Abstract
Rationale
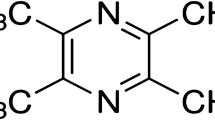
Evidences indicate that methylglyoxal, a highly reactive metabolite of hyperglycemia, can enhance protein glycation, oxidative stress, or inflammation. Mangiferin, a polyphenol compound of C-glucoside, has many beneficial biological activities, including anti-inflammation, anti-oxidation, neuroprotection, cognitive enhancement, etc. Whether mangiferin alleviates diabetes-associated cognitive impairment is still unclear.
Objectives
The present study was designed to investigate the effects of mangiferin on the behavioral deficits of diabetic rats induced by streptozotocin; the mechanisms associated with methylglyoxal toxicity are especially investigated.
Methods
Diabetic rats were treated with mangiferin (15, 30, and 60 mg/kg, p.o.) for 9 weeks. Cognitive performances were evaluated with the Morris water maze. Hippocampus and blood were obtained for evaluation of the effects of mangiferin on protein glycation, oxidative stress, and inflammation in diabetic state.
Results
Mangiferin significantly improved the behavioral performances of diabetic rats, evidenced by a decrease in escape latency as well as increases in numbers of crossing the platform and percentage of time spent in the target quadrant, which were accompanied by decreases in the levels of advanced glycation end-products and their receptor (RAGE), interleukin-1β, TNF-α, and malondialdehyde and increases in the activity and expression of glyoxalase 1 as well as glutathione level in the hippocampus of diabetic rats. Furthermore, mangiferin produced a significant decrease in malondialdehyde level and increased glutathione level and superoxide dismutase activity in the serum of diabetic rats.
Conclusions
This study demonstrates that mangiferin can markedly ameliorate diabetes-associated cognitive decline in rats, which is done likely through suppressing methylglyoxal hyperactivity (promoting protein glycation, oxidative stress, and inflammation) mediated noxious effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic encephalopathy is an emerging diabetic complication (Sima 2010), and diabetes-associated cognitive decline (DACD) is the core component (Biessels et al. 2008; Wrighten et al. 2009). Chronic hyperglycemia is thought to be a primary cause of diabetic complications, and the downstream consequences of hyperglycemia include multiple pathophysiological processes including oxidative stress, protein glycation, and inflammation (Calcutt et al. 2009). The pathogenic role of protein glycation or advanced glycation end-product (AGE) formation in diabetic vascular complications is widely recognized, and protein glycation may be a key bond linking oxidative stress and inflammation in the development and progression of diabetic complications.
Methylglyoxal (MG), an alpha-carbonyl aldehyde, is one of the most powerful glycating agents of proteins and other important cellular components, with modification directed mainly to the guanidino group of arginine residues (Thornalley 2007). In the diabetic context, an increase in the concentration of MG has been observed in human body fluids and tissues that seem to be responsible for diabetic complications (Fosmark et al. 2009; Lu et al. 2011; van Deutekom et al. 2008). Without clearance, MG can accumulate in the body and accelerates the formation of AGEs. Cognitive problems, common to aged or diabetic patients, may be related to the accumulation of toxic alpha-oxoaldehydes such as MG. For example, a recent report showed that the serum concentration of MG was associated with increased cognitive decline in elderly individuals (Beeri et al. 2011). Reports suggest that diabetes-causing impairment of cognitive processes may be involved in AGE formation (van Deutekom et al. 2008; Wang et al. 2009). Thus, it is possible that MG or MG-mediated glycation could be, at least in part, responsible for the diabetes-associated cognitive impairment. Furthermore, MG also has the ability to increase both oxidative stress and inflammation (Di Loreto et al. 2004, 2008; Rabbani and Thornalley 2008; Sena et al. 2012; Yamawaki et al. 2008), and both are often implicated in the pathogenesis of diabetic complications as well as DACD (Kuhad and Chopra 2007; Kuhad et al. 2009; Liu et al. 2012b). Together, as one of important metabolic intermediates of hyperglycemia, MG may be a central player in the pathogenesis of diabetes-associated cognitive deficiency. Hippocampus is known to exert a vital role in learning and memorizing, and hippocampal neurons were highly susceptible to MG (Di Loreto et al. 2004, 2008), where AGE levels were also increased, accompanied by hippocampal dysfunction and diabetes-associated depression (Wang et al. 2009).
