Abstract
Rationale
Numerous case reports have suggested that aripiprazole can worsen psychotic symptoms in schizophrenia.
Objectives
We reviewed reported cases which have suggested that aripiprazole can worsen psychotic symptoms in schizophrenia and evaluated each regarding quality of the causal relationship.
Methods
A systematic literature search was conducted on August 18, 2012, using the PubMed and the EMBASE. Twenty-two cases met the following inclusion criteria: (1) diagnosis of schizophrenia or schizoaffective disorder, (2) worsening of psychotic symptoms associated with aripiprazole, and (3) aripiprazole dose ≤30 mg/day. Information about the causal relationship between aripiprazole and increased psychotic symptoms was extracted. The quality of the causal relationship was evaluated according to the modified guidelines for evaluation of drug-associated events and classified as “questionable,” “moderately suggestive,” or “highly suggestive.”
Results
Patients were chronic in at least 15 cases, and prior antipsychotic dose exceeded recommended guidelines in 19 cases. Psychotic symptoms worsened after simply adding aripiprazole to the current regimen in eight cases. Besides psychotic symptoms, increasing agitation (nine cases), aggression (11 cases), and/or activation (seven cases) were reported. Clinical resolution occurred after aripiprazole discontinuation in eight cases. Regarding causal relationship, 11 cases were classified as “highly suggestive,” three as “moderately suggestive,” and eight as “questionable”.
Conclusions
Clinicians should be vigilant when adding aripiprazole to patients with chronic schizophrenia also receiving relatively high doses of other antipsychotics, and discontinuation of aripiprazole should be considered if psychotic symptoms and/or agitation/aggression/activation increase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a partial dopamine agonist, aripiprazole is pharmacologically unique amongst existing antipsychotic drugs (Burris et al. 2002). It is postulated that this bestows benefits from the standpoint of side effects related to dopamine blockade, including extrapyramidal symptoms (EPS) and hyperprolactinemia in patients with schizophrenia (Marder et al. 2003). Aripiprazole also has a favorable profile in terms of weight gain and associated metabolic disturbances (e.g., disturbances in lipids and glucose) (Marder et al. 2003).
In view of its unique pharmacological and clinical profile, randomized controlled trials have been conducted to investigate the impact of adding, or switching to, aripiprazole to address problems such as hyperprolactinemia (Shim et al. 2007) or metabolic side effects in patients with schizophrenia (Fleischhacker et al. 2010; Newcomer et al. 2008; Stroup et al. 2011). While these studies have reported benefits with such a strategy, there have also been reports of worsening of psychotic symptoms in patients with schizophrenia, for example after adding aripiprazole compared to placebo (Fleischhacker et al. 2010), or switching to aripiprazole versus (L69) continuing olanzapine (Newcomer et al. 2008). The possibility that aripiprazole may worsen psychotic symptoms in patients with schizophrenia dovetails with a body of research suggesting such an association for agents that increase dopaminergic activity (Jaskiw and Popli 2004; Lieberman et al. 1987).
On the other hand, these clinical trials did not provide detailed clinical information on individual patients who experienced a worsening of psychotic symptoms, and thus cannot allow for conclusions regarding aripiprazole and a causal relationship. To date, there have been a number of case reports of psychotic symptoms worsened by aripiprazole in patients with schizophrenia since the first such case was reported in 2004 (Ramaswamy et al. 2004). Contrary to the clinical trials, these cases supply enough information to not only evaluate the quality of the causal relationship between aripiprazole and worsening of psychotic symptoms but also identify the potential risk factors of this phenomenon. Based on these objectives, we carried out a systematic review of reported cases.
Method
A literature search was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement (Moher et al. 2009) on August 18, 2012, in the PubMed and the EMBASE using the following search terms: “aripiprazole AND schizophreni*”. Our literature search was limited to “case reports,” “humans,” and “English” in the PubMed, and “letters,” “human,” and “English language” in the EMBASE. The reference lists of the relevant reports were also examined.
Inclusion criteria were as follows: (1) diagnosis of schizophrenia or schizoaffective disorder; (2) worsening of psychotic symptoms in conjunction with aripiprazole, including hallucinations, delusions, disorganization, and/or bizarre behavior; and (3) aripiprazole ≤30 mg/day (i.e., maximum recommended therapeutic dose).
