds provide, for the first time, an opportunity to identify the precise role of 3(α4)2(β2) nAChR subtypes in nicotine addiction.
The present study investigated the effects of NS9283, a stoichiometry-selective PAM of low-sensitivity 3(α4)2(β2) nAChRs, on nicotine self-administration and the reinstatement of nicotine-seeking behavior, an animal model of smoking relapse, in rats. In addition to examining the effects of NS9283 on nicotine taking and seeking, these experiments also assessed the role of NS9283 in modulating sucrose self-administration and reinstatement in order to examine the specificity of this drug treatment in appetitive/reinforced behaviors. To assess a potential abuse liability, the ability of NS9283 to support self-administration behavior on its own was assessed in nicotine-experienced rats. We hypothesized that NS9283 administration would attenuate nicotine, but not sucrose, taking and seeking. Since NS9283 does not activate 3(α4)2(β2) nAChRs on its own (Timmermann et al. 2012) or substitute for the discriminative stimulus properties of nicotine (Mohler et al. 2014), we also hypothesized that rats would not self-administer NS9283.
Materials and methods
Animals and housing
Male Sprague Dawley rats (Rattus norvegicus) weighing 225–250 g were obtained from Taconic laboratories (Germantown, NY, USA). All rats were mildly food restricted (20–25 g chow daily) to ∼90% of their free-feeding body weight following a recovery from surgery. Water was available ad libitum in the home cage. Rats were housed in a colony maintained on a 12-h/12-h reverse light/dark cycle, with lights off at 7:00 a.m. All behavioral tests were conducted during the dark phase. All experimental protocols were in accordance with the guidelines set forth by the National Institutes of Health and were approved by the University of Pennsylvania School of Medicine Institutional Animal Care and Use Committee.
Materials
All self-administration experiments were conducted in ventilated, sound-attenuating operant conditioning chambers purchased from Med-Associates Inc. (East Fairfield, VT, USA). Each operant conditioning chamber was equipped with both active and inactive response levers, a sucrose pellet dispenser, cue lights, tone generator, as well as an automated injection pump for administering drug or vehicle solutions intravenously.
Surgery
Rats were handled daily and allowed 1 week to acclimate to their home cages upon arrival. Prior to surgery, rats were anesthetized with 80 mg/kg ketamine (Midwest Veterinary Supply, Valley Forge, PA, USA) and 12 mg/kg xylazine (Sigma Aldrich/RBI, St. Louis, MO, USA). For rats self-administering nicotine, an indwelling silastic catheter (SAI Infusion Technologies, Lake Villa, IL, USA) was inserted into the right external jugular vein and sutured securely in place. The catheter was connected to a mesh backmount that was implanted subcutaneously above the shoulder blades. To prevent infection and maintain patency, catheters were flushed daily with 0.2 ml of a solution of the antibiotic Timentin (0.93 mg/ml; Fisher, Pittsburgh, PA, USA) dissolved in heparinized 0.9% saline (Butler Schein, Dublin, OH, USA). When not in use, catheters were sealed with plastic obturators.
Nicotine self-administration
Rats were allowed 7 days to recover from the surgery before behavioral testing commenced. Nicotine self-administration was performed as described previously (Hopkins et al. 2012; Kimmey et al. 2012; Lee et al. 2014). Initially, rats were placed in operant conditioning chambers and allowed to lever press for intravenous nicotine (0.03 mg/kg nicotine/59 μl saline, infused over 5 s) on a fixed-ratio 1 (FR1) schedule of reinforcement. Each nicotine infusion was paired with a contingent 10-s light/tone cue. The light cue was illuminated directly above the active lever (i.e., drug-paired lever). Stable responding on the FR schedules of reinforcement was defined as < 20% variation in total active lever responses over three consecutive self-administration days. After stable responding on FR1 was achieved (∼8–10 days), the schedule of reinforcement was increased to FR3 for 3–5 days and then finally increased to an FR5 schedule. Rats continued to respond for nicotine on an FR5 schedule for ∼14 more days prior to behavioral testing (i.e., a total of 28 days of nicotine self-administration). A 20-s timeout period followed each nicotine infusion, during which time the chamber light was turned off, and active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during the operant sessions and were used as a measure of nonspecific behavioral activation. All nicotine self-administration sessions were 2 h in duration. Rats that failed to acquire nicotine self-administration or respond stably on FR5 were excluded from further behavioral testing.
