Abstract
Rationale
The influences of the serotonergic system on dopamine (DA) neuron activity have received considerable attention during the last three decades due to the real opportunity to improve disorders related to central DA neuron dysfunctions such as Parkinson’s disease, schizophrenia, or drug abuse with serotonergic drugs. Numerous biochemical and behavioral data indicate that serotonin (5-HT) affects dopaminergic terminal function in the striatum.
Objective
The authors propose a thorough examination of data showing controversial effects induced by striatal 5-HT on dopaminergic activity.
Results
Inhibitory and excitatory effects of exogenous 5-HT have been reported on DA release and synthesis, involving various striatal 5-HT receptors. 5-HT also promotes an efflux of DA through reversal of the direction of DA transport. By analogy with the mechanism of action described for amphetamine, the consequences of 5-HT entering DA terminals might explain both the excitatory and inhibitory effects of 5-HT on presynaptic DA terminal activity, but the physiological relevance of this mechanism is far from clear. The recent data suggest that the endogenous 5-HT system affects striatal DA release in a state-dependent manner associated with the conditional involvement of various 5-HT receptors such as 5-HT2A, 5-HT2C, 5-HT3, and 5-HT4 receptors.
Conclusion
Methodological and pharmacological issues have prevented a comprehensive overview of the influence of 5-HT on striatal DA activity. The distribution of striatal 5-HT receptors and their restricted influence on DA neuron activity suggest that the endogenous 5-HT system exerts multiple and subtle influences on DA-mediated behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of the interaction between serotonin (5-HT) and dopamine (DA) systems in the brain is crucial to understanding of the mechanisms of action of several psychoactive drugs. For instance, atypical antipsychotics, drugs able to reduce psychosis with minimal extrapyramidal side effects, block more efficiently 5-HT2A receptors than DA-D2 receptors (Deutch et al. 1991; Meltzer 1999; Meltzer and Nash 1991). This property has contributed to highlighting the involvement of the 5-HT system in the efficacy of DA drugs in humans. Similarly, the 5-HT system may directly and indirectly participate in the mechanisms of action of l-DOPA, the metabolic precursor of DA and the gold standard medication of Parkinson’s disease, and numerous data have shown that 5-HT drugs may ameliorate the motor and psychotic side effects induced by l-DOPA (Carta et al. 2007; 2008; Jenner et al. 1983; Melamed et al. 1996; Pact and Giduz 1999; Zoldan et al. 1995). The 5-HT drugs constitute a real hope for improving current DA therapies to treat extrapyramidal symptoms and knowledge of the influence of 5-HT in the control of DA neuron activity appears as a critical point.
The striatum is part of the basal ganglia, a group of subcortical structures involved in the control of motor behavior. The striatum receives a substantial DA innervation coming from midbrain DA cell bodies mainly located in the substantia nigra pars compacta (Lindvall and Björklund 1978; Gerfen 1987) and, to a lesser extent, a 5-HT innervation coming from midbrain 5-HT cell bodies located in the dorsal raphe nucleus (DRN) (Azmitia and Segal 1978; Steinbusch 1984). Several behavioral, electrophysiological, and biochemical studies have shown that 5-HT may affect striatal DA transmission. However, 40 years after the discovery by Besson et al. (1969) that 5-HT enhances DA release from rat striatal slices, the mechanisms elicited by 5-HT in the control of DA neuron activity are still mysterious. Several factors may account for the inconsistent results. First, a major difficulty in studying such an interaction comes from the existence of multiple receptor subtypes for 5-HT as revealed by molecular biology or retrograde pharmacological approaches. Most of these receptors have been described in the striatum (Barnes and Sharp 1999). Selective pharmacological agents for particular 5-HT receptor subtype(s) were lacking until recently, and it is still difficult to assess their respective roles in the control of nigrostriatal DA function. Besides the pharmacological overlap of 5-HT drugs on 5-HT receptors, numerous studies have also shown that 5-HT and some 5-HT compounds may directly act within DA terminals to enhance DA release in a powerful fashion. This action, similar to that described for amphetamine, relies on the release of DA by plasmalemmal DA transporters. Such a mechanism of action is not observed when endogenous 5-HT is released in the striatum, raising the issue of its physiological relevance. Finally, as postulated many years ago, the influence of 5-HT system on DA function could change when central DA transmission is altered.
The purpose of this review is to present a thorough examination of the biochemical material published until now. This review will briefly explore the anatomical basis of the 5-HT system as it relates to the action of 5-HT in the striatum. We will then review biochemical data showing that 5-HT modifies striatal DA function. Excitatory and inhibitory effects will be discussed as well as the 5-HT receptors thought to mediate these effects. Then, we will focus on evidence indicating that 5-HT, by entering DA terminals, may elicit a carrier-mediated release of DA or inhibit enhanced DA release. Based on evidence obtained in the last few years using in vivo methods, we will discuss the physiological relevance of such a mechanism. Finally, we will present the hypothesis that striatal endogenous 5-HT and 5-HT receptors operate in a state-dependent manner to modulate DA function. This work extends the reviews by Alex and Pehek (2007) and Fink and Göthert (2007).