Each of the cases meeting inclusion criteria were screened for the following information: age and gender, diagnosis, onset or history of illness/treatment, rationale for initiating aripiprazole, previous antipsychotic medication(s)/dose(s), concomitant psychotropic medication(s)/dose(s), switching strategies to aripiprazole until psychotic worsening commences, duration until psychotic worsening commences since initiation of aripiprazole, nature of psychotic/non-psychotic symptoms that increased, effective/ineffective strategies for managing increased psychotic symptoms, and duration until psychotic worsening resolves.
Psychotic symptoms were defined by the following symptoms: hallucinations, delusions, disorganization, and bizarre behavior. Increased anxiety, hostility, and psychomotor activity were also recorded under the following categories, respectively: agitation, aggression, and activation.
The quality of the causal relationship between aripiprazole and increased psychotic symptoms was systematically evaluated for each case according to “the guidelines for evaluation of drug-associated events” developed by Aubry et al. (2000). These guidelines, developed to evaluate the relationship between atypical antipsychotics and mania/hypomania, were modified to specifically evaluate psychotic symptoms and are detailed in Table 1.
Items 1–7 of the original guidelines were maintained, with item 8 excluded based on the original definition (Aubry et al. 2000). In line with the original scale, the total number of fulfilled items was used to categorize each case as follows: 1–3 as “questionable,” 4 as “moderately suggestive,” and 5–7 as “highly suggestive” (Aubry et al. 2000).
Results
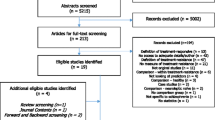
A total of 22 cases in 16 reports (Adan-Manes and Garcia-Parajua 2009; Ahuja and Lloyd 2007; Avari et al. 2011; Barnas et al. 2005; Burke and Lincoln 2006; Chiu et al. 2011; DeQuardo 2004; Glick et al. 2006; Grover et al. 2006; Kapusta et al. 2007; Lea et al. 2007; Letmaier et al. 2012; Ponde and Novaes 2007; Raja 2007; Ramaswamy et al. 2004; Reeves and Mack 2004) were identified (Fig. 1); a summary is provided in Table 2. Case reports have been published almost yearly since 2004 when aripiprazole became available in the USA.
Case characteristics
Age ranged from 23 to 72 years (median, 48), and cases were evenly split based on gender. Most cases (15 cases) were diagnosed with schizophrenia, with the remainder (seven cases) schizoaffective disorder. Patients were characterized as being chronic and/or ill >10 years in most cases (15 cases).
The reasons for introducing aripiprazole included persistent symptoms (12 cases), side effects (seven cases), both (one case), or other reasons (two cases). Previous antipsychotic treatment included atypical antipsychotic monotherapy (15 cases), typical antipsychotic monotherapy (four cases), combinations of atypical and typical antipsychotics (two cases), and combinations of atypical antipsychotics (one case). The prior antipsychotic dose exceeded the recommended maximum therapeutic dose (Gardner et al. 2010) in seven cases, and did so by one daily defined dose (DDD) (WHO Collaborating Centre for Drug Statistics Methodology, available at http://www.whocc.no/atc_ddd_index/) in most cases (15 cases). Mood stabilizers, used in eight cases of 12 cases, represented the most frequent concomitant medication class. Anticholinergic drugs were used in two cases.
Psychotic symptoms worsened after adding aripiprazole to current treatment, left unchanged, in eight cases. In most of these (six cases), psychotic symptoms worsened at a dose before/without aripiprazole titration. Doses of aripiprazole associated with a worsening of psychotic symptoms ranged from 5 to 30 mg/day (mode, 15 mg/day), which suggests no clear relationship between aripiprazole dose and worsening of psychotic symptoms. Duration of aripiprazole exposure until increased psychotic symptoms ranged from days to months (median and mode, 2 weeks). Specifically identified symptoms included hallucinations (11 cases), delusions (19 cases), disorganization (two cases), and/or bizarre behavior (two cases). Intensity of increased psychotic symptoms was described as “significantly worsened/increased” (case 2, 4, and 20), “outbreak” (case 15), “dramatically increased” (case 17 and 21), “severely exacerbated” (case 22), “intensely paranoid” (case 5), or “overtly paranoid” (case 6). Other symptoms noted were agitation (10 cases), aggression (11 cases), and/or activation (seven cases).
