Abstract
Rationale
Obsessive-compulsive disorder (OCD) implicates dysfunction of orbitofrontal and insula-related circuitry and of the serotonin system. There is an on-going search in psychiatry for intermediate biological markers, termed ‘endophenotypes’, that exist not only in patients with a given disorder but also in their clinically unaffected first-degree relatives.
Objective
Pharmacological challenge is recognized as a means of eliciting an endophenotype, but this strategy has yet to be used in OCD.
Methods
Twenty-three OCD patients without comorbidities (12 [52.2 %] female), 13 clinically asymptomatic first-degree relatives of OCD patients (11 [84.6 %] female) and 27 healthy controls (16 [59.3 %] female) received single-dose escitalopram (20 mg) and placebo in a randomized double-blind crossover design. Effects of treatment on decision-making were quantified using the Cambridge Gamble Task (CGT) in conjunction with a mixed model analysis of covariance (ANCOVA).
Results
There was a significant interaction between serotonergic challenge and group for risk adjustment on the CGT (F = 4.1406; p = 0.02). Only controls showed a significant placebo-drug change in risk adjustment (p = 0.02; versus p > 0.10). Numerically, escitalopram was associated with increase in risk adjustment in controls and reductions in the other groups. Change in risk adjustment was similar in OCD patients and relatives (p = 0.806) and differed significantly from controls (p = 0.007; p = 0.041, respectively).
Conclusions
Individuals with OCD, and first-degree relatives, showed an altered cognitive response to serotonin challenge. This is the first demonstration of a candidate pharmacological challenge endophenotype for the disorder. Future work should confirm these findings in a larger sample size and ideally extend them to other cognitive paradigms, utilizing functional neuroimaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obsessive-compulsive disorder (OCD) is characterized by recurrent and persistent ego-dystonic thoughts that are usually followed by repetitive and time-consuming behaviours aimed at reducing the distress or undertaken according to rigid rules (American Psychiatric Association 2013). The prevalence of this disabling condition is between 2 and 3 % (e.g. Fontenelle et al. 2006; Ruscio et al. 2010). Generally, studies of OCD in adults have found either an equal distribution of men and women or a slight predominance of women with this disorder.
Serotonin reuptake inhibitors (SRIs) are considered safe and relatively effective first-line pharmacological treatments for OCD in adults (Lochner and Stein 2015). Response to pharmacotherapy in OCD has been mixed however, with 40–60 % of patients exhibiting a lack of significant response to SRI (Bloch et al. 2006; Erzegovesi et al. 2001). Nevertheless, there is a myriad of studies that have supported the hypothesis that the serotonin system may be dysfunctional in OCD (Pauls 2010). Although serotonin is the neurotransmitter most commonly implicated in OCD, direct support for a role for serotonin in the pathophysiology of OCD remains elusive (Goodman et al. 2014).
It is increasingly recognized that in order to optimize diagnostic classification systems and treatments for mental disorders, it is necessary to ‘look beyond’ top level symptoms and to consider neurobiological vulnerability markers. Complex mental disorders such as OCD cannot easily be subgrouped into genetically homogeneous subtypes using symptoms or signs (phenotypical markers). Hence, there is ongoing search in the neurosciences for endophenotypes that represent internal, intermediate traits or vulnerability markers for disease development and which lie closer to the genetic origins of the disorder under scrutiny (Gottesman and Gould 2003). So, it can be said that an endophenotype is a heritable quantitative trait that is therefore present in both patients and their clinically unaffected relatives (Bearden and Freimer 2006). It cannot be seen ‘by the unaided eye’ and therefore requires technology to be accessed (Gottesman and Gould 2003). While the original definition of ‘endophenotypes’ recognized that they could be elicited by a behavioural (Gottesman and Gould 2003) or a pharmacological challenge such as one of the SRIs (Corregiari et al. 2012; Miller and Rockstroh 2013) this latter strategy has been largely overlooked in OCD to date.