5-HT system in the neostriatum
5-HT nerve terminals
The 5-HT neurons originating from the dorsal (DR) and median raphe (MR) nuclei represent the major afferents to DA systems at both somato-dendritic and nerve terminal regions (Azmitia and Segal 1978). The 5-HT innervation of mammalian neostriatum arises primarily from the dorsal raphe nucleus (Azmitia and Segal 1978; Bobillier et al. 1976). The presence of 5-HT terminals in these regions has been confirmed by autoradiographic studies assessing the binding distribution of [3H]-imipramine or [3H]-citalopram, two 5-HT uptake site ligands (D’Amato et al. 1987; Dewar et al. 1991), as well as antibodies directed against 5-HT (Steinbusch et al. 1981; Steinbusch 1984), 5-HT reuptake sites (Hrdina et al. 1990), or tryptophan hydroxylase (Saavedra 1977). Consistently, post-mortem studies have shown that the striatum contains a substantial amount of tissue 5-HT or its main metabolite 5-hydroxyl-indolacetic acid. More recent studies have provided evidence for a functional release of 5-HT from striatal 5-HT terminals in vivo (Kalén et al. 1988; Kreiss et al. 1993).
Serotonergic axon terminals are closely apposed to a variety of structures including axon terminals, dendritic spines, and shafts, but rarely neuronal somata. The incidence of direct contact between DA and 5-HT axon terminals is lower than that found in the nucleus accumbens (Soghomonian et al. 1989; Van Bockstaele and Pickel 1993). Based on synaptic and apposition features of 5-HT axon terminals in the striatum and also in various brain regions, it has been proposed that 5-HT may exert its various effects on striatal neurons through diffusion processes (Descarries et al. 1991; Descarries and Mechawar 2000; Umbriaco et al. 1995). It may be hypothesized that 5-HT alters the function of striatal DA terminals through both direct and indirect mechanisms.
5-HT receptors present in the striatum
Serotonin, released in the striatum, may act on a variety of 5-HT receptors. To date, 16 5-HT receptors have been described and several of those have been described in the striatum with the use of different anatomical techniques: 5-HT1A, 5-HT1B, 5-HT1E, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT6, and 5-HT7 (Barnes and Sharp 1999; Hoyer et al. 2002; Zifa and Fillion 1992). Generally, 5-HT can affect most of the intracellular second messenger pathways by acting on various receptor subtypes. The distribution and the relative expression of 5-HT receptors are different for each receptor in the striatum and the basal ganglia.
5-HT1A receptors
The striatum displays very few binding sites for 5-HT1A radioligands such as [3H]-8-OH-DPAT or [3H]-WAY100635 (Gozlan et al. 1983; Hall et al. 1997; Laporte et al. 1994). Moreover, mRNAs encoding 5-HT1A receptor are almost undetectable in the rat striatum or the substantia nigra (SN) (Pompeiano et al. 1992). Although some biochemical data have suggested their presence on DA terminals (Johnson et al. 1993; see below), it has been shown that the binding of 8-OH-DPAT persisted in rats after lesion of DA neurons (Gozlan et al. 1983). In addition, using immunocytochemistry, some 5-HT1A receptor-labeled cells were also positive for the gliosis factor-associated protein, suggesting that a proportion of striatal 5-HT1A receptors are produced by glial cells (Whitaker-Azmitia et al. 1993). Using another immunohistochemical approach, Frechilla et al. (2001) have described labeling of interneurons that were not calbindin-positive in the striatum of primates. Some investigators have postulated the presence of 5-HT1A receptors on some terminals of corticostriatal fibers (Antonelli et al. 2005; Mignon and Wolf 2007), but the anatomical description of this hypothesis is still lacking. Thus, 5-HT1A receptors are poorly expressed in the striatum and are likely not located on DA neurons.
5-HT1B receptors
5-HT1B receptors have been well described in the basal ganglia of mammals. 5-HT1B receptor mRNA is highly concentrated in the striatum and the nucleus accumbens (Bonaventure et al. 1998; Boschert et al. 1994; Hen 1992; Varnas et al. 2005). Although presenting a two- to threefold-lower density of binding sites than in the SN, ventral tegmental area (VTA), or globus pallidus, 5-HT1B receptors have been detected in the striatum, the nucleus accumbens, and the cortex using various radioligands (Doménech et al. 1997; Hamon et al. 1990; Radja et al. 1991) or immunohistochemistry (Sari et al. 1997; 1999). Their expression is higher in the ventral striatum (Compan et al. 1998; Varnas et al. 2001).
5-HT1B receptors could be expressed at several striatal sites. Usually found at presynaptic terminals, they could be present on GABAergic collateral fibers of striatofugal neurons (Sari et al. 1999). They are likely present on 5-HT terminals as 5-HT autoreceptors modulating the release of 5-HT (Engel et al. 1986; see Hen 1992; Kreiss et al. 1993) although the contribution of this labeling appears to be extremely low compared to the total labeling of 5-HT1B receptors in the striatum (Vergé et al. 1986). The presence of 5-HT1B receptors on thalamostriatal and/or corticostriatal neurons has also been suggested (Bonaventure et al. 1998). On the other hand, numerous anatomical studies have reported that 5-HT1B receptors are not located on central DA neurons. Indeed lesions of DA neurons do not alter the density of [3H]-5-HT striatal binding sites whereas kainic acid lesion of striatal neurons decreases density (>80%) (Quirion and Richard 1987). In addition, a loss of density of these receptors was observed in the post-mortem analysis of striata from Huntington’s disease patients, a degenerative disease associated with destruction of striatal neurons, but not from parkinsonian patients (Waeber and Palacios 1989). Furthermore, mRNAs encoding 5-HT1B receptor are not found in the SNc (Bonaventure et al. 1998). These data suggest that 5-HT1B receptors could be placed to control the neuronal activity of some striatal efferent neurons.