Sucrose self-administration
Reinforcer-specificity and potential nonspecific rate-suppressing effects of NS9283 were evaluated by assessing the influence of NS9283 on sucrose taking and seeking in separate cohorts of rats. Initially, rats were trained to lever press for 45 mg sucrose pellets (Research Diets, Inc., New Brunswick, NJ, USA) on an FR1 schedule of reinforcement. Once animals achieved stable responding for sucrose (defined as < 20% variation in responding over three consecutive sessions) on the FR1 schedule of reinforcement, the response requirement was increased to an FR5 schedule of reinforcement. Animals were limited to 30 sucrose pellets during each daily one-hour operant session. Subjects were mildly food restricted in order to maintain consistency with the nicotine self-administration experiments (i.e., to ensure similar motivational states). Each successful completion of the response requirement resulted in delivery of a sucrose pellet as well as contingent presentation of light/tone cues. A total of 14–16 daily sucrose self-administration sessions were conducted prior to behavioral testing.
Experiment 1: effects of acute NS9283 on nicotine and sucrose self-administration behaviors
The effects of acute NS9283 administration were examined in rats that acquired stable nicotine or sucrose self-administration on an FR5 schedule of reinforcement. A between-session, within-subject design was used to test the effects of NS9283 on voluntary nicotine- and sucrose-taking behaviors. Each test day was separated by at least 2 days of nicotine or sucrose self-administration to ensure that nicotine or sucrose taking had stabilized between test sessions. In all experiments, rats were pretreated with NS9283 (0, 0.35, and 3.5 mg/kg, i.p.) 20 min prior to the beginning of the operant session. All doses of NS9283 were counterbalanced and tested in each rat to avoid rank order effects of drug pretreatment. Doses and time course of administration were selected based on previous in vivo rat studies demonstrating behavioral and neurochemical responses following systemic NS9283 administration (Grupe et al. 2013b; Mohler et al. 2014; Rode et al. 2012; Timmermann et al. 2012).
A separate cohort of rats was used to study the effects of NS9283 on nicotine self-administration maintained on a progressive ratio (PR) schedule of reinforcement. Initially, rats were allowed to self-administer nicotine (0.03 mg/kg/infusion) on an FR5 schedule as described above. Once rats achieved stable responding on the FR5 schedule, they were switched to a PR schedule of reinforcement. Under a PR schedule, the response requirement for each subsequent drug delivery increases until the subject fails to meet a requirement. In the current experiments, the response requirement for the ith reinforcement was given by R(i) = [5e0.2i-5] and the session expired when an animal took more than 30 min to receive an injection similar to our previous studies (Hopkins et al. 2012; Schmidt et al. 2016). The breakpoint was operationally defined as the last response requirement completed before the termination of the PR test session. PR schedules of reinforcement are commonly used to assess the reinforcing efficacy of drugs and natural rewards (Hodos 1961). With regard to the present study, lower breakpoints reflect decreased reinforcing efficacy of nicotine (Richardson and Roberts 1996). The effects of NS9283 administration nicotine self-administration were evaluated using a within-subject design. Rats were pretreated with vehicle or 3.5 mg/kg NS9283 (i.p.) 20 min prior to a PR test session. Once all doses were tested, rats were allowed to self-administer a higher unit dose of nicotine (0.06 mg/kg/infusion) on an FR5 schedule. Once rats achieved stable responding for the new unit dose of nicotine, they were switched to a PR schedule. The effects of vehicle and 3.5 mg/kg NS9283 (i.p.) pretreatment on self-administration of nicotine (0.06 mg/kg/infusion) were then tested. Vehicle and NS9283 infusions were counterbalanced across test sessions.
Experiment 2: effects of repeated NS9283 on nicotine and sucrose self-administration behaviors
The effects of repeated NS9283 administration on nicotine and sucrose taking were investigated in a separate cohort of rats that acquired stable nicotine or sucrose self-administration on an FR5 schedule of reinforcement. Rats were divided into different treatment groups and a between-subject design was used to test the effects of repeated NS9283 on voluntary nicotine and sucrose taking. Rats were pretreated with NS9283 (0, 0.35, and 3.5 mg/kg, i.p.) 20 min prior to the beginning of the operant test sessions. Rats were pretreated with NS9283 daily for 10 consecutive days similar to the previous studies of repeated drug administration on nicotine self-administration in rats (Ashare et al. 2016; Levin et al. 2011).