OCD has been conceptualized by some as a disorder of decision-making (e.g. Sachdev and Malhi 2005), in part due to the centrality of obsessive doubting and uncertainty in this condition (Nikodijevic et al. 2015). The cognitive process of doubt in OCD has been shown as the result of the dynamic interplay between the impacts of possibility- and reality-based information, with OCD patients being significantly more affected by possibility-based information (Aardema et al. 2009). Moreover, OCD patients seem to require more information and spend more time deliberating over scenarios before making a decision, compared to healthy controls (Banca et al. 2015; Foa et al. 2003). It has been argued that the excessive doubt and uncertainty mediate such difficulties in decision-making (Foa et al. 2003). In the study by Banca et al. (2015) there was a differential influence of high and low uncertainty contexts on specific components of decision-making in OCD. Patients required more evidence to reach a decision under conditions of high uncertainty. In contrast, under conditions of low uncertainty, decision-making was slowed owing to poorer quality of evidence entering the decision process (Banca et al. 2015).
Decision-making can be assessed using the Cambridge Gamble Task (CGT), which is sensitive to abnormal decision-making in substance abuse, mania and frontal lesions (Manes et al. 2002; Murphy et al. 2001; Rogers et al. 1999; Watkins et al. 2005). Using the CGT, patients with comorbidity-free OCD, and their clinically asymptomatic first-degree relatives, did not show significant overt behavioural impairments on the CGT compared to healthy controls (Chamberlain et al. 2007a, b; Watkins et al. 2005). This was contrary to expectations, because the task is dependent on orbitofrontal circuitry, and abnormalities of orbitofrontal circuitry are commonly reported in OCD (Menzies et al. 2008). It should also be noted that one study did find decision-making deficits in OCD using this task (Dittrich and Johansen 2013) while other literature has reported deficits in OCD using other types of decision-making paradigms (e.g. Cavedini et al. 2002; da Rocha et al. 2011). It may be argued that deficits detected by CGT are either subtle or variable in OCD and may be more clearly seen if elicited with a pharmacological probe or conceivably during symptom provocation.
Several tiers of evidence suggest that serotonin manipulations, including over the short term, can impact aspects of decision-making (e.g. Merens et al. 2007; Rogers 2011) (for discussion, see Faulkner and Deakin 2014). The direction of behavioural effect is likely to depend on many factors, including baseline serotonin status. In a comprehensive review of the role of serotonin in various types of decision-making processes, it was suggested that serotonin promotes adaptive behaviour, with high central serotonin levels being associated with decision-making related behaviours (Homberg 2012). In previous work, acute tryptophan depletion—a technique that transiently diminishes brain serotonin—reduced quality of decision-making on the CGT in healthy volunteers (Rogers et al. 2003). Elsewhere, it was shown that acute citalopram resulted in impaired reversal learning (ability to learn from feedback) in healthy volunteers, using a different type of task (Chamberlain et al. 2006). Here, we were interested in exploring vulnerability markers for OCD using acute serotonin challenge and the CGT, given this task’s dependence on orbitofrontal and insula integrity and serotonergic neurotransmission.
Methods
The study comprised three groups of participants: patients with OCD (n = 23, with 12 [52.2 %] on chronic SRI treatment; all free from current clinically significant comorbid psychiatric disorders), clinically unaffected first-degree relatives of OCD patients (n = 13, none on psychotropic medication; with no history of OCD) and healthy controls (n = 27; with no current psychiatric disorder, nor any history of OCD). The study was approved by the Institutional Review Board of the University of Stellenbosch, and all participants gave informed written consent to participate after risks and benefits had been explained.
Clinical assessments
OCD patients were referred to our research unit from a wide range of sources (including the OCD Association of South Africa, community-based primary care practitioners and psychiatrists). They were screened telephonically and subsequently interviewed by a clinical psychologist. Patients met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (American Psychiatric Association 2000) criteria for a primary diagnosis of non-comorbid OCD. The Mini International Neuropsychiatric Interview Plus (MINI Plus) version 5 (Sheehan et al. 1998) a structured diagnostic interview developed for DSM-IV and ICD-10 psychiatric disorders, was used for diagnostic purposes. All patients were also interviewed with the Structured Clinical Interview for Obsessive-Compulsive Spectrum Disorders (SCID-OCSD) to determine the presence of comorbid obsessive-compulsive spectrum disorders (du Toit et al. 2001).
Patients were included however if they were either psychotropic medication free or on psychotropic medication that was (1) limited to a single psychotropic medication from the selective SRI class of agents, (2) administered at a steady dose that was not higher than the optimal dose for OCD for the particular agent (e.g. 60 mg of fluoxetine), (3) taken for at least 2 months (8 weeks) and (4) stabilized according to the treating psychiatrist. Referring clinicians were contacted to help establish, where possible, a longitudinal expert assessment and diagnosis. Patients with any clinically significant current psychiatric comorbidity or past history of significant substance or alcohol abuse were excluded from participation (n = 31).
