Abstract
Rationale
Past research has demonstrated that when an animal changes from a previously drug-naive to an opiate-dependent and withdrawn state, morphine's motivational effects are switched from a tegmental pedunculopontine nucleus (TPP)-dependent to a dopamine-dependent pathway. Interestingly, a corresponding change is observed in ventral tegmental area (VTA) GABAA receptors, which change from mediating hyperpolarization of VTA GABA neurons to mediating depolarization.
Objectives
The present study investigated whether pharmacological manipulation of VTA GABAA receptor activity could directly influence the mechanisms underlying opiate motivation.
Results
Using an unbiased place conditioning procedure, we demonstrated that in Wistar rats, intra-VTA administration of furosemide, a Cl− cotransporter inhibitor, was able to promote a switch in the mechanisms underlying morphine's motivational properties, one which is normally observed only after chronic opiate exposure. This behavioral switch was prevented by intra-VTA administration of acetazolamide, an inhibitor of the bicarbonate ion-producing carbonic anhydrase enzyme. Electrophysiological recordings of mouse VTA showed that furosemide reduced the sensitivity of VTA GABA neurons to inhibition by the GABAA receptor agonist muscimol, instead increasing the firing rate of a significant subset of these GABA neurons.
Conclusions
Our results suggest that the carbonic anhydrase enzyme may constitute part of a common VTA GABA neuron-based biological pathway responsible for controlling the mechanisms underlying opiate motivation, supporting the hypothesis that VTA GABAA receptor hyperpolarization or depolarization is responsible for selecting TPP- or dopamine-dependent motivational outputs, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine neurotransmission has long been proposed to be critical for drug motivation (Robinson and Berridge 1993; Wise 1982). However, multiple drugs of abuse have been shown to possess positive motivational properties even in the absence of dopaminergic signaling (Cunningham et al. 1992; Hnasko et al. 2005; Laviolette and van der Kooy 2003; Olmstead and Franklin 1993; Pettit et al. 1984). For example, past research suggests that in previously drug-naive animals, opiate reinforcement requires the brainstem tegmental pedunculopontine nucleus (TPP), and dopamine is critical only in animals receiving substantial opiate exposure (Bechara and van der Kooy 1992; Olmstead and Franklin 1993; Olmstead et al. 1998). This “switch” in substrates is hypothesized to be under the control of ventral tegmental area (VTA) GABAergic GABAA receptor activity. Specifically, it has been suggested that GABAA receptor-dependent hyperpolarization of VTA GABA neurons is responsible for selecting a TPP-dependent opiate motivational pathway, and conversely, GABAA receptor-dependent depolarization of VTA GABA neurons is responsible for selecting a dopamine-dependent pathway (Laviolette et al. 2004; Vargas-Perez et al. 2009).
Considerable evidence suggests that activation of GABAA receptors can produce depolarization in lieu of its more traditional hyperpolarizing response (Coull et al. 2003; Hubner et al. 2001; Kaila et al. 1993; Rivera et al. 1999; Staley et al. 1995). One hypothesis to explain this concerns the neuronal KCC2 cotransporter, which removes intracellular Cl− and thereby maintains an inward-directed hyperpolarizing Cl− flow (Rivera et al. 2002; Thompson et al. 1988; Viitanen et al. 2010). Consequently, blockade of KCC2 should result in a build-up of intracellular Cl− and a reduction in Cl− ion influx after long-term GABAA receptor activation, allowing other ion flows (such as a depolarizing bicarbonate efflux) to dominate (Coull et al. 2003; Kaila et al. 1993; Rivera et al. 1999; Staley et al. 1995; Sun et al. 2012; Sun and Alkon 2001). If VTA GABAA receptor signaling activity is directly responsible for controlling the mechanisms underlying opiate motivation, artificial manipulation of this signaling should allow for the selection of either TPP- or dopamine-dependent mechanisms of opiate motivation, regardless of past drug history.
To address this question, we used infusions of furosemide (KCC2 inhibitor) and acetazolamide (inhibitor of the enzyme responsible for generating bicarbonate) in conjunction with a place conditioning paradigm to investigate whether the mode of VTA GABAA receptor signaling is directly responsible for determining the substrates underlying opiate motivation (Fig. 1). Additionally, we performed electrophysiological recordings of VTA GABA and dopamine neurons to examine the actions of furosemide and acetazolamide. An ability to artificially induce a switch to a dopamine-dependent system, in lieu of the normal requirement for extensive drug exposure, would provide compelling evidence for a VTA GABAA receptor-based switching mechanism.
A proposed (and testable) model for the change of an inhibitory GABAA receptor to an excitatory GABAA receptor. In the drug-naive state (left), VTA GABAA receptor activation of GABA neurons produces an inward Cl− ion flow that overshadows the outward-directed HCO3 − (bicarbonate) ion flow. This gradient is maintained by intracellular Cl− removal via the KCC2 cotransporter. Drug-dependent animals (right) are hypothesized to have excitatory VTA GABAA receptors caused by a breakdown of the Cl− gradient. One potential explanation for this is blockade of the KCC2 cotransporter (for example, as occurs with furosemide), resulting in a decreased inward-directed Cl− ion flow due to a lack of Cl− clearance. Now, the flow of bicarbonate ions out of the cell is able to produce a shift in the signaling properties of VTA GABAA receptors, from inhibitory (hyperpolarizing) to excitatory (depolarizing). The enzyme carbonic anhydrase catalyzes the formation of bicarbonate ions; therefore, inhibition of this enzyme using acetazolamide is hypothesized to decrease the production of bicarbonate and thereby prevent a switch to an excitatory GABAA receptor
Materials and methods
Animals
Male Wistar rats (Charles River; 350–450 g at the start of experiments) were utilized for all behavioral experiments at the University of Toronto. Subjects were singly housed in Plexiglas cages at 22 °C (lights on 7:00 a.m. to 7:00 p.m.). Access to standard rodent chow was ad libitum. Water was always freely available. All behavioral experiments were approved by the University of Toronto Animal Care Committee in accordance with the Canadian Council on Animal Care guidelines. A total of 296 animals were used for the behavioral analyses. All electrophysiology experiments were performed at Brigham Young University.