Experiment 3: effects of acute NS9283 on the reinstatement of nicotine- and sucrose-seeking behaviors
The effects of NS9283 on nicotine and sucrose seeking were studied in separate cohorts of rats that were not previously tested with NS9283 during the self-administration phase of the experiment. Following ∼28 daily nicotine self-administration sessions, drug-taking behavior was extinguished by replacing the nicotine solution with 0.9% saline. Light/tone cues that were paired with nicotine infusions during the self-administration phase were turned off. A daily 2-hour extinction sessions continued until responding on the active lever was < 20% of the total active lever responses completed during the last nicotine self-administration session. Typically, it took 3–7 days for rats to meet this criterion. Once nicotine self-administration was extinguished, the ability of an acute priming injection of nicotine (0.2 mg/kg, s.c.) plus light/tone cues to reinstate nicotine seeking was assessed. Satisfaction of the response requirement (i.e., every five active lever responses) resulted in a saline infusion along with contingent presentation of the light/tone cues that were previously paired with nicotine infusions during the self-administration phase of the experiment. On subsequent reinstatement test days, NS9283 (0, 0.35, and 3.5 mg/kg, i.p.) was administered 20 min prior to an acute priming injection of nicotine. Rats were placed immediately into the operant conditioning chambers following the priming injection of nicotine and a 2-hour reinstatement test session began. Using a between-session design, each reinstatement test session was followed by extinction days until responding was < 20% of the maximum number of responses maintained by nicotine self-administration.
The effect of NS9283 on the reinstatement of sucrose-seeking behavior was examined in a separate cohort of rats. Sucrose taking was extinguished by inactivating the sucrose pellet dispenser and turning off the light/tone cues that were paired with sucrose delivery. Once active lever responding decreased to < 20% of the maximum number of response completed during sucrose self-administration, rats proceeded to reinstatement testing. During the reinstatement test sessions, rats were allowed to respond for light/tone cues that were previously paired with sucrose pellet delivery during the self-administration phase. Rats were pretreated with vehicle or 3.5 mg/kg NS9283 (i.p.) 20 min prior to the beginning of the reinstatement session. The experimenter remotely administered one sucrose pellet along with a 10-s noncontingent presentation of the light/tone cue every 2 min for the first 10 min of the reinstatement session. A between-session design was used such that each daily reinstatement test session was followed by extinction sessions on subsequent days until responding was again < 20% of the total active lever responses maintained by sucrose. A within-subject design was used for all reinstatement tests and doses of NS9283 were counterbalanced across test sessions.
Experiment 4: potential abuse liability of NS9283
The reinforcing efficacy of NS9283 was assessed into two separate experiments. Rats that stably self-administered nicotine on an FR5 schedule of reinforcement were randomly divided into separate treatment groups and a between-subject design was used to investigate the ability of NS9283 or a vehicle to support self-administration behavior. In the first experiment, nicotine was replaced with a vehicle, 0.125 or 1.25 μg/kg/infusion NS9283. In the second experiment, nicotine was replaced with vehicle or 2.5 μg/kg/infusion NS2983. Solubility limitations prevented testing NS9283 doses higher than 2.5 μg/kg/infusion. NS9283 doses were based on previous reports demonstrating that systemic NS9283 administration potentiates nicotine-evoked glutamate release in the brain (Grupe et al. 2013b) and acetylcholine-evoked peak current amplitude (Timmermann et al. 2012). Light/tone cues that were previously paired with nicotine delivery were turned off during the substitution phase. Substitution tests lasted for five consecutive sessions. Contingent light/tone cues become conditioned stimuli that are reinforcing in their own right when paired with nicotine (Chaudhri et al. 2006). To determine if rats limited their responding due to NS9283 substitution alone and not omission of the cues, a control group was allowed to continue self-administer nicotine in the absence of cues.
Drugs
(−) Nicotine hydrogen tartrate salt (Sigma Aldrich, St. Louis, MO, USA) was dissolved in a sterile 0.9% saline (pH was adjusted to 7.4 ± 0.5 with sodium hydroxide). Nicotine doses are reported as free base concentrations. NS9283 [3-(3-Pyridinyl)-1,2,4-oxadiazol-5-yl benzonitrile] was synthesized and gifted by Saniona (Ballerup, Denmark). NS9283 was dissolved in a sterile 0.9% saline containing 35% hydroxyproyl beta-cyclodextrin (MP Biomedicals, Fountain Pkwy, Solon, OH).
Statistical analyses
Data are presented as means and standard errors of the mean (S.E.M.). Paired t tests were used to analyze PR data. For all other experiments, total active lever responses, inactive lever responses, and infusions were analyzed with separate two-way repeated measure analyses of variances (ANOVAs). All pairwise comparisons were made with Bonferroni post hoc tests following two-way ANOVAs (p < 0.05).
Results
Acute NS9283 administration dose-dependently attenuated nicotine, but not sucrose, self-administration in rats.