First-degree relatives of OCD patients and controls were included if they had no current DSM-IV-TR disorder, nor any history of OCD. Participants with OCD including those that we excluded from participation gave consent for a first-degree relative (parent or sibling) to be contacted, and these first-degree relatives were enrolled into the study on the basis of freedom from psychiatric disorders including OCD. Controls were recruited through advertisements in the local community and university intranet and were entered into the study on the basis of freedom from psychiatric disorders including OCD and having no known first-degree family members with OCD. Two family members and 26 controls were excluded due to diagnoses of current psychiatric disorders.
Across all study participants, the 14-item Hamilton Anxiety Rating Scale (HAM-A) (Hamilton 1959) a measure of global anxiety, and the ten-item Montgomery-Asberg Depression Rating Scale (MADRS) (Davidson et al. 1986) a measure of severity of depressive symptomology, were administered to all participants. In the OCD group, the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al. 1989a, b) was administered to assess the severity of OCD symptoms.
Pharmacological challenge
As noted earlier, serotonin manipulations may impact aspects of decision-making on the CGT and conceptually related tasks. Participants received a single dose of one of the SSRIs, i.e. escitalopram (20 mg), and placebo, in a double-blind, randomized, cross-over design. The two study visits were separated by a minimum of 7 days in order to allow ample washout of escitalopram. Three hours after administration of escitalopram or placebo, neurocognitive assessment of decision-making was undertaken as outlined below. The rationale for using escitalopram was based on its pharmacokinetic profile, with peak plasma concentration levels (PPCLs) being expected 3–4 h after oral dosing (Rao 2007). PPCLs of the other SSRIs (all except citalopram) are generally much longer (e.g. sertraline, at 6–8 h, fluoxetine, at 6–8 h, and fluvoxamine, at 4–12 h). Cognitive assessments took place during the PPCL phase (i.e. approximately 3–4 h after administration).
Cognitive assessment
Dissociable aspects of decision-making were investigated using the CGT, from the Cambridge Neuropsychological Test Automated Battery (CANTAB: www.cambridgecognition.com) (for detailed descriptions of the task, the reader is referred to previous validation studies) (Clark et al. 2008; Manes et al. 2002). The task was administered using a touch-screen computer in a quiet testing environment, supervised by a trained test administrator. Although other tasks from the CANTAB were also performed, our analyses focused on CGT, as performance on this type of task is sensitive to serotonin manipulations in healthy volunteers (Merens et al. 2007) and it may tap into neural substrates that tie in closely with those often implicated in OCD (the orbitofrontal cortices and insula, for example) (e.g. Chamberlain et al. 2008; Menzies et al. 2008; Saxena et al. 2001).
On the CGT, for each trial, subjects were presented with a row of ten boxes across the top of the screen, each box being blue or red. Subjects were informed that the computer had hidden a ‘token’ behind one of these boxes and that they had to first decide whether they believed the token to be hidden behind a red or blue box. This decision was made by pressing a blue or red rectangle towards the bottom of the screen. After making this choice, the participant had to gamble a proportion of their points as to whether they had made the correct colour choice.
Over the course of the task, the aim was to accumulate as many points as possible. The proportion of red and blue boxes was varied pseudo-randomly, so that decision-making tendencies could be summarized across a spread of risk levels. The outcome measures of interest on this task were as follows:
-
1.
Overall proportion of points gambled;
-
2.
Overall proportion of rational decisions made (the extent to which the participant indicated that the token was likely hidden behind a red box when red was in the majority and behind the blue box when blue was in the majority); and
-
3.
Risk adjustment (the extent to which participants modulated the amount gambled depending on the probability of making correct choices or by the ratio of boxes, with higher scores indicating greater sensitivity to risk).
Statistical analysis
Statistical analysis was undertaken using STATISTICA 12 (www.statsoft.com). Potential baseline group differences in clinical and demographic characteristics were explored using analysis of variance (ANOVA), with Fisher’s LSD tests used to explore significant main effects of group where identified (or equivalent non-parametric tests where appropriate, as indicated in the text).
