Abstract
Rationale
Obsessive-compulsive disorder (OCD) patients show overactivation of the orbitofrontal cortex and deficits in cognitive tasks that require proper orbitofrontal functioning including delayed alternation tests of spatial working memory. We recently showed that OCD-like behavior is induced in mice by activating orbitofrontal serotonin 1B receptors (5-HT1Bs). However, the role of 5-HT1Bs in delayed alternation remains unclear.
Objectives
We examined the effects of 5-HT1B receptor activation on delayed alternation task (DAT) performance. We also assessed the ability of an effective OCD treatment, fluoxetine, to prevent 5-HT1B-induced deficits in DAT performance.
Methods
Mice were tested on the DAT after acute treatment with saline, 3 or 6 mg/kg RU24969 (5-HT1B/1A agonist), 0.3 or 3 mg/kg 8-OH-DPAT (5-HT1A agonist), or co-injection with 3 mg/kg RU24969 and 5 mg/kg GR127935 (5-HT1B/1D antagonist). Separate mice were pretreated chronically (28 days) with 10 mg/kg fluoxetine and then tested on the DAT after acute treatment with 3 mg/kg RU24969, 0.3 mg/kg 8-OH-DPAT, or saline.
Results
Both doses of RU24969 decreased accuracy and increased latency on the DAT, and GR127935 blocked RU24969-induced effects on accuracy. The 0.3 mg/kg 8-OH-DPAT did not affect the DAT performance, whereas 3 mg/kg increased omissions on the DAT. Finally, RU24969-induced DAT deficits were absent in fluoxetine-pretreated mice.
Conclusions
We show that 5-HT1B receptor activation disrupts DAT performance in mice, and chronic fluoxetine pretreatment blocks these 5-HT1B-induced deficits. Our findings suggest that 5-HT1B receptors play an important role in modulating orbitofrontal-dependent delayed alternation. Moreover, 5-HT1B-induced DAT deficits may provide a mouse model for DAT deficits in OCD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alterations in orbitofrontal cortex (OFC) function have been implicated in the pathophysiology of obsessive-compulsive disorder (OCD). OCD patients show excessive activity of the OFC at rest (Baxter et al. 1988; Nordahl et al. 1989; Swedo et al. 1989) and following symptom provocation (Breiter et al. 1996; Cottraux et al. 1996) that is reversed after successful treatment (Benkelfat et al. 1990; Swedo et al. 1992; Saxena et al. 1999). Moreover, OCD patients show deficits on neuropsychological tests of orbitofrontal functioning such as the delayed alternation task (Abbruzzese et al. 1995, 1997; Gross-Isseroff et al. 1996; Moritz et al. 2001).
The delayed alternation task (DAT) is a well-established measure of spatial working memory (Butters and Rosvold 1968; Goldman et al. 1970; Goldman-Rakic 1987) that has been used across species, including rodents (Zahrt et al. 1997), cats (Markowitsch et al. 1980), pigeons (Gagliardo et al. 1996), monkeys (Goldman-Rakic 1987), and humans (Abbruzzese et al. 1995, 1997; Gross-Isseroff et al. 1996; Moritz et al. 2001). Delayed alternation tasks require participants to alternate between spatially distinct (e.g., left and right) choices on consecutive trials in order to gain rewards. Participants must learn this alternation rule through trial and error, and a delay is implemented between trials to increase task difficulty. The DAT is sensitive to frontal lobe damage in humans (Freedman and Oscar-Berman 1986) and non-human primates (Warren et al. 1964) and has been shown to be a measure of orbitofrontal functioning in studies of patients with brain lesions (Freedman et al. 1998). Moreover, deficits in DAT performance are seen in OCD, but not in other psychiatric conditions with working memory deficits such as schizophrenia (Abbruzzese et al. 1995, 1997).