Surgery
Surgery was performed under isoflurane anesthesia (5 % induction, 1–3 % maintenance). Ketoprofen (5 mg/kg) was administered as an analgesic. VTA 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA, USA) were implanted bilaterally at a 10° angle using the following coordinates relative to bregma: AP, −5.3 mm; ML, ±2.3 mm; and DV, −8.0 mm from the dural surface. Dorsal controls used DV, −7.0. TPP and nucleus accumbens (NAc) cannulae were implanted at 10° angles (AP, −7.6; ML, ±3.1; DV, −6.6 and AP, +1.8; ML, ±3.1; DV, −6.8, respectively). Animals were allowed to recover for at least 1 week prior to conditioning.
Drugs
Furosemide (0.1–1 mM), acetazolamide (50 μM), imidazole (2.5 mM), lidocaine (4 %), and alpha-flupenthixol (0.01 M; Sigma) were dissolved in Ringer's solution (pH = 7.4) and infused bilaterally (0.5 μL per side). Doses were chosen based on previous studies (Laviolette and van der Kooy 2004; Sun and Alkon 2001; Viitanen et al. 2010). Diamorphine hydrochloride (heroin; 0.5 mg/kg s.c.), morphine sulfate (10 mg/kg i.p.; 500 ng per hemisphere intra-VTA; Almat Pharmachem Inc., Concord, ON, Canada), and alpha-flupenthixol (0.8 mg/kg i.p.) were dissolved in a 0.9 % saline solution.
Place conditioning apparatus
Conditioning took place in one of two distinct environments that differed in color, texture, and smell. One environment (41 × 41 × 38 cm) was black with a smooth black Plexiglas floor and was scented with 0.3 mL of a 10 % acetic acid solution prior to each conditioning session. The other environment had identical dimensions and was white with a metallic mesh floor.
Place conditioning procedure
Rats were conditioned using a fully counterbalanced (an equal number of rats were administered morphine in both conditioning environments, and an equal number of rats received morphine or saline for the first injection), unbiased place conditioning procedure (Mucha et al. 1982). All groups underwent one conditioning session (40 min) during the light cycle each day until a total of eight sessions (four morphine and saline pairings) were completed. Morphine injections directly preceded exposure to the conditioning environments. Pretreatments of either saline or alpha-flupenthixol occurred 2.5 h prior to conditioning (15 min prior in the case of intra-NAc alpha-flupenthixol).
Intra-cranial infusions (0.5 μL/hemisphere) occurred over 1 min (plus an additional minute to allow for drug diffusion from the injector tip) prior to i.p. injections and conditioning, for all conditioning sessions. For experiments involving both furosemide and acetazolamide, the drugs were dissolved in the same solution and infused simultaneously.
For studies involving opiate-dependent and withdrawn rats, heroin (0.5 mg/kg, s.c., once per day) was administered for four consecutive days prior to conditioning to induce a state of dependence. This treatment regimen produces strong locomotor sensitization and conditioned place aversions (Laviolette et al. 2004). Conditioning occurred approximately 21 h after a heroin injection, and maintenance doses were administered 3.25 h after conditioning (to dissociate the heroin from the conditioning process). Eight conditioning sessions were spaced evenly over 16 days, with rats receiving heroin each day.
In all cases, testing occurred 1 week after conditioning was completed. Rats (in a drug-free state) were placed in a neutral gray zone (41 × 10 cm) separating the two conditioning environments and were allowed to freely explore all three distinct areas for a 10-min period. The time spent in all three compartments was recorded. This procedure produces robust place preferences for the morphine-paired environment as compared with the vehicle-paired or neutral environment (Supplementary Figure 1).
Histology
Animals that underwent surgery were deeply anesthetized with sodium pentobarbital (0.8 mg/ml, i.p.; Animal Resources Centre, Montreal, QC, Canada) and perfused transcardially with 200 ml each of saline and 4 % formaldehyde. Brains were rapidly removed and stored for 24 h in a 25 % sucrose/4 % formaldehyde post-fixative, sliced into 30-μm-thick sections, and mounted on gelatin-coated slides. Correct placements were verified with cresyl violet staining and light microscopy (Paxinos and Watson 1986). Investigators were blind to the behavioral performance of the animals during analyses. Subjects were excluded if either of their placements were situated outside of the targeted area. There were no observed effects on brain tissue (e.g., increases in cell death at the injection sites) due to drug infusions.
Preparation of tissue slices for electrophysiological recordings
Experiments were performed in adult (>28 days) male GAD-GFP knock-in mice in order to positively identify VTA GABA neurons by visual inspection of fluorescence. For brain extraction, mice were anesthetized with isoflurane (5 %) and by i.p. injection of ketamine (60 mg/kg) and decapitated. The brain was quickly removed and glued onto a cutting stage. Slicing was performed using a sapphire blade (Electron Microscopy Sciences, Hatfield, PA, USA) on a vibratome in an ice-cold cutting solution (in millimolar: 220 sucrose, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 12 MgSO4, 10 glucose, 0.2 CaCl2, and 0.4 ketamine) perfused with 95 % O2 and 5 % CO2. Horizontal slices (210-μm-thick) containing the VTA were cut and transferred into an incubation chamber containing artificial cerebral spinal fluid (ACSF; in millimolar: 124 NaCl, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 12 glucose, 1.2 MgSO4, and 2 CaCl2) perfused with 95 % O2 and 5 % CO2 at 32 °C for at least 30 min. Following incubation, slices were transferred to a recording chamber with continuous flow of ACSF maintained at 36 °C throughout the experiment.
Characterization of VTA neurons in vitro
In GAD-GFP knock-in mice (Tamamaki et al. 2003), GABA neurons were studied in horizontal brain slices with the aid of fluorescence microscopy. The VTA was visualized by first locating the substantia nigra reticulata (SNr) in the horizontal slice preparation under low power (×4) with fluorescence illumination. The SNr has a characteristic glow under low magnification with GFP fluorescence optics, likely due to dense GABA terminal innervation. Substantia nigra compacta (SNc) was then identified medial to SNr. GABA neurons in the VTA were studied by visualizing GAD+ neurons in an area medial to the glowing SNr, posterior to the fasciculus retroflexus and mammillothalamic tract, anterior to the decussation of the superior cerebellar peduncle, and dorsal to the interpeduncular nucleus (Allison et al. 2011; Steffensen et al. 2011). Neurons in the VTA of GAD-GFP mice that did not fluoresce (i.e., GAD−) but exhibited a non-cation specific inward rectifying current (I h) in whole-cell mode following cell-attached mode recordings of firing rate (see below), and had relatively low input resistance and regular, slow spike activity were assumed to be dopamine neurons (Allison et al. 2006, 2011; Johnson and North 1992b; Margolis et al. 2006; Steffensen et al. 2011).
