Abstract
Rationale
Partial dopamine receptor agonists have been proposed as candidate pharmacotherapies for cocaine dependence.
Objective
This 42-day, within-subject, human laboratory study assessed how maintenance on aripiprazole, a partial D2 receptor agonist, influenced smoked cocaine self-administration, cardiovascular measures, subjective effects, and cocaine craving in nontreatment-seeking, cocaine-dependent volunteers.
Methods
In order to achieve steady-state concentrations, participants (n = 8 men) were administered placebo and aripiprazole (15 mg/day) capsules in counter-balanced order for 21 days. A smoked cocaine dose–response curve (0, 12, 25, 50 mg) was determined twice under placebo and aripiprazole maintenance. Sessions comprised a “sample” trial, when participants smoked the cocaine dose available that session, and five choice trials, when they responded on a progressive-ratio schedule of reinforcement to receive the cocaine dose or receive $5.00.
Results
Cocaine’s reinforcing, subjective, and cardiovascular effects were dose-dependent. Aripiprazole significantly increased cocaine (12, 25 mg) self-administration. Following a single administration of cocaine (25 mg), aripiprazole decreased ratings of how much participants would pay for that dose. Following repeated cocaine (50 mg) self-administration, aripiprazole decreased ratings of cocaine quality, craving, and good drug effect as compared to placebo.
Conclusions
These data suggest that aripiprazole may have increased self-administration to compensate for a blunted subjective cocaine effect. Overall, the findings do not suggest aripiprazole would be useful for treating cocaine dependence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are currently no medications approved by the Food and Drug Administration to facilitate treatment for cocaine dependence, although a vast number has been evaluated (see Vocci and Ling 2005; Grabowski et al. 2004). Among patients seeking treatment for their cocaine use, psychosocial treatments are clearly superior to no treatment (Crits-Christoph et al. 1999; see Dutra et al. 2008), yet overall rates of relapse to cocaine use remain high. Having the option of supplementing behavioral treatment with a medication that targets selective aspects of compulsive cocaine use, such as its direct reinforcing effects or the likelihood of relapse could markedly improve cocaine treatment outcome.
Research on cocaine pharmacotherapy has often focused on dopaminergic medications. Although cocaine blocks the reuptake of several monoamines, cocaine-induced elevations in synaptic levels of dopamine, in particular, appear most essential for maintaining cocaine self-administration (see Howell and Kimmel 2008) and for producing cocaine “high” (Volkow et al. 1997). However, dopamine receptor antagonists can be both poorly tolerated (e.g., cardiovascular effects; extrapyramidal symptoms; see Newcomer 2005) and ineffective in decreasing cocaine self-administration (see Bergman 2008; Amato et al. 2010). An alternative approach not well tested yet in humans is partial dopaminergic agonism, in which a compound binds to the dopamine receptor with high affinity but low efficacy.
One such medication is aripiprazole, a partial dopamine (D2) receptor agonist used as an atypical antipsychotic to treat schizophrenia and bipolar disorder, irritability associated with autistic disorder, and as an adjunct treatment of major depressive disorder (Lieberman 2004; Keck et al. 2003; Owen et al. 2009; Berman et al. 2007). As a partial agonist, aripiprazole is hypothesized to function as a dopamine D2 receptor antagonist when extracellular dopamine levels are high (e.g., shortly after cocaine use) and as a D2 agonist when extracellular dopamine levels are low (e.g., during abstinence after cocaine use), which are characteristics that may be useful in attenuating both cocaine intoxication and relapse (Pulvirenti and Koob 2002; Platt et al. 2002). Cocaine users have lower D1 and D2 dopamine receptor density, and lower dopamine release in response to a stimulant than healthy controls (e.g., Volkow et al. 2004; Martinez et al. 2007, 2009). In fact, less dopamine release following amphetamine administration predicts greater smoked cocaine self-administration (Martinez et al. 2007). Taken together, these findings suggest that a partial dopamine agonist could reduce cocaine use.