The worsening of psychotic symptoms was not improved by increasing or decreasing aripiprazole, while leaving other medications unchanged, in approximately half the cases (six cases out of 13 cases). Further to this point, increasing aripiprazole dose was implicated in further worsening in four cases. On the other hand, discontinuation of aripiprazole, without changing other medications, resulted in resolution in most instances where this was implemented (eight cases). Just as time to onset of psychotic worsening was quite variable, duration until improvement of psychotic symptoms after effective intervention ranged widely, from days to months (median, 8 days; mode, 1 week). Table 2 provides details of each case.
Besides the 22 cases, 4 cases were identified as rechallenge trials (Table 2). In all, psychotic symptoms worsened after simply adding aripiprazole across a wide range of doses (2.5–15 mg/day) to the current regimen and resolved shortly after discontinuation of aripiprazole.
Quality of causal relationship between aripiprazole and psychotic worsening for cases
Causal relationship between aripiprazole and worsening of psychotic symptoms is summarized as follows: 11 cases, “highly suggestive”; three cases, “moderately suggestive”; and eight cases, “questionable” (Table 2). All four rechallenge cases were classified as “highly suggestive.”
Discussion
Our main findings from this systematic review are as follows: (1) approximately half of reported cases demonstrated a robust causal relationship between aripiprazole and a worsening of psychotic symptoms, (2) approximately one third of cases reported psychotic symptoms worsened after simply adding aripiprazole to the current treatment regimen, and (3) approximately one third of cases showed resolution of psychotic worsening was achieved by discontinuing aripiprazole without changing other medications. Aripiprazole also exacerbated psychotic symptoms across a wide range of doses. Taken together, the evidence seems to substantiate a causal relationship between aripiprazole and a worsening of psychotic symptoms in a proportion of patients with schizophrenia or schizoaffective disorder.
What is less clear is whether there are predictors of those at risk; to date, there have been few studies examining risk factors for aripiprazole-induced psychotic worsening. One study reported 40 % of patients worsened following a switch to aripiprazole, and patients already receiving relatively high antipsychotic doses may be at greater risk compared to those on lower doses (727 vs. 382 mg/day, respectively) (Takeuchi et al. 2009). A second study indicated that approximately 30 % of patients did not complete a switch to aripiprazole; descriptively, they received more typical antipsychotics and higher doses (≥1 DDD), were more ill, and had a longer course of illness compared to those who successfully completed the switch (Lin et al. 2009). Summarizing, it would seem that increased risk occurs in patients with chronic schizophrenia who are more ill and, as a result, are receiving relatively high antipsychotic doses. However, we are also reminded that these studies involved individuals undergoing a switch to aripiprazole, in contrast to reports where aripiprazole is added to existing treatment. Data drawn from switching studies must also factor in the possibility that the new drug, in this case aripiprazole, is not as effective as the antipsychotic the individual had been taking.
Differences were identified between cases where aripiprazole was added to current treatment (eight cases) and those where individuals were switched to aripiprazole from current treatment (14 cases). The main reason for introducing aripiprazole was efficacy (six cases) in the former, while efficacy (six cases) and side effects (six cases) were equally represented in the latter. In addition, number of cases where prior antipsychotic dose exceeded 1 DDD was slightly higher in cases where aripiprazole was added to existing antipsychotic treatment (six of eight cases) versus cases where individuals were being switched to aripiprazole (nine of 14 cases). Not surprisingly, it would appear that aripiprazole is added to current treatment in patients with treatment-resistant schizophrenia.
In terms of mechanism of action, exacerbation of psychotic symptoms by aripiprazole is most readily explained by its partial dopamine agonist properties (Burris et al. 2002); indeed, all 14 case reports that discuss the possible mechanism also implicate it. Notably, aripiprazole has a very high affinity for D2 receptors (Burris et al. 2002), in contrast to low affinities for other receptors other than 5-HT1A and 5-HT2A (DeLeon et al., 2004). That the cases occur in chronically treated individuals also calls into question the impact of long-term antipsychotic administration and dopamine upregulation (Silvestri et al. 2000), possibly resulting in increased sensitivity to dopaminergic perturbations (i.e., supersensitivity psychosis) as may occur with partial agonist activity via the addition of aripiprazole. To this point, 11 case reports posit a role for upregulation of postsynaptic dopamine receptors or supersensitivity psychosis (Moncrieff 2006).
The worsening of psychosis associated with aripiprazole, as detailed in these case reports, was not improved by altering the dose of aripiprazole, although resolution occurred frequently after aripiprazole discontinuation. This certainly offers at least indirect evidence supporting a causal relationship between aripiprazole and psychotic worsening.