Cognitive data were analyzed using separate mixed model analysis of covariance (ANCOVA) for each of the three outcome measures of interest. The within-subject factors were order (placebo first or escitalopram first) and challenge (placebo or escitalopram), and the between-subject factor was group (OCD, OCD relatives, controls). We subsequently compared the above outcome measures of interest between OCD patients taking SSRIs chronically and those not on any chronic psychotropic medication at the time of the assessments. When significant effects were identified in an ANCOVA model, the nature of these effects was further explored using post hoc tests as appropriate (one-way ANOVA, t tests, including for change scores). Due to age differing between groups at baseline (see “Results” section), age was entered as a covariate in the ANCOVA models.
Statistical significance was defined as p < 0.05 uncorrected.
Results
Twenty-three OCD patients (n = 23, 12 [52.2 %] female), 13 unaffected first-degree relatives of OCD patients (11 [84.6 %] female) and 27 healthy controls (16 [59.3 %] female) participated in the study. Most of the relatives were from independent families, and three were relatives of the OCD patients that participated in this study. All participants were Caucasian. The age range of participants was 18–59 years (median 33 years).
Demographic and clinical variables
As depicted in Table 1, the three groups did not differ significantly in terms of gender or IQ (both p > 0.10). However, groups differed in terms of age at entry (p < 0.001); post hoc t tests indicated that this was due to OCD relatives being older than the two other groups (versus OCD (p < 0.001) and healthy controls (p = <0.001)).
In terms of severity levels of anxiety and depressive symptomatology (Table 1), the groups differed significantly on both, as expected (both p < 0.001). Post hoc tests indicated that this was due to OCD patients reporting significantly higher anxiety and depression levels than OCD relatives (p < 0.001) and healthy controls (p < 0.001). OCD relatives and controls did not differ significantly from each other on these measures (anxiety: p = 0.73; depression: p = 0.89).
OCD patients’ scores on the YBOCS ranged from 16 to 32, with a mean (SD) of 23.4 (4.5). None of the controls nor the relatives reported any obsessions/compulsions; thus, their scores (all 0) were not compared to that of the OCD group.
Twelve out of the 23 OCD patients were receiving SSRI treatment at the time of study participation. According to participant reports and feedback from their treating psychiatrists, the SSRI treatment effects had stabilized for all of these participants. However, patients were symptomatic enough in terms of their OCD to be included in the study (i.e. YBOCS total score ≥16), and all OCD relatives were free of any psychiatric diagnosis.
Cognitive results
Proportion of points gambled
On ANCOVA, there was no significant main effect of group (F = 3.0029; p = 0.058) nor of challenge (F = 0.3594; p = 0.551) on this measure. The group-by-challenge interaction term was not significant (F = 1.9733; p = 0.149) (for raw data, see Supplementary online Table 1).
Proportion of rational decision-making
On ANCOVA, there was no significant main effect of group (F = 0.3624; p = 0.698) nor of challenge (F = 2.627; p = 0.111) on this measure. The group-by-challenge interaction term was not significant (F = 1.5768; p = 0.216) (for raw data, see Supplementary online Table 2).
Risk adjustment
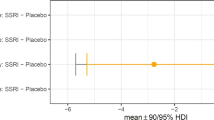
On ANCOVA, there was no main effect of group (F = 1.0924; p = 0.342) nor of challenge (F = 0.1322; p = 0.717) on this measure. However, there was a significant group-by-challenge interaction (F = 4.1406; p = 0.02). As can be seen in Fig. 1, this significant interaction between group and treatment was due to risk adjustment reducing numerically in OCD patients and OCD relatives when on escitalopram, while the opposite effect was seen in the controls. The ANCOVA change within each group was significant only for controls (p < 0.03; vs both other p > 0.10) (for raw data, see Supplementary online Table 3).
Using the change scores (i.e. the values obtained after administration of escitalopram minus the values that were obtained with placebo, per participant) to further characterize the causes of the interaction effect (group × treatment), it was found that the effect of escitalopram challenge on the change scores for risk adjustment differed significantly across diagnostic groups (F(2.56) = 3.512, p = 0.037) (Fig. 2). Post hoc t tests indicated that change scores did not differ significantly between OCD patients and their relatives (p = 0.806), while the differences between these two groups and controls were significant (OCD vs. controls: p = 0.007, effect size 0.79: OCD relatives vs. controls: p = 0.041, effect size 0.77).