Notably, the 5-HT1D receptors that have for a long time been confused with 5-HT1B receptors from a pharmacological point of view are present in the striatum. Their mRNA and binding sites have been clearly identified in the striatum. Their pattern of expression is almost the same as that described for 5-HT1B receptors, but their density is lower (Bruinvels et al. 1994; Mengod et al. 1996; Waeber and Moskowitz 1995).
Other 5-HT1 receptors
The 5-HT1E receptors were described by Lovenberg et al. (1993). This receptor is negatively coupled to adenylyl cyclase activity (Lovenberg et al. 1993). It has been shown, using northern blot analysis, that striatum expresses detectable levels of 5-HT1E mRNA. In addition, it has been shown that 5-HT binds to a site sensitive to various 5-HT1 receptor ligands, with the exception of 5-carboxytryptamine (5-CT), a non-selective 5-HT1/7 agonist (Bruinvels et al. 1993). For these authors, this 5-HTnon1Anon1Bnon2C receptor corresponds to the 5-HT1E site and is expressed by striatal neurons (Bruinvels et al. 1993). 5-HT1F receptors are poorly expressed in the striatum (Waeber and Moskowitz 1995).
5-HT2 receptor family
Three 5-HT receptors, namely, 5-HT2A, 5-HT2B, and 5-HT2C receptors, are coupled to G proteins, leading to an intracellular increase in IP3 through the stimulation of phospholipase C. On the basis of the pharmacological and molecular similarities of 5-HT2 and 5-HT1C receptors, the 5-HT2 receptor has been renamed as the 5-HT2A subtype, while the 5-HT1C subtype was renamed 5-HT2C (Hoyer et al. 1994). The 5-HT2F, first described in the stomach fundus, corresponds to the 5-HT2B subtype. These three receptors are present in the brain (for reviews, see Barnes and Sharp 1999; Saudou and Hen 1994).
5-HT2A receptors
The presence of 5-HT2A receptors has been revealed by autoradiographic procedures using different radioligands such as [3H]-ketanserin (Pazos et al. 1985), [3H]-RP62203 (Malgouris et al. 1993), [3H]-DOI (Waeber and Palacios 1994; Compan et al. 1998), and more recently with [3H]-M100907 (López-Giménez et al. 1998). Typically, the density is maximal in the ventrocaudal region and the dorsomedial (periventricular) parts of the striatum. The density of 5-HT2A binding sites is higher in rodents compared to primates and humans (Lopez-Gimenez et al. 1999, 1998, 2001). In agreement with this, mRNA encoding 5-HT2A receptors is found in the striatum of rodents (Pompeiano et al. 1994; Ward and Dorsa 1996) while their expression appears to be low and even undetectable in human caudate putamen (Burnet et al. 1995; López-Giménez et al. 2001; Pasqualetti et al. 1996).
In rodents, some immunocytochemical and in situ hybridization studies indicate that 5-HT2A receptors are present mostly in the extrastriosomal “matrix” compartment of the striatum, though striosomal labeling has been reported in humans (Lopez-Gimenez et al. 1999). The striosomes, or “patches”, and the extrastriosomal “matrix” represent a functional organization of the striatum, with the striosomes being low in acetylcholinesterase, rich in mu opioid receptors, and containing the cell bodies of the GABAergic neurons projecting to the SNc (Gerfen 1984, 1985; Graybiel 1991). The 5-HT2A receptors are localized on striatal efferent GABAergic neurons (Morilak et al. 1993; Ward and Dorsa 1996), on striatal interneurons (Morilak et al. 1993) in low density (Bonsi et al. 2007), as well as on afferents putatively of cortical origin (Bubser et al. 2001). Both striatopallidal and striatonigral neurons express 5-HT2A receptors (Laprade et al. 1996; Ward and Dorsa 1996) mostly at the level of proximal dendrites (Cornea-Hébert et al. 1999). Their presence on DA neuron terminals remains unclear. In some studies, the binding of 5-HT2A receptors in the striatum is not modified in the striatum of rats with a lesion of DA neurons (Leysen et al. 1988; Muramatsu et al. 1988). Conversely, it has been shown that destruction of nigrostriatal DA neurons in rats as neonates increased 5-HT2A binding sites (Radja et al. 1993), and this effect is paralleled by an increase in the levels of the mRNA encoding this receptor in the striatum (Laprade et al. 1996).
5-HT2B receptors
The presence of 5-HT2B receptors in the brain has been uncertain for several years (Loric et al. 1992; Pompeiano et al. 1994). Using an antibody directed against the receptor and in situ hybridization, it has been shown that 5-HT2B receptors are expressed in the central nervous system (Duxon et al. 1997). Whereas the expression of both the mRNA and the protein has been consistently detected in the hippocampus, the amygdala, and the cortex, the striatum and structures related to basal ganglia function are devoid of 5-HT2B receptors (Duxon et al. 1997).
