Abstract
Rationale
Performance on the Cambridge Neuropsychological Test Automated Battery touchscreen paired-associates learning (PAL) test is predictive of Alzheimer’s disease and impaired in schizophrenia and chronic drug users. An automated computer touchscreen PAL task for rats has been previously established. A pharmacologically validated PAL task for mice would be a highly valuable tool, which could be useful for a number of experimental aims including drug discovery.
Objectives
This study sought to investigate the effects of systemic administration of cholinergic agents on task performance in C57Bl/6 mice.
Methods
Scopolamine hydrobromide (0.02, 0.2, and 2.0 mg/kg), dicyclomine hydrochloride (M1 receptor antagonist; 2.0, 4.0, and 8.0 mg/kg), and donepezil hydrochloride (cholinesterase inhibitor; 0.03, 0.1, and 0.3 mg/kg) were administered post-acquisition in C57Bl/6 mice performing the PAL task.
Results
Scopolamine (0.2 and 2.0 mg/kg) and dicyclomine (at all administered doses) significantly impaired PAL performance. A significant facilitation in PAL was revealed in mice following donepezil administration (0.3 mg/kg).
Conclusions
The present study shows that mice can acquire the rodent PAL task and that the cholinergic system is important for PAL task performance. M1 receptors in particular are likely implicated in normal performance of PAL. The finding that mouse PAL can detect both impairments and improvements indicates that this task could prove to be a highly valuable tool for a number of experimental aims including drug discovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal models of human disease are essential for drug discovery. However, rodents are often assessed in behavioral tests that do not closely resemble the cognitive tasks that are currently used to examine human subjects. Creating more translatable behavioral tasks for testing rodents might provide results that are more interpretable, yielding more useful information regarding which treatments and therapeutic agents might aid human patients. This was part of the motivation behind the development of a touchscreen testing method for rats and mice (Bussey et al. 1994; Bussey et al. 1997; Bussey et al. 2001b). This method allows the presentation of stimuli on a touch-sensitive computer screen; the rodent can then select an object or location by poking its nose toward the screen. In this way, tests can be administered in which the stimuli and responses are comparable to touchscreen neuropsychological tasks used in humans (e.g., Cambridge Neuropsychological Test Automated Battery (CANTAB); Robbins et al. 1994). Furthermore, like the human touchscreen paradigm, the nature of the stimulus materials, responses, feedback, and testing environment are consistent across tasks, whether those tasks measure learning, memory, perception, behavioral inhibition, or other aspects of cognition. This feature of the method allows better comparison between tasks than is possible in other task batteries in which different aspects of cognition are assessed using very different apparatus, stimuli, responses, and feedback. Furthermore, food reward is used as the reinforcer in all tasks, avoiding the use of an aversive, stressful testing situation.
The object-in-place paired-associates learning (PAL) task, part of the CANTAB, requires the subject to learn to associate a given object with a particular location. CANTAB PAL has been shown effectively to identify and predict conversion from mild cognitive impairment to Alzheimer’s disease (AD; Sahakian et al. 1988; Swainson et al. 2001; Blackwell et al. 2004). This impressive sensitivity may be due to the requirement for the use of both place and object information, thought to depend on structures such as the hippocampus and perirhinal cortex that are amongst the first affected in the disease (Braak and Braak 1997). PAL is also impaired in first-episode psychosis, chronic schizophrenics, and chronic drug users (Wood et al. 2002; Barnett et al. 2005; Ersche et al. 2006). Furthermore, the performance of AD patients in CANTAB PAL has been shown to be significantly improved following oral administration of a cholinesterase inhibitor (Greig et al. 2005). A touchscreen-automated object-in-place PAL task has been recently adapted for rats and has been shown to be sensitive to manipulation of glutamatergic systems within the hippocampus (Talpos et al. 2009).
Although the touchscreen testing method has been successfully adapted for mice (Bussey et al. 2001b; Brigman et al. 2005; Izquierdo et al. 2006; Morton et al. 2006; Brigman et al. 2008), the utility of a mouse version of the PAL task has not been demonstrated. Since mice are commonly used for the screening of therapeutic agents and for understanding human cognitive disorders, a validated touchscreen-automated PAL task for the mouse would be a highly valuable tool for a number of experimental aims including drug discovery. Therefore, in the present investigation, the same touchscreen-automated PAL task used for rats (Talpos et al. 2009) was used with mice. Since CANTAB PAL measures retention of paired associates, rather than acquisition of these pairs across trials, the present study examined retention of paired associates that had been previously acquired. Furthermore, since CANTAB PAL is commonly impaired in AD and schizophrenic patients and AD, and to a lesser extent schizophrenia, has been associated with cholinergic dysfunction (Bartus et al. 1982; Raedler et al. 2007; Scarr and Dean 2009), our initial studies focused on cholinergic agents. Scopolamine (a competitive non-selective muscarinic acetylcholine antagonist) was predicted to impair PAL task performance. Dicyclomine (M1 receptor antagonist) was used to begin to understand the specific cholinergic mechanisms involved in PAL. Finally, the effect of systemic administration of one of the most commonly prescribed therapeutic drugs for AD, donepezil (Aricept®), was also examined and was predicted to have a facilitatory effect on performance of the PAL task.