The changes in risk adjustment scores (means, SDs) on the CGT are summarized in Table 2. Post hoc t tests suggested that escitalopram challenge had a similar effect in OCD patients and OCD relatives (p = 0.804), i.e. to decrease risk adjustment, compared to the increase noted in the healthy controls.
Given the relatively uneven distribution of genders across groups (i.e. 52.2 % of OCD patients, 59.3 % of controls and 84.6 % of relatives were female, respectively; p = 0.121), analyses of risk adjustment data were repeated with males excluded. Smaller sample size reduced power; the results nevertheless showed similar tendencies than in the whole sample although these were not significant (p = 0.21) (see Supplementary online Figs. 1 and 2).
Subsequent analyses showed that the extent and direction of changes on the CGT remained the same when the above outcome measures of interest were compared between OCD patients taking SSRIs chronically and those not on any chronic psychotropic medication at the time of the assessments. More specifically, prior medication status did not impact risk adjustment or the effect of escitalopram challenge on risk adjustment in OCD patients.
Discussion
As noted earlier, endophenotypes, as quantifiable traits lying on the causal chain between a clinical phenotype and aetiology (Shaw et al. 2015) can be elicited by behavioural and/or pharmacological challenge. Here, we applied both strategies. We found that patients with OCD and unaffected first-degree relatives of patients with OCD showed a similar, altered response to acute serotonin challenge. Specifically, escitalopram (SSRI) challenge led to significant increases in risk adjustment on the gambling task in healthy controls, and this effect was absent in the OCD patients and relatives. We believe this to be the first demonstration of a candidate endophenotype for OCD that can be elicited by pharmacological challenge.
Finding stable endophenotypes is valuable in that it makes genetic and biological studies of the aetiologies for disease categories more manageable than using heterogeneous psychiatric symptoms or phenotypes (Gould and Gottesman 2006). A fundamental assumption of the endophenotype concept is that the proposed marker is genetically heritable (Gottesman and Gould 2003). Our findings here suggest that patients with OCD and unaffected first-degree relatives of patients with OCD showed a similar discrepancy from controls in the effect of escitalopram on risk adjustment, alluding to the heritability of the identified endophenotype. It may, on the other hand, be argued that variance (between OCD patients/OCD relatives and controls) could be due to environmental influences with OCD patients and relatives sharing environments. Future work would need to replicate this result and show whether the absence of response to serotonin challenge is significantly heritable.
The CGT and other decision-making tasks are dependent on distributed neural circuitry linked with the processing of aversive outcomes and reward. Using the CGT, it has been demonstrated that patients with lesions to the insular cortex failed to adjust their bets by the odds of winning, i.e. showed dampened risk adjustment (Clark et al. 2008). This pattern of abnormal performance on the task was different from that observed in patients with damage to other cortical regions including the ventromedial/lateral prefrontal cortices (e.g. Clark et al. 2003; Clark et al. 2008). It should be noted, however, that insula damage affected not only risk adjustment but also betting behaviour, in that insular damage was also associated with participants gambling a greater proportion of their points overall (Clark et al. 2008). The dorsal and median raphe nuclei represent the source of most serotonergic projections in the brain and show strong functional connectivity with regions including the insula (Beliveau et al. 2015). The insula, and other regions including the lateral orbitofrontal cortices, play a cardinal role in evaluating negative outcomes (Liu et al. 2007). Data from a functional neuroimaging pharmaco-functional MRI (fMRI) challenge study in healthy controls indicates that serotonin 2A receptors modulate the evaluation of ‘missed’ rewards, i.e. one type of negative outcome (Macoveanu et al. 2013). In the recent study by Worbe et al. (2015) reducing serotonin neurotransmission in healthy controls using acute dietary tryptophan depletion led to impairment of specific goal-directed aspects of decision-making related to gaining a reward, whereas these aspects of decision-making were promoted under the risk of punishment. The authors suggested that this differential effect on appetitive and aversive goal-directed behaviour is likely mediated by alteration of the representation of reward produced by serotonin depletion.