Cell-attached, voltage-clamp recording of spike activity in brain slices
Electrodes were pulled from borosilicate glass capillaries and then filled with 150 mM NaCl (3–5 MΩ). Positive pressure was applied to the electrode when approaching the neuron. By applying suction to the electrode, a seal (10 MΩ–1 GΩ) was created between the cell membrane and the recording pipette. Spontaneous GABA spike activity was recorded in voltage-clamp mode with an Axon Instruments Multiclamp 700B amplifier and sampled at 10 kHz using an Axon 1440A digitizer, and collected and analyzed using pClamp10 software. Neurons were voltage-clamped at 0 mV throughout the experiment. A stable baseline recording of firing activity was obtained for 5–10 min. Two methods were used to assess the effects of muscimol on VTA GABA neuron firing rate. In some slices, only one dose of muscimol (Sigma-Aldrich, St. Louis, MO, USA; 0.01–10 μM) was studied on one cell in each slice. In other slices, a wash was performed between superfusions of different concentrations of muscimol on the same cell (5–10 min at each dose with 10 min between doses). Regardless, no more than one cell was studied/slice, and no more than three slices were studied/animal.
Statistical analyses
Data were analysed using two-tailed Student's t tests or analysis of variance (ANOVA) where appropriate (alpha = 0.05), followed by post hoc Tukey's HSD or Dunnett's method tests as required. Where appropriate, results are expressed as means ± standard error of the mean (SEM).
Results
Intra-VTA furosemide switches systemic opiate motivation from dopamine-independent to dopamine-dependent in non-deprived animals
In drug-naive, non-deprived rats receiving intra-VTA vehicle and pretreated with either saline or alpha-flupenthixol (a broad-spectrum dopamine receptor antagonist; N = 6–7), a 2 × 2 ANOVA (pretreatment × drug conditioning) revealed a significant effect of drug conditioning (morphine vs. saline) [F(1, 22) = 21.5, p < 0.05], suggesting that non-deprived rats demonstrated morphine place preferences irrespective of alpha-flupenthixol pretreatment (Fig. 2a).
In non-deprived rats, systemic morphine place preferences are attenuated by dopamine receptor antagonism only after intra-VTA furosemide infusion. a Systemic morphine induced conditioned place preferences (gray bars) in rats receiving intra-VTA vehicle irrespective of pretreatment with alpha-flupenthixol. b Conversely, in rats receiving intra-VTA furosemide, systemic morphine place preferences were attenuated by alpha-flupenthixol pretreatment (left). In alpha-flupenthixol pretreated rats infused with furosemide 1 mm dorsal to the VTA, strong morphine place preferences were observed, indicating that furosemide is likely producing its effects in the VTA (right). In rats infused with intra-VTA furosemide, significant systemic morphine-conditioned place preferences were present even in alpha-flupenthixol pretreated rats after intra-VTA infusion of acetazolamide (right). c Systemic morphine did not produce conditioned place preferences in rats receiving intra-TPP lidocaine. Conversely, rats receiving intra-VTA furosemide demonstrated significant systemic morphine place preferences even after intra-TPP lidocaine. Data represent means ± SEMs of time spent in the morphine- or saline-paired environment
We next examined the effect of intra-VTA furosemide on systemic morphine's motivational properties in non-deprived rats. If an excitatory mode of VTA GABAA receptor signaling activity is responsible for selecting a dopamine-dependent mechanism of opiate motivation, we hypothesized that intra-VTA infusion of furosemide would promote such a switch. Rats were pretreated with either saline or alpha-flupenthixol (N = 6–7). A 2 × 2 ANOVA (pretreatment × drug conditioning) revealed a significant effect of drug conditioning (morphine vs. saline) [F(1, 22) = 18.4, p < 0.05] and a significant interaction [F(1, 22) = 9.0, p < 0.05]. This suggested that after intra-VTA furosemide, non-deprived rats demonstrated systemic morphine place preferences that were now sensitive to alpha-flupenthixol pretreatment (Fig. 2b, left). A similar effect was observed for intra-VTA as opposed to systemic morphine place preferences, and a similar effect also was seen at a tenfold lower dose of furosemide (Supplementary Figure 2).
To confirm the VTA as furosemide's site of action, the previous experiment was repeated with cannulae targeted 1 mm dorsal to the VTA. A t test indicated a significant effect of morphine [t(1, 10) = 13.8, p < 0.05], suggesting that furosemide was acting in the VTA and not via diffusion up the cannula tract (Fig. 2b, right; N = 6).
Past work has demonstrated that the positive motivational effects of morphine in non-deprived animals are blocked by intra-TPP infusions of lidocaine (Vargas-Perez et al. 2009). According to our hypothesis, intra-VTA furosemide should allow for the expression of a morphine place preference even in the presence of intra-TPP lidocaine, as opiate motivation will be switched to a dopamine-dependent system. Therefore, we examined the effects of either intra-VTA vehicle or furosemide (N = 7–9) on morphine's motivational properties in non-deprived rats receiving intra-TPP lidocaine. A 2 × 2 ANOVA (VTA treatment × drug) revealed a significant effect of drug conditioning (morphine vs. saline) [F(1, 28) = 10.2, p < 0.05] and a significant interaction [F(1, 28) = 8.8, p < 0.05]. This suggested that after intra-VTA furosemide (but not vehicle), non-deprived rats were in fact capable of demonstrating morphine place preferences even after intra-TPP lidocaine (Fig. 2c).
In opiate-dependent and withdrawn animals, intra-VTA acetazolamide switches systemic opiate motivation from dopamine-dependent to dopamine-independent
We first examined whether intra-VTA saline vehicle had any effect on systemic morphine's motivational properties in opiate-dependent and withdrawn (deprived) rats. Rats were pretreated with either saline or alpha-flupenthixol (N = 13–16). A 2 × 2 ANOVA (pretreatment × drug conditioning) revealed a significant effect of drug conditioning (morphine vs. saline) [F(1, 54) = 6.09, p < 0.05] and a significant interaction [F(1, 54) = 5.04, p < 0.05], indicating that morphine's motivational effects in opiate-deprived rats were blocked by alpha-flupenthixol (Fig. 3a).