Materials and methods
Subjects
The subjects were ten male C57Bl/6 mice (Harlan Olac, Bicester, UK) aged 7–10 weeks old at the start of behavioral testing. In Experiment 1, one mouse did not reach criterion on the task before the drug studies and was excluded from all analyses. In addition, one mouse died after the first drug study; therefore, nine mice were included in data analysis for drug study A and eight mice were included for drug studies B and C. Mice were housed in groups in a room with a 12-h light/dark cycle (lights off at 7:00 p.m.). All behavioral testing was conducted during the light phase of the cycle. Mice were maintained on a restricted diet and kept at 85% of free-feeding body weight during behavioral testing. Mice were fed wet food mash in home cages following behavioral testing. Water was available ad libitum throughout the experiment. All experimentation was conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act (1986).
Apparatus
Testing was conducted in a touchscreen-based automated operant system for mice (e.g., Bussey et al. 2001b; Morton et al. 2006; Brigman et al. 2008). The apparatus consisted of a standard modular testing chamber housed within a sound- and light-attenuating box (40 × 34 × 42 cm; Med Associates Inc., St. Albans, Vermont). The inner operant chamber consisted of a metal frame, clear Perspex walls, and a stainless steel grid floor. The box was fitted with a fan (for ventilation and masking of extraneous noise) and a pellet receptacle (magazine), which was illuminated by a 3-W light bulb and fitted with a photocell head entry detector attached to 14 mg pellet dispenser (situated outside of the box). A 3-W houselight and tone generator (Med Associates) were fitted to the back wall of the chamber. Behavioral testing programs were controlled with a computer software program written in Visual Basic (Microsoft, Redmond, WA).
At the end of the box opposite the magazine was a flat-screen monitor equipped with an infrared “touchscreen” (16 cm high and 21.20 cm wide; Craft Data Limited, Chesham, UK) mediated by ELO touchscreen software (ELO Touchsystems Inc.). Since the “touchscreen” uses infrared photocells, the mouse was not required to exert any pressure on the monitor screen in order for a nose-poke to be detected. Furthermore, black Perspex “masks” with response windows were placed over the screen, through which the mouse could make a nose-poke toward the screen. The mask (h 11.80 cm × w 22.8 cm) had three response windows (h 5.80 cm × w 5.0 cm) which were positioned 0.8 cm apart from one another and located 3.0 cm from the sides of the mask.
Pretraining
Prior to testing, mice were handled daily for 1 week, and body weights were closely monitored. During the first session, mice were habituated to the testing chamber for two consecutive daily sessions. The houselight remained on during the entire session and food pellets were placed in the magazine and the mice were left in the testing chamber for 15 min. After the habituation stage, mice were trained to collect pellets delivered under a variable interval 30 s schedule, which coincided with illumination of the magazine light and presentation of a tone. Training stimuli (40 stimuli varying in brightness, shape, and pattern) were presented (one per trial) for 30 s on the touchscreen located on one of the three response windows. A large set of training stimuli were used to minimize subsequent generalization from a particular stimulus feature. A single reward pellet was delivered immediately following stimulus offset. If the mouse touched the stimulus, the stimulus immediately disappeared from the touchscreen and the mouse was rewarded with the tone, magazine light, and three food pellets. Completion of this stage was not dependent on the mouse touching the stimuli on the screen; therefore, the mice were removed from the testing chamber after the 30 min session, regardless of the number of trials completed.
The next training session required the mouse to respond to the stimulus presented on the touchscreen in order to receive a pellet reward. On each trial, a training stimulus was shown in one of the three response windows and remained on the screen until the mouse responded to the stimulus. The mouse was rewarded with a pellet, tone, and illumination of the magazine light, followed by a 20-s ITI after which the next trial commenced, and a new stimulus was presented on the touchscreen. Once a mouse successfully completed 30 trials in a 1-h session, the mouse was then required to initiate trials in the next training stage. During this stage, after collecting a reward following a response to the stimulus, and exiting the food magazine, the mouse was required to again poke their head in the magazine in order for the next stimulus array to be presented on the screen. Once the mouse was successfully initiating trials and had completed 30 trials in 1 h, the last training stage began.