Preclinical and human laboratory data demonstrate that acute aripiprazole administration can attenuate both the discriminative stimulus and reinforcing effects of amphetamine and cocaine. In rodents, acute aripiprazole administration produced a downward or rightward shift in the dose–response curve for cocaine (SØrensen et al. 2008; Thomsen et al. 2008; but see Feltenstein et al. 2007) and methamphetamine (Wee et al. 2007) self-administration. In non-human primates, acute aripiprazole administration shifted the dose–response function for methamphetamine discrimination to the right, although there was no shift in the reinforcing effects of cocaine (Bergman 2008). In humans, acute aripiprazole administration (10, 20 mg) decreased oral d-amphetamines (2.5–15 mg) and oral cocaines (25–100 mg) subjective, discriminative stimulus, and cardiovascular effects (Lile et al. 2005, 2010;Stoops 2006).
Although the effects of acute aripiprazole pretreatment appear promising, medications to treat cocaine dependence would be given repeatedly, so it is essential to evaluate how aripiprazole maintenance influences cocaine's effects. Medication effects on cocaine self-administration can vary as a function of the duration of medication administration (see Haney and Spealman 2008). For example, in rats, acute aripiprazole administration decreased cocaine self-administration, yet continuous aripiprazole infusion (0.32–0.56 mg/kg/h) for 5 days or longer did not, and in fact tended to shift the dose–response curve leftward (Thomsen et al. 2008). In humans, aripiprazole administration (10, 15 mg/day for >7 days) tended to increase the effects of intranasal cocaine (Stoops et al. 2007; Lile et al. 2008), and in methamphetamine users, aripiprazole (15 mg/day for 10 days) increased ratings of i.v. methamphetamine-induced craving (Newton et al. 2008) compared to placebo maintenance. Further, a clinical trial investigating aripiprazole maintenance (15 mg p.o.) as a treatment for i.v. amphetamine dependence was terminated earlier because patients maintained on aripiprazole were more than twice as likely to have amphetamine-positive urines than the placebo group (Tiihonen et al. 2007). Thus, the objective of the present human laboratory study was to determine how aripiprazole maintenance (for 21 days) altered smoked cocaine's reinforcing, subjective effects, and cardiovascular effects.
Materials and methods
Participants
Twenty-seven participants, solicited through word-of-mouth referral and newspaper advertisement in New York, NY, USA were enrolled. Volunteers, who were explicitly not interested in treatment for their cocaine use, met DSM-IV criteria for cocaine dependence and tested positive for benzoylecgonine during screening. None met criteria for dependence on any other illicit drug or on alcohol, or had any major psychiatric or medical disorders. Participants signed a consent form approved by The New York State Psychiatric Institute Institutional Review Board which described the study, outlined the possible risks, and indicated that varying doses of aripiprazole (including placebo) and smoked cocaine would be tested. Volunteers were compensated for their participation.
Study schedule
As shown in Table 1, this within-groups study comprised two, 21-day phases: each phase tested a different aripiprazole dose (0, 15 mg/day). Participants were outpatient for the first 16 days of each phase to allow for aripiprazole dose build-up or clearance. During the outpatient phase, they were instructed to take their medication once per day in the early evening. Medication compliance during the outpatient phase was ensured and verified by having participants take their daily capsule under staff observation 3 days/week; testing urine for the presence of riboflavin using ultraviolet detection when participants took capsules at home; and measuring plasma aripiprazole once per outpatient week to compare to titers obtained during the inpatient phase, when participants took medication under observation.
Participants moved into Columbia University's Irving Institute for Clinical and Translational Research at New York-Presbyterian Hospital on day 17, and had two cocaine self-administration sessions per day on days 18–21. They received oral aripiprazole (or placebo) at 5 pm daily. Participants had access to television, radio-, and videotaped movies, and could smoke cigarettes except during laboratory sessions. No one was permitted to leave the unit unescorted and visitors were prohibited. On day 22, the medication condition (aripiprazole vs. placebo) dose was switched, and participants moved out of the laboratory.
Cocaine sessions
Cocaine sessions (2.5 h duration) occurred twice each day for 4 days during both active and placebo–aripiprazole maintenance conditions. Four doses of smoked cocaine (0, 12, 25, 50 mg) were each tested twice during each maintenance condition; order of cocaine dosing was systematically varied between and within-subjects except, as a precaution, the lower doses (12, 25 mg) preceded the higher dose (50 mg) for the first active cocaine administration of the study. Procedures for the morning (9:00 am) and afternoon sessions (3:00 pm) were identical, except for the dose of cocaine tested.