The glyoxalase system is the major detoxication system for dicarbonyl compounds in the human body, and glyoxalase 1 (Glo-1) is the rate-limiting enzyme of alpha-carbonyl aldehyde metabolism. With reduced glutathione (GSH) as a cofactor, Glo-1 can catalyze the conversion of reactive acyclic alpha-carbonyl aldehydes into the corresponding alpha-hydroxyacids (Thornalley 2007). Glo-1 can promptly clear alpha-carbonyl aldehydes, such as MG, and then inhibits the formation of AGEs; additionally, Glo-1 also exerted a direct inhibition on AGE formation (Shinohara et al. 1998). Hyperglycemia-induced reactive oxygen species (ROS) increased the expression of AGEs and the receptor for AGEs (RAGE), which was mediated by MG, while the overexpression of Glo-1 normalized this, suggesting that Glo-1 knockdown mimicked the effect of high glucose (Yao and Brownlee 2010). The overexpression of Glo-1 in Caenorhabditis elegans decreased dicarbonyl glycation of mitochondrial proteins, the formation of ROS, proteome markers of dicarbonyl glycation and markers of oxidative and nitrosative damage with concomitant life extension (Rabbani and Thornalley 2008). Most importantly, the overexpression of Glo-1 reduces hyperglycemia-induced levels of AGEs and oxidative stress in diabetic rats (Brouwers et al. 2011). Thus, Glo-1-mediated MG removal has a leading role in the pathogenesis of diabetic complications, including DACD, and Glo-1 may be a preventative and therapeutic target.
Mangiferin (MF), also called chinonin, is a major glucoside of xanthone in Rhizome Anemarrhena, a well-known Chinese Materia Medica in China. Mangiferin has many beneficial biological activities, including anti-inflammatory (Marquez et al. 2012), anti-oxidative (Marquez et al. 2012; Martinez Sanchez et al. 2001; Prabhu et al. 2006), and antidiabetic (Miura et al. 2001; Muruganandan et al. 2005) effects. Moreover, mangiferin has neuroprotective effects. For example, mangiferin is neuroprotective in both in vitro and in vivo models of forebrain ischemia in rats (Gottlieb et al. 2006). Mangiferin reduced ischemia-induced neuronal loss and oxidative damage in the gerbil brain (Martinez Sanchez et al. 2001). Importantly, mangiferin significantly ameliorated scopolamine-induced learning deficits in mice (Jung et al. 2009) and long-term object recognition memory in rats (Pardo Andreu et al. 2010). Mangiferin also improved diabetic complications in the heart and kidney (Li et al. 2010; Muruganandan et al. 2002). Furthermore, evidences indicate that mangiferin can traverse the blood–retina barrier and blood–brain barrier and reach therapeutic concentrations (Hou et al. 2010; Martinez Sanchez et al. 2001). Our latest report indicated that the alcohol extract of Rhizoma Anemarrhenae ameliorated DACD in rats due to decreases of amyloid-beta peptide and TNF-α in the brain (Liu et al. 2012a). However, there is no report on whether mangiferin can affect the cognitive impairment caused by diabetes. Therefore, the aims of the current study were to investigate the effects of mangiferin on DACD in rats as well as the noxious effects of methylglyoxal (promotions in protein glycation, oxidative stress, and inflammation) in the hippocampus in diabetic condition.
Materials and methods
Animals
Male Sprague–Dawley rats (10 weeks of age) were bred in the Center of Experimental Animal, Xuzhou Medical College. All rats were housed under a humidity- (50 ± 10 %) and temperature-controlled (24 ± 1 °C) room. They were maintained under controlled light (12-h day/night cycle) with free access to water and rodent chow. All animal experiments were granted approval by the Animal Ethics Committee of Xuzhou Medical College. All experiments conformed to the Guidelines for Ethical Conduct in the Care and Use of Animals. Every effort was made to minimize stress to the animals.
Experimental design
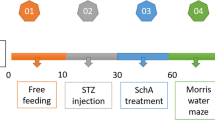
The rats fasted for 12 h were subjected to a single intraperitoneal injection of streptozotocin (STZ), 55 mg/kg, freshly dissolved in 0.1 mol/L sodium citrate buffer at pH 4.5. Age-matched normal rats received sodium citrate buffer only. The development of diabetes was confirmed by fasting blood glucose (FBG) level using a reagent kit (Jiancheng Bioengineering Institute, Nanjing, China). Rats with FBG levels higher than 11.1 mmol/L were considered to be diabetic after 72 h of STZ injection (Liu et al. 2007). Then, the diabetic rats were randomly divided into four groups with ten animals each group viz. diabetic rats, diabetic rats treated with three doses of mangiferin, i.e., 15, 30, and 60 mg/kg (p.o.). Mangiferin (purity > 97 %, Fengshanjian Medicinal Research Co. Ltd., Kunming, China) was suspended in distilled water. Diabetic rats and age-matched normal rats (n = 10) both received distilled water. Blood glucose and body weight of the rats were examined monthly and weekly, respectively. Blood glucose was measured for the rats fasted for 7 h.
After treatment for 8 weeks, animals were tested for learning and memory tasks in the Morris water maze for five consecutive days, still being administrated intragastrically with mangiferin as before. The animals were killed under ethyl ether anesthesia, blood was collected by femoral vein bleeding, and the serum separated. The brain was rapidly removed and both sides of the hippocampus were isolated. The samples were stored at −80 °C until processed for biochemical assays.