Elucidation of the neural substrates that underlie behavior in the DAT may lead to a better understanding of orbitofrontal dysfunction in OCD. The serotonergic system has been implicated in the pathophysiology of OCD due in part to the fact that serotonin reuptake inhibitor (SRI) antidepressants provide the only effective pharmacological monotherapy for OCD (Pigott et al. 1990; Tollefson et al. 1994). However, very little is known about the role of the serotonergic system in delayed alternation. Pharmacological challenge with serotonin 1B receptor (5-HT1B) agonists exacerbates symptoms in OCD patients (Stein et al. 1999; Koran et al. 2001; Gross-Isseroff et al. 2004), and we recently provided preclinical evidence that orbitofrontal 5-HT1B receptors may underlie this effect. Specifically, we reported a novel mouse model of aspects of OCD in which acute injection of serotonin 1B receptor (5-HT1B) agonist induces OCD-like behavior, including perseverative locomotion and prepulse inhibition deficits in mice (Shanahan et al. 2009, 2011). These 5-HT1B-induced OCD-like behaviors are blocked by effective (SRIs), but not ineffective, OCD treatments with a time course that closely mimics the human therapeutic response (Shanahan et al. 2011). Moreover, activation of orbitofrontal 5-HT1B receptors is both necessary and sufficient to produce OCD-like behavior in this model (Shanahan et al. 2011). Since the DAT requires proper orbitofrontal functioning and is disrupted in OCD, we hypothesized that DAT performance could be disrupted by 5-HT1B receptor activation, and this deficit could be blocked by chronic SRI treatment.
The present study investigated the effects of 5-HT1B receptor activation on delayed alternation. Moreover, we examined the ability of fluoxetine, a SRI that provides first-line treatment for OCD, to block 5-HT1B-induced deficits in DAT performance. We used RU24969 to activate 5-HT1B receptors in this study because RU24969 is a selective agonist (K i = 0.38 nM) for rodent 5-HT1B receptors that crosses the blood–brain barrier (Glennon et al. 2000). Since RU24969 also has affinity for 5-HT1A receptors (K i = 2.5 nM) (Glennon et al. 2000), we examined the ability of GR127935, a selective 5-HT1B/1D receptor antagonist, to block RU24969-induced behavior. Moreover, we examined the effects of 8-OH-DPAT, a highly selective 5-HT1A agonist, on DAT performance.
Methods
Animals
Female C57BL/6 J mice (Jackson Laboratories, Bar Harbor, Maine) 7–11 weeks of age were used for all experiments. Mice were housed in a temperature-controlled colony room on a 12-h light/dark schedule with food and water available ad libitum. Behavioral testing occurred during the dark phase. Animal testing was conducted in accordance with the National Institutes of Health Laboratory Animal Care Guidelines and with the Institutional Animal Care and Use Committee approval.
Chemicals
RU24969 and 8-OH-DPAT (Tocris Bioscience, Minneapolis, MN, USA) were dissolved in 0.9 % saline as salt doses and injected IP and SC, respectively. GR127935 (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in distilled water and injected SC. All injected drugs were prepared at a volume of 5-mL/kg body weight with 1-cc syringes and 27 gauge needles. Fluoxetine was administered in the drinking water at a concentration of 80 mg/L to achieve a 10-mg/kg/day dose (Dulawa et al. 2004).
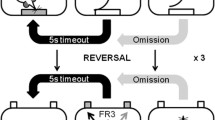
Delayed alternation
For these experiments, mice were food-restricted and were maintained at 75–85 % of their individual starting weight. Body weights were monitored daily. Delayed alternation tests were performed using a T-maze (each arm 31.5-cm long × 8.3-cm wide × 17.5-cm high) and were based on previously described methods (Zahrt et al. 1997). Upon reaching the weight criterion, mice were habituated to a T-maze in two phases.