Each self-administration session consisted of six trials. Participants were instructed that they would be repeatedly given the choice to smoke a dose of cocaine or receive $5.00. Sessions began with a baseline period in which participants completed computerized subjective-effects questionnaires, and blood was withdrawn through an 18-gauge catheter (Quik-Cath®, Travenol Laboratories, Deerfield, IL, USA). The session began with one “sample” trial, where participants responded on a keyboard under a fixed ratio schedule (FR200; participants were required to press the spacebar 200 times to receive the option). Subsequently, there were five “choice” trials, spaced 14-min apart, in which participants had the opportunity to self-administer the same dose of cocaine as the sample dose, or to receive money. In each choice trial, participants were asked to choose between two options on their monitor; they were instructed that selecting the left option would be associated with cocaine and the right option would be associated with money; they were given actual cash that was then collected at the end of the session for security purposes, and given to participants upon move-out. They selected the left or right option and pressed the spacebar until they completed the response requirement and the message “Left (or Right) Option Chosen” appeared. A modified progressive ratio of responding was used, in which the first response requirement was a FR200. Following each choice, the response requirement for the chosen option increased by 400, while the response requirement for the non-chosen option did not change. Thus, if a participant only chose cocaine or only chose money, the response requirements would escalate progressively through 600, 1,000, 1,400, 1,800, and 2,200 bar presses.
Research nurses monitored participants via a one-way mirror, and an intercom system provided two-way communication. Electrocardiograms were continuously monitored via limb leads (MAC PC®, Marquette Electronics, Milwaukee, WI, USA), while heart rate and systolic and diastolic blood pressure were recorded every 2 min (Sentry II-Model 6100 automated vital signs monitor, NBS Medical, Costa Mesa, CA, USA) beginning at 20 min prior to cocaine administration and ending at 30 min following the last reinforcer delivery. Subjective effects were measured at baseline, 4 min after each dose, and 30 min after the last option delivery. Blood for plasma cocaine assays was drawn at baseline, 4 min and 12 min after the “sample” cocaine administration and at the end of the session. Plasma aripiprazole levels were measured once per inpatient phase.
Neither cocaine nor money was given if cardiovascular activity exceeded vital signs criteria for at least 6 min [SP > 160 mmHg, DP > 100 mmHg, or HR > (220-participant's age)*0.85], or if changes on the electrocardiogram were deemed unsafe [e.g., >10 premature ventricular contractions/session, evidence of cardiac ischemia]. If two consecutive sessions were terminated due to abnormal vital signs, participants were counseled, referred to appropriate medical follow-up, and discharged from the study.
Subjective-effects battery
A computerized subjective-effects questionnaire, comprising a series of 100-mm visual analog scales (VAS) labeled “Not at all” (0 mm) at one end and “Extremely” at the other, was completed five times per session. Cluster analysis on the 18 mood ratings has yielded four clusters (Evans et al., 2002). The good drug effect cluster comprised: “Good Drug Effect,” “High,” and “Stimulated.” The bad drug effect cluster comprised: “Anxious,” “Bad Drug Effect,” “Confused,” “Depressed,” “Irritable,” “Sedated,” and “Tired.” The social cluster comprised: “Social,” “Talkative,” “Self-confident,” “Alert,” and “Friendly.” The focused/calm cluster comprised “Able to Concentrate” and “Calm.” An additional cluster analysis was done for three adjectives rating the quality of the dose. The cocaine quality cluster comprised: “The choice was …” “Good Quality,” “Potent,” and “I Liked the Choice.” Three VAS operationalized drug craving: “I want…” “Cocaine,” “Alcohol,” and “Tobacco.” The last VAS asked how much participants would pay for the dose (0–$25).
Drugs
Cocaine
The doses of cocaine base (0, 12, 25, 50 mg) were derived from cocaine hydrochloride (Mallinckrodt) by pharmacists in the New York State Psychiatric Institute (NYSPI). Participants were presented with cocaine in a glass pipe stem fitted with a screen. A nurse held a butane flame on the cocaine and participants were instructed to take one large inhalation and hold it as long as they would outside the laboratory. Participants wore eye masks during cocaine administration to be blinded to the dose. The placebo condition was an empty pipe stem.