Morris water maze test
The Morris water maze test was employed for testing learning and memory according to our previous reports (Liu et al. 2012a, b). A place navigation test was performed wherein the extent of learning was assessed. The rats received four consecutive daily training trials, with each trial having a ceiling time of 90 s and a trial interval of approximately 30 s. On the day before the start of the training days, all the rats were put into the water for 90 s to adapt to the environment. For each trial, each rat was put into the water at one of four start locations, the sequence of which being selected randomly. During the test trials, rats were placed into the tank at the same start location, with their heads facing the tank wall. The rat had to swim until it climbed onto the platform submerged underneath the water. After climbing onto the platform, the animal remained there for 20 s before the commencement of the next trial. The escape platform was kept in the same position relative to the distal cues. If the rat failed to reach the escape platform within the maximally allowed time of 90 s, it was promptly conducted to climb onto the platform and allowed to remain there for the same amount of time. The time to reach the platform (latency in seconds) was measured.
A spatial probe test was performed wherein the extent of memory was assessed. On day 5, the rats were then given a memory test. The time spent in the target quadrant indicates the degree of memory that has taken place after learning training. In the probe trial, the hidden platform was removed from the pool and the rat was placed into the pool from the start location at the quadrant opposite to the former platform quadrant. The number of times of crossing the former platform and the percentage of time spent in the former platform quadrant were recorded for 90 s.
Determination of AGE levels in the hippocampus by fluorometry
After weighing, the preserved rat hippocampus was homogenized in 10 volumes (w/v) of 100 mmol/L PBS extraction buffer (pH 7.4) with a motor-operated homogenizer (FLUKO Equipment Shanghai Co. Ltd., China) in an ice bath. The homogenate was centrifuged at 4 °C, 10,000×g for 15 min, and the supernatant was collected for Glo-1 activity and other biochemical assays. The residual pellets were washed three times with distilled water and then added with 1.0 ml of CHCl3–MeOH (1:1) and shaken overnight at room temperature. After that, 0.5 mL of MeOH–H2O (4:1) was added and centrifuged at 4 °C, 4,000×g for 5 min. The residual pellets were washed twice with MeOH and distilled water in turn, respectively; then, the pellets were washed twice with 0.02 mol/L HEPES buffer (pH 7.5) containing 0.1 mol/L CaCl2. The pellets were suspended in 1.0 mLl of HEPES buffer overnight at 4 °C. After removing the buffer by centrifugation, the pellets were suspended in 1.0 mL of HEPES buffer containing 290 U of type I collagenase (Sigma-Aldrich Co. LLC.), and 2.0 μL of the antiseptics methylbenzene and chloroform each were added. The mixture system was shaken for 24 h at 37 °C and centrifuged. Then, the supernatant was collected for the determination of fluorescence intensity using a fluorescent instrument (9203-941, Promega Biosystems, Inc., Sunnyvale, CA) at an excitation wavelength 370 nm and emission wavelength of 440 nm, with the HEPES buffer only containing type I collagenase as the standard. AGE levels in the hippocampus were expressed as the enzyme activity of type I collagenase (in units) per milligram of protein.
Glo-1 activity assay in hippocampus by ultraviolet spectrophotometry
The Glo-1 assay was performed using a spectrophotometric method monitoring the increase in absorbance at 240 nm due to the formation of S-d-lactoylglutathione for 2 min at 25 °C (Maher et al. 2011). The standard assay mixture contained 8 mmol/L MG, 2 mmol/L GSH, 10 mmol/L magnesium sulfate, and 50 mmol/L phosphate potassium (pH 6.6). Before initiating the reaction by adding the supernatant of the brain homogenate (10–30 μg protein) to the assay mixture, the mixture was allowed to stand for at least 2 min to ensure the equilibration of hemithioacetal formation. Glo-1 activity was expressed as the percentage of the production of S-d-lactoylglutathione per minute per milligram protein in the normal rats (100 %). Protein concentration was determined by the bicinchoninic acid (BCA) assay.
Western blot analysis for Glo-1 and RAGE expressions
After weighing, the preserved hippocampus was homogenized using a sonicator with 10 volumes (w/v) of 50 mmol/L (pH 7.4) Tris-buffered saline containing 0.6 mmol/L phenylmethylsulphonyl fluoride, 1 mmol/L Na3VO4, and 50 mmol/L NaF in an ice bath. After being placed at 4 °C for at least 60 min, the homogenates were centrifuged at 4 °C, 10,000×g for 15 min, and the supernatant was collected for Western blot studies. The protein concentration in the supernatant was determined using the BCA protein assay kit (Beyotime Institute of Biotechnology, Nanjing, China).
The protein samples (80 μg) were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to BiotraceTM nitrocellulose membranes. The membrane was blocked with 5 % blocking buffer for 120 min and incubated overnight at 4 °C with primary antibodies including goat anti-Glo-1 (R&D System) and rabbit anti-RAGE (Santa Cruz Biotechnology). The proteins were detected using alkaline phosphatase-conjugated affinipure donkey anti-goat or goat anti-rabbit secondary antibodies. The membranes were exposed to BCIP/NBT alkaline phosphatase color developing reagent (Beyotime Institute of Biotechnology) for 15 min. Densities of signals on the blots were measured with ImageJ software and normalized using rabbit anti-GAPDH (Bioworld Technology Inc., USA) as an internal control (optical densitydetected protein/optical densityinternal control).