5-HT2C receptors
The distribution of 5-HT2C receptors in the striatum was described in 1985 by studying the binding of [3H]-mesulergine (Pazos et al. 1985). This study shows that 5-HT2C sites were located throughout the striatum, with the ventrolateral part being more enriched. The distribution has been found to be similar in other species (Saudou and Hen 1994). It is noteworthy that a patchy distribution of [3H]-DOI and [3H]-mesulergine has been reported in the striatum of humans and monkeys, but not rodents (Waeber and Palacios 1994). The presence of 5-HT2C receptors has been confirmed by immunocytochemistry and by in situ hybridization (Abramowski et al. 1995; Clemett et al. 2000; Eberle-Wang et al. 1997; Mengod et al. 1990; Molineaux et al. 1989; Pompeiano et al. 1994). Most neurons expressing 5-HT2C receptor mRNA are medium-sized spiny neurons of striatopallidal and striatonigral pathways (Ward and Dorsa 1996). Recent reverse transcriptase polymerase chain reaction (RT-PCR) studies have shown that 5-HT2C receptor mRNA is present in cholinergic interneurons and that it shows an interesting patchy distribution (Ward and Dorsa 1996).
The 5HT2C receptors are presumably not located on DA terminals. Indeed it has been shown that SN neurons that synthesized mRNA encoding 5-HT2C receptors coexpress glutamic acid decarboxylase, the rate-limiting step of GABA synthesis, but not tyrosine hydroxylase, the rate-limiting step of DA biosynthesis (Eberle-Wang et al. 1997). Similar conclusions have been recently proposed from studies performed on human brain tissues (Pasqualetti et al. 1999). More recent techniques of laser capture microdissection allow the determination of mRNA expression in selected cell populations with quantitative PCR, a technique that is much more sensitive than in situ hybridization histochemistry. With this approach, 5HT2C receptor mRNA can be detected in tyrosine hydroxylase-positive neurons of the SNc (Mortazavi and Chesselet, unpublished observations). These data are compatible with results from a recent study reporting immunostaining with an antibody raised against 5-HT2C receptors in both GABAergic and DA neurons of the VTA (Bubar and Cunningham 2007). Even though such a colocalization appears to exist in the SN based on the PCR data, it is not known whether a small proportion of the receptors are found on terminals of DA neurons. This proportion should be extremely low because the lesion of DA neurons does not decrease the densities of mRNA encoding 5-HT2C receptors or [3H]-mesulergine binding along the nigrostriatal axis (Fox and Brotchie 2000; Numan et al. 1995; Radja et al. 1993).
5-HT3 receptors
The 5-HT3 receptor differs from the other 5-HT receptors by the efferent intracellular coupling system. Indeed this receptor is a Ca2+/K+/Na+ channel similar to the nicotinic receptors (Davies et al. 1999; Maricq et al. 1991). The first distribution of 5-HT3 receptors was provided in 1987 by Kilpatrick et al. who described the binding of the preferential 5-HT3 antagonist [3H]-zacopride throughout the brain. These authors found that the striatum was poor in 5-HT3 receptor sites, in contrast to the cortex, the hippocampus, or the amygdala. These data have been confirmed later with several [3H]-ligands, antibodies raised against the receptor, or in situ hybridization (Barnes et al. 1989; 1990; Kilpatrick et al. 1989; Miyake et al. 1995; Morales et al. 1996; Perry 1990; Tecott et al. 1993; Waeber and Palacios 1989). The density of binding sites is dependent on the radioligands used and varies among species, with the human striatum displaying higher densities of 5-HT3 receptor binding sites to the rat striatum (Barnes et al. 1990).
Notably, two subunits have been cloned (Davies et al. 1999), namely, the 5-HT3A and 5-HT3B subunits, and their association can lead to 5-HT3 receptors with distinct functional and pharmacological properties. The 5-HT3A receptor subunit is highly expressed in the brain compared to the 5-HT3B (Doucet et al. 2000), with the striatum of rodents being devoid of the 5-HT3B subunit (Morales and Wang 2002).
The 5-HT3 receptors are likely not present on DA terminals in the striatum. In humans, the binding of 5-HT3 receptors is markedly reduced in putamen homogenates from patients with Huntington’s disease, but not those with Parkinson’s disease (Steward et al. 1993). Similarly, it has been shown that the binding of [3H]-(r,s)-zacopride disappeared in rats with a lesion of striatal neurons but persisted in rats with a lesion of DA neurons (Kidd et al. 1993). It has been suggested that 5-HT3 receptors are expressed by interneurons in the brain (Barnes et al. 2009; Mikics et al. 2009; Morales and Bloom 1997; Morales et al. 1996), which is in agreement with the assumption that they are located on GABAergic interneurons in the striatum (Waeber and Palacios 1989).