Intra-VTA acetazolamide switches systemic morphine motivation from dopamine-dependent to dopamine-independent in opiate-dependent and withdrawn animals. a In opiate-dependent and withdrawn rats receiving intra-VTA saline vehicle, systemic morphine-conditioned place preferences (gray bars) were blocked in rats pretreated with alpha-flupenthixol. b (Left) This block was reversed by intra-VTA acetazolamide. (Right) Intra-NAc alpha-flupenthixol also blocked morphine-conditioned place preferences in opiate-dependent and withdrawn rats, and this also was reversed by intra-VTA acetazolamide. Data represent means ± SEMs of time spent in the morphine- or saline-paired environment
According to our hypothesis, bicarbonate ions are crucial for the shift to an excitatory/depolarizing mode of GABAA receptor signaling and, therefore, a shift to a dopamine-dependent mechanism of opiate motivation. Consequently, we examined the effect of intra-VTA acetazolamide on systemic morphine's motivational properties in opiate-dependent and withdrawn rats. Rats were pretreated with either saline or alpha-flupenthixol (N = 8–13). A 2 × 2 ANOVA (pretreatment × drug conditioning) revealed a significant effect of drug conditioning (morphine vs. saline) [F(1, 38) = 16.3, p < 0.05] only. This suggested that after intra-VTA acetazolamide, opiate-deprived rats demonstrated morphine place preferences irrespective of alpha-flupenthixol pretreatment (Fig. 3b, left).
To determine whether dopaminergic signaling within the NAc was critical for the motivational properties of morphine in opiate-deprived rats, we repeated the above experiment using intra-NAc infusions of alpha-flupenthixol (N = 6–10). A 2 × 2 ANOVA (VTA treatment × drug conditioning) revealed a significant effect of drug conditioning (morphine vs. saline) [F(1, 28) = 13.2, p > 0.05] and a significant interaction [F(1, 28) = 7.02, p < 0.05]. This suggested that opiate-deprived morphine place preferences were blocked by intra-NAc alpha-flupenthixol, but this was reversed by intra-VTA acetazolamide (Fig. 3b, right). Intra-VTA acetazolamide had no effect in drug-naive animals (Supplementary Figure 3).
Intra-VTA acetazolamide reverses the effects of intra-VTA furosemide
Since acetazolamide is able to prevent the switch to a dopamine-dependent opiate motivational system in opiate-dependent and withdrawn animals (Fig. 3), we tested whether acetazolamide also would reverse the effect of furosemide. In non-deprived animals pretreated with alpha-flupenthixol, we co-infused furosemide and acetazolamide into the VTA of animals conditioned with systemic morphine (N = 7). A t test revealed a significant effect of morphine [t(1, 12) = 11.3, p < 0.05] (Fig. 2b, right), indicating that acetazolamide was able to fully reverse the effect of furosemide (in non-deprived rats) and prevent a switch to a dopamine-dependent motivational system.
In opiate-dependent and withdrawn animals receiving intra-VTA acetazolamide, morphine place preferences are blocked by intra-TPP lidocaine
We next examined whether intra-TPP lidocaine would block systemic morphine place preferences in opiate-dependent and withdrawn rats that also received intra-VTA acetazolamide. All rats received acetazolamide and either intra-TPP vehicle or lidocaine (N = 6–12). A 2 × 2 ANOVA (TPP treatment × drug conditioning) revealed a significant effect of drug conditioning (morphine vs. saline) [F(1, 32) = 16.6, p < 0.05] and a significant interaction [F(1, 32) = 9.4, p < 0.05]. This suggested that opiate-deprived subjects receiving acetazolamide were switched to a TPP-dependent pathway, and therefore, intra-TPP lidocaine was now capable of blocking this morphine place preference (Fig. 4).
TPP lidocaine blocks morphine-conditioned place preferences in opiate-dependent and withdrawn animals that receive intra-VTA acetazolamide. In opiate-dependent and withdrawn animals receiving intra-VTA acetazolamide, systemic morphine-conditioned place preferences (gray bars) were blocked by intra-TPP lidocaine. Data represent means ± SEMs of time spent in the morphine- or saline-paired environment
Histological analysis
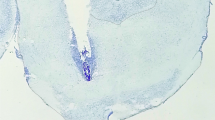
Representative intra-VTA morphine infusion cannulae placements are shown (N = 12–13) (Fig. 5). A one-way ANOVA revealed no differences between morphine infusions into the rostral, middle, or caudal VTA over all experiments [F(2, 34) = 0.76, p > 0.05] (inset). Similarly, no behavioral variation was observed with NAc placements (N = 16). There were no consistent rostral-caudal effects observed for acetazolamide, furosemide, imidazole, or TPP lidocaine infusions, and infusion misses behaved no differently from controls.
Histological analysis of intra-VTA bilateral cannulae placements. (Left) Schematic representation of rostral, middle, and caudal intra-VTA infusion placements (closed circles). For clarity, only a few bilateral cannulae placements are shown, for experiments involving acetazolamide, furosemide, or food deprivation, in addition to morphine. Rostral placements (−4.8 mm from bregma or before) are plotted on a −4.8-mm section, middle placements (between sections −4.8 and −5.8 mm) are plotted on a −5.3-mm section, and caudal placements (−5.8 mm or more) are plotted on a −5.8-mm section. Placements that were not located bilaterally within the VTA were excluded from analyses and are not shown. No differences were found between bilateral morphine infusions into the rostral, middle, or caudal VTA. Data represent means ± SEMs of time spent in the morphine-paired minus vehicle-paired environment. (Right) Histological analysis of intra-NAc bilateral cannulae placements. Schematic representation of rostral, middle, and caudal intra-NAc infusion placements. Placements are 2.2, 1.7, or 1.0 mm rostral to bregma. The majority of placements were located in the nucleus accumbens core. No behavioral variation was observed in these placements
Furosemide reduces muscimol inhibition of VTA GABA neurons
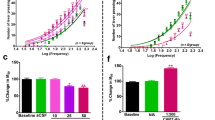
VTA GABA neurons in GAD GFP mice were positively identified by fluorescence imaging. The average firing rate of VTA GABA neurons recorded in control mice in vitro was 12.7 ± 1.7 Hz (N = 18), and in the presence of 100 μM furosemide was 12.2 ± 2.2 Hz (N = 13). Furosemide did not significantly affect the firing rate of VTA GABA neurons [F(1, 34) = 0.03, p > 0.05]. Muscimol (0.01–10.0 μM) briskly and markedly inhibited VTA GABA neuron firing rates in control mice (Fig. 6 (A)), with an approximate IC50 of 60 nM, but enhanced firing rates in furosemide-treated slices (Fig. 6 (B)). Figure 6 (C) summarizes the effects of muscimol (0.01–10.0 μM) on VTA GABA neurons in control vs. furosemide. A two-way ANOVA (treatment vs. muscimol concentration) revealed significant main effects of treatment (control vs. furosemide) [F(1, 68) = 8.45, p < 0.05] and muscimol concentration [F(3, 68) = 11.4, p < 0.05], as well as a significant treatment × concentration interaction [F(3, 68) = 5.9, p < 0.05]. Post-hoc Tukey's HSD test revealed that the furosemide treatment was significantly different from the control treatment only at the 0.1- and 1-μM concentrations of muscimol (p < 0.05). On the other hand, furosemide had no significant effect on muscimol-induced inhibition of putative VTA dopamine neurons, which were much less sensitive to muscimol than VTA GABA neurons (Supplementary Figures 4 and 5).