Habituation to reward
During each session, mice were allowed to consume an unlimited amount of milk chocolate (Hershey’s, PA, USA) rewards (which were placed in each T-maze arm and continually replenished). Mice underwent one 8-min session per day and were subjected to this phase of habituation until ≥10 rewards were consumed per session in two consecutive sessions.
Habituation to injections and handling
Mice continued the above habituation schedule but were administered a saline injection 5 min prior to each session and were picked up and placed back in the start box immediately following the consumption of two consecutive rewards. Mice underwent one session per day until ≥10 rewards were consumed in 5 min for two consecutive sessions.
Testing
Sessions consisted of eleven 30-s trials with approximately 5 s between trials. Each trial began with a mouse placed in the start box. On the first trial, mice were rewarded for entering either arm (i.e., both arms were baited with reward). Thereafter, mice were rewarded only if they entered the maze arm that was not chosen previously (correct choice). After each arm entry (correct or incorrect), mice were placed back in the start box. The training period ended after the mice made ≥80 % correct choices on two consecutive sessions. Mice were then tested for their performance on the DAT under experimental conditions. Arm entry was defined as placement of all four paws into an arm. In all experiments, the T-maze was located in the same position so that potential spatial cues never changed.
Experiments
Experiments 1 and 2
Mice (n = 13) were tested on the DAT after acute drug treatments (saline, 3 mg/kg RU24969, or 0.3 mg/kg 8-OH-DPAT). Separate mice (n = 17) were tested on the DAT after acute drug treatments (saline, 6 mg/kg RU24969, or 3 mg/kg 8-OH-DPAT). In both experiments, mice were tested on three separate test days in a counterbalanced fashion. One day of rest separated each test day. On test day, mice received one acute injection before the first trial, and the session lasted under 7 min. Thus, the drugs were active throughout the entire DAT session.
Experiment 3
Mice (n = 16) were tested on the DAT after acute co-injection with saline/saline, saline/3 mg/kg RU24969, saline/5 mg/kg GR127935, or 3 mg/kg RU24969/5 mg/kg GR127935.
Experiment 4
All aspects were identical to experiment 1, except that mice were pretreated chronically (28 days average) with 0 or 10 mg/kg/day fluoxetine (n = 12/group).
Statistical analysis
For experiments 1–3, ANOVAs with acute drug treatment (vehicle, RU24969, 8-OH-DPAT, or GR127935) as a within-subject factor were applied to three DAT measures: accuracy (% of trials on which a correct choice was made), latency (duration of time prior to arm entry), and omissions (% of trials on which no arm was entered). Significance was set at p < 0.05.
For experiment 3, ANOVAs with SRI pretreatment (vehicle or fluoxetine) as a between-subject factor and acute drug treatment (vehicle, RU24969, or 8-OH-DPAT) as a within-subject factor were applied to three DAT measures: accuracy, latency, and omissions. Significant interactions were resolved using post hoc ANOVAs for within-subject factors and/or Newman Keuls post hoc tests for between-subject factors. Significance was set at p < 0.05, and p values for post hoc ANOVAs were adjusted using the Bonferroni correction.
For all experiments, only successful (i.e., no omission) trials were analyzed for accuracy and latency. Effects of acute drug treatments were analyzed with separate overall ANOVAs.
Results
Activation of 5-HT1B receptors induces spatial working memory deficits in mice
Mice treated with 3 mg/kg RU24969 performed worse on the DAT than vehicle-treated mice as revealed by a main effect of 3 mg/kg RU24969 on accuracy (F(1,12) = 13.27, p < 0.01) and latency (F(1,12) = 24.54, p < 0.001) (Fig. 1). No effects of 3 mg/kg RU24969 on omissions were found. Mice treated with 0.3 mg/kg 8-OH-DPAT were not different from vehicle-treated mice on any DAT measure (Fig. 1). In a follow-up study, mice were treated with higher doses of RU24969 and 8-OH-DPAT. Main effects of 6 mg/kg RU24969 were found on accuracy (F(1,16) = 6.37, p < 0.05), latency (F(1,16) = 17.822, p < 0.001), and omissions (F(1,16) = 4.92, p < 0.05) (Fig. 2). Furthermore, main effects of 3 mg/kg 8-OH-DPAT were found on accuracy (F(1,16) = 51.48, p < 0.001), latency (F(1,16) = 48.47, p < 0.001), and omissions (F(1,16) = 14.79, p < 0.01) (Fig. 2).