Aripiprazole
Aripiprazole (Abilify®) was provided by Bristol-Myers Squibb and was encapsulated by the NYSPI pharmacy with a riboflavin marker (50 mg) to monitor medication compliance by urine fluorescence. Dosing was double blind and counterbalanced. Participants were maintained on two doses of aripiprazole (0, 15 mg/day) for 21 days each. Doses were escalated in increments of 5 mg, and the maintenance dose was achieved on the sixth study day (Table 1). Aripiprazole has a long half-life (75 h), so we allowed 17 days of dose build-up and clearance prior to inpatient cocaine sessions.
Data analysis
The effects of aripiprazole maintenance on the number of choices to smoke cocaine within a session, and the subjective effects, cardiovascular measures, and plasma cocaine levels were analyzed using planned comparisons generated using a repeated measures analysis of variance. There were four within-subjects factors: aripiprazole dose (0, 15 mg/day), cocaine dose (0, 12, 25, 50 mg), replication (first and second cocaine dose–response determination within each maintenance condition), and time of measurement. The planned comparisons were single degree of freedom comparisons that used the error term for the aripiprazole dose × cocaine dose × time interaction. Four planned comparisons were completed for each measure to compare placebo maintenance to aripiprazole maintenance for each dose of cocaine (0, 12, 25, 50 mg). P values less than 0.05 were considered statistically significant. Huynh–Feldt corrections were used, when appropriate.
Results
Participant characteristics
Table 2 portrays demographic data for eight male research volunteers who completed the study; although a ninth participant completed the study, his data were not used (see below) so his demographic information was not included in Table 2. All met DSM-IV criteria for cocaine dependence and tested positive for urinary benzoylecgonine during screening. No volunteer was interested in drug treatment, and none met criteria for dependence on other illicit drugs or alcohol or had a major affective illness, schizophrenia, hypertension, or a significant history of heart disease. Eighteen additional participants were enrolled in the protocol but did not complete it: 3 were discharged because they exceeded our cardiovascular criteria for cocaine administration while inpatient; 1 was arrested; 1 was found to be participating in another research study; and 13 failed to reliably make their appointments during the outpatient phases: The majority (69%) of those who failed to make their appointments were in the aripiprazole phase of the study, and had been taking the medication for a mean duration of 11 days (±5 days).
Plasma cocaine
Figure 1 portrays mean plasma cocaine levels 4 and 12 min after a single administration of each cocaine dose, i.e., the “sample dose,” as a function of aripiprazole and cocaine dose. Cocaine produced significant and dose-dependent increases in plasma cocaine levels [F(3,21) = 10.46, p < 0.001]. Aripiprazole had no significant influence on cocaine plasma levels compared to placebo. Data from one participant (S099), portrayed separately, demonstrates that he did not inhale the cocaine vapor at any dose. Thus, his data were excluded from the subsequent analysis.
Mean plasma cocaine levels 4 and 12 min following a single administration of cocaine (the “sample dose”); the data were averaged because plasma cocaine levels were closely similar at these timepoints. The large figure represents eight participants; the ninth participant (S099) is portrayed separately. Error bars represent ± SEM
Self-administration
Figure 2 portrays cocaine self-administration as a function of aripiprazole and cocaine dose. Cocaine was significantly and dose-dependently self-administered [F(3,21) = 56.21, p < 0.0001]. Aripiprazole increased the number of times cocaine (12, 25 mg) was self-administered, as compared to placebo maintenance.
Mean number of cocaine choices as a function of cocaine dose and aripiprazole dose (n = 8). Participants sampled the dose of cocaine available for the session and then had five choices to respond for money ($5.00) or cocaine using a modified progressive-ratio schedule. Error bars represent ± SEM. Asterisks denote a significant difference between active and placebo aripiprazole at that dose of cocaine (*p < 0.05, **p < 0.01)
Subjective effects measures
Figure 3 portrays select subjective effects and cocaine craving as a function of aripiprazole and cocaine dose following a single administration of each cocaine dose, i.e., the “sample” dose. Cocaine significantly increased ratings of cocaine quality [F(3,21) = 8.35, p < 0.05], pay for dose [F(3,21) = 10.81, p < 0.008], good drug effect [F(3,21) = 12.09, p < 0.002], while decreasing ratings of focused/calm by about 10% [F(3,21) = 8.91, p < 0.005; data not shown]. Cocaine also increased cocaine craving, but the effect was not significant (p < 0.06). Aripiprazole had no significant effect on any subjective effects measure at baseline (prior to cocaine administration) compared to placebo. Following a single administration of cocaine, aripiprazole significantly decreased the amount participants would pay for the 25 mg dose.