Furosemide effects on muscimol inhibition of VTA GABA neurons. VTA GABA neurons in GAD GFP mice were visualized with fluorescent optics and recorded in cell-attached mode in voltage clamp. A (a, b) These are representative 5-s traces of GABA neuron spike activity recorded before and after muscimol; (c) this ratemeter shows the firing rate of this neuron (traces in a, b recorded at times indicated on graph), which was approximately 10 Hz, before and after application of 0.01–10.0 μM muscimol, which markedly inhibited the firing rate of this VTA GABA neuron. B (a, b) These are representative 5-s traces of the spike activity of another VTA GABA neuron spike activity recorded before and after muscimol in the presence of 100 μM furosemide; (c) this ratemeter shows the firing rate of this neuron (traces in a, b recorded at times indicated on graph), which was approximately 8 Hz, before and after application of 0.01–10.0 μM muscimol. Only 10 μM muscimol inhibited the firing rate of this neuron in the presence of furosemide. C Comparison of muscimol (0.01–10.0 μM) effects on VTA GABA neuron firing rate for control and furosemide treatment conditions. Muscimol significantly inhibited the firing rate of VTA GABA neurons, which was significantly reduced by furosemide. D Whereas in control conditions 100 % of VTA GABA neurons were inhibited in response to muscimol (0.1 μM), furosemide treatment produced muscimol-induced excitation in a significant population of cells. A higher dose of muscimol (1 μM) produced similar results (data not shown)
While 7/7 (100 %) VTA GABA neurons were significantly inhibited after application of muscimol (0.1 and 1 μM) in control subjects (as compared to their baseline responses at 10 nM), fewer than 50 % of VTA GABA neurons were inhibited in response to muscimol after furosemide treatment (Fig. 6 (D)). Instead, 4/8 (50 %) (0.1 μM) and 3/9 (33 %) (1 μM) GABA neurons increased their firing rates (>25 %). Additionally, approximately 25 % of the GABAergic cells showed no significant hyperpolarization in response to muscimol after the furosemide treatment, while another 25 % showed a similar degree of inhibition in response to muscimol as in controls (Fig. 6 (D)). Furthermore, co-administration of acetazolamide (50 μM) and furosemide partially attenuated the effect of furosemide (100 nM muscimol firing rate, 68.9 ± 21.1; 1 μM firing rate, 75.2 ± 41.9), resulting in the inhibition of 4/6 (66 %) GABA neurons in response to muscimol application. At both concentrations of muscimol, one-way ANOVAs (control vs. furosemide vs. furosemide + acetazolamide) revealed significant main effects of treatment {100 nM [F(2, 22) = 4.04, p < 0.05] and 1 μM [F(2, 28) = 4.52, p < 0.05]}. In both cases, Dunnett's method revealed a significant difference between control and furosemide, and not between control and furosemide + acetazolamide treatments.
Discussion
The mechanisms underlying the transformation from a drug-naive animal to one consumed by drug-seeking and drug-taking behavior are crucial for our understanding of addiction. Our work supports the hypothesis that during this transformation, a change in VTA GABAA receptor signaling activity produces a corresponding change in the mechanisms responsible for opiate motivation (Laviolette et al. 2004; Vargas-Perez et al. 2009). Our work indicates a novel significance for depolarizing VTA GABAA receptor activity, adding to their growing number of reported functions (Auger et al. 2001; Ben-Ari 2002; Cherubini et al. 1991; Wagner et al. 1997).
The carbonic anhydrase enzyme is necessary for the switch to a dopamine-dependent motivational system in opiate-deprived animals
In the VTA, the majority of GABAA receptors reside on GABA cells (Churchill et al. 1992; Kalivas 1993), although some also are found on dopamine cells (Schwarzer et al. 2001). Functionally, it has been suggested that those located on the GABA cells are most behaviorally relevant (Laviolette and van der Kooy 2004). Although they are generally regarded as Cl− ion channels, GABAA receptors are also permeable to bicarbonate ions. Indeed, the efflux of negatively charged bicarbonate ions is one proposed mechanism behind GABAA receptor depolarization (Kaila 1994; Staley et al. 1995; Sun and Alkon 2001). Consistent with this idea, intra-VTA infusion of acetazolamide, an inhibitor of the carbonic anhydrase enzyme responsible for the generation of bicarbonate, was able to prevent a switch to a dopamine-dependent mechanism of opiate motivation. As this was true for systemic injections of morphine, even though opiates elicit motivational effects in areas other than the VTA (Goeders et al. 1984; Olds 1982; Wise 1989), our work suggests that VTA GABAA receptors mediate a “global” switching mechanism capable of gating the motivational properties of opiates towards either TPP- or dopamine-dependent pathways.
Intra-VTA furosemide switches the substrates mediating opiate motivation
Furosemide blocks the activity of KCC2 cotransporters that remove intracellular Cl− (Martina et al. 2001; Viitanen et al. 2010), an effect that should reduce the influx of Cl− ions and magnify the importance of the corresponding flow of bicarbonate out of the cell (Fig. 1). Therefore, we hypothesized that intra-VTA infusion of furosemide should precipitate a change in GABAA receptor activity and a corresponding switch in the mechanisms underlying opiate motivation, even in non-deprived animals with limited exposure to morphine. We observed precisely this result, supporting a VTA GABAA receptor switch model. Intra-VTA infusion of imidazole, a drug which is proposed to shift VTA GABAA receptor activity from hyperpolarizing to depolarizing via a different mechanism of action to furosemide (i.e., increased production of bicarbonate ions through carbonic anhydrase enzyme activation), produced a similar effect, further supporting our interpretation (Supplementary Figure 6).