Thus, one plausible explanation for our findings in healthy controls is that acute blockade of serotonin reuptake by a single dose of escitalopram led to enhanced sensitivity to negative outcomes, which in turn contributed to greater adjustment of gambling behaviour as a function of risk of loss. In the absence of fMRI, the neural mechanisms of the effects of serotonin challenge on this component of decision-making that we observed are unclear but could reflect serotonergic modulation of the insula. This could be explored in future pharmacofMRI work. The absence of a significant effect of serotonin reuptake inhibition on risk adjustment common to OCD patients and asymptomatic relatives is indicative of a preexisting abnormality of the serotonin system that may predispose towards OCD. Data from studies showing the selective response to treatment with SRIs, the transient exacerbation of OCD symptoms when challenged with serotonergic agonists such as mCPP and sumatriptan and a role for biological markers of serotonergic function in OCD, for example, have, over the years, reinforced the idea that this condition is associated with a disruption in the serotonin neurocircuitry (e.g. Barr et al. 1992). Chronic treatment with SSRIs has been associated with remediation of some neuropsychological deficits in some OCD (Andres et al. 2008) however, here, administration of a serotonergic agent was associated with the absence of a significant change in risk adjustment in OCD patients and relatives which may reflect on the compromised serotonin neurocircuitry not responding to acute challenge.
In terms of limitations, the study focused on a few aspects of decision-making which may be affected by serotonin manipulations. Additional related cognitive variables could have been added to the analyses; however, it was decided to include three outcome measures of interest on the CGT in order to reduce the number of comparisons. The relatively small sample size constitutes a limitation; however, the relatively large effect sizes (e.g. using the change scores [Fig. 2; Table 2]: OCD vs. controls; OCD relatives vs. controls) were comforting. The OCD relative group was smaller than the other two study groups but still exhibited a significant difference versus controls in terms of change scores, suggesting that the group was nonetheless large enough to detect group differences (i.e. was adequately powered). While the OCD relative group had numerically a higher proportion of females (not statistically significant), we did not find any significant effect of gender on CGT performance overall. In terms of age, the decline in cognitive function with aging is well established (e.g. De Luca et al. 2003; Sliwinski and Buschke 1999). Some of the CANTAB tests (not the CGT) have also revealed significant cognitive decline as a function of age (e.g. Rabbitt and Lowe 2000). In our analyses, age was entered as a covariate in the analyses given the fact that the relatives were much older than the other two study groups; therefore, the key findings appeared robust to this confound, as far as could be ascertained. Another potential limitation is the fact that our OCD sample was heterogeneous in terms of their OC symptoms; i.e. patients with specific OC symptom subtypes may be more prone to risk taking or vice versa (Lochner et al. 2005). The fact that OCD patients receiving SSRI medication were not withdrawn from participation can also be construed as a limitation. However, our results indicated that prior medication status did not impact risk adjustment or the effect of escitalopram challenge on risk adjustment in OCD patients. Finally, here, we focused on the CGT, but other decision-making tasks are available, some of which have shown abnormal cognitive functioning in relatives of OCD patients (Cavedini et al. 2010). It would be valuable for future work to address the impact of serotonin challenge on cognition in OCD and relatives of patients using alternative tasks.
Conclusion
In summary, there are very few studies that have investigated the effects of acute SSRI administration on selected cognitive functions in humans, and most of these have focused on healthy volunteers. Our study is, to our knowledge, the first to investigate the effects of acute SSRI administration on decision-making in OCD patients, first-degree OCD relatives and healthy controls in an attempt to accurately identify another useful OCD endophenotype. This is important given that the discovery of novel candidate brain function endophenotypes may increase the power of candidate gene and genome-wide association studies. Future studies should aim to replicate these findings, in larger cohorts, and examine the stability and utility of the hypothesized endophenotype in OCD and OCD-related conditions, ideally incorporating fMRI.