Mean VAS ratings as a function of cocaine dose and aripiprazole dose 4 min following a single administration of cocaine (the “sample dose”) at the start of the session (n = 8). See Fig. 2 for details
Determining how aripiprazole influenced the subjective effects of repeated cocaine administration is confounded by the self-administration design: for certain doses (12, 25 mg), the number of cocaine doses smoked within a session varied as a function of medication condition, so it is not possible to determine if aripiprazole influenced cocaine's subjective effects during repeated administration of these doses. However, participants self-administered closely similar amounts of the placebo and high-dose cocaine (50 mg) across the two medication conditions (see Fig. 2: 3.9 vs. 3.7 choices), and the pattern of self-administration within a session was closely similar in each medication condition. Comparing the effects of aripiprazole to placebo on subjective effects ratings for these doses revealed that aripiprazole decreased ratings of good drug effect, cocaine quality, and cocaine craving following cocaine (50 mg) relative to placebo maintenance. Aripiprazole also significantly decreased ratings of cigarette craving (by about 18%) and self-confidence (by about 8%) over the course of repeated cocaine administration (50 mg) relative to placebo medication. Further, aripiprazole produced small (6–9%) but significant decreases in ratings of focused/calm following repeated placebo or active (50 mg) cocaine administration.
Cardiovascular measures
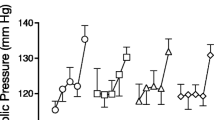
Cocaine significantly increased heart rate [F(3,21) = 68.78, p < 0.0001], diastolic pressure [F(1,21) = 62.07, p < 0.0001], and systolic pressure [F(3,21) = 47.04, p < 0.0001]. Aripiprazole alone produced a small (4 bpm) but significant increase in baseline heart rate [F(1,7) = 9.21, p < 0.02]. Aripiprazole increased the effects of a single administration of cocaine (25 mg) on diastolic pressure by about 4 mmHg [F(1,147) = 5.06, p < 0.05]. The cardiovascular effects of aripiprazole on repeated administration of the lower cocaine doses (12, 25 mg) could not be assessed because of the self-administration design. In combination with repeated placebo–cocaine administration, aripiprazole significantly increased diastolic pressure (5 mmHg) and systolic pressure (6 mmHg). Aripiprazole did not significantly alter the effects of repeated administration of the 50-mg cocaine dose.
Outpatient assessments
In the two outpatient weeks preceding each inpatient phase, participants reported spending $283 ± 58 per week on cocaine during placebo maintenance, while averaging $414 ± 92 per week during aripiprazole maintenance, although the effect was not significant (p < 0.11). There was no difference in the percentage of urines testing positive for cocaine metabolites as a function of medication condition.
Plasma aripiprazole levels during the outpatient phase were comparable to levels obtained while participants were inpatient, demonstrating that participants reliably took their medication (outpatient 124 + 23 ng/ml; inpatient 159 + 26 ng/ml); plasma aripiprazole levels were negligible during the placebo phase (<9 ng/ml). Regarding side effects, during aripiprazole maintenance, five out of the nine participants reported symptoms of fatigue and sluggishness in at least half of their outpatient laboratory visits. By contrast, there were occasional reports of fatigue or insomnia during placebo maintenance, but no one reported these symptoms frequently.
Discussion
The present study demonstrated that administration of the partial dopamine agonist, aripiprazole (15 mg/day), for several weeks increased smoked cocaine (12, 25 mg) self-administration. This increase in cocaine's reinforcing effects occurred in the absence of any change in baseline measures of cocaine craving. Following a single administration of cocaine (25 mg), aripiprazole decreased ratings of how much participants would pay for that dose, and following repeated cocaine (50 mg) administration, aripiprazole decreased ratings of cocaine quality, craving, and good drug effect as compared to placebo. These data suggest that aripiprazole may have increased self-administration to compensate for a blunted subjective cocaine effect.