High dose of 5-HT1B or 5-HT1A agonist disrupts performance and increases omissions on the DAT. Mice received acute injection with 6 mg/kg RU24969 or 3 mg/kg 8-OH-DPAT and were tested for accuracy (a), latency (b), and omissions (c) on the DAT. Results are presented as mean ± SEM. Asterisk denotes p < 0.05 compared to saline-treated mice
GR127935 blocks RU24969-induced spatial working memory deficits
Mice treated with 3 mg/kg RU24969 performed worse on the DAT than vehicle-treated mice as revealed by a main effect of 3 mg/kg RU24969 on accuracy (F(1,15) = 5.78, p < 0.05), latency (F(1,15) = 32.08, p < 0.001), and omissions (F(1,15) = 16.98, p < 0.001) (Fig. 3). Mice co-injected with 3 mg/kg RU24969 and 5 mg/kg GR127935 were not different from vehicle-treated mice on accuracy (p = 0.52) and exhibited increased latency in comparison to vehicle-treated mice (F(1,15) = 21.42, p < 0.001) (Fig. 3). RU24969/GR127935 significantly increased the number of omissions in comparison to vehicle-treated mice (F(1,15) = 32.09, p < 0.001). Mice treated with GR127935 alone were not different from vehicle-treated mice on any DAT measure.
RU24969-induced decreases in accuracy are mediated by 5-HT1B receptors. Mice received acute co-injection with saline/saline, saline/3 mg/kg RU24969, saline/5 mg/kg GR127935, or 3 mg/kg RU24969/5 mg/kg GR127935, and were tested for accuracy (a), latency (b), and omissions (c) on the DAT. Results are presented as mean ± SEM. Asterisk denotes p < 0.05 compared to saline-treated mice
Chronic fluoxetine treatment blocks RU24969-induced spatial working memory deficits
Chronic fluoxetine treatment blocked the ability of RU24969 to disrupt accuracy on the DAT as revealed by a pretreatment × treatment interaction (F(1,22) = 7.96, p < 0.01). RU24969 decreased accuracy in vehicle-pretreated mice (F(1,11) = 8.83, p < 0.05), but not fluoxetine-pretreated mice (F(1,11) = 0.071, p = 0.79). Moreover, Newman Keuls post hoc tests revealed that fluoxetine-pretreated mice showed increased accuracy on the DAT compared to vehicle-pretreated mice following RU24969 (Fig. 4). No main effects of fluoxetine or 8-OH-DPAT on DAT accuracy were found (Fig. 4).
Chronic fluoxetine treatment blocks RU24969-induced DAT deficits. Following 4 weeks of treatment with 0 or 10 mg/kg fluoxetine, mice received acute injection with 3 mg/kg RU24969 or 0.3 mg/kg 8-OH-DPAT and were immediately tested for accuracy (a) and latency (b) on the DAT. Results are presented as mean ± SEM. Asterisk denotes p < 0.05 compared to saline-treated mice. Number sign denotes p < 0.05 compared to vehicle-treated mice
Chronic fluoxetine treatment diminished the ability of RU24969 to increase latency on the DAT as revealed by a pretreatment × treatment interaction (F(1,22) = 9.61, p < 0.01). RU24969 increased latency in both vehicle-pretreated (F(1,11) = 20.78, p < 0.001) and fluoxetine-pretreated mice (F(1,11) = 12.73, p < 0.01). However, Newman Keuls post hoc tests revealed that fluoxetine-pretreated mice showed decreased latency on the DAT compared to vehicle-pretreated mice (Fig. 4). Main effects were found for 8-OH-DPAT to increase latency (F(1,22) = 5.35, p < 0.05) and fluoxetine pretreatment to decrease latency (F(1,22) = 5.20, p < 0.05). However, no interaction of pretreatment × treatment was found (F(1,22) = 1.87, p = 0.19) (Fig. 4).