Determination of interleukin-1β and TNF-α levels in the hippocampus by ELISA
The levels of interleukin-1β (IL-1β) and TNF-α in the hippocampus, reflections of inflammation in the brain, were determined using rat IL-1β and TNF-α ELISA kit, respectively (ExCell Biology, Inc., Shanghai, China) according to the manufacturer’s instructions. Tissue protein was estimated using the BCA method. The level of brain IL-1β or TNF-α was expressed as picograms of IL-1β or TNF-α per milligram of protein.
Determination of GSH and malondialdehyde levels in serum and hippocampus
GSH and malondialdehyde (MDA) levels, evaluations of the oxidative stress, were determined according to our previous report (Liu et al. 2012b). Briefly, the compound 3-carboxy-4-nitrophenyl disulfide can react with sulfhydryl compounds (such as GSH), which forms a yellow compound with stronger absorption at 420 nm. MDA can be condensed with thiobarbituric acid, which forms a pink product with maximal absorption at 532 nm. The determination of GSH or MDA level was performed with a corresponding commercial kit (Jiancheng Bioengineering Institute). Serum GSH or DMA was expressed as micromoles of GSH or MDA per liter and the brain GSH or MDA content as nanomoles of GSH or MDA per milligram of protein.
Superoxide dismutase activity assay in serum
Superoxide dismutase (SOD) activity, an important anti-oxidative enzyme, was determined according to the method of Sun et al. (1988). The principle of the method is based on the inhibition of nitroblue tetrazolium reduction by the xanthine–xanthine oxidase system as a superoxide generator. SOD activity assay was assessed using a commercial kit (Jiancheng Bioengineering Institute). One unit of SOD was defined as the amount of enzyme causing 50 % inhibition in the rate of nitroblue tetrazolium reduction.
Statistical analysis
Results were expressed as the mean ± SEM. The intergroup variation was measured by one-way analysis of variance followed by Tukey’s test. Statistical significance was considered at P < 0.05. Statistical analysis was done using the SPSS Statistical software, version 13.0.
Results
Effects of mangiferin on fasting blood glucose and body weight of diabetic rats
Serum glucose levels of the experimental rats were measured at weeks 0, 4, and 9 after treatment with mangiferin. Compared with age-matched normal rats, diabetic rats had higher FBG levels throughout the treatment [F(4,49) = 52.208, P < 0.01]. However, the FBG levels were still high in diabetic rats treated with mangiferin both at weeks 4 and 9, and no significant differences were found between the treated and untreated groups (Table 1). Body weights of the experimental rats were also measured from week 1 to week 9 following treatment with mangiferin. Compared with those of the age-matched normal rats, the body weights of diabetic rats always declined after STZ induction [F(4,49) = 88.037, P < 0.01]. A low-dose mangiferin treatment had no obvious effects on the body weights of diabetic rats. Mid-dose mangiferin treatment had a significant increment on the body weights of diabetic rats only at weeks 5 and 9; for the high dose, at weeks 6 and 9 (Table 2).
Effects of mangiferin on the learning and memory abilities of diabetic rats in the Morris water maze
The cognitive function was assessed in the Morris water maze test (Fig. 1). In the place navigation test (on days 1–4), the mean escape latency in diabetic rats was always significantly longer than that of normal rats during 4 days of learning. Chronic mangiferin treatment decreased the mean escape latency of diabetic animals [F(4,44) = 5.256, P < 0.01] (Fig. 1a). The low-dose group showed a significant decrease on the second, third, and fourth days of training, the middle-dose group on the first and second days, and the high-dose group on the first, third, and fourth days (Fig. 1a).
Effects of mangiferin on diabetes-associated cognitive decline in rats in the Morris water maze test. Escape latency (a), the percentage of time spent in the target quadrant (b), and numbers of crossing the platform (c) in groups of the normal (Cont.), STZ-induced diabetic (DM), and diabetic rats treated with low-dose (DM+MF-L), middle-dose (DM+MF-M), and high-dose (DM+MF-H) mangiferin. Mean ± SEM, n = 8–10. *P < 0.05, **P < 0.01 (vs. Cont. group); # P < 0.05, ## P < 0.01 (vs. DM group)
In the probe trial (on day 5) of the Morris water maze study, which assesses how well the animals have learned and consolidated the platform location during the 4 days of training, the animals showed a significant difference. The percentage of time spent in the target quadrant [F(4,43) = 4.652, P < 0.01] (Fig. 1b) and the numbers of crossing the platform [F(4,43) = 2.434, P < 0.05] (Fig. 1c) were significantly lower in diabetic animals as compared to those of the normal group. However, mangiferin may dose-dependently increase the percentage of time spent in the target quadrant in diabetic animals (Fig. 1b). Nevertheless, only the high-dose group performed a significant improvement for the numbers of crossing the platform compared to the untreated group (Fig. 1c).