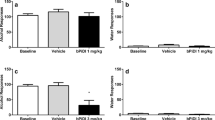
Total lever response for rats self-administering nicotine on an FR5 schedule of reinforcement are shown in Fig. 1a (n = 11/treatment). These data were analyzed with a two-way repeated measure ANOVA, which revealed a significant main effect of treatment [F(2,40) = 51.94, p < 0.0001] and lever [F(1,20) = 62.24, p < 0.0001] as well as significant treatment × lever interaction [F(2,40) = 38.58, p < 0.0001]. Subsequent pairwise analyses showed that the total active lever responses were significantly different between rats pretreated with vehicle and 3.5 mg/kg NS9283 (Bonferroni, p < 0.05). Total nicotine infusions are shown in Fig. 1b. These data were analyzed with a one-way repeated measures ANOVA, which revealed a significant main effect of treatment [F(2,20) = 68.16, p < 0.0001]. Subsequent pairwise analyses showed that total nicotine infusions were significantly different between rats pretreated with vehicle and 3.5 mg/kg NS9283 (Bonferroni, p < 0.05). The behaviorally relevant dose of NS9283 (i.e., 3.5 mg/kg) that attenuated nicotine self-administration had no effect on sucrose taking (Fig. 1c). Total lever responses were analyzed with a two-way repeated measure ANOVA in rats that were pretreated with NS9283 (n = 11/treatment). No significant differences in lever responding were found between treatments.
Acute administration of NS9283 dose-dependently attenuated nicotine, but not sucrose, self-administration in rats. Total number of responses (mean ± S.E.M.) on the active and inactive levers (a) and total nicotine infusions (b) in animals pretreated with 0, 0.35, or 3.5 mg/kg NS9283 during nicotine self-administration test sessions (n = 11/treatment). The asterisks represent a significant decrease in active lever responding in rats treated with 3.5 mg/kg NS9283 when compared to vehicle-treated controls (Bonferroni, p < 0.05). c Total number of lever responses (mean ± S.E.M.) in rats self-administering sucrose pellets on an FR5 schedule of reinforcement. No significant differences in responding were noted between subjects pretreated with vehicle or 3.5 mg/kg NS9283 (n = 11/treatment)
Total active lever responses, total infusions, and breakpoints (mean ± S.E.M.) for rats self-administering nicotine on a PR schedule of reinforcement are shown in Fig. 2 (n = 12/treatment). NS9283 pretreatment significantly reduced total active lever responses [t(11) = 3.18, p < 0.01], total infusions [t(11) = 3.42, p < 0.01], and breakpoints [t(11) = 4.38, p < 0.01] in rats self-administering 0.03 mg/kg nicotine. Consistent with these findings, NS9283 pretreatment also reduced total active lever responses [t(11) = 2.47, p < 0.05], total infusions [t(11) = 2.37, p < 0.05], and breakpoints [t(11) = 2.61, p < 0.05] in rats self-administering 0.06 mg/kg nicotine. No significant differences in inactive lever responding were noted between treatments in rats responding for 0.03 or 0.06 mg/kg nicotine on a PR schedule of reinforcement (data not shown).
Acute administration of NS9283 decreased nicotine self-administration maintained on a PR schedule of reinforcement. Total number of active lever responses (a), total nicotine infusions (b), and breakpoints (c) were significantly attenuated in rats pretreated with 3.5 mg/kg NS9283 when compared to vehicle-treated controls during PR test sessions in which 0.03 and 0.06 mg/kg/infusion nicotine was available for self-administration (n = 12/treatment; *p < 0.05; **p < 0.01)
Repeated NS9283 administration dose-dependently attenuated nicotine, but not sucrose, self-administration in rats
Rats that maintained stable responding for nicotine were assigned to one of the three treatment groups. Rats that were designated to receive vehicle (n = 12), 0.35 mg/kg NS9283 (n = 9), or 3.5 mg/kg NS9283 (n = 5) self-administered the same number of nicotine infusions (mean ± S.E.M.) 3 days prior to the treatment phase of the experiment: 21.47 ± 1.65, 19.41 ± 1.55, and 18.67 ± 2.73, respectively (Fig. 3b, day 0). The total active lever responses and the total nicotine infusions (mean ± S.E.M.) for rats pretreated with vehicle or NS9283 daily for 10 consecutive days are shown in Fig. 3a, b, respectively (days 1–10). Analysis of the total active lever data revealed significant main effects of treatment [F(2,25) = 8.36, p < 0.01]. Analysis of the total nicotine infusions revealed significant main effects of treatment [F(2,25) = 9.22, p < 0.01]. Subsequent pairwise analyses showed that the total active lever responses and the total nicotine infusions were significantly decreased in rats pretreated with 3.5 mg/kg NS9283 when compared to vehicle-treated controls for each day of the 10-day treatment phase (Bonferroni, p < 0.05). Post hoc analyses also showed that the total active lever responses and the total nicotine infusions were significantly decreased in rats pretreated with 3.5 mg/kg NS9283 when compared to 0.35 mg/kg NS9283 on days 5, 6, 8, and 10 of treatment (Bonferroni, p < 0.05). There was also a significant main effect of treatment [F(2,25) = 4.55, p < 0.05] on the total inactive lever responses (Fig. 3c). Pairwise analyses showed that inactive lever responses were significantly increased in rats pretreated with vehicle or 0.35 mg/kg NS9283 when compared to 3.5 mg/kg NS9283 on days 8 and 9 of treatment (Bonferroni, p < 0.05). Separate cohorts of rats were pretreated with vehicle or 3.5 mg/kg NS9283 (n = 6/treatment) daily prior to 10 consecutive days of sucrose self-administration (Fig. 3d). Analysis of these data showed no effect of treatment on sucrose taking.