Finally, from a clinical standpoint, it is also important to recognize that the symptoms related to the addition of aripiprazole were not confined to psychotic symptoms per se, but also included agitation, aggression, and activation. Indeed, cases have been reported where the central features were agitation (Cho and Lindenmayer 2009) or mania (Ducroix et al. 2008; Padala et al. 2007; Traber et al. 2007). Regarding mania induced by atypical antipsychotics, some reviews (Aubry et al. 2000; Benyamina and Samalin 2012; Michalopoulou and Lykouras 2006; Rachid et al. 2004) have suggested all types of atypical antipsychotics can induce mania or hypomania; thus, this phenomenon is not specific to aripiprazole. Notwithstanding, clinicians must also be vigilant of these symptoms and the possible need to consider aripiprazole discontinuation should they appear.
Unfortunately, a series of case reports does not allow us to establish the exact risk of aripiprazole for worsening psychotic symptoms, and predicting who is liable also remains an unanswered question. Any interpretation of the data is qualified by the diversity of the cases (e.g., illness severity, types and doses of prior antipsychotics, or concomitant medications) and variables beyond pharmacotherapy. For example, there is the possibility that factors such as natural course of illness or environmental stress play a role, although the quality of the causal relationship between aripiprazole and increased psychotic symptoms was evaluated according to the modified guidelines for evaluation of drug-associated events.
In conclusion, evidence suggests that at least a small number of patients with schizophrenia or schizoaffective disorder risk an exacerbation of psychotic symptoms if aripiprazole is added to existing antipsychotic treatment. Many of the cases reported involve patients who are quite ill and have been exposed to long-term antipsychotic treatment, but it may also be that this population is simply more likely to be exposed to augmentation strategies. There appears to be no relationship between its occurrence and age or gender; similarly, it can occur across all doses of aripiprazole and anytime from days to weeks after treatment commences. At this point, clinicians must simply be cognizant of the risk, vigilant of clinical worsening in the context of such a scenario, and aware that aripiprazole discontinuation appears the preferred strategy should it occur.
References
Adan-Manes J, Garcia-Parajua P (2009) Aripiprazole in combination with other antipsychotic drugs may worsen psychosis. J Clin Pharm Ther 34:245–246
Ahuja N, Lloyd AJ (2007) Aripiprazole and worsening of psychosis: a case report. J Clin Psychiatry 68:805–806
Aubry JM, Simon AE, Bertschy G (2000) Possible induction of mania and hypomania by olanzapine or risperidone: a critical review of reported cases. J Clin Psychiatry 61:649–655
Avari J, Mahgoub N, Alexopoulos GS (2011) Adding aripiprazole to clozapine worsened delusions and hallucinations: a case report. J Clin Psychopharmacol 31:528–531
Barnas ME, Hussain N, Petrides G (2005) Treatment-emergent psychosis with aripiprazole. J Clin Psychiatry 66:1339
Benyamina A, Samalin L (2012) Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract 16:2–7
Burke MJ, Lincoln J (2006) Aripiprazole and haloperidol: a clinically relevant interaction with a dopamine antagonist and partial agonist. Ann Clin Psychiatry 18:129–130
Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB (2002) Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302:381–389
Chiu YH, Chen CH, Lu ML (2011) Worsening psychosis after adding aripiprazole to clozapine. Prog Neuropsychopharmacol Biol Psychiatry 35:291–292
Cho DY, Lindenmayer JP (2009) Aripiprazole-induced agitation after clozapine discontinuation: a case report. J Clin Psychiatry 70:141–143
DeLeon A, Patel NC, Crismon ML (2004) Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther 26:649–666
DeQuardo JR (2004) Worsened agitation with aripiprazole: adverse effect of dopamine partial agonism? J Clin Psychiatry 65:132–133
Ducroix C, Beghelli F, Rousset I, Vacheron MN (2008) Emergent mania with atypical antipsychotics: review of the literature about one case. Therapie 63:153–154
Fleischhacker WW, Heikkinen ME, Olie JP, Landsberg W, Dewaele P, McQuade RD, Loze JY, Hennicken D, Kerselaers W (2010) Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 13:1115–1125
Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ (2010) International consensus study of antipsychotic dosing. Am J Psychiatry 167:686–693