5-HT4 receptors
The 5-HT4 receptors were discovered in 1988 by Dumuis et al. (1988) on mouse colliculus slices. In particular, it was the first described 5-HT receptor positively linked to adenylyl cyclase activity with a unique pharmacology (Bockaert et al. 1990; Dumuis et al. 1989). Using the 5-HT4 antagonist [3H]-GR113808, Grossman et al. (1993) reported that 5-HT4 receptors are concentrated in human basal ganglia, especially in the striatum, the nucleus accumbens, and the olfactory tubercles. This distribution has been largely confirmed and, to some extent, is quite similar in several species (Jakeman et al. 1994; Schiavi et al. 1994). An increasing gradient of binding site density from the ventrolateral to the dorsolateral striatum has been reported in rodents (Vilaró et al. 1996; Waeber et al. 1994), but not in primates or humans (Bonaventure et al. 2000; Vilaro et al. 2005). The 5-HT4 receptor was cloned in 1995 (Gerald et al. 1995). From this date, several splice variants (5-HT4a,b,c,d,e,f,g, and h) diverging in the sequence encoding the C-terminal region of the receptor have been described (Bender et al. 2000; Blondel et al. 1998; Gerald et al. 1995). Although both the structure and the efficiency to adenylyl cyclase coupling are modified, no major difference in the affinity of several 5-HT4 ligands has yet been noticed between the different products.
Whatever the isoform considered, mRNAs of 5-HT4 receptors are expressed by efferent striatal neurons (Bender et al. 2000; Vilaró et al. 1996). These data suggest that the 5-HT4 receptor binding sites in the globus pallidus or the SN are due to striatal efferent neurons (Compan et al. 1996). In addition, the binding of [3H]-GR113808 is abolished by an exocytotic lesion of striatal neurons with kainic acid, but not by a 6-hydroxydopamine lesion of nigrostriatal DA neurons (Compan et al. 1996; Patel et al. 1995). In line with these data, the binding of [3H]-GR113808 is strongly diminished in the striatum in Huntington’s disease, but not in Parkinson’s disease (Reynolds et al. 1995). Despite the recent report that the 5-HT4a subunit mRNA is expressed by neurons in the SN (Vilaro et al. 2005), it is far from clear if the expression is related to DA neurons. Consequently, 5-HT4 receptors are likely not present on DA neurons in the striatum.
5-HT5 receptors
The 5-HT5 receptors were discovered in 1993 and two subtypes, namely, the 5-HT5A and the 5-HT5B, have been distinguished on the basis of their primary sequence (Erlander et al. 1993). These receptors, highly concentrated in cortical areas, are, however, barely expressed in the striatum of various species (Saudou and Hen 1994; Barnes and Sharp 1999) as reported by in situ hybridization and binding studies (Rees et al. 1994; Thomas 2006; Waeber and Moskowitz 1995; Wesolowska 2002). Nonetheless, a faint immunohistochemical labeling has been reported in the rat striatum that could be associated with medium-sized spiny neurons (Oliver et al. 2000).
5-HT6 receptors
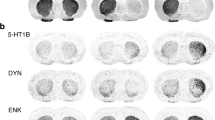
The 5-HT6 receptor was first described from its cloning from rat striatal slices cDNA (Monsma et al. 1993; Ruat et al. 1993a). The 5-HT6 receptor has been shown to stimulate adenylyl cyclase activity in both HEK-293 cells (Monsma et al. 1993), pig caudate membranes (Schoeffter and Waeber 1994), and mouse embryonic striatal neurons (Sebben et al. 1994). The highest level of 5-HT6 receptor mRNAs are detected in the olfactory tubercles, nucleus accumbens, striatum, and hippocampus (Gérard et al. 1996; Monsma et al. 1993; Ruat et al. 1993a; Ward et al. 1995). In the striatum, the mRNA encoding 5-HT6 receptors is synthesized by GABAergic neurons expressing both dynorphin and enkephalin and may colocalize with either 5-HT2A or 5-HT2C receptor mRNA (Ward and Dorsa 1996). The distribution and the high quantitative expression have been confirmed in the striatum using various [3H]-ligands such as [3H]-SB258584 (Roberts et al. 2002). Gérard et al. (1997) raised polyclonal antibodies against a synthetic peptide corresponding to part of the C-terminal domain of the receptor. They confirmed that 5-HT6 receptors were present in the striatum and showed that this receptor was expressed on dendritic shafts of striatal neurons (Gérard et al. 1997; Hamon et al. 1999).
Although 5-HT6 receptor mRNAs have not been detected in the SN in prior studies using RT-PCR on nigral tissue (Bourson et al. 1998; Monsma et al. 1993), other studies provide evidence for the expression of 5-HT6 receptors by some nigral neurons (Gérard et al. 1996). The presence of the protein in the SN was further confirmed by immunohistochemistry (Gérard et al. 1997). Nevertheless, the presence of 5-HT6 receptors on DA neurons remains doubtful because the binding of [3H]-SB258585 is not affected by a 6-hydroxydopamine lesion of nigral DA neurons (Roberts et al. 2002).