Discussion
We found that RU24969, a 5-HT1B/1A agonist, disrupted performance on the DAT. Furthermore, we show that this effect is mediated by 5-HT1B and not 5-HT1A receptors since GR127935, a 5-HT1B/1D antagonist, blocked RU24969-induced decreases in accuracy on the DAT. In addition to reducing accuracy on the DAT, RU24969 increased latency and omissions on the DAT. However, these effects were likely mediated by 5-HT1A receptors, since GR127935 did not block these RU24969-induced effects. Moreover, chronic (4 weeks) pretreatment with the SRI fluoxetine blocked RU24969-induced reductions in DAT accuracy and latency. Our results suggest that activation of 5-HT1B receptors reduces performance on the DAT.
Our present findings suggest that DAT measures are sensitive to lower doses of RU24969 than other OCD-related measures in mice. We chose the doses of 3 and 6 mg/kg RU24969 based on our previous behavioral studies in which 10 mg/kg RU24969 induced OCD-like behavior in mice (Shanahan et al. 2009, 2011). In these studies, 10 mg/kg RU24969 induced perseverative hyperlocomotion and prepulse inhibition deficits, which were attenuated by chronic fluoxetine treatment. To minimize the hyperlocomotive effects of RU24969 in the DAT, we administered lower doses of RU24969 in the present study. Our present findings show that doses of RU24969 as low as 3 mg/kg induce behavioral deficits with relevance to OCD in mice.
Doses of 8-OH-DPAT were also chosen based on previous experiments showing 8-OH-DPAT-induced behavioral and physiological effects (Dulawa et al. 2000; Shanahan et al. 2009, 2011). The 0.3 mg/kg dose of 8-OH-DPAT did not disrupt DAT performance, suggesting that RU24969-induced DAT deficits are not likely mediated by 5-HT1A receptors. However, the 3-mg/kg dose of 8-OH-DPAT increased latency and omissions, suggesting that this higher dose may produce non-specific effects in this behavioral test.
To directly assess the role of 5-HT1B receptors in RU24969-induced DAT deficits, we co-injected RU24969 with GR127935, a highly-selective 5-HT1B/1D receptor antagonist. Co-injection of GR127935 blocked the reductions in accuracy induced by RU24969 but did not block RU24969-induced increases in latency and omissions. Thus, 5-HT1B receptors specifically mediate accuracy, but not latency, in the DAT. Conversely, 5-HT1A receptors may specifically mediate latency, but not accuracy, in the DAT. 5-HT1B receptor-induced reductions in DAT accuracy may model the DAT deficits in OCD patients. Indeed, DAT deficits in OCD patients are characterized by reduced accuracy (Abbruzzese et al. 1995, 1997; Gross-Isseroff et al. 1996; Moritz et al. 2001). Moreover, 5-HT1B receptor activation exacerbates symptoms in OCD patients (Stein et al. 1999; Koran et al. 2001; Gross-Isseroff et al. 2004). To our knowledge, latency on the DAT has not been examined in OCD patients.