Effects of mangiferin on AGE levels and RAGE expression in the hippocampus of diabetic rats
To investigate whether the decrease of MG hyperactivity by mangiferin could result in the inhibition of the AGE–RAGE axis, we examined the AGE levels as well as the protein expression of their receptor (RAGE) in the hippocampus. A significant increment of AGE levels and RAGE expression occurred in the hippocampus of diabetic rats (Fig. 2). Mangiferin significantly decreased the AGE levels [F(4,30) = 11.275, P < 0.01] (Fig. 2a) and RAGE [F(4,17) = 34.559, P < 0.01] (Fig. 2b) in diabetic rats.
Effects of mangiferin on the level of advanced glycation end-products (a) and the protein expression of the receptor for AGEs (RAGE) (b) in the hippocampus in groups of the normal (Cont), STZ-induced diabetic (DM), and diabetic rats treated with low-dose (DM+MF-L), middle-dose (DM+MF-M), and high dose (DM+MF-H) mangiferin. Mean ± SEM, n = 6 (AGEs), n = 3–4 (RAGE). **P < 0.01 (vs. Cont group); ## P < 0.01 (vs. DM group)
Effects of mangiferin on the activity and expression of Glo-1 in the hippocampus of diabetic rats
To investigate whether cognition-enhancing effects of mangiferin were related to the decrease in MG level, we examined the enzymatic activity and protein expression of Glo-1, the rate-limiting enzyme of MG clearance. It was found that the activity [F(4,32) = 10.809, P < 0.01] (Fig. 3a) and protein expression [F(4,18) = 15.529, P < 0.01] (Fig. 3b) of Glo-1 were both significantly reduced in the hippocampus of diabetic rats. Mangiferin significantly enhanced Glo-1 activity and protein expression (Fig. 3).
Effects of mangiferin on glyoxalase 1 (Glo-1) activity (a) and protein expression (b) in the hippocampus in groups of the normal (Cont), STZ-induced diabetic (DM), and diabetic rats treated with low-dose (DM+MF-L), middle-dose (DM+MF-M), and high-dose (DM+MF-H) mangiferin. Mean ± SEM, n = 6–7 (activity), n = 3–4 (expression). *P < 0.05, **P < 0.01 (vs. Cont group); # P < 0.05, ## P < 0.01 (vs. DM group)
Effects of mangiferin on oxidative stress and inflammation caused by diabetes
To investigate whether the decrease of MG hyperactivity by mangiferin had inhibitory effects on oxidative stress and inflammation in the brain, we examined the levels of MDA and GSH as well as IL-1β and TNF-α in the hippocampus. Diabetes remarkably caused oxidative stress damage evidenced by the increased MDA level [F(4,33) = 22.273, P < 0.01] (Fig. 4a) and the declined GSH level [F(4,34) = 5.877, P < 0.01] (Fig. 4b) in the hippocampus. Besides oxidative stress, neuroinflammation also occurred in the brain of diabetic rats, reflected by the decreases in IL-1β [F(4,32) = 17.415, P < 0.01] (Fig. 5a) and TNF-α levels [F(4,32) = 10.864, P < 0.01] (Fig. 5b) in the hippocampus. Nevertheless, chronic treatment with mangiferin significantly reversed the above alterations (Figs. 4 and 5).
Effects of mangiferin on the levels of interleukin-1β (IL-1β) (a) and TNF-α (b) in the hippocampus in groups of the normal (Cont), STZ-induced diabetic (DM), and diabetic rats treated with low-dose (DM+MF-L), middle-dose (DM+MF-M), and high-dose (DM+MF-H) mangiferin. Mean ± SEM, n = 6–7. **P < 0.01 (vs. Cont group); ## P < 0.01 (vs. DM group)
Effects of mangiferin on the levels of malondialdehyde (MDA) (a) and reduced glutathione (GSH) (b) in the hippocampus in groups of the normal (Cont), STZ-induced diabetic (DM), and diabetic rats treated with low-dose (DM+MF-L), middle-dose (DM+MF-M), and high-dose (DM+MF-H) mangiferin. Mean ± SEM, n = 6–7. **P < 0.01 (vs. Cont group); ## P < 0.01 (vs. DM group)
To further demonstrate the inhibitory effects of mangiferin on oxidative stress, we also examined SOD activity as well as MDA and GSH levels in serum. Diabetes significantly decreased SOD activity [F(4,32) = 3.440, P < 0.05] (Fig. 6a) and GSH level [F(4,42) = 3.015, P < 0.05] (Fig. 6c) and increased the MDA level [F(4,42) = 3.094, P < 0.05] (Fig. 6b) in the serum of rats, but chronic treatment with mangiferin markedly improved the above changes (Fig. 6).