5-HT7 receptors
5-HT7 receptors are poorly expressed in the striatum. The study by Ruat et al. (1993b) reporting an undetectable level of 5-HT7 receptor mRNA in the striatum has been challenged by the use of [3H]-5-CT in the presence of the cold 5-HT1 ligands sumatriptan and (−)pindolol in order to label selectively 5-HT7 receptors (To et al. 1995) or by the use of [3H]-mesulergine in a specific procedure (Martín-Cora and Pazos 2004). Using the more specific ligand [3H]-SB269970, the density of binding sites appears to be extremely low in the striatum and could be species dependent (Varnas et al. 2004). An immunohistochemical study also reports the weak labeling of 5-HT7 receptors in the striatum (Neumaier et al. 2001). The failure to detect mRNA for 5-HT7 receptors in the striatum (Mengod et al. 1996; Ruat et al. 1993b) has been challenged because of the later discovery of distinct splice variants encoding this receptor. It has been reported that the 5-HT7 (a) isoform, common in humans and rats, could be present in the striatum (Heidmann et al. 1997). According to Muneoka and Takigawa (2003), the expression of 5-HT7 receptors in the striatum appears in the early stage of development and tends to disappear in adults.
Concluding remarks
In conclusion, the presence of a variety of 5-HT receptors in the striatum has been demonstrated repeatedly and Fig. 1 summarizes these findings. Taken together, most of the anatomical data aimed at determining the distribution of 5-HT receptors with respect to nigrostriatal DA neurons conclude that the 5-HT receptors are not located on DA terminals. The receptors are rather expressed by striatal neurons, including GABAergic striatofugal neurons and interneurons, and cholinergic interneurons as well as afferents. The use of immunohistochemistry using powerful antibodies or laser capture microdissection in selected cell populations of the SNc coupled to Q-PCR might challenge this first assumption. For instance, using this latter approach, 5HT2C receptor mRNA can be detected in tyrosine hydroxylase-positive neurons of the SNc (Mortazavi and Chesselet, unpublished observations). These data are compatible with results from a recent study reporting immunostaining with an antibody raised against 5-HT2C receptor in both GABAergic and DA neurons of the VTA (Bubar and Cunningham 2007). In any case, however, it appears that the hypothetical presence of 5-HT receptors on DA terminals in the striatum or the nucleus accumbens would be extremely low compared to their total expression in the striatum. This is important to consider when looking at the biochemical data.
Drawing representing the expression of the different 5-HT receptor subtypes in the striatum. The plus and minus symbols refer to their putative influence on their cellular targets. Several 5-HT receptors are expressed by striatal cells including the medium-sized spiny neurons (MS), the cholinergic interneurons (Ach), and the other interneurons (IN). 5-HT receptors are also postulated to be present on thalamo- and corticostriatal fibers. Some 5-HT receptors (5-HT1B/5-HT4) are addressed at extrastriatal regions. Their presence on 5-HT fibers coming from the dorsal raphe nucleus (DRN) or DA fibers coming from the substantia nigra pars compacta (SNc) is not established anatomically. Of note, several 5-HT receptors are present also in the substantia nigra (for reviews, see Di Giovanni et al. 2008; Di Matteo et al. 2008). GPi and GPe, internal and external globus pallidus; SNr, substantia nigra pars reticulata
Effect of 5-HT on striatal DA terminal function
The study of the influence of 5-HT system on DA terminals has been undertaken by applying 5-HT itself (exogenous) or agonists able to target 5-HT receptors in various models. One of the most complex features of the 5-HT–DA interaction is that both excitatory and inhibitory effects have been reported.
Excitatory and inhibitory effects of 5-HT
Excitatory effects
Several in vitro studies have reported an excitatory influence of exogenous 5-HT on basal DA release in the striatum (Andrews et al. 1978; Besson et al. 1969; Jacocks and Cox 1992; Yi et al. 1991; Zhou et al. 2005). This effect has also been observed in the nucleus accumbens (Jacocks and Cox 1992; Nurse et al. 1988), the olfactory tubercle (Zazpe et al. 1994), the SN (Williams and Davies 1983), and the VTA (Beart and McDonald 1982). The excitatory effect of 5-HT on basal DA release occurs either on striatal slices or on synaptosomes, a model allowing the study of the presynaptic regulation of synthesis and release (De Belleroche and Bradford 1980). It has been also reported that 5-HT further enhances preloaded [3H]-DA or endogenous DA release in rat striatal slices or synaptosomes stimulated electrically or with high K+ concentration (Blandina et al. 1989; De Belleroche and Bradford 1980; Kamal et al. 1983; Zhou et al. 2005), a depolarizing stimulus mostly involving exocytotic processes (Table 1).
The excitatory effect of 5-HT on striatal DA release has been observed in vivo by using intracerebral microdialysis. This in vivo technique permits the application of pharmacological compounds through the microdialysis probe to the site at which the neurotransmitter is measured (Di Chiara 1990). Using this pharmacological approach, it has been consistently shown that striatal infusion of 5-HT enhances striatal DA release in a concentration-dependent manner in the rat (Benloucif and Galloway 1991; Benloucif et al. 1993; Bonhomme et al. 1995; Yadid et al. 1994) (Fig. 2). Similar results have been also reported in rat nucleus accumbens (Parsons and Justice 1993; Zangen et al. 2001), cortex (Iyer and Bradberry 1996), and SN (Cheramy et al. 1981; Thorré et al. 1998). Striatal DA release is enhanced above the threshold concentration of 0.5–1 μM 5-HT in vivo (Benloucif and Galloway 1991; Bonhomme et al. 1995; Zangen et al. 2001) and may reach the huge enhancement of 2,000% of baseline values when 5-HT is infused at 100 μM (Benloucif and Galloway 1991). The increase in DA release induced by a 10-μM 5-HT concentration progressively diminishes during its perfusion (West and Galloway 1996).