Glick ID, Duggal V, Hodulik C (2006) Aripiprazole as a dopamine partial agonist: positive and negative effects. J Clin Psychopharmacol 26:101–103
Grover S, Sharan P, Gupta N (2006) Aripiprazole worsens psychosis: a case report. Prim Care Companion J Clin Psychiatry 8:380–381
Jaskiw GE, Popli AP (2004) A meta-analysis of the response to chronic l-dopa in patients with schizophrenia: therapeutic and heuristic implications. Psychopharmacology (Berl) 171:365–374
Kapusta ND, Mossaheb N, Barnas C, Fischer P (2007) Aripiprazole-induced psychosis: a case report of reexposure by stepwise up-titration. J Clin Psychiatry 68:1445–1446
Lea JW, Stoner SC, Lafollette J (2007) Agitation associated with aripiprazole initiation. Pharmacotherapy 27:1339–1342
Letmaier M, Painold A, Holl AK, Grohmann R, Vergin H (2012) Severe psychotic exacerbation during combined treatment with aripiprazole/haloperidol after prior treatment with risperidone. Int J Psychiatry Clin Pract 16:153–156
Lieberman JA, Kane JM, Alvir J (1987) Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 91:415–433
Lin HC, Chong MY, Lee Y, Yeh WC, Lin PY (2009) Switching of antipsychotics to aripiprazole in the treatment of schizophrenia. Chang Gung Med J 32:409–416
Marder SR, McQuade RD, Stock E, Kaplita S, Marcus R, Safferman AZ, Saha A, Ali M, Iwamoto T (2003) Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 61:123–136
Michalopoulou PG, Lykouras L (2006) Manic/hypomanic symptoms induced by atypical antipsychotics: a review of the reported cases. Prog Neuropsychopharmacol Biol Psychiatry 30:549–564
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Moncrieff J (2006) Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse. Acta Psychiatr Scand 114:3–13
Newcomer JW, Campos JA, Marcus RN, Breder C, Berman RM, Kerselaers W, L’Italien GJ, Nys M, Carson WH, McQuade RD (2008) A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry 69:1046–1056
Padala PR, Wengel SP, Petty F (2007) Manic episode during treatment with aripiprazole. Am J Psychiatry 164:172–173
Ponde MP, Novaes CM (2007) Aripiprazole worsening positive symptoms and memantine reducing negative symptoms in a patient with paranoid schizophrenia. Rev Bras Psiquiatr 29:92
Rachid F, Bertschy G, Bondolfi G, Aubry JM (2004) Possible induction of mania or hypomania by atypical antipsychotics: an updated review of reported cases. J Clin Psychiatry 65:1537–1545
Raja M (2007) Improvement or worsening of psychotic symptoms after treatment with low doses of aripiprazole. Int J Neuropsychopharmacol 10:107–110
Ramaswamy S, Vijay D, William M, Sattar SP, Praveen F, Petty F (2004) Aripiprazole possibly worsens psychosis. Int Clin Psychopharmacol 19:45–48
Reeves RR, Mack JE (2004) Worsening schizoaffective disorder with aripiprazole. Am J Psychiatry 161:1308
Shim JC, Shin JG, Kelly DL, Jung DU, Seo YS, Liu KH, Shon JH, Conley RR (2007) Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry 164:1404–1410
Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, Kapur S, Zipursky RB, Wilson AA, Christensen BK, Seeman P (2000) Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 152:174–180
Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, Rosenheck RA, Perkins DO, Nussbaum AM, Lieberman JA (2011) A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry 168:947–956
Takeuchi H, Uchida H, Suzuki T, Watanabe K, Kashima H (2009) Predictors of clinical worsening after a switch to aripiprazole in patients with schizophrenia: a 1-year naturalistic follow-up study. J Clin Psychopharmacol 29:394–395
Traber R, Schneiter R, Modestin J (2007) A case of aripiprazole-induced mania. Pharmacopsychiatry 40:37–38
Conflict of interest
Dr. Takeuchi has received fellowship grants from the Japanese Society of Clinical Neuropsychopharmacology and Astellas Foundation for Research on Metabolic Disorders, speaker’s honoraria from Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji Seika Pharma, and Otsuka Pharmaceutical, and manuscript fees from Dainippon Sumitomo Pharma within the past 5 years. Dr. Remington has received research support from Novartis, Medicure, and Neurocrine Bioscience, consultant fees from Roche, and speaker’s fees from Novartis. He holds no commercial investments in any pharmaceutical company within the past 5 years.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeuchi, H., Remington, G. A systematic review of reported cases involving psychotic symptoms worsened by aripiprazole in schizophrenia or schizoaffective disorder. Psychopharmacology 228, 175–185 (2013). https://doi.org/10.1007/s00213-013-3154-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3154-1