Given the efficacy of partial agonists for treating opioid (Johnson et al. 2000) and nicotine dependence (Cahill et al. 2009), the rationale for testing a partial dopamine agonist as a treatment for cocaine dependence was to diminish the impact of cocaine-induced increases in extracellular dopamine without incurring the risk and adverse side effects associated with dopaminergic antagonists (Lieberman 2004; Keck et al. 2003). The dose of aripiprazole tested (15 mg) occupies over 85% of striatal D2 receptors (Yokoi et al. 2002; Mamo et al. 2007). Thus, the blunting of cocaine's subjective effects is consistent with the hypothesis that aripiprazole functions as a dopamine receptor antagonist under conditions of elevated extracellular dopamine.
Although aripiprazole increased cocaine self-administration in the present study, the antagonist strategy for drug treatment is based on the assumption that cocaine-taking behavior would eventually extinguish as patients repeatedly experienced a blunted cocaine effect. This is the primary hypothesis in an ongoing multi-site clinical trial, testing a therapeutic cocaine vaccine in cocaine-dependent patients. In the laboratory, individuals who developed sufficient anti-cocaine antibody titers had a blunted subjective response to controlled administration of smoked cocaine (Haney et al. 2010), and in a clinical treatment trial, cocaine use was decreased when participants had high antibody titers (Martell et al. 2009). Yet, unlike the present findings, cocaine-selective antibodies produced an immediate (within 4 min of cocaine smoking) and robust (55–81%) reduction in ratings of good drug effect and cocaine quality (Haney et al. 2010). A similar pattern has been demonstrated for the treatment of opioid dependence: depot naltrexone, which robustly antagonizes opioid effects, decreased heroin use and improved treatment retention without evidence of increased use to overcome the blockade (Comer et al. 2006). By contrast, aripiprazole tended to blunt cocaine's effects after repeated self-administration, and the effect was not large enough to suggest cocaine taking would eventually extinguish, even if individuals seeking treatment were to be tested. In a clinical study of i.v. methamphetamine treatment, aripiprazole increased methamphetamine use relative to placebo (Tiihonen et al. 2007), consistent with our predictions for cocaine treatment.
Similar to aripiprazole, maintenance on the D1 receptor antagonist, ecopipam, increased cocaine self-administration in the human laboratory (Haney et al. 2001). However, unlike aripiprazole, ecopipam also enhanced cocaine's subjective effects. We hypothesized that chronic ecopipam administration produced neuroadaptations associated with continuous dopamine receptor blockade, resulting in enhanced sensitivity to cocaine's reinforcing and subjective effects. The present findings do not support a similar explanation because aripiprazole: (1) attenuated smoked cocaine's subjective effects, and (2) does not appear to readily produce neuroadaptations, such as receptor D2 receptor upregulation (Inoue et al. 1996; Lawler et al. 1999; Lieberman 2004), which could account for heightened subjective effects.
It is important to note that other human laboratory studies have reported that aripiprazole maintenance increased the subjective effects of psychostimulants. Aripiprazole (15 mg) administered for 10 days increased craving for methamphetamine, following i.v. administration (15, 30 mg). However, craving was also increased following i.v. saline administration, so aripiprazole's effects were not specific to methamphetamine (Newton et al. 2008). Aripiprazole (10, 15 mg) maintenance also enhanced certain subjective effects of intranasal cocaine (Lile et al. 2008; Stoops et al. 2007). Yet intranasal cocaine alone produced quite modest subjective effects in these studies. Given that the influence of a partial agonist varies as a function of receptor activation, perhaps aripiprazole enhances subjective ratings when cocaine levels are low, while blunting the effects of more intensive dosing.
Although the present findings do not suggest that aripiprazole would be an effective treatment for patients who continue to use cocaine, the study did not assess whether the medication would be useful in preventing relapse in abstinent cocaine users. In rats, aripiprazole administration (0.25–1 mg/kg i.p. for 3 days) decreased cue- and cocaine-induced reinstatement (Feltenstein et al. 2009). Yet aripiprazole did not decrease baseline cocaine craving in the present study, and had few effects following a single administration of cocaine, which does not suggest that a cocaine-induced relapse would be averted. That is, if a patient used some cocaine, there is little to indicate that aripiprazole would decrease the effects of an acute cocaine dose sufficiently to prevent that lapse from abstinence from progressing to more cocaine use.