Effects of mangiferin on superoxide dismutase (SOD) activity (a), levels of malondialdehyde (MDA) (b), and reduced glutathione (GSH) (c) in serum in groups of the normal (Cont), STZ-induced diabetic (DM), and diabetic rats treated with low-dose (DM+MF-L), middle-dose (DM+MF-M), and high-dose (DM+MF-H) mangiferin. Mean ± SEM, n = 6–9. **P < 0.01 (vs. Cont group); # P < 0.05, ## P < 0.01 (vs. DM group)
Discussion
In human diabetes, chronic hyperglycemia is associated with a high incidence of progressive cognitive deficits (Ryan et al. 2003). The potential mechanisms for this include not only the direct effect of hyperglycemia but also the indirect effects of hyperglycemia and its intermediate metabolic products causing damages in cerebral constitution and vessels. MG is a major metabolic intermediate of hyperglycemia, with highly reactive properties. This study analyzed the effects of mangiferin on the behavioral deficits of diabetic rats; the mechanisms associated with MG toxicity were widely investigated. STZ-induced diabetes produced a marked impairment to the cognitive function of rats, which was coupled with significant increases in protein glycation, oxidative stress, and inflammation in brain, with MG hyperactivity as the core factor. Mangiferin significantly ameliorated the cognitive impairment and MG hyperactivity-mediated damages in the hippocampus of diabetic rats.
MG is mainly from triosephosphates (including glyceraldehyde-3-phosphate and dihydroxyacetonephosphate) produced in the process of glycolysis. MG has much higher intrinsic reactivity toward glycation than glucose, 10,000- to 50,000-fold higher (Thornalley 2005). Under diabetic or hyperglycemic condition, much more production of MG occurred in body fluids and tissues (Fosmark et al. 2009; Lu et al. 2011; van Deutekom et al. 2008). The hippocampus is known to play a crucial role in learning and memory, and hippocampal neurons had a high susceptibility to MG through an oxidation-dependent mechanism (Di Loreto et al. 2004). Moreover, MG is a key precursor of AGE formation, which may be involved in the cognitive impairment caused by diabetes (van Deutekom et al. 2008; Wang et al. 2009). Therefore, it is possible that MG could be, at least in part, responsible for diabetes-associated cognitive impairment. In the present study, significant functional impairments in cognition occurred in diabetic rats, evidenced by an increase in escape latency as well as decreases in the numbers of crossing the platform and percentage of time spent in the target quadrant in the Morris water maze test, coupled with the elevated levels of AGEs and their receptor RAGE in the hippocampus. Thus, MG-mediated glycation and AGE formation may be one of the reasons for the pathogenesis of diabetes-associated cognitive impairment. Nonetheless, chronic treatment with mangiferin markedly improved the cognitive decline of diabetic rats, accompanied by remarkable decreases in AGE levels and RAGE expression in the hippocampus. Additionally, an in vitro study showed that mangiferin had an inhibitory effect on AGE formation (Guo et al. 2009). Thus, the inhibition of AGEs/RAGE interaction could be one of the reasons for the amelioration of DACD by mangiferin.
As the major detoxifying enzyme of MG, Glo-1 was significantly downregulated in both activity and protein expression in the cerebral cortex of Akita mice (a kind of spontaneously type 1 diabetic mice), coupled with an increase in protein glycation by MG (Maher et al. 2011). MG caused a dramatic depletion of GSH and a significant inhibition of both Glo-1 and glutathione peroxidase activities in hippocampal neurons (Di Loreto et al. 2008). In the present study, the activity and protein expression of Glo-1 were both markedly declined in the hippocampus of STZ-induced diabetic rats, accompanied by a remarkable decrease of GSH, an essential cofactor for Glo-1. These suggested that the accumulation of MG damaged Glo-1 in diabetic conditions, possibly by GSH deficiency and protein glycation. Our study demonstrated that mangiferin treatment could significantly increase the activity and expression of Glo-1 as well as GSH level in the hippocampus of diabetic rats. Additionally, a report showed that mangiferin increased the GSH level in neuroblastoma cells (Das et al. 2011). To our knowledge, it is the first report of mangiferin being able to enhance the function of Glo-1, indicating that Glo-1 may be an action target for mangiferin.