During the final stage, a cue to signal incorrect responses (responses to a window in which no stimulus was displayed) and a correction procedure were introduced. A stimulus was presented in one of the three response windows and the mouse was required to nose-poke the stimulus. Correct responses were followed by stimulus offset, presentation of the pellet, tone, and illumination of the food magazine (followed by a 20-s ITI). Incorrect responses resulted in the houselight being extinguished, disappearance of the stimulus, and a time-out period of 5 s, which was followed by the 20 s ITI. A correction procedure was implemented whereby the trial was repeated until the mouse made a correct choice. Once the mouse completed this stage at 70% correct (correction trials were not included; the 70% correct measure pertains to non-correction trials only; 21/30) over two consecutive sessions, mice began the PAL task.
PAL task
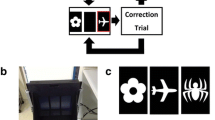
After successful completion of pretraining, mice were trained on the PAL task (see Fig. 1a; Clelland et al. 2009). At the beginning of a session, the mouse was required to initiate the first trial. Then, a pair of stimuli would appear on the screen in two of the three locations: left, middle, and right; one stimulus was the correct S+ and the other was the incorrect S−. There were six possible trial types and three different stimuli were used (flower, plane, and spider). Within the six trial types, the flower was rewarded only when presented in the left location, the plane was rewarded only when presented in the middle location, and the spider was rewarded only when presented in the right location (see Fig. 1b). Therefore, the mouse was required to learn the paired-association of stimulus and location. A nose-poke to the correct S+ resulted in a tone, magazine light, and reward pellet. Incorrect responses resulted in a 5-s time-out period, followed by correction procedure. Nose-pokes to response windows in which no stimulus was presented were ignored. The ITI during the task was fixed at 20 s. During the first 60 sessions, each mouse was required to complete 72 trials (each of the six trial types occurred 12 times, and the maximum number of times a trial type could occur consecutively was 2) or complete as many trials as possible in 1 h. However, some mice were not completing the 72 trials within a 1-h session. Therefore, starting at session 60, mice were required to complete 36 trials in 1 h (each trial type could occur six times, and the maximum number of times a trial could occur consecutively was 2). Mice were then required to complete all 36 trials with 80% accuracy over three consecutive days (chance level is 50%) to reach criterion. After a mouse reached criterion, testing of that mouse stopped. After all mice reached criterion (all mice reached criterion within 35 sessions), mice were re-baselined on the task for 4 days for stable performance, and then the first drug study began. Following the drug study A, mice were re-baselined on the PAL task for 7 days prior to drug study B. Following drug study B, mice were re-baselined on the task for 7 days prior to drug study C. During all drug studies, two washout days were used between treatments, and on these days mice were baselined on the PAL task.
Illustration of the PAL task. a) Figure depicting trial type 1, in which flower is the S+ and plane is the S−. b) Figure depicting three of the six possible trial types that could occur in the PAL task. Flower is the S+ when it is located in the left location, plane is the S+ when located in the middle location, and spider is the S+ when located in the right location. From Clelland et al. (2009). Reprinted with permission from AAAS
Pharmacological challenges
The role of the cholinergic system in the PAL task was assessed using a variety of cholinergic drugs. All drugs were administered intraperitoneally with injection volumes of 0.1 ml per 20 g of the weight of the mouse. Drugs were dissolved in physiological saline (0.9% sodium chloride, pH 7.0; Aquapharm; Animalcare, York, UK) to create appropriate stock solutions. The first pharmacological study (drug study A) used a Latin square design to examine the effects of administering the commonly used acetylcholinesterase inhibitor used to treat AD, donepezil (Aricept, donepezil hydrochloride, Toronto Research Chemicals, Canada). Donepezil was also given at three doses (0.03, 0.1, and 0.3 mg/kg) and was administered 1 h prior to behavioral testing. Previous studies have utilized a wide range of pharmacological doses of donepezil in order to improve behavior in mice (e.g., Spowart-Manning and van der Staay 2004; Csernansky et al. 2005; Dong et al. 2005; Riedel et al. 2009). However, treatment of donepezil using a 0.3 mg/kg dose has been shown to reverse behavioral deficits in middle-aged mice completing an episodic contextual memory task (Béracochéa et al. 2007), improve Morris Water Maze performance in a APP23 mutant mouse model of AD (Van Dam et al. 2005), and improve short-term working memory in aged mice (Marighetto et al. 2008). Pirenzepine (0.02, 0.2, and 2.0 mg/kg) was also given to these animals in a crossed Latin square design; however, there were no effects of this drug (data not shown), likely due to its suspected inability to cross the blood-brain