References
Aardema F, O’Connor KP, Pelissier M, Lavoie ME (2009) The quantification of doubt in obsessive-compulsive disorder. Int J Cogn Ther 2:188–205
American Psychiatric Association (2000) American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 4th edition—text revision (DSM-IV-TR). American Psychiatric Association, Washington DC
American Psychiatric Association (2013) DSM-5: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Washington DC
Andres S, Lazaro L, Salamero M, Boget T, Penades R, Castro-Fornieles J (2008) Changes in cognitive dysfunction in children and adolescents with obsessive-compulsive disorder after treatment. J Psychiatr Res 42:507–514
Banca P, Vestergaard MD, Rankov V, Baek K, Mitchell S, Lapa T, Castelo-Branco M, Voon V (2015) Evidence accumulation in obsessive-compulsive disorder: the role of uncertainty and monetary reward on perceptual decision-making thresholds. Neuropsychopharmacology 40:1192–1202
Barr LC, Goodman WK, Price LH, McDougle CJ, Charney DS (1992) The serotonin hypothesis of obsessive compulsive disorder: implications of pharmacologic challenge studies. J Clin Psychiatry 53(Suppl):17–28
Bearden CE, Freimer NB (2006) Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet 22:306–313
Beliveau V, Svarer C, Frokjaer VG, Knudsen GM, Greve DN, Fisher PM (2015) Functional connectivity of the dorsal and median raphe nuclei at rest. Neuroimage 116:187–195
Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF (2006) A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry 11:622–632
Cavedini P, Riboldi G, D’Annucci A, Belotti P, Cisima M, Bellodi L (2002) Decision-making heterogeneity in obsessive-compulsive disorder: ventromedial prefrontal cortex function predicts different treatment outcomes. Neuropsychologia 40:205–211
Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L (2010) Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol Psychiatry 67:1178–1184
Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311:861–863
Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2007a) A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia 45:654–662
Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ (2007b) Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 164:335–338
Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, Robbins TW, Sahakian BJ (2008) Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science 321:421–422
Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW (2003) The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia 41:1474–1483
Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW (2008) Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain 131:1311–1322
Corregiari FM, Bernik M, Cordeiro Q, Vallada H (2012) Endophenotypes and serotonergic polymorphisms associated with treatment response in obsessive-compulsive disorder. Clinics (Sao Paulo) 67:335–340
da Rocha FF, Alvarenga NB, Malloy-Diniz L, Correa H (2011) Decision-making impairment in obsessive-compulsive disorder as measured by the Iowa Gambling Task. Arq Neuropsiquiatr 69:642–647
Davidson J, Turnbull CD, Strickland R, Miller R, Graves K (1986) The Montgomery Asberg Depression Scale: reliability and validity. Acta Psychiatr Scand 73:544–548
De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C (2003) Normative data from the CANTAB. I: development of executive function over the lifespan. J Clin Exp Neuropsychol 25:242–254
Dittrich WH, Johansen T (2013) Cognitive deficits of executive functions and decision-making in obsessive-compulsive disorder. Scand J Psychol 54:393–400
du Toit PL, van Kradenburg J, Niehaus D, Stein DJ (2001) Comparison of obsessive-compulsive disorder patients with and without comorbid putative obsessive-compulsive spectrum disorders using a structured clinical interview. Compr Psychiatry 42:291–300
Erzegovesi S, Cavallini MC, Cavedini P, Diaferia G, Locatelli M, Bellodi L (2001) Clinical predictors of drug response in obsessive-compulsive disorder. J Clin Psychopharmacol 21:488–492
Faulkner P, Deakin JF (2014) The role of serotonin in reward, punishment and behavioural inhibition in humans: insights from studies with acute tryptophan depletion. Neurosci Biobehav Rev 46(Pt 3):365–378
Foa EB, Mathews A, Abramowitz JS, Amir N, Przeworski A, Riggs DS, Filip JC, Alley A (2003) Do patients with obsessive-compulsive disorder have deficits in decision-making? Cogn Ther Res 27:431–445
Fontenelle LF, Mendlowicz MV, Versiani M (2006) The descriptive epidemiology of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 30:327–337
Goodman WK, Price L, Rasmussen SA, Mazure C, Fleischmann R, Hill C et al (1989a) The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 46:1006–1011
Goodman W, Price L, Rasmussen S, Mazure C, Delgado P, Heninger G et al (1989b) The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry 46:1012–1016
Goodman WK, Grice DE, Lapidus KA, Coffey BJ (2014) Obsessive-compulsive disorder. Psychiatr Clin N Am 37:257–267