Oxidative stress has been implicated in the pathogenesis of diabetes and its complications (Brownlee 2005), including diabetic encephalopathy (Kuhad and Chopra 2007; Liu et al. 2012b; Saxena et al. 2007). AGEs synergize and potentiate oxidative stress and contribute to the development of diabetic complications (Ceriello 1999). Also, MG has the ability to increase oxidative stress (Di Loreto et al. 2008; Rabbani and Thornalley 2008; Sena et al. 2012). Moreover, the overexpression of Glo-1 has been shown to reduce hyperglycemia-induced levels of AGEs and oxidative stress in diabetic rats (Brouwers et al. 2011). These suggest that oxidative stress and protein glycation mediated by MG could play central roles in the pathogenesis of diabetes-associated cognitive impairment, and Glo-1 becomes a preventative and therapeutic target. In this study, diabetic rats showed a marked impairment in learning and memory abilities, coupled with significant increases in oxidative stress damages evidenced by an increase of MDA level, an important marker for lipid peroxidation, and a reduction of the GSH level, a potent endogenous antioxidant that is the first line of defense against free radicals, as well as a decrease of SOD activity, a potent endogenous radical scavenger. As expected, mangiferin treatment significantly ameliorated the above alterations in diabetic state. The literature indicated that mangiferin improved oxidative damage in rat brain after stress (Marquez et al. 2011), ischemia-induced neuronal loss and oxidative damage in the gerbil brain (Martinez Sanchez et al. 2001), and antioxidant status in isoproterenol-induced myocardial infarction in rats (Prabhu et al. 2006). Furthermore, mangiferin-pretreated neuroblastoma cells showed a significant increase in the GSH and glutathione-S-transferase levels followed by a significant decrease in MDA formation (Das et al. 2011). These results demonstrate that the inhibitory effect of mangiferin on oxidative stress damage is a very important reason for mangiferin improving DACD.
Inflammation is frequently implicated with oxidative stress in diabetic complications, including diabetic encephalopathy. For example, cognitive decline in diabetic animals was usually associated with the presence of inflammation, besides oxidative stress (Kuhad and Chopra 2007; Kuhad et al. 2009; Liu et al. 2012b). Moreover, diabetes led to the production of pro-inflammatory cytokines such as TNF-α and IL-1β, which was medicated by the activation of NF-κB signaling, coupled with diabetes-associated cognitive deficits (Kuhad et al. 2009; Liu et al. 2012b). Reports showed that MG had the ability to increase inflammation (Sena et al. 2012; Yamawaki et al. 2008), also causing a significant increase in both transcript and protein expression of IL-1β in hippocampal neurons (Di Loreto et al. 2004). Together with the above discussion that MG has the ability to increase oxidative stress, these indicated that the accumulation of MG was an important source not only for oxidative stress damage but also inflammatory responses in diabetic brain. Plenty of evidences proved mangiferin a potent anti-inflammatory agent (Garrido et al. 2004; Leiro et al. 2004; Marquez et al. 2012). In the current study, the levels of IL-1β and TNF-α were dramatically elevated in the hippocampus of diabetic rats, while mangiferin treatment significantly prevented the elevation of IL-1β and TNF-α levels. Thus, the anti-inflammatory efficacy of mangiferin may at least be an important contribution to the improvement of cognitive impairment.
Conclusion
The present study demonstrates that under diabetic condition, MG hyperactivity promotes protein glycation, oxidative stress, and inflammation, which contributes to diabetes-associated cognitive decline, with protein glycation as the core of the three factors. Mangiferin can markedly ameliorate diabetes-associated cognitive impairment in rats, which is done likely through enhancing the function of Glo-1, a main detoxifying enzyme of MG, and Glo-1 may be an action target of mangiferin.
References
Beeri MS, Moshier E, Schmeidler J et al (2011) Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech Ageing Dev 132:583–587
Biessels GJ, Deary IJ, Ryan CM (2008) Cognition and diabetes: a lifespan perspective. Lancet Neurol 7:184–190
Brouwers O, Niessen PM, Ferreira I et al (2011) Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem 286:1374–1380
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Calcutt NA, Cooper ME, Kern TS, Schmidt AM (2009) Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 8:417–429
Ceriello A (1999) Hyperglycaemia: the bridge between non-enzymatic glycation and oxidative stress in the pathogenesis of diabetic complications. Diabetes Nutr Metab 12:42–46
Das S, Nageshwar Rao B, Satish Rao BS (2011) Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chem Biol Interact 93:129–140
Di Loreto S, Caracciolo V, Colafarina S et al (2004) Methylglyoxal induces oxidative stress-dependent cell injury and up-regulation of interleukin-1beta and nerve growth factor in cultured hippocampal neuronal cells. Brain Res 1006:157–167
Di Loreto S, Zimmitti V, Sebastiani P et al (2008) Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int J Biochem Cell Biol 40:245–257
Fosmark DS, Berg JP, Jensen AB et al (2009) Increased retinopathy occurrence in type 1 diabetes patients with increased serum levels of the advanced glycation endproduct hydroimidazolone. Acta Ophthalmol 87:498–500
Garrido G, Delgado R, Lemus Y et al (2004) Protection against septic shock and suppression of tumor necrosis factor alpha and nitric oxide production on macrophages and microglia by a standard aqueous extract of Mangifera indica L. (VIMANG). Role of mangiferin isolated from the extract. Pharmacol Res 50:165–172