barrier (Piper 1995). The second pharmacological study (drug study B) was carried out using a Latin square design to investigate the role of the non-specific muscarinic antagonist, scopolamine. Twenty minutes prior to behavioral testing, scopolamine (scopolamine hydrobromide, Sigma-Aldrich, UK) was administered at three doses (0.02, 0.2, and 2.0 mg/kg) and methylscopolamine (Sigma-Aldrich, UK) was administered at the highest scopolamine dose (2.0 mg/kg). Previous studies have used identical (Humby et al. 1999) and similar doses (0.3, 1, and 3.0 mg/kg; Dodart et al. 1997) to investigate behavior in mice in automated and object recognition tasks, respectively; therefore, we believe our dose range is ideal for the present study. Methylscopolamine was used to dissociate the central from peripherally mediated effects of scopolamine, since this drug cannot cross the blood-brain barrier. The final pharmacological study (drug study C) examined the effects of the M1-preferring antagonist dicyclomine (dicyclomine hydrochloride, Sigma-Aldrich, UK; Giachetti et al. 1986; Doods et al. 1987) in the PAL task. Dicyclomine was administered 20 min prior to behavioral testing and was given at three doses (2.0, 4.0, and 8.0 mg/kg). Although higher doses of dicyclomine (16, 32, and 64 mg/kg; Soares et al. 2006) have been used to investigate behavior in rats, recent studies have shown behavioral impairments in mice in spatial tasks following systemic dicyclomine treatment at lower doses (8.0 mg/kg; Caccamo et al. 2006).
Data analysis
Group means of accuracy (percent correct) were analyzed for each drug study. Reaction time and magazine latency were analyzed if a drug study resulted in a main effect on accuracy. Group means of reaction time (the length of time between an initiation (nose-poke to magazine at the back of the chamber) and nose-poke to the touchscreen) were analyzed and incorrect trials (not including correction trials). Group means of magazine latency (the length of time between a correct choice nose-poke to the touchscreen and a nose-poke for food in magazine) were analyzed for correct trials (including correction trials). Group means of the total number of correction trials committed during each drug study were analyzed in order to determine whether the drugs had a perseverative effect on mice in PAL. Data were excluded from analysis when a mouse completed less than 80% of trials during a session. Means were submitted to one-way ANOVA with repeated measures for drug. Planned comparisons t tests were used when hypotheses predicted a greater effect of drug with the highest experimental dose. Paired-samples t tests were used for post hoc analyses of within-subject effects of drug. When data were significantly non-normal, appropriate transformations were performed (Howell 2002). All statistical analyses were conducted with a significance level of p = 0.05.
Results
Drug study A
Donepezil a selective AChE inhibitor, was administered systemically at three dose levels: low (0.03 mg/kg), middle (0.1 mg/kg), and high (0.3 mg/kg) and was predicted to produce an improvement in performance in the PAL task.
Accuracy
There was no overall effect of drug following administration of donepezil in the PAL task (F (3,24) = 2.43, p = 0.09). We predicted the greatest improvement in PAL performance would be revealed following administration of the high dose (0.3 m/kg) of donepezil since recent studies have reported behavioral improvements in mice using this dose. In the present study, planned comparisons revealed that the high dose of donepezil (0.3 mg/kg) produced significantly higher performance in PAL as compared to saline trials (t (8) = 2.84, p = 0.02; see Fig. 2 for graph and Table 1A for paired-samples t tests post hoc comparisons of accuracy).
Reaction time
Reaction times for correct trials (F (3,24) = 1.47) and incorrect trials (F (3,24) = 2.12) were not affected by donepezil (see Table 1B for mean reaction times ± SEM for the correct trials and incorrect trials).
Magazine latency
Magazine latency for correct trials was also not affected by injections of donepezil (F (3,24) = 1.59; see Table 1B for mean magazine latencies ± SEM).
Correction trials
Administration of donepezil significantly affected the number of correction trials committed by mice in the PAL task (F (3,24) = 3.34, p = 0.04; see Fig. 1b for mean correction trials ± SEM and Fig. 1c for paired-samples t test post hoc comparisons).
Drug study B
Following drug study A, which showed that donepezil can produce facilitations in the PAL task, drug study B was designed to examine whether antagonizing the effect of cholinergic muscarinic receptors would produce deficits in the PAL task. Therefore, three doses of the non-selective muscarinic antagonist scopolamine: low (0.02 mg/kg), middle (0.2 mg/kg), and high (2.0 mg/kg), methylscopolamine (2.0 mg/kg), and saline were injected prior to behavioral testing. Since increasing ACh activity produced an improvement in performance following donepezil injections, administration of scopolamine was predicted to decrease accuracy in the PAL task.
Accuracy
Administration of scopolamine produced a highly significant effect on performance in the PAL task (F (4,28) = 14.22, p < 0.0001; see Fig. 3 for graph and Table 2A for paired-samples t tests post hoc comparisons of accuracy).