The study also did not address whether aripiprazole would be beneficial to cocaine-dependent individuals being treated for a psychiatric comorbidity. Although aripiprazole did not alter cocaine use in bipolar or schizoaffective patients with cocaine-related disorders (Brown et al. 2005), an open-label trial showed that aripiprazole decreased cocaine use in cocaine-dependent schizophrenic patients (Beresford et al. 2005). Given aripiprazole's widespread clinical use, controlled studies are needed to determine its effects on cocaine use for those using the medication to treat a psychiatric disorder.
In terms of study weaknesses, a large number of participants did not complete the study. The majority of those who were discontinued for failure to make their appointments (69%) were taking aripiprazole at the time, which may have contributed to more cocaine use and less reliable behavior. Aripiprazole had intrinsic effects, such as small but significant increases in blood pressure and heart rate (e.g., BP, 5–6 mmHg; HR, 3 bpm) and outpatient reports of fatigue and sluggishness, which could have influenced compliance. Yet participants did not report being distressed about these symptoms, and the medication was well tolerated during inpatient phases, e.g., no effect on ratings of bad drug effect, so we do not suspect that side effects from the medication had a substantial effect on completion rates. Rather, we hypothesize that the factor most likely affecting study completion was the lengthy design, requiring considerable diligence in taking daily medication and making thrice weekly outpatient appointments.
To conclude, maintenance on aripiprazole increased cocaine self-administration while reducing the effects of repeated cocaine administration on measures of craving and intoxication. Although an immediate and robust attenuation of cocaine's effects might reduce cocaine use over time, aripiprazole's effects were mainly apparent only after several administrations of cocaine, and would not likely extinguish cocaine use clinically. Thus, this human laboratory study does not support the potential efficacy of aripiprazole for the treatment of cocaine dependence.
References
Amato L, Minozzi S, Pani PP, Davoli M (2010) Antipsychotic medications for cocaine dependence (Review). Cochrane Libr 3:1–37
Beresford TP, Clapp L, Martin B, Wiberg JL, Alfers J, Beresford HF (2005) Aripiprazole in schizophrenia with cocaine dependence: a pilot study. J Clin Psychopharmacol 25:363–366
Bergman J (2008) Medications for stimulant abuse: agonist-based strategies and preclinical evaluation of the mixed-action D2 partial agonist aripiprazole (Abilify®). Exp Clin Psychopharmacol 16:475–483
Berman RM, Marcus RN, Swanink R, McQuade R, Carson WH, Corey-Lisle PK, Khan A (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatr 68:843–853
Brown ES, Jeffress J, Liggin JD, Garza M, Beard L (2005) Switching outpatients with bipolar or schizoaffective disorders and substance abuse from their current antipsychotic to aripiprazole. J Clin Psychiatr 66:756–760
Cahill K, Stead L, Lancaster T (2009) A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Saf 2:119–135
Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP (2006) Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatr 63:210–218
Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT (1999) Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatr 56:493–502
Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW (2008) A meta-analytic review of psychosocial interventions for substance abuse use disorders. Am J Psychiatr 165:179–187
Evans SM, Haney M, Foltin RW (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159:397–406
Feltenstein MW, Altar CA, See RE (2007) Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatr 61:582–590
Feltenstein MW, Do PH, See RE (2009) Repeated aripiprazole administration attenuates cocaine seeking in a rat model of relapse. Psychopharmacology 207:401–411
Grabowski JA, Shearer J, Merrill J, Negus SS (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464
Haney M, Spealman R (2008) Controversies in translational research: drug self-administration. Psychopharmacology 199:403–419
Haney M, Ward AS, Foltin RW, Fischman MW (2001) Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology 155:330–337
Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW (2010) Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatr 67:59–65
Howell LL, Kimmel HL (2008) Monoamine transporters and psychostimulant addition. Biochem Pharmacol 75:196–217
Inoue T, Domae M, Yamada K, Furukawa T (1996) Effects of the novel antipsychotic agent 7-(4-[4-(2, 3-dichlorophenyl)-1-piperazinyl]butyloxy)-3, 4-dihydro−2(1H)-quinolinone (OPC-14597) on prolactin release from the rat anterior pituitary gland. J Pharmacol Exp Ther 277:137–143
Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE (2000) A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med 343:1290–1297
Keck PE, Marcus R, Tourkodimitris S, Ali M, Liebeskind A, Saha A, Ingentio G (2003) A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatr 160:1651–1658
Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB (1999) Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20:612–627
Lieberman JA (2004) Dopamine partial agonists: a new class of antipsychotic. CNS Drugs 18:251–267
Lile JA, Stoops WW, Vansickel AR, Glaser PE, Hays LR, Rush CR (2005) Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of D-amphetamine in humans. Neuropsychopharmacology 30:2103–2114
Lile JA, Stoops WW, Hays LR, Rush CR (2008) The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance II: increased aripipirazole dose and maintenance period. Am J Drug Alcohol Abuse 34:721–729
Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR (2011). Discriminative stimulus, subject-rated and cardiovascular effects of cocaine alone and in combination with aripiprazole in humans. J Psychopharmacology October 15 (in press)
Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S (2007) Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatr 164:1411–1417
Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR (2009) Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatr 66:1116–1123
Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M (2007) Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatr 164:622–629
Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD (2009) Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatr 166:1170–1177
Newcomer JW (2005) Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19(Suppl 1):1–93
Newton TF, Reid MS, De La Garza R, Mahoney JJ, Abad A, Condos R, Palamar J, Halkitis PN, Mojisak J, Anderson A, Li SH, Elkashef A (2008) Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol 11:1037–1045
Owen L, Sikich RN, Marcus et al (2009) Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 124:1533–1540
Platt DM, Rodefer JS, Rowlett JK (2002) Suppression of cocaine- and food-maintained behavior by the D2-like receptor partial agonist terguride in squirrel monkeys. Psychopharmacology (Berl) 166:298–305
Pulvirenti L, Koob GF (2002) Being partial to psychostimulant addiction therapy. Trends in Pharmacol Sci 23:151–153
Sørensen G, Sager TN, Petersen JH, Brennum LT, Thøgersen P, Hee Bengtsen C, Thomsen M, Wörtwein G, Fink-Jensen A, Woldbye DP (2008) Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology 199:37–46
Stoops WW (2006) Aripiprazole as a potential pharmacotherapy for stimulant dependence: human laboratory studies with d-amphetamine. Exp Clin Psychopharmacol 4:413–421
Stoops WW, Lile JA, Lofwall MR, Rush CR (2007) The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance. Am J Drug Alcohol Abuse 33:769–776
Thomsen M, Fink-Jensen A, Woldbye DP, Wörtwein G, Sager TN, Holm R, Pepe LM, Caine SB (2008) Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology 201:43–53
Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorma H, Sokero P, Haukka J, Meririnne E (2007) A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatr 164:160–162
Vocci FJ, Ling WW (2005) Medications development: successes and challenges. Pharmacol Ther 108:94–108
Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Pappas N, Hitzermann R, Shea CE (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830
Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatr 9:557–569
Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF (2007) Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology 32:2238–2247
Yokoi F, Gründer G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF (2002) Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology 27:248–259
Acknowledgments
The authors would also like to thank the nurses, physicians, and research assistants working in the Marian W. Fischman Cocaine Research Laboratory, and appreciate the contributions of their fellow investigators: Drs. Carl L. Hart, Suzanne Vosburg, Nehal Vadhan, Suzette Evans, and Stephanie Collins Reed.
Financial disclosures
Dr. Haney received consultant fees from Celtic Pharmaceuticals in 2007. Dr. Foltin currently receives research support for an investigator-initiated protocol from Aztra Zeneca. Dr. Rubin reported no biomedical financial interests or potential conflicts of interest. This research was supported by both NIDA (DA10755/Haney; DA006234/Foltin) and funding from Bristol-Meyers Squibb. Participants resided on the Irving Institute for Clinical and Translational Research of The New York-Presbyterian Medical Center, supported by Grant No. MOI-RR-00645 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haney, M., Rubin, E. & Foltin, R.W. Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology 216, 379–387 (2011). https://doi.org/10.1007/s00213-011-2231-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2231-6