Accuracy on the DAT reflects the ability to remember which of the two T-maze arms most recently contained a reward based on spatial cues. In addition, cognitive flexibility is required to adapt to the changing requirements for accuracy on the task, and motor inhibition is required to refrain from choosing the arm that was previously rewarded. We show here that 5-HT1B receptor activation disrupts accuracy on the DAT. Mice administered with RU24969 also showed increased latency to make a choice on the DAT. This effect is unlikely to be due to hypolocomotion, since 5-HT1B receptor agonists such as RU24969 produce a dose-dependent increase in locomotor activity (Green et al. 1984; Shanahan et al. 2009, 2011). Moreover, RU24969-induced increases in latency appear to be mediated by 5-HT1A receptors, but not 5-HT1B receptors, as increased latency was not blocked by the 5-HT1B/1D antagonist GR127935. The effects of RU24969 on latency in the DAT may be relevant to the psychomotor slowing exhibited by OCD patients on tests of executive function subserved by frontostriatal circuitry (Roth et al. 2004; Remijnse et al. 2009). For example, OCD patients show longer reaction times but normal accuracy on reversal learning tasks (Remijnse et al. 2009).
Fluoxetine pretreatment prevented RU24969-induced reductions in DAT accuracy and latency. The mechanisms for these effects likely involve desensitization by fluoxetine of specific populations of 5-HT1B or 5-HT1A receptors, respectively. We recently showed that 4 weeks of treatment with fluoxetine reduces the expression of 5-HT1B receptors in the orbitofrontal cortex, but not the substantia nigra, globus pallidus, dorsal striatum, or dorsofrontal cortex, and that this population of 5-HT1B receptors mediates OCD-like perseveration and sensorimotor gating deficits in mice (Shanahan et al. 2009, 2011). Thus, reduced expression of orbitofrontal 5-HT1B receptors may underlie the ability of fluoxetine to prevent 5-HT1B-induced deficits in DAT performance. Chronic fluoxetine pretreatment has also been shown to desensitize presynaptic 5-HT1A receptors (Hervas et al. 2001; El Mansari and Blier 2005). Since chronic fluoxetine treatment blocked RU24969-induced increases in latency on the DAT, this effect might be mediated through the desensitization of presynaptic 5-HT1A receptors.
Impairments in spatial working memory, cognitive flexibility, and motor inhibition are putative endophenotypes for OCD (Chamberlain et al. 2007). Specifically, deficits in delayed alternation have been proposed as a novel endophenotype for OCD since they appear to be state independent (Rao et al. 2008) and are also exhibited by non-affected relatives of OCD patients (Viswanath et al. 2009). DAT deficits observed in OCD patients are thought to result from perseveration rather than strategic deficits (Moritz et al. 2009). A number of reports have used reversal learning to model perseverative behavior in OCD (for example Andersen et al. 2010). However, it should be noted that deficits in delayed alternation (Abbruzzese et al. 1995, 1997; Gross-Isseroff et al. 1996; Moritz et al. 2001), but not reversal learning (Remijnse et al. 2006, 2009), have been consistently found in OCD.
To our knowledge, this is the first evidence of 5-HT1B receptor control over delayed alternation. In summary, we show that 5-HT1B receptor activation disrupts DAT accuracy, and chronic fluoxetine pretreatment prevents these 5-HT1B-induced deficits. Our previous work showing that chronic fluoxetine treatment downregulates 5-HT1B receptor expression in the orbitofrontal cortex (Shanahan et al. 2011) provides a likely mechanism for fluoxetine’s prevention of 5-HT1B-induced deficits in DAT performance. Moreover, 5-HT1B-induced deficits in DAT performance may provide a mouse model for DAT deficits in OCD patients since OCD patients exhibit delayed alternation deficits, and chronic SRI treatment alleviates OCD symptoms. Future studies will test the hypothesis that orbitofrontal 5-HT1B receptors modulate DAT performance.