Gottesman II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160:636–645
Gould TD, Gottesman II (2006) Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav 5:113–119
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55
Homberg JR (2012) Serotonin and decision making processes. Neurosci Biobehav Rev 36:218–236
Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE (2007) Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci 27:4587–4597
Lochner C, Stein DJ (2015) Treatment of obsessive-compulsive and related disorders. In: Goodman W, Murrough J (eds) Anxiety, obsessive compulsive and related disorders. Springer, Philadelphia, USA
Lochner C, Hemmings SM, Kinnear CJ, Niehaus DJ, Nel DG, Corfield VA, MoolmanSmook JC, Seedat S, Stein DJ (2005) Cluster analysis of obsessive-compulsive spectrum disorders in patients with obsessive-compulsive disorder: clinical and genetic correlates. Compr Psychiatry 46:14–19
Macoveanu J, Rowe JB, Hornboll B, Elliott R, Paulson OB, Knudsen GM, Siebner HR (2013) Serotonin 2A receptors contribute to the regulation of risk-averse decisions. Neuroimage 83:35–44
Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T (2002) Decision-making processes following damage to the prefrontal cortex. Brain 125:624–639
Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET (2008) Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 32:525–549
Merens W, Willem Van der Does AJ, Spinhoven P (2007) The effects of serotonin manipulations on emotional information processing and mood. J Affect Disord 103:43–62
Miller GA, Rockstroh B (2013) Endophenotypes in psychopathology research: where do we stand? Annu Rev Clin Psychol 9:177–213
Murphy FC, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES, Sahakian BJ (2001) Decision-making cognition in mania and depression. Psychol Med 31:679–693
Nikodijevic A, Moulding R, Anglim J, Aardema F, Nedeljkovic M (2015) Fear of self, doubt and obsessive compulsive symptoms. J Behav Ther Exp Psychiatry 49:164–72
Pauls DL (2010) The genetics of obsessive-compulsive disorder: a review. Dialogues Clin Neurosci 12:149–163
Rabbitt P, Lowe C (2000) Patterns of cognitive ageing. Psychol Res 63:308–316
Rao N (2007) The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet 46:281–290
Rogers RD (2011) The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology 36:114–132
Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW (1999) Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20:322–339
Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS (2003) Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology 28:153–162
Ruscio AM, Stein DJ, Chiu WT, Kessler RC (2010) The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 15:53–63
Sachdev PS, Malhi GS (2005) Obsessive-compulsive behaviour: a disorder of decision making. Aust N Z J Psychiatr 39:757–763
Saxena S, Bota RG, Brody AL (2001) Brain-behavior relationships in obsessive-compulsive disorder. Semin Clin Neuropsychiatry 6:82–101
Shaw P, Sharp W, Sudre G, Wharton A, Greenstein D, Raznahan A, Evans A, Chakravarty MM, Lerch JP, Rapoport J (2015) Subcortical and cortical morphological anomalies as an endophenotype in obsessive-compulsive disorder. Mol Psychiatry 20:224–231
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33
Sliwinski M, Buschke H (1999) Cross-sectional and longitudinal relationships among age, cognition, and processing speed. Psychol Aging 14:18–33
Watkins LH, Sahakian BJ, Robertson MM, Veale DM, Rogers RD, Pickard KM, Aitken MR, Robbins TW (2005) Executive function in Tourette’s syndrome and obsessive-compulsive disorder. Psychol Med 35:571–582
Worbe Y, Palminteri S, Savulich G, Daw ND, Fernandez-Egea E, Robbins TW, Voon V (2015) Valence-dependent influence of serotonin depletion on model-based choice strategy. Mol Psychiatry. doi:10.1038/mp.2015.46
Acknowledgments
This work was supported by the Medical Research Council of South Africa, the Obsessive-Compulsive Foundation (Prof Stein), the National Research Foundation of South Africa (Prof Lochner), an unrestricted grant from Lundbeck H/S and by a Starter Grant for Clinical Lecturers from the Academy of Medical Sciences UK (Dr Chamberlain). Dr Chamberlain consults for Cambridge Cognition. We would like to acknowledge the contribution of our research assistants.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(PDF 87 kb)
Supplementary Figure 2
(PDF 85 kb)
Supplementary Table 1
(PDF 17 kb)
Supplementary Table 2
(PDF 17 kb)
Supplementary Table 3
(PDF 17 kb)
Rights and permissions
About this article
Cite this article
Lochner, C., Chamberlain, S.R., Kidd, M. et al. Altered cognitive response to serotonin challenge as a candidate endophenotype for obsessive-compulsive disorder. Psychopharmacology 233, 883–891 (2016). https://doi.org/10.1007/s00213-015-4172-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4172-y