However, while furosemide and acetazolamide were effective, we cannot rule out the possibility that they acted via alternative mechanisms of action or in a non-specific manner. For example, although this concentration of furosemide has been reported to have negligible effects on other Cl− cotransporters, or presumably, on the carbonic anhydrase enzyme itself (Viitanen et al. 2010), its potential actions on GABAA receptor subunit compositions (Fritschy et al. 1994) or on other VTA cell types (e.g., dopamine or glial cells) cannot be ruled out and warrant further investigation. It should be noted, however, that neither an infusion of furosemide dorsal to the VTA (Fig. 2b) nor an infusion of acetazolamide into drug-naive rats (Supplementary Figure 3) had any other observable effects.
Muscimol inhibition of VTA GABA neurons is significantly reduced by furosemide
We previously have demonstrated, in vivo, that a subpopulation of VTA GABA neurons switches their response to muscimol from inhibition to excitation in association with chronic morphine treatment (Laviolette et al. 2004) and with BDNF injections into the VTA (Vargas-Perez et al. 2009). In the latter study, the baseline firing rates of VTA GABA neurons were enhanced significantly in BDNF-treated rats. The baseline firing rates of VTA GABA neurons recorded ex vivo are significantly lower than in vivo. However, it is worth mentioning that the slice preparation is clearly a different environment from the intact animal. For example, we have previously demonstrated that the VTA GABA neuron firing rates in mice average approximately 32 Hz in vivo (Steffensen et al. 2011) compared to approximately 14 Hz ex vivo. We assume this is due to severing of excitatory synaptic inputs from the prefrontal cortex in vitro that typically drive VTA GABA neuron excitability in vivo. Nonetheless, muscimol robustly inhibited the firing rates of VTA GABA neurons at low concentrations (<1.0 μM) in GAD GFP mice, which was markedly reduced by superfusion of furosemide. This is consistent with our previous studies demonstrating a switch in GABA neuron response to muscimol in BDNF-treated rats (Vargas-Perez et al. 2009). Putative dopamine neurons were inhibited by muscimol, but only at the 10-μM concentration (Supplementary Figure 4). The approximate IC50 for GABA neurons was 60 nM while that for dopamine neurons was 5 μM—nearly a 100× difference in sensitivity—suggesting that dopamine neuron GABAA receptors are less sensitive to muscimol activation, or that muscimol's inhibition of GABA neurons is having a marked disinhibitory effect on dopamine neurons. The predominant GABAA receptor subunits expressed in the VTA are α1–6, β1, β3, and γ2 (Okada et al. 2004). While GABA neurons express α1 GABAA receptors, dopamine neurons do not (Tan et al. 2010), which might explain why GABA neurons in the VTA are more sensitive to muscimol than dopamine neurons. Most recently, we and others have shown that GABA neurons receive more GABA receptor-mediated inhibitory drive than dopamine neurons (Allison et al. 2011; Tan et al. 2010). In the horizontal slice preparation used in this study, GABA input may be from local circuit GABA neurons or, possibly, from GABA input from the rostromedial tegmental nucleus (RMTg, also called the tail of the VTA), an area that contains μ-opioid receptor + GABA neurons projecting to the VTA (Jhou et al. 2009). However, we have shown that VTA GABA neurons express μ-opioid receptors and are sensitive to local application of opioids (Steffensen et al. 2006), suggesting that GABA inhibitory drive to GABA neurons is most likely from neighboring μ-opioid receptor + VTA GABA neurons.
Inhibition of bicarbonate ion production can reverse the effect of furosemide
The behavioral effect of furosemide was reversed by administration of acetazolamide into the VTA. This suggests that the inhibitory effect of acetazolamide on the carbonic anhydrase enzyme is downstream of furosemide's proposed actions on KCC2 (Fig. 1). Supporting this interpretation are electrophysiological data showing that co-infusion of acetazolamide is able to attenuate the increase in GABA excitation induced by furosemide superfusion.
If furosemide blockade of KCC2 results in a decreased inward-directed Cl− ion flow, it is plausible that the outward-directed bicarbonate ion flow would now take precedence, leading to a shift in the signaling activity of VTA GABAA receptors. Conversely, administration of acetazolamide inhibits the activity of the carbonic anhydrase enzyme and thereby prevents both the generation of bicarbonate and the corresponding switch to an excitatory (depolarizing) GABAA receptor. In other words, treatment with acetazolamide returns the mechanisms underlying opiate motivation to a TPP-dependent state, presumably by returning the activity of VTA GABAA receptors to a hyperpolarizing state—even though furosemide is still blocking Cl− cotransporter extrusion. While hypothetical, this return to a hyperpolarizing mode of GABAA receptor activity may be due in part to an increased inward-directed Cl− ion driving force made possible by an overall decrease in intracellular negatively charged ions (due to acetazolamide inhibition of bicarbonate production).
Previous work has demonstrated that in food-deprived (but opiate-naive) rats, morphine motivation is not TPP-dependent but rather dopamine-dependent (Nader and van der Kooy 1994). That is, food deprivation is sufficient to induce a switch in the system underlying opiate motivation, even though the rats are previously drug-naive. We found that infusion of intra-VTA acetazolamide was sufficient to prevent this switch, suggesting that food deprivation may produce a similar effect on VTA GABAA receptors as opiate deprivation (Supplementary Figure 7). Although speculative, it is possible that both opiate and food deprivation produce a “highly stressed” state, leading to an increased release of brain-derived neurotrophic factor (BDNF) which is known to play a role in at least some types of stress (Berton et al. 2006; Sarkar et al. 2011). Indeed, BDNF levels are increased in opiate-deprived subjects (Vargas-Perez et al. 2009). The mechanisms underlying BDNF's actions are not fully known but may involve inhibition of KCC2, similar to furosemide (Rivera et al. 2002; Wake et al. 2007).