Repeated administration of NS9283 dose-dependently attenuated nicotine, but not sucrose, self-administration in rats. Daily 3.5 mg/kg NS9283 pretreatment (n = 5) significantly attenuated total active lever responses (a) and total nicotine infusions (b) in rats stably self-administering nicotine on an FR5 schedule of reinforcement when compared to vehicle-treated controls (n = 12) over 10 consecutive days (*p < 0.05; # p < 0.01) between vehicle and 3.5 mg/kg NS9283 treatments. c Total inactive responses were significantly increased on days 8 and 9 of vehicle and 0.35 mg/kg NS9283 administration when compared to 3.5 mg/kg NS9283 treatments (*p < 0.05). d There were no effects of repeated NS9283 administration on sucrose self-administration (n = 6/treatment, p > 0.05)
Administration of NS9283 dose-dependently attenuated the reinstatement of nicotine, but not sucrose, seeking behavior in rats
Total lever responses following systemic administration of NS9283 prior to the nicotine reinstatement test session are shown in Fig. 4a (n = 10/treatment). These data were analyzed with a two-way repeated measure ANOVA which revealed significant main effects of treatment [F(2,36) = 11.08, p < 0.001] and lever [F(1,18) = 34.22, p < 0.0001] as well as a significant treatment × lever interaction [F(2,36) = 5.34, p < 0.01]. Subsequent post hoc analysis showed a significant decreased in active lever responding in rats pretreated with 3.5 mg/kg NS9283 compared to vehicle-treated controls and rats pretreated with 0.35 mg/kg NS9283 (Bonferroni, p < 0.05). The effects of systemic NS9283 administration on sucrose reinstatement are shown in Fig. 4b (n = 8/treatment). There were no effects of NS9283 pretreatment on sucrose seeking.
Systemic administration of NS9283 dose-dependently attenuated the reinstatement of nicotine, but not sucrose, seeking behavior in rats. a Total number of responses on the active and inactive levers in rats pretreated with 0, 0.35, or 3.5 mg/kg NS9283 (n = 10/treatment). NS9283 dose-dependently attenuated drug seeking induced by an acute priming injection of nicotine and cues previously associated with nicotine self-administration. The asterisk represents a significant decrease in responding on the active lever in rats pretreated with 3.5 mg/kg NS9283 when compared to vehicle-treated controls and rats pretreated with 0.35 mg/kg NS9283 (*p < 0.05). No significant differences in inactive lever responding were noted between treatments. b Total number of lever responses completed during sucrose reinstatement test sessions in rats pretreated with vehicle or 3.5 mg/kg NS9283 (n = 8/treatment). No differences in lever responses were noted
Substitution of nicotine for NS9283 did not support self-administration behavior in rats
Rats stably self-administering nicotine were randomly divided into multiple groups. Total active lever responses (Fig. 5a, d, g), total nicotine infusions (Fig. 5b, e, h), and total inactive lever responses (Fig. 5c, f, i) did not differ between these groups over the last 3 days of nicotine self-administration (days −3, −2, and −1, respectively). In one experiment, rats were then allowed to self-administer vehicle (n = 5), 0.125 (n = 6), or 1.25 (n = 6) μg/kg/infusion NS9283 for five consecutive days (Fig. 5d–i, days 1–5). In the second experiment, rats were allowed to self-administer vehicle (n = 5) or 2.5 μg/kg/infusion NS9283 (n = 6) for five consecutive days (Fig. 5a–c, days 1–5). A control group (n = 6) was allowed to continue self-administering nicotine in the absence of the light/tone cues to determine the magnitude of responding due to nicotine alone versus the conditioned stimuli. There were no effects of vehicle or NS9283 substitution on total active lever responses (Fig. 5a, d, g), total nicotine infusions (Fig. 5b, e, h), and total inactive lever responses (Fig. 5c, f, i). In contrast, significant main effects of treatment were noted between the nicotine control group and vehicle or NS9283 substitution for total active lever responses (Fig. 5a, d, g) and total infusions (Fig. 5b, e, h), but not total inactive lever responses (Fig. 5c, f, i). These results clearly indicate that NS9283 alone does not support self-administration behavior in rats and suggests no potential abuse liability of NS9283 in human clinical trials.