Effect of the intrastriatal application of 5-HT on striatal DA release. 5-HT has been applied at 1, 3, or 10 μM by reverse microdialysis in the striatum of anesthetized rats (adapted from De Deurwaerdère et al. 1996; courtesy of the International Society of Neurochemistry)
Inhibitory effects on DA release
The inhibitory effects of 5-HT on DA release have also been reported in vitro. Among all in vitro approaches employed, the inhibitory effect of 5-HT has been essentially observed on the stimulated release of [3H]-DA in [3H]-DA-preloaded preparations. Thus, 5-HT is able to negatively modulate the electrical- or K+-stimulated striatal [3H]-DA release in striatal slices or synaptosomes (Ennis et al. 1981; Muramatsu et al. 1988; Nurse et al. 1988; Sarhan et al. 1999; Westfall and Tittermary 1982). The inhibitory effect of 5-HT appears at concentrations of 5-HT lower or equal to 1 μM (Sarhan et al. 1999) and its maximal magnitude usually reaches 50% inhibition of [3H]-DA released by the depolarizing stimulus (Ennis et al. 1981; Muramatsu et al. 1988). Using cyclic voltammetry on mice striatal slices, an inhibitory effect of 5-HT on endogenous DA release has been observed in the presence of a DA uptake blocker (Zhou et al. 2005).
Inhibitory effects on DA synthesis
It has been shown that 5-HT inhibited DA synthesis in vitro on rat brain synaptosomes (Andrews et al. 1978; De Belleroche and Bradford 1980; Johnson et al. 1993). This effect can occur independently of a decrease of [3H]-DA release (De Belleroche and Bradford 1980). It is observed with low concentrations of 5-HT.
Concluding remarks
The excitatory effect of 5-HT on basal DA release has been reported in all studies in vitro or in vivo, including those reporting an inhibitory effect of 5-HT on stimulated [3H]-DA released for in vitro preparations preloaded with [3H]-DA (Ennis et al. 1981; Sarhan et al. 1999). In general, the inhibitory effect of 5-HT can be observed at lower concentrations compared to the excitatory effects. These effects can occur concomitantly at higher concentrations of 5-HT, depending on the experimental parameter used to reveal their occurrence.
5-HT receptors involved in the excitatory effects of 5-HT on DA release
Several researchers have attempted to determine the 5-HT receptors involved in the excitatory effects of exogenous 5-HT using 5-HT agonists and antagonists directed to 5-HT receptor subtypes. Despite the availability of a growing number of selective pharmacological tools, a clear picture concerning the 5-HT receptors involved in the excitatory effect of 5-HT on striatal DA release is lacking. To date, the involvement of 5-HT1B, 5-HT2A/2C, 5-HT3, and 5-HT4 receptors has been proposed. Some of the data in the literature are summarized in Table 1.
5-HT1A receptors
Some data have shown that the intrastriatal infusion of high concentrations of the 5-HT1A/7 agonist 8-OHDPAT enhanced striatal DA release in vivo (Benloucif and Galloway 1991; Santiago et al. 1998). However, a role for 5-HT1A/7 receptors in the effects of 8-OHDPAT remains unlikely given that 5-HT1A antagonists did not reduce this effect (Benloucif and Galloway 1991; Santiago et al. 1998).
5-HT1B receptors
The involvement of 5-HT1B receptors in the facilitatory control exerted by 5-HT was postulated 20 years ago by the finding that the striatal application in vivo of several non-selective 5-HT1B agonists such as RU 24969, TFMPP, and m-CPP enhanced striatal DA (Benloucif and Galloway 1991). More recently, similar results have been observed following the application of the 5-HT1B agonists CP93129 and CGS20654 or the non-selective 5-HT1/7 agonist 5-CT in vitro and in vivo (Bentué-Ferrer et al. 1998; Galloway et al. 1993; Ng et al. 1999; Sarhan et al. 1999; Yadid et al. 1994). This effect has been reported in vivo in various structures such as the cortex (Iyer and Bradberry 1996), the nucleus accumbens (Hållbus et al. 1997), or the SN (Thorré et al. 1998). Some 5-HT1B antagonists such as (−)pindolol, at high concentrations only (100 μM), are able to reduce the facilitatory effect of 5-HT or 5-HT1B agonists (Benloucif and Galloway 1991). Also, high concentrations of the 5-HT1B antagonist isamoltane reduced the excitatory effects of 5-CT (Ng et al. 1999). In the nucleus accumbens or the cortex, the mixed 5-HT1B/1D antagonist GR127935 infused at 10 μM reduced 5-HT-induced DA release (Hållbus et al. 1997; Iyer and Bradberry 1996). Nevertheless, some 5-HT antagonists able to block 5-HT1B receptors, such as methiotepine, did not affect the release of striatal DA induced by 5-HT (De Deurwaerdère et al. 1997). In addition, lower concentrations of (−)pindolol, although blocking efficiently the decrease in endogenous 5-HT release elicited by 5-HT1B agonists (Thorré et al. 1998), are unable to affect the excitatory effect of 5-HT or CP 93129 (10 μM) on DA release in the striatum and the SN (Bonhomme et al. 1995, personal communication; Thorré et al. 1998). The excitatory effect of CP 93129 was also not affected in 5-HT1B receptor knock-out mice (De Groote et al. 2003). Recently, it has been reported that the intrastriatal administration of CP 93129 reduced jaw movements induced by apomorphine, but this effect was not blocked by the 5-HT1B antagonist GR55562 (Fujita et al. 2008), confirming that CP 93129 has multiple sites of action other than solely 5-HT1B receptors. These data show that 5-HT1B antagonists lost their selectivity toward 5-HT1B receptors at high concentrations and, in fact, their perfusion at high concentrations increased DA release (Benloucif and Galloway 1991; Bonhomme et al. 1995; Thorré et al. 1998). These results detract from the hypothesis that striatal 5-HT1B receptors are critically involved in an excitatory control of striatal DA release.