VTA GABAA receptors form a motivational switching mechanism for opiates
GABAA receptors on VTA GABA neurons are anatomically well positioned to mediate a switch between TPP- and dopamine-dependent systems (Kalivas 1993; Laviolette et al. 2004; Laviolette and van der Kooy 2004; Swanson 1982). Our work suggests that this switch is produced by changes in VTA GABAA receptor signaling activity.
According to the model, opiates bind to presynaptic μ-opioid receptors located on GABA terminals in the VTA, inhibiting activation of GABAA receptors (and have more limited functional activity at μ-opioid receptors located directly on VTA GABA cells) (Garzon and Pickel 2001; Laviolette et al. 2004; Omelchenko and Sesack 2009; Svingos et al. 2001; Xia et al. 2011). Additional research will be needed to clarify which specific populations of GABAergic inputs are critical, although recent work has suggested the NAc (Xia et al. 2011) and RMTg (Balcita-Pedicino et al. 2011; Jhou et al. 2009) as possibilities. Our work is more supportive of a NAc input, as GABA terminals from that region appear to synapse preferentially on VTA GABA cells, as opposed to dopamine cells (Jalabert et al. 2011; Lecca et al. 2011). In any event, tonic activation of GABAA receptors in opiate-naive animals hyperpolarizes GABA cells (Laviolette et al. 2004), and therefore, opiate blockade of this activation will depolarize GABAergic neurons. This results in continued GABAergic inhibition of VTA dopamine neurons and the generation of a TPP-dependent motivational signal (Heinmiller et al. 2009; Fig. 7, left).
A hypothetical representation of opiate motivation in the non-deprived and drug-dependent and withdrawn motivational states. In non-deprived animals (left), activation of VTA GABAA receptors produces hyperpolarization (inhibition). Opiate binding to presynaptic μ-opioid receptors reduces GABAA receptor activation. Consequently, VTA GABA cells are depolarized as a result of the loss of an inhibitory input. In turn, VTA dopamine cells remain inhibited, and an opiate reinforcement signal is sent to the TPP. Conversely, in opiate-deprived animals (right), activation of VTA GABAA receptors produces depolarization (excitation). Opiate binding to presynaptic μ-opioid receptors again reduces VTA GABAA receptor activation. In turn, VTA GABA cells are hyperpolarized due to the loss of an excitatory input, and VTA dopamine cells are disinhibited, leading to the production of a dopamine-dependent opiate motivational signal initiated in the VTA and terminating in the NAc. The change to a drug-deprived state can be accomplished by extensive opiate exposure (enough to produce withdrawal), intra-VTA furosemide administration, or food deprivation, and can be prevented by intra-VTA acetazolamide administration
Conversely, if an opiate-deprived state is synonymous with VTA GABAA receptors that produce depolarization, opiate administration would have the exact opposite effect: prevention of VTA GABAA receptor activation would hyperpolarize VTA GABA cells and therefore disinhibit VTA dopamine cells, producing a dopamine-dependent motivational signal that is transmitted from the VTA to the NAc (Fig. 7, right). Additionally, it is possible that morphine binding to μ-opiate receptors located on the VTA GABA neurons themselves—which also would disinhibit the dopamine cells and produce dopamine-dependent opiate reward—plays a factor here (Johnson and North 1992a).
While this proposed model is based on local VTA GABA neuron projections to dopamine cells, it is well established that the anatomy of the VTA is far more complex. It remains unclear whether the various other subpopulations of VTA GABA and dopamine neurons —with their diverse cellular inputs and outputs—might fit into this model (Jhou et al. 2009; Kalivas 1993; Omelchenko and Sesack 2005; Sesack and Pickel 1992; Thierry et al. 1980; Van Bockstaele and Pickel 1995). Future work is needed to determine whether the depolarizing actions of glutamate might be related to the VTA GABAA receptor switch model (Dobi et al. 2010; Kalivas 2009). For example, glutamatergic signaling in rat hippocampal neurons has been reported to downregulate KCC2 channel activity, resulting in reduced GABAergic hyperpolarization (Lee et al. 2011). Similarly, it would be interesting to ascertain how (if at all) the results reported here might fit in with reports of withdrawal-induced GABA release (Bonci and Williams 1997) and long-term potentiation (Niehaus et al. 2010) in the VTA, all of which result in an increase in VTA dopamine cell firing after increased opiate exposure.
In summary, the current findings are consistent with the hypothesis that GABAA receptors on VTA GABA neurons form a switching mechanism that controls the substrates underlying opiate motivation, irrespective of the animal's previous drug history. As muscimol inhibition of VTA GABA neurons can be reversed by furosemide, and treatment with acetazolamide is sufficient to partially attenuate this reversal and prevent the occurrence of a behavioral switch, our findings suggest that carbonic anhydrase may constitute the downstream portion of a common VTA GABA neuron-based biological pathway responsible for controlling the mechanisms underlying opiate motivation.