NS9283 did not support self-administration behavior in rats. Total active lever responses (a, d, g), infusions (b, e, h), and inactive lever responses (c, f, i) after substitution of nicotine for vehicle or NS9283. A control group that continued to self-administer nicotine in the absence of the light/tone cues was also run (0.03 mg/kg/infusion nicotine w/o cues). No significant differences in any measure were noted between rats given access to vehicle (n = 5), 0.125 (n = 6), or 1.25 (n = 6) μg/kg/infusion NS9283 in experiment one. No significant differences in any measure were noted between rats given access to vehicle (n = 5) or 2.5 (n = 6) μg/kg/infusion NS9283 in experiment two. The asterisks represent significant decreases in active lever responding and total infusions between the substitution groups (i.e., vehicle or NS9283) and the group that continued to self-administer nicotine in the absence of cues (*p < 0.05)
Discussion
To the best of our knowledge, the present study is the first to investigate the efficacy of NS9283, a PAM of low-sensitivity 3(α4)2(β2) nAChRs, in rodent models of nicotine-taking and -seeking behaviors. Here, we show that acute NS9283 administration dose-dependently attenuated nicotine self-administration and reinstatement in rats. Consistent with these effects, repeated NS2983 administration reduced daily nicotine consumption over 10 consecutive days. In contrast, acute and repeated NS9283 administration had no effect on sucrose taking or seeking suggesting that the effects of NS9283 were reinforcer specific and not due to general motor impairments. NS9283 also failed to support self-administration behavior indicating that the abuse liability of this compound in humans is low (O’Connor et al. 2011). Taken together, these findings indicate a novel role for low-sensitivity 3(α4)2(β2) nAChR subtypes in voluntary nicotine-taking and -seeking behaviors and support therapeutic strategies aimed at developing stoichiometry-selective PAMs of 3(α4)2(β2) nAChRs for smoking cessation.
The current findings contribute to and expand upon a large literature demonstrating a critical role of α4β2* nAChRs in nicotine addiction. Here, we show that administration of NS9283, a stoichiometry-selective PAM of 3(α4)2(β2) nAChRs, is sufficient to attenuate nicotine taking and seeking in rats. These results are the first to identify a role for low-sensitivity 3(α4)2(β2) nAChRs in preclinical models of nicotine addiction and are consistent with a previous study demonstrating that acute administration of dFBr, a nonselective PAM of α4β2* nAChRs, reduces nicotine, but not sucrose, self-administration in rats (Liu 2013). The exact behavioral mechanisms by which NS9283 reduces nicotine taking and seeking are unclear. NS9283 readily crosses the blood-brain barrier following systemic administration and potentiates nicotine-induced 3(α4)2(β2) nAChR signaling (Timmermann et al. 2012). Taken together with the present study, these findings suggest that decreased nicotine self-administration and reinstatement in rats pretreated with NS9283 may reflect an increase in the reinforcing efficacy of nicotine. This hypothesis is supported by a recent study demonstrating that NS9283 increases the nicotine-like discriminative stimulus effects of subthreshold doses of nicotine (Mohler et al. 2014).
NS9283 has also been shown to bind to α2- and β4-containing nAChRs in vitro (Timmermann et al. 2012; Wang et al. 2015). Although we cannot exclude the contributions of α2- and β4-containing nAChR subtypes to our findings, their limited expression in the rodent brain strongly suggest that these contributions are minimal (Ishii et al. 2005; Winzer-Serhan and Leslie 1997). Interestingly, α2-containing nAChRs are expressed at higher levels in the primate versus rodent brain (Han et al. 2000; Wada et al. 1989). This raises the intriguing possibility that drugs targeting α2-containing nAChR receptor subtypes, including NS9283, may be more efficacious in humans. Further studies are required to determine whether the effects of NS9283 on nicotine reinforcement are due to positive allosteric modulation of low-sensitivity 3(α4)2(β2) nAChRs alone or in combination with α2-containing nAChRs.