References
Abbruzzese M, Bellodi L, Ferri S, Scarone S (1995) Frontal lobe dysfunction in schizophrenia and obsessive-compulsive disorder: a neuropsychological study. Brain Cogn 27:202–212
Abbruzzese M, Ferri S, Scarone S (1997) The selective breakdown of frontal functions in patients with obsessive-compulsive disorder and in patients with schizophrenia: a double dissociation experimental finding. Neuropsychologia 35:907–912
Andersen SL, Greene-Colozzi EA, Sonntag KC (2010) A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry 68:741–747
Baxter LR Jr, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fairbanks L (1988) Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am J Psychiatry 145:1560–1563
Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM (1990) Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients treated with clomipramine. Arch Gen Psychiatry 47:840–848
Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD et al (1996) Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry 53:595–606
Butters N, Rosvold HE (1968) Effect of caudate and septal nuclei lesions on resistance to extinction and delayed-alternation. J Comp Physiol Psychol 65:397–403
Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ (2007) Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 164:335–338
Cottraux J, Gerard D, Cinotti L, Froment JC, Deiber MP, Le Bars D, Galy G et al (1996) A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Res 60:101–112
Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA (2000) Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology 22:650–659
Dulawa SC, Holick KA, Gundersen B, Hen R (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29:1321–1330
El Mansari M, Blier P (2005) Responsiveness of 5-HT(1A) and 5-HT2 receptors in the rat orbitofrontal cortex after long-term serotonin reuptake inhibition. J Psychiatry NeuroSci 30:268–274
Freedman M, Black S, Ebert P, Binns M (1998) Orbitofrontal function, object alternation and perseveration. Cereb Cortex 8:18–27
Freedman M, Oscar-Berman M (1986) Bilateral frontal lobe disease and selective delayed response deficits in humans. Behav Neurosci 100:337–342
Gagliardo A, Bonadonna F, Divac I (1996) Behavioural effects of ablations of the presumed ‘prefrontal cortex’ or the corticoid in pigeons. Behav Brain Res 78:155–162
Glennon RA, Dukat M, Westkaemper RB American College of Neuropsychopharmacology (2000) Psychopharmacology: the fourth generation of progress. Raven Press, New York
Goldman-Rakic P (1987) Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory. Handbook of physiology, the nervous system, higher functions of the brain. Plum F (ed). Bethesda, MD, American Physiological Society. V:373–417
Goldman PS, Rosvold HE, Mishkin M (1970) Evidence for behavioral impairment following prefrontal lobectomy in the infant monkey. J Comp Physiol Psychol 70:454–463
Green AR, Guy AP, Gardner CR (1984) The behavioural effects of RU 24969, a suggested 5-HT1 receptor agonist in rodents and the effect on the behaviour of treatment with antidepressants. Neuropharmacology 23:655–661
Gross-Isseroff R, Cohen R, Sasson Y, Voet H, Zohar J (2004) Serotonergic dissection of obsessive compulsive symptoms: a challenge study with m-chlorophenylpiperazine and sumatriptan. Neuropsychobiology 50:200–205
Gross-Isseroff R, Sasson Y, Voet H, Hendler T, Luca-Haimovici K, Kandel-Sussman H, Zohar J (1996) Alternation learning in obsessive-compulsive disorder. Biol Psychiatry 39:733–738
Hervas I, Vilaro MT, Romero L, Scorza MC, Mengod G, Artigas F (2001) Desensitization of 5-HT(1A) autoreceptors by a low chronic fluoxetine dose effect of the concurrent administration of WAY-100635. Neuropsychopharmacology 24:11–20
Koran LM, Pallanti S, Quercioli L (2001) Sumatriptan, 5-HT(1D) receptors and obsessive-compulsive disorder. Eur Neuropsychopharmacol 11:169–172
Markowitsch HJ, Pritzel M, Kessler J, Guldin W, Freeman RB Jr (1980) Delayed-alternation performance after selective lesions within the prefrontal cortex of the cat. Behav Brain Res 1:67–91