Impairments in accuracy is produced following systemic administration of scopolamine in the PAL task. Data are presented as mean accuracy ± SEM, paired-samples: methylscopolamine—middle scopolamine, **p = 0.003; methylscopolamine—high scopolamine, ***p < 0.0001; saline—middle scopolamine, **p = 0.001; saline—high scopolamine, ***p < 0.0001; low scopolamine—middle scopolamine, *p = 0.02; low scopolamine—high scopolamine, *p = 0.03
Reaction times
Reaction times for correct trials (F (4,28) = 4.10, p = 0.01) and incorrect trials (F (4,28) = 4.86, p = 0.004) were significantly affected by scopolamine (see Table 2B for mean reaction times ± SEM for the correct trials and incorrect trials and Table 2C for paired-samples t tests post hoc comparisons of mean reaction times for correct trials and Table 2D for paired-samples t tests post hoc comparisons of mean reaction times for incorrect trials).
Magazine latency
Magazine latency was significantly affected by injections of scopolamine (F (4,28), p = 0.01; Table 2B for mean reaction times ± SEM and Table 2E for paired-samples t tests of mean reaction times for latency).
Correction trials
Administration of scopolamine significantly affected the number of correction trials committed by mice in the PAL task (F (4,28) = 16.90, p < 0.0001; see Table 2B for mean correction trials ± SEM and Table 2F for paired-samples t test analyses of correction trials).
Drug study C
Since drug study B revealed that cholinergic muscarinic receptors are necessary for PAL, drug study C was designed to investigate if M1 receptors, in particular, are involved in the PAL task. Therefore, drug study C examined the potential effect an M1 preferring antagonist (dicyclomine) might have on the PAL task. Three doses of dicyclomine: low (2.0 mg/kg), middle (4.0 mg/kg), and high (8.0 mg/kg) were injected prior to behavioral testing. Since scopolamine produced profound deficits in PAL, administration of dicyclomine was predicted to also decrease accuracy in the PAL task.
Accuracy
Administration of dicyclomine produced a highly significant effect on performance in the PAL task (F (3,21) = 6.96, p = 0.002; see Fig. 4 for graph and Table 3A for paired-samples t tests post hoc comparisons of accuracy).
Reaction time
Reaction time for correct trials (F (3,21) = 14.77, p = 0.00002) and incorrect trials (F (3,21) = 15.34, p < 0.0001) was significantly affected by injections of dicyclomine (see Table 3B for mean reaction times ± SEM for the correct trials and incorrect trials and Table 3C for paired-samples t tests post hoc comparisons of mean reaction times for correct trials and Table 3D for paired-samples t tests comparisons for reaction times for incorrect trials.
Magazine latency
Magazine latency was also significantly affected by injections of dicyclomine (F (3,21) = 5.37, p = 0.007; see Table 3B for mean magazine latencies ± SEM and Table 3E for paired-samples t tests post hoc comparisons of mean magazine latencies).
Correction trials
Administration of dicyclomine did not significantly affect the number of correction trials committed by mice in the PAL task (F (3,21) = 2.11; see Table 3B for mean correction trials ± SEM).
Discussion
The present study sought to test the utility of a mouse version of the rodent touchscreen PAL task (Talpos et al. 2009) for pharmacological investigations. We found that dose-dependent effects of a variety of agents could be detected using mouse PAL. Specifically, the current series of experiments implicates the central cholinergic system in mediating PAL performance. Administration of scopolamine impaired accuracy of mice performing the PAL task at the middle doses (0.2 and 2.0 mg/kg), and administration of the M1 preferring antagonist, dicyclomine, also impaired accuracy. Performance was impaired at even the lowest dose of dicyclomine, thus this study did not identify a dose at which performance was unaffected. Nevertheless, these results suggest that cholinergic muscarinic receptors, and more specifically M1 receptors, may be involved in performance of rodent PAL.
In addition, we found that donepezil (Aricept) at a dose of 0.3 mg/kg was able to produce improvements in accuracy on mouse PAL. Donepezil is in a class of AChE inhibitor drugs that are commonly prescribed for mild, moderate, and severe AD (Birks and Harvey 2006; Lleo et al. 2006). Previous studies have shown that donepezil treatment in AD patients (Rogers et al. 1998; Burns et al. 1999; Winblad et al. 2001), transgenic mouse models of AD (Dong et al. 2005; Van Dam et al. 2008), and aged monkeys (Buccafusco and Terry 2004) improves cognition and behavior. Donepezil has also been used to reverse scopolamine-induced behavioral impairments in healthy animals (Buccafusco et al. 2008; Cachard-Chastel et al. 2008). Furthermore, an in vivo imaging study has revealed that AD patients taking donepezil have a better preservation of M1 receptor binding than AD patients receiving placebo treatment (Kemp et al. 2003; consistent with our finding of a role for M1 receptors in the performance of PAL; see below). The current study indicates that systemic administration of donepezil can also produce behavioral facilitations in mouse PAL in normal, healthy mice and indeed, because the facilitation was observed at the highest dose tested, still greater improvements in performance might be observed at higher doses.