Systemic NS9283 administration in vivo potentiates nicotine-evoked glutamate release in the rat medial prefrontal cortex (mPFC) (Grupe et al. 2013b). In contrast, NS9283 does not potentiate nicotine-evoked dopamine release from striatal minces in vitro (Rode et al. 2012). These findings are consistent with studies showing that only high-sensitivity 2(α4)3(β2), but not low-sensitivity 3(α4)2(β2), nAChRs are expressed in the striatum (Anderson et al. 2009). Thus, it is likely that the effects of NS9283 on nicotine self-administration and reinstatement are mediated by glutamatergic mechanisms in the mPFC and not dopamine signaling in the striatum. This hypothesis is supported by studies demonstrating that glutamate signaling in the mPFC is altered by nicotine self-administration (Wang et al. 2008) and that the mPFC plays an important role in nicotine seeking (Lubbers et al. 2014). In order to more fully realize the neurochemical mechanisms underlying the effects of systemic NS9283 on nicotine taking and seeking, future studies should aim to identify the neurocircuits mediating these behavioral responses as well as the cellular phenotypes that express low-sensitivity 3(α4)2(β2) nAChRs, and whether these receptors are located on presynaptic or postsynaptic sites.
Recent strategies have focused on reversing or normalizing nicotine withdrawal-induced cognitive deficits in order to reduce smoking relapse rates (Ashare and Schmidt 2014). Systemic administration of NS9283 increases cognitive performance in drug-naïve rats suggesting that PAMs of low-sensitivity 3(α4)2(β2) nAChRs may increase cognitive performance during nicotine withdrawal (Timmermann et al. 2012). While our study clearly indicates that NS9283 reduces nicotine seeking in abstinent rats, it does not assess the effects of NS9283 on nicotine withdrawal-induced cognitive deficits. It will be interesting to note in future studies whether NS9283 has beneficial effects on cognition during nicotine withdrawal and whether these cognitive-enhancing properties promote abstinence in treatment-seeking smokers.
The present findings indicate that acute and repeated NS9283 administration attenuates voluntary nicotine taking and seeking in rats. These are the first data demonstrating that a stoichiometry-selective PAM of 3(α4)2(β2) nAChRs can modulate addiction-like behaviors in rats and support the hypothesis that PAMs targeting low-sensitivity 3(α4)2(β2) nAChRs may reduce chronic smoking behavior and relapse in humans. NS9283 alone does not support self-administration behavior (present study) or produce subjective effects similar to nicotine (Mohler et al. 2014) indicating no potential abuse liability in humans. While it will be important to determine the efficacy of NS9283 and other PAMs of low-sensitivity 3(α4)2(β2) nAChRs in human smokers, future clinical studies should also examine the therapeutic benefit of these PAMs in combination with agonists of α4β2* nAChRs. Co-administration of NS9283 and a subthreshold dose of an α4β2* nAChR agonist produces nicotine-like discriminative stimulus-like effects in rats suggesting that lower doses of α4β2* nAChR agonists could be used to treat smoking relapse (Mohler et al. 2014). In summary, our results identify a novel role of low-sensitivity 3(α4)2(β2) nAChRs in nicotine addiction and support further studies of stoichiometry-selective PAMs of nAChRs for smoking cessation.
References
Anderson DJ, Malysz J, Gronlien JH, El Kouhen R, Hakerud M, Wetterstrand C, Briggs CA, Gopalakrishnan M (2009) Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combination. Biochem Pharmacol 78:844–851
Ashare RL, Schmidt HD (2014) Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opin Drug Discov 9:579–594
Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, Schmidt HD (2016) Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Transl Psychiatry 6:e713
CDC CfDCaP (2015) Tobacco use among middle and high school students—United States, 2011–2014. Morb Mortal Wkly Rep 64:381–385
CDCP CfDCaP (2015) Current cigarette smoking among adults—United States, 2005–2014. Morb Mortal Wkly Rep 64:1244–1240
Changeux JP (2010) Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11:389–401
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology 184:353–366
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD 3rd, O’Neill BT (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477
Grupe M, Jensen AA, Ahring PK, Christensen JK, Grunnet M (2013a) Unravelling the mechanism of action of NS9283, a positive allosteric modulator of (alpha4)3(beta2)2 nicotinic ACh receptors. Br J Pharmacol 168:2000–2010
Grupe M, Paolone G, Jensen AA, Sandager-Nielsen K, Sarter M, Grunnet M (2013b) Selective potentiation of (alpha4)3(beta2)2 nicotinic acetylcholine receptors augments amplitudes of prefrontal acetylcholine- and nicotine-evoked glutamatergic transients in rats. Biochem Pharmacol 86:1487–1496
Han ZY, Le Novere N, Zoli M, Hill JA Jr, Champtiaux N, Changeux JP (2000) Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci 12:3664–3674
Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134:943–944
Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD (2012) Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37:2310–2321
Ishii K, Wong JK, Sumikawa K (2005) Comparison of alpha2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J Comp Neurol 493:241–260
Kimmey BA, Rupprecht LE, Hayes MR, Schmidt HD (2012) Donepezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats. Addiction biology.