Gottlieb M, Leal-Campanario R, Campos-Esparza MR et al (2006) Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol Dis 23:374–386
Guo ZH, Xi RG, Wang XB, Wu LJ, Gao HY (2009) A new trincallane derivative from Salacia hainanensis Chun et How. Yao Xue Xue Bao 44:1123–1126 (in Chinese)
Hou Y, Fan S, Zhang H et al (2010) Pharmacokinetic study of mangiferin in rat plasma and retina using high-performance liquid chromatography. Mol Vis 16:1659–1668
Jung K, Lee B, Han SJ, Ryu JH, Kim DH (2009) Mangiferin ameliorates scopolamine-induced learning deficits in mice. Biol Pharm Bull 32:242–246
Kuhad A, Chopra K (2007) Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur J Pharmacol 576:34–42
Kuhad A, Bishnoi M, Tiwari V, Chopra K (2009) Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav 92:251–259
Leiro J, Arranz JA, Yanez M et al (2004) Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int Immunopharmacol 4:763–778
Li X, Cui X, Sun X et al (2010) Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother Res 24:893–899
Liu YC, Liu HY, Yang HW et al (2007) Impaired expression and function of breast cancer resistance protein (Bcrp) in brain cortex of streptozocin-induced diabetic rats. Biochem Pharmacol 74:1766–1772
Liu YW, Zhu X, Lu Q et al (2012a) Total saponins from Rhizoma Anemarrhenae ameliorate diabetes-associated cognitive decline in rats: involvement of amyloid-beta decrease in brain. J Ethnopharmacol 139:194–200
Liu YW, Zhu X, Li W et al (2012b) Ginsenoside Re attenuates diabetes-associated cognitive deficits in rats. Pharmacol Biochem Behav 101:93–98
Lu J, Randell E, Han Y et al (2011) Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin Biochem 44:307–311
Maher P, Dargusch R, Ehren JL et al (2011) Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS One 6:e21226
Marquez L, Garcia-Bueno B, Madrigal JL, Leza JC (2012) Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur J Nutr 51:729–739
Martinez Sanchez G, Candelario-Jalil E, Giuliani A et al (2001) Mangifera indica L. extract (QF808) reduces ischaemia-induced neuronal loss and oxidative damage in the gerbil brain. Free Radic Res 35:465–473
Miura T, Ichiki H, Iwamoto N et al (2001) Antidiabetic activity of the rhizoma of Anemarrhena asphodeloides and active components, mangiferin and its glucoside. Biol Pharm Bull 24:1009–1011
Muruganandan S, Gupta S, Kataria M, Lal J, Gupta PK (2002) Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology 176:165–173
Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J (2005) Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol 97:497–501
Pardo Andreu GL, Maurmann N, Reolon GK et al (2010) Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur J Pharmacol 635:124–128
Prabhu S, Jainu M, Sabitha KE, Devi CS (2006) Role of mangiferin on biochemical alterations and antioxidant status in isoproterenol-induced myocardial infarction in rats. J Ethnopharmacol 107:126–133
Rabbani N, Thornalley PJ (2008) Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress. Biochem Soc Trans 36:1045–1050
Ryan CM, Geckle MO, Orchard TJ (2003) Cognitive efficiency declines over time in adults with type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia 46:940–948
Saxena G, Singh SP, Pal R, Singh S, Pratap R, Nath C (2007) Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav 86:797–805
Sena CM, Matafome P, Crisostomo J et al (2012) Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res 65:497–506
Shinohara M, Thornalley PJ, Giardino I et al (1998) Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest 101:1142–1147
Sima AA (2010) Encephalopathies: the emerging diabetic complications. Acta Diabetol 47:279–293
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Thornalley PJ (2005) Dicarbonyl intermediates in the Maillard reaction. Ann N Y Acad Sci 1043:111–117
Thornalley PJ (2007) Endogenous alpha-oxoaldehydes and formation of protein and nucleotide advanced glycation endproducts in tissue damage. Novartis Found Symp 285:229–243, discussion 243–226
van Deutekom AW, Niessen HW, Schalkwijk CG, Heine RJ, Simsek S (2008) Increased Nepsilon-(carboxymethyl)-lysine levels in cerebral blood vessels of diabetic patients and in a (streptozotocin-treated) rat model of diabetes mellitus. Eur J Endocrinol 158:655–660
Wang SH, Sun ZL, Guo YJ, Yuan Y, Yang BQ (2009) Diabetes impairs hippocampal function via advanced glycation end product mediated new neuron generation in animals with diabetes-related depression. Toxicol Sci 111:72–79
Wrighten SA, Piroli GG, Grillo CA, Reagan LP (2009) A look inside the diabetic brain: contributors to diabetes-induced brain aging. Biochim Biophys Acta 1792:444–453
Yamawaki H, Saito K, Okada M, Hara Y (2008) Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am J Physiol Cell Physiol 295:C1510–C1517
Yao D, Brownlee M (2010) Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 59:249–255
Acknowledgment
The work was supported by the Natural Science Foundation of Jiangsu Province (BK2011208), China, China Postdoctoral Science Foundation (201150M1576), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China. The experiments comply with the current laws of the People’s Republic of China.
Conflict of interest
The authors have declared that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yao-Wu Liu and Xia Zhu contributed equally to this project.
Rights and permissions
About this article
Cite this article
Liu, YW., Zhu, X., Yang, QQ. et al. Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology 228, 585–594 (2013). https://doi.org/10.1007/s00213-013-3061-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3061-5