References
Allison DW, Ohran AJ, Stobbs SH, Mameli M, Valenzuela CF, Sudweeks SN, Ray AP, Henriksen SJ, Steffensen SC (2006) Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse 60:20–31
Allison DW, Wilcox RS, Ellefsen KL, Askew CE, Hansen DM, Wilcox JD, Sandoval SS, Eggett DL, Yanagawa Y, Steffensen SC (2011) Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: a physiologic role for connexin-36 GAP junctions. Synapse 65:804–813
Auger AP, Perrot-Sinal TS, McCarthy MM (2001) Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci U S A 98:8059–8064
Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR (2011) The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol 519:1143–1164
Bechara A, van der Kooy D (1992) A single brain stem substrate mediates the motivational effects of both opiates and food in nondeprived rats but not in deprived rats. Behav Neurosci 106:351–363
Ben-Ari Y (2002) Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3:728–739
Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868
Bonci A, Williams JT (1997) Increased probability of GABA release during withdrawal from morphine. J Neurosci 17:796–803
Cherubini E, Gaiarsa JL, Ben-Ari Y (1991) GABA: an excitatory transmitter in early postnatal life. Trends Neurosci 14:515–519
Churchill L, Dilts R, Kalivas P (1992) Autoradiographic localization of gamma-aminobutyric acid-A receptors within the ventral tegmental area. Neurochem Res 17:101–106
Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424:938–942
Cunningham CL, Malott DH, Dickinson SD, Risinger FO (1992) Haloperidol does not alter expression of ethanol-induced conditioned place preference. Behav Brain Res 50:1–5
Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M (2010) Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci 30:218–229
Fritschy J-M, Paysan J, Enna A, Mohler H (1994) Switch in the expression of rat GABA-A-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci 14:5302–5324
Garzon M, Pickel VM (2001) Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse 41:311–328
Goeders NE, Lane JD, Smith JE (1984) Self-administration of methionine enkephalin into the nucleus accumbens. Pharmacol Biochem Behav 20:451–455
Heinmiller A, Ting-A-Kee R, Vargas-Perez H, Yeh A, van der Kooy D (2009) Tegmental pedunculopontine glutamate and GABA-B synapses mediate morphine reward. Behav Neurosci 123:145–155
Hnasko TS, Sotak BN, Palmiter RD (2005) Morphine reward in dopamine-deficient mice. Nature 438:854–857
Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ (2001) Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 30:515–524
Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F (2011) Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A 108:16446–16450
Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800
Johnson SW, North RA (1992a) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Johnson SW, North RA (1992b) Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 450:455–468
Kaila K (1994) Ionic basis of GABA-A receptor channel function in the nervous system. Prog Neurobiol 42:489–537
Kaila K, Voipio J, Paalasmaa P, Pasternack M, Deisz RA (1993) The role of bicarbonate in GABA-A receptor-mediated IPSPs of rat neocortical neurones. J Physiol 464:273–289
Kalivas PW (1993) Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev 18:75–113
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572
Laviolette SR, van der Kooy D (2003) Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry 8:50–59
Laviolette SR, van der Kooy D (2004) GABAA receptors signal bidirectional reward transmission from the ventral tegmental area to the tegmental pedunculopontine nucleus as a function of opiate state. Eur J Neurosci 20:2179–2187
Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D (2004) Opiate state controls bi-directional reward signaling via GABA-A receptors in the ventral tegmental area. Nat Neurosci 7:160–169
Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M (2011) Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology 36:589–602
Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ (2011) NMDA receptor activity downregulates KCC2 resulting in depolarizing GABA(A) receptor-mediated currents. Nat Neurosci 14:736–743
Margolis EB, Lock H, Hjelmstad GO, Fields HL (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577:907–924
Martina M, Royer S, Pare D (2001) Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol 86:2887–2895
Mucha RF, van der Kooy D, O'Shaughnessy M, Bucenieks P (1982) Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243:91–105
Nader K, van der Kooy D (1994) The motivation produced by morphine and food is isomorphic: approaches to specific motivational stimuli are learned. Psychobiology 22:68–76
Niehaus JL, Murali M, Kauer JA (2010) Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci 32:108–117
Okada H, Matsushita N, Kobayashi K (2004) Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem 89:7–14
Olds ME (1982) Reinforcing effects of morphine in the nucleus accumbens. Brain Res 237:429–440
Olmstead MC, Franklin KB (1993) Effects of pedunculopontine tegmental nucleus lesions on morphine-induced conditioned place preference and analgesia in the formalin test. Neuroscience 57:411–418
Olmstead MC, Munn EM, Franklin KB, Wise RA (1998) Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci 18:5035–5044
Omelchenko N, Sesack SR (2005) Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol 483:217–235
Omelchenko N, Sesack SR (2009) Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse 63:895–906
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic, Toronto
Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984) Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berlin) 84:167–173
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K (1999) The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255
Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M (2002) BDNF-induced TrkB activation down-regulates the K+–Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol 159:747–752
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J (2011) Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci 31:18198–18210
Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G (2001) Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol 433:526–549
Sesack SR, Pickel VM (1992) Synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol 320:145–160
Staley KJ, Soldo BL, Proctor WR (1995) Ionic mechanisms of neuronal excitation by inhibitory GABA-A receptors. Science 269(5226):977–981
Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ (2006) Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp Neurol 202:139–151
Steffensen SC, Bradley KD, Hansen DM, Wilcox JD, Wilcox RS, Allison DW, Merrill CB, Edwards JG (2011) The role of connexin-36 gap junctions in alcohol intoxication and consumption. Synapse 65:695–707
Sun M-K, Alkon DL (2001) Pharmacological enhancement of synaptic efficacy, spatial learning, and memory through carbonic anhydrase activation in rats. J Pharmacol Exp Ther 297:961–967
Sun L, Yu Z, Wang W, Liu X (2012) Both NKCC1 and anion exchangers contribute to Cl(−) accumulation in postnatal forebrain neuronal progenitors. Eur J Neurosci 35:661–672
Svingos AL, Garzon M, Colago EEO, Pickel VM (2001) mu-opioid receptors in the ventral tegmental area are targeted to presynaptically and directly modulate mesocortical projection neurons. Synapse 41:221–229
Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study. Brain Res Bull 9:321–353
Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T (2003) Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467:60–79
Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C (2010) Neural bases for addictive properties of benzodiazepines. Nature 463:769–774
Thierry AM, Deniau JM, Herve D, Chevalier G (1980) Electrophysiological evidence for non-dopaminergic mesocortical and mesolimbic neurons in the rat. Brain Res 201:210–214
Thompson SM, Deisz RA, Prince DA (1988) Outward chloride/cation co-transport in mammalian cortical neurons. Neurosci Lett 89:49–54
Van Bockstaele EJ, Pickel VM (1995) GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res 682:215–221
Vargas-Perez H, Ting-A-Kee R, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D (2009) Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science 324:1732–1734
Viitanen T, Ruusuvuori E, Kaila K, Voipio J (2010) The K+–Cl− cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol 588:1527–1540
Wagner S, Castel M, Gainer H, Yarom Y (1997) GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387:598–603
Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, Asai K, Ojika K, Hirata M, Nabekura J (2007) Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci 27:1642–1650
Wise RA (1982) Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci 5:39–87
Wise RA (1989) Opiate reward: sites and substrates. Neurosci Biobehav Rev 13:129–133
Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO (2011) Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci 31:7811–7816
Acknowledgments
This work was funded by the Canadian Institutes of Health Research (CIHR), a Natural Sciences and Engineering Research Council of Canada (NSERC) graduate training award, and by a NIH grant AA020919 to SCS.
Conflicts of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 676 kb)
Rights and permissions
About this article
Cite this article
Ting-A-Kee, R., Vargas-Perez, H., Mabey, J.K. et al. Ventral tegmental area GABA neurons and opiate motivation. Psychopharmacology 227, 697–709 (2013). https://doi.org/10.1007/s00213-013-3002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3002-3