The findings that scopolamine and dicyclomine impair mouse PAL are consistent with the finding that scopolamine impairs performance of CANTAB PAL (Rusted and Warburton 1988; Robbins et al. 1997) and a non-human primate version of PAL (Taffe et al. 2002). It should be noted, however, that the effect in humans appears to be relatively mild unless the task is made more challenging, for example by removing features such as colors on which the stimuli can be discriminated (Rusted and Warburton 1988). This suggests that at least part of the impairment seen on CANTAB PAL and in the present study may be due to difficulties in visual discrimination. It is likely that the visual discrimination component of rodent PAL is more challenging for rodents than the visual discrimination component of CANTAB PAL is for humans. This idea is consistent with the very robust effects of scopolamine on mouse PAL in the present study. Important targets for future studies using mouse PAL include testing with other drugs that affect paired-associate learning in humans, for example diazepam (Robbins et al. 1997).
Where are these drugs having their effects? We know that hippocampal dysfunction impairs rodent PAL; however, intra-hippocampal administration of scopolamine in rats leaves PAL performance intact (Talpos et al. 2009). This finding suggests that in the present study, systemically delivered scopolamine and dicyclomine produced impairments due to actions of the drug outside of the hippocampus. Results from other object-in-place associative learning tasks (Gaffan and Parker 1996; Bussey et al. 2001a) indicate roles for both the hippocampus and perirhinal cortex. Whereas the hippocampus may contribute to the location component of the task, perirhinal cortex may contribute “object” information. Consistent with this suggestion and with the present findings, muscarinic receptors in the perirhinal cortex have been implicated in object learning and memory (e.g., Turchi et al. 2005; Winters et al. 2007) and object-in-place associative recognition memory (Barker and Warburton 2008).
The rodent PAL task was inspired by the PAL task that is part of the CANTAB cognitive testing battery, which is highly sensitive to, and indeed predictive of, AD (Swainson et al. 2001; Blackwell et al. 2004). Although the rodent PAL task has similarities in to the CANTAB PAL task, a major difference is that in CANTAB PAL, a subject is given an acquisition phase, in which pairings of stimulus and location are presented, followed by a recall phase. In rodent PAL, there are not two phases; instead object–place associations are learned across trials. The question is whether this difference is important. We would argue that it is likely that a critical factor contributing to the sensitivity of CANTAB PAL to neurodegenerative diseases such as AD is that the task requires simultaneous activation of both object and spatial representations. These representations are thought to be mediated by medial temporal lobe structures, which are the first to show pathology in AD. Consistent with this interpretation, imaging studies indicate that these structures are recruited in CANTAB PAL (M. De Rover, V.A. Pironti, P.C. Fletcher, T.W. Robbins and B.J. Sahakian, unpublished observations). Furthermore, the idea that the critical factor may be the representations necessary for solution of the task, and not the rate of learning, is supported by experiments showing that the rate of learning has no bearing on whether a task recruits temporal lobe regions. Instead, the critical factor is the material that is being learned and indeed, slowly acquired tasks can be the ones that most strongly require the temporal lobe (Bussey et al. 2001a, b; Barense et al. 2005). Thus, it seems likely that the object-in-place learning requirement of the task is perhaps the most critical factor in the sensitivity of the task to AD. This requirement is present in both the human and rodent versions of the task.
At some doses, some of the drugs used in the present study, such as scopolamine, affected choice reaction times, as well as magazine latency (although numbers of trials completed was not affected in any case). The relationship between the altered reaction times and choice accuracy is unclear. It could be that reaction times and choice accuracy are unrelated, perhaps mediated by non-overlapping brain systems mediating motor behavior and cognition, respectively. Alternatively, the slower reaction times could have been secondary to the primary effect of the drug on cognition, which resulted in fewer correct trials and therefore lack of motivation. It seems somewhat less likely that taking longer to respond might have detrimentally affected choice accuracy. The first interpretation seems most likely, as impairments in PAL are not always accompanied by slower reaction times, and intra-hippocampal scopolamine lengthens reaction times but leaves accuracy unaffected on PAL in rats (Talpos et al. 2009).