Lee AM, Arreola AC, Kimmey BA, Schmidt HD (2014) Administration of the nicotinic acetylcholine receptor agonists ABT-089 and ABT-107 attenuates the reinstatement of nicotine-seeking behavior in rats. Behav Brain Res 274:168–175
Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA (2007) Translational research in medication development for nicotine dependence. Nat Rev Drug Discov 6:746–762
Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE (2011) Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther 338:890–896
Liu X (2013) Positive allosteric modulation of alpha4beta2 nicotinic acetylcholine receptors as a new approach to smoking reduction: evidence from a rat model of nicotine self-administration. Psychopharmacology 230:203–213
Lubbers BR, van Mourik Y, Schetters D, Smit AB, De Vries TJ, Spijker S (2014) Prefrontal gamma-aminobutyric acid type a receptor insertion controls cue-induced relapse to nicotine seeking. Biol Psychiatry 76:750–758
Mohler EG, Franklin SR, Rueter LE (2014) Discriminative-stimulus effects of NS9283, a nicotinic alpha4beta2* positive allosteric modulator, in nicotine-discriminating rats. Psychopharmacology 231:67–74
Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I (2006) alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70:755–768
Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J (2003) Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63:332–341
O’Connor EC, Chapman K, Butler P, Mead AN (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912–938
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Rode F, Munro G, Holst D, Nielsen EO, Troelsen KB, Timmermann DB, Ronn LC, Grunnet M (2012) Positive allosteric modulation of alpha4beta2 nAChR agonist induced behaviour. Brain Res 1458:67–75
Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR (2016) Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41:1917–1928
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology 117:2–10 discussion 14-20
Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8:733–750
Timmermann DB, Sandager-Nielsen K, Dyhring T, Smith M, Jacobsen AM, Nielsen EO, Grunnet M, Christensen JK, Peters D, Kohlhaas K, Olsen GM, Ahring PK (2012) Augmentation of cognitive function by NS9283, a stoichiometry-dependent positive allosteric modulator of alpha2- and alpha4-containing nicotinic acetylcholine receptors. Br J Pharmacol 167:164–182
Tuesta LM, Fowler CD, Kenny PJ (2011) Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol 82:984–995
Uteshev VV (2014) The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol 727:181–185
Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW (1989) Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol 284:314–335
Wang F, Chen H, Sharp BM (2008) Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. J Neurochem 106:943–956
Wang J, Kuryatov A, Sriram A, Jin Z, Kamenecka TM, Kenny PJ, Lindstrom J (2015) An accessory agonist binding site promotes activation of alpha4beta2* nicotinic acetylcholine receptors. J Biol Chem 290:13907–13918
Williams DK, Wang J, Papke RL (2011) Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 82:915–930
Winzer-Serhan UH, Leslie FM (1997) Codistribution of nicotinic acetylcholine receptor subunit alpha3 and beta4 mRNAs during rat brain development. J Comp Neurol 386:540–554
Acknowledgements
HDS was supported in part by a K01 training grant (DA030445), an R01 (DA037897) and an R21 (DA039393) from the National Institutes of Health (NIH), a pilot grant (P50-CA-143187) from the Center for Interdisciplinary Research on Nicotine Addiction (CIRNA) at UPenn, and an Institutional Research Grant (IRG-78-002-31) from the American Cancer Society and the Abramson Cancer Center at UPenn. The authors would like to thank Aniona for generously providing NS9283 for these studies. The authors also extend a note of gratitude to Adrian Arreola and Duncan Van Nest for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contribution
HDS was responsible for the study concept and design, supervised and contributed to the acquisition of data, analyzed the data, and drafted the manuscript. JJM contributed to the acquisition of animal data, analyzed the data, and helped draft the manuscript. KS-N also provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
Rights and permissions
About this article
Cite this article
Maurer, J.J., Sandager-Nielsen, K. & Schmidt, H.D. Attenuation of nicotine taking and seeking in rats by the stoichiometry-selective alpha4beta2 nicotinic acetylcholine receptor positive allosteric modulator NS9283. Psychopharmacology 234, 475–484 (2017). https://doi.org/10.1007/s00213-016-4475-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4475-7