Moritz S, Fricke S, Wagner M, Hand I (2001) Further evidence for delayed alternation deficits in obsessive-compulsive disorder. J Nerv Ment Dis 189:562–564
Moritz S, Hottenrott B, Randjbar S, Klinge R, Von Eckstaedt FV, Lincoln TM, Jelinek L (2009) Perseveration and not strategic deficits underlie delayed alternation impairment in obsessive-compulsive disorder (OCD). Psychiatry Res 170:66–69
Nordahl TE, Benkelfat C, Semple WE, Gross M, King AC, Cohen RM (1989) Cerebral glucose metabolic rates in obsessive compulsive disorder. Neuropsychopharmacology 2:23–28
Pigott TA, Pato MT, Bernstein SE, Grover GN, Hill JL, Tolliver TJ, Murphy DL (1990) Controlled comparisons of clomipramine and fluoxetine in the treatment of obsessive-compulsive disorder. Behavioral and biological results. Arch Gen Psychiatry 47:926–932
Rao NP, Reddy YC, Kumar KJ, Kandavel T, Chandrashekar CR (2008) Are neuropsychological deficits trait markers in ocd? Prog Neuropsychopharmacol Biol Psychiatry 32:1574–1579
Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ (2006) Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry 63:1225–1236
Remijnse PL, Nielen MM, van Balkom AJ, Hendriks GJ, Hoogendijk WJ, Uylings HB, Veltman DJ (2009) Differential frontal-striatal and paralimbic activity during reversal learning in major depressive disorder and obsessive-compulsive disorder. Psychol Med 39:1503–1518
Roth RM, Baribeau J, Milovan DL, O'Connor K (2004) Speed and accuracy on tests of executive function in obsessive-compulsive disorder. Brain Cogn 54:263–265
Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, Phelps ME et al (1999) Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology 21:683–693
Shanahan NA, Holick Pierz KA, Masten VL, Waeber C, Ansorge M, Gingrich JA, Geyer MA et al (2009) Chronic reductions in serotonin transporter function prevent 5-HT1B-induced behavioral effects in mice. Biol Psychiatry 65:401–408
Shanahan NA, Velez LP, Masten VL, Dulawa SC (2011) Essential role for orbitofrontal serotonin 1B receptors in obsessive-compulsive disorder-like behavior and serotonin reuptake inhibitor response in mice. Biol Psychiatry 70:1039–1048
Stein DJ, Van Heerden B, Wessels CJ, Van Kradenburg J, Warwick J, Wasserman HJ (1999) Single photon emission computed tomography of the brain with Tc-99 m HMPAO during sumatriptan challenge in obsessive-compulsive disorder: investigating the functional role of the serotonin auto-receptor. Prog Neuropsychopharmacol Biol Psychiatry 23:1079–1099
Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, Rapoport SI et al (1992) Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Arch Gen Psychiatry 49:690–694
Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R et al (1989) Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry 46:518–523
Tollefson GD, Birkett M, Koran L, Genduso L (1994) Continuation treatment of OCD: double-blind and open-label experience with fluoxetine. J Clin Psychiatry 55(Suppl: 69–76):69–76, discussion 77–68
Viswanath B, Janardhan Reddy YC, Kumar KJ, Kandavel T, Chandrashekar CR (2009) Cognitive endophenotypes in OCD: a study of unaffected siblings of probands with familial OCD. Prog Neuropsychopharmacol Biol Psychiatry 33:610–615
Warren JM, Akert K, University PS (1964) The frontal granular cortex and behavior. McGraw-Hill, New York
Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997) Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17:8528–8535
Conflict of interest
The authors of this paper have no potential conflict of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the following grants: R01 MH079424, R24 MH080022, T32 MH020065, and T32 GM07839.
Rights and permissions
About this article
Cite this article
Woehrle, N.S., Klenotich, S.J., Jamnia, N. et al. Effects of chronic fluoxetine treatment on serotonin 1B receptor-induced deficits in delayed alternation. Psychopharmacology 227, 545–551 (2013). https://doi.org/10.1007/s00213-013-2985-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-2985-0