Donepezil and scopolamine significantly affected the mean number of correction trials committed in PAL as compared to saline (and the overall effect of drug was significantly greater in the scopolamine study). However, perseveration (mice committing a greater number of correction trials after systemic administration of cholinergic drugs) was not observed in the dicyclomine drug study. Although there is evidence to suggest that lesions to the ventrolateral prefrontal cortex produce perseverative interference in object-in-place scene learning (Baxter et al. 2008), further investigation is necessary to examine whether cholinergic manipulations in mouse PAL produce similar perseveration impairments.
The present investigation introduces the touchscreen-automated mouse PAL task as a valuable tool for pharmacological investigations in mice. As many other tests of cognition can be run in the touchscreen apparatus (e.g., (Bussey et al. 1997; Bussey et al. 2001b; Chudasama et al. 2001; Morton et al. 2006; Brigman et al. 2008; Bussey et al. 2008; McTighe et al. 2009), mouse PAL can be used as part of a cognitive battery in which stimulus material, responses, reinforcers, and testing context can be held constant across all tasks. Such a battery may prove particularly useful in drug discovery applications.
References
Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS (2005) Functional specialization in the human medial temporal lobe. J Neurosci 25:10239–10246
Barker GR, Warburton EC (2008) NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci 28:2837–2844
Barnett JH, Sahakian BJ, Werners U, Hill KE, Brazil R, Gallagher O, Bullmore ET, Jones PB (2005) Visuospatial learning and executive function are independently impaired in first-episode psychosis. Psychol Med 35:1031–1041
Bartus RT, Dean RL 3rd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Baxter MG, Browning PG, Mitchell AS (2008) Perseverative interference with object-in-place scene learning in rhesus monkeys with bilateral ablation of ventrolateral prefrontal cortex. Learn Mem 15:126–132
Béracochéa D, Philippin JN, Meunier S, Morain P, Bernard K (2007) Improvement of episodic contextual memory by S 18986 in middle-aged mice: comparison with donepezil. Psychopharmacology (Berl) 193:63–73
Birks J, Harvey RJ (2006) Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev: CD001190
Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR (2004) Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dement Geriatr Cogn Disord 17:42–48
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357
Brigman JL, Bussey TJ, Saksida LM, Rothblat LA (2005) Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav Neurosci 119:839–842
Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A (2008) Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem 15:50–54
Buccafusco JJ, Terry AV (2004) Donepezil-induced improvement in delayed matching accuracy by young and old rhesus monkeys. J Mol Neurosci 24:85–91
Buccafusco JJ, Terry AV Jr, Webster SJ, Martin D, Hohnadel EJ, Bouchard KA, Warner SE (2008) The scopolamine-reversal paradigm in rats and monkeys: the importance of computer-assisted operant-conditioning memory tasks for screening drug candidates. Psychopharmacology (Berl) 199:481–494
Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Moller HJ, Rogers SL, Friedhoff LT (1999) The effects of donepezil in Alzheimer’s disease—results from a multinational trial. Dement Geriatr Cogn Disord 10:237–244
Bussey TJ, Muir JL, Robbins TW (1994) A novel automated touchscreen procedure for assessing learning in the rat using computer graphic stimuli. Neurosci Res Commun 15:103–110
Bussey TJ, Muir JL, Everitt BJ, Robbins TW (1997) Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci 111:920–936
Bussey TJ, Dias R, Amin E, Muir JL, Aggleton JP (2001a) Perirhinal cortex and place–object conditional learning in the rat. Behav Neurosci 115:776–785
Bussey TJ, Saksida LM, Rothblat LA (2001b) Discrimination of computer-graphic stimuli by mice: a method for the behavioral characterization of transgenic and gene-knockout models. Behav Neurosci 115:957–960
Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM (2008) The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Mem 15:516–523
Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM (2006) M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 49:671–682
Cachard-Chastel M, Devers S, Sicsic S, Langlois M, Lezoualc’h F, Gardier AM, Belzung C (2008) Prucalopride and donepezil act synergistically to reverse scopolamine-induced memory deficit in C57Bl/6j mice. Behav Brain Res 187:455–461
Chudasama Y, Bussey TJ, Muir JL (2001) Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci 14:1009–1020
Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325:210–213
Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H (2005) Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology 30:2135–2143
Dodart JC, Mathis C, Ungerer A (1997) Scopolamine-induced deficits in a two-trial object recognition task in mice. NeuroReport 8:1173–1178
Dong H, Csernansky CA, Martin MV, Bertchume A, Vallera D, Csernansky JG (2005) Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology (Berl) 181:145–152
Doods HN, Mathy MJ, Davidesko D, van Charldorp KJ, de Jonge A, van Zwieten PA (1987) Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther 242:257–262
Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ (2006) Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology 31:1036–1047
Gaffan D, Parker A (1996) Interaction of perirhinal cortex with the fornix-fimbria: memory for objects and “object-in-place” memory. J Neurosci 16:5864–5869
Giachetti A, Giraldo E, Ladinsky H, Montagna E (1986) Binding and functional profiles of the selective M1 muscarinic receptor antagonists trihexyphenidyl and dicyclomine. Br J Pharmacol 89:83–90
Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK (2005) An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res 2:281–290
Howell D (2002) Statistical methods for psychology, 5th edn. Duxbury, Duxbury
Humby T, Laird FM, Davies W, Wilkinson LS (1999) Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci 11:2813–2823
Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A (2006) Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res 171:181–188
Kemp PM, Holmes C, Hoffmann S, Wilkinson S, Zivanovic M, Thom J, Bolt L, Fleming J, Wilkinson DG (2003) A randomised placebo controlled study to assess the effects of cholinergic treatment on muscarinic receptors in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 74:1567–1570
Lleo A, Greenberg SM, Growdon JH (2006) Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med 57:513–533
Marighetto A, Valerio S, Desmedt A, Philippin JN, Trocmé-Thibierge C, Morain P (2008) Comparative effects of the alpha7 nicotinic partial agonist S 24795, and the cholinesterase inhibitor, donepezil, against aging-related deficits in declarative and working memory in mice. Psychopharmacology (Berl) 197:499–508
McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM (2009) A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport 20:881–885
Morton AJ, Skillings E, Bussey TJ, Saksida LM (2006) Measuring cognitive deficits in disabled mice using an automated interactive touchscreen system. Nat Meth 3:767
Piper DW (1995) A comparative overview of the effects of antinuclear drugs. Drug Saf 12:120–138
Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B (2007) Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry 12:232–246
Riedel G, Kang SH, Choi DY, Platt B (2009) Scopolamine-induced deficits in social memory in mice: reversal by donepezil. Behav Brain Res 204:217–225
Robbins TW, James M, Owen AM, Sahakian BJ, McInnes LPR (1994) Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 5:266–281
Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K (1997) Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 134:95–106
Rogers SL, Doody RS, Mohs RC, Friedhoff LT (1998) Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 158:1021–1031
Rusted JM, Warburton DM (1988) The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology (Berl) 96(2):145–152
Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW (1988) A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain 111(Pt 3):695–718
Scarr E, Dean B (2009) Role of the cholinergic system in the pathology and treatment of schizophrenia. Expert Rev Neurother 9:73–86
Soares JC, Fornari RV, Oliveira MG (2006) Role of muscarinic M1 receptors in inhibitory avoidance and contextual fear conditioning. Neurobiol Learn Mem 86:188–196
Spowart-Manning L, van der Staay FJ (2004) The T-maze continuous alternation task for assessing the effects of putative cognitive enhancers in the mouse. Behav Brain Res 151:37–46
Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ (2001) Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord 12:265–280
Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH (2002) Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 160:253–262
Talpos JC, Winters BD, Dias R, Saksida LM, Bussey TJ (2009) A novel touchscreen-automated paired-associate learning (PAL) task sensitive to pharmacological manipulation of the hippocampus: a translational rodent model of cognitive impairments in neurodegenerative disease. Psychopharmacology (Berl) 205:157–168
Turchi J, Saunders RC, Mishkin M (2005) Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proc Natl Acad Sci USA 102:2158–2161
Van Dam D, Abramowski D, Staufenbiel M, De Deyn PP (2005) Symptomatic effect of donepezil, rivastigmine, galantamine, and memantine on cognitive deficits in the APP23 model. Psychopharmacology (Berl) 180:177–190
Van Dam D, Coen K, De Deyn PP (2008) Cognitive evaluation of disease-modifying efficacy of donepezil in the APP23 mouse model for Alzheimer’s disease. Psychopharmacology (Berl) 197:37–43
Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P (2001) A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57:489–495
Winters BD, Bartko SJ, Saksida LM, Bussey TJ (2007) Scopolamine infused into perirhinal cortex improves object recognition memory by blocking the acquisition of interfering object information. Learn Mem 14:590–596
Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, Stuart GW, Velakoulis D, McGorry PD, Pantelis C (2002) Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med 32:429–438
Acknowledgments
This work was supported by a grant from the Alzheimer’s Research Trust. S.J.B. was additionally supported by a Ruth L. Kirschstein Predoctoral Fellowship from the National Institutes of Mental Health. We also thank Jamie Simon for assistance with Figure 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartko, S.J., Vendrell, I., Saksida, L.M. et al. A computer-automated touchscreen paired-associates learning (PAL) task for mice: impairments following administration of scopolamine or dicyclomine and improvements following donepezil. Psychopharmacology 214, 537–548 (2011). https://doi.org/10.1007/s00213-010-2050-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2050-1