5-HT2A/2C receptors
The influence of striatal 5-HT2A and 5-HT2C receptors in the control of striatal DA release still remains enigmatic. This is attributable in part to the long period during which 5-HT2 compounds overlapped on both receptors (Baxter et al. 1995) and the absence of selective agonists for each subtype. Thus, local infusion of the 5-HT2A/2C agonist DOI by reverse microdialysis has been shown to slightly enhance striatal DA release in some studies (Balcioglu and Wurtman 1998; Lucas and Spampinato 2000) but not in others (Benloucif et al. 1993; Ng et al. 1999). DOI did not modulate [3H]-DA release in striatal slices (Jacocks and Cox 1992). The facilitatory effect of 1 μM DOI in vivo is blocked by the 5-HT2B/2C receptor antagonist SB 206553 but not by the 5-HT2A receptor antagonist SR46349 (Lucas and Spampinato 2000), suggesting a preferential role of striatal 5-HT2C receptors in this effect. Intrastriatal infusion of SB 206553 has been shown to effect per se striatal DA release in vivo but the data are contradictory, reporting either an inhibitory (Lucas and Spampinato 2000) or an excitatory effect (Alex et al. 2005; Navailles and De Deurwaerdère 2011). In the nucleus accumbens, the infusion of SB 206553 induced an increase in DA release (Navailles et al. 2006) while 5-HT2C antagonists enhance the expression of the proto-oncogene c-fos in the striatum (De Deurwaerdère et al. 2010). It is noteworthy, however, that 5-HT2 antagonists such as ritanserin, methiotepine, ketanserin, or cinanserin did not reduce the facilitatory effect of 5-HT on striatal DA release in vitro and in vivo (Benloucif et al. 1993; Bonhomme et al. 1995; De Belleroche and Bradford 1980; De Deurwaerdère et al. 1997; Jacocks and Cox 1992).
It has been well established for almost 15 years that 5-HT2A receptors enhance both DA release and DA synthesis (Schmidt et al. 1992). Indeed selective 5-HT2A antagonists such as MDL100907 or SR 46349B, mixed 5-HT2A/2C antagonists, but not 5-HT2B/2C antagonist, are able to reduce the enhancement of DA release induced by MDMA or amphetamine (Porras et al. 2002b; Schmidt et al. 1992; Yamamoto et al. 1995). Similar findings have been observed with the non-selective DA antagonists haloperidol and raclopride (Andersson et al. 1995; Lucas et al. 2000b). On the one hand, the facilitatory effect of MDMA or haloperidol in vivo can be reduced by the intrastriatal administration of 5-HT2A antagonists (Lucas and Spampinato 2000; Schmidt et al. 1994; Yamamoto et al. 1995). These data strongly suggested that striatal 5-HT2A receptors stimulate striatal DA release. On the other hand, the effect elicited by the intrastriatal administration of MDMA was not reduced by the systemic administration of ketanserin (Nash and Brodkin 1991). Furthermore, on rat striatal slices in vitro, MDL 100907 did not affect the excitatory effect of MDMA on DA release (Schmidt et al. 1994). Koch and Galloway (1997) have reported also that the MDMA-induced DA release was sensitive to endogenous 5-HT only when MDMA was administered peripherally. These data, in apparent contradiction, are however in perfect agreement with the fact that the intrastriatal application of 5-HT elicited an increase in DA release independently of 5-HT2 receptor stimulation (Benloucif et al. 1993; Bonhomme et al. 1995). These data add a higher level of complexity in the interaction between 5-HT and DA systems in which the involvement of 5-HT receptors in the control of striatal DA function is dependent on extrastriatal influences (see the paragraph on the state-dependent involvement of striatal 5-HT receptors).
5-HT3 receptors
Blandina et al. (1989) have found that the enhancement of endogenous DA elicited by 5-HT or the 5-HT3 agonist 2-methyl-5-HT in rat striatal slices was suppressed by the 5-HT3 antagonist ICS205930. These data, suggesting the involvement of 5-HT3 receptors in the control of striatal DA release, were soon contradicted. Indeed an action of the 5-HT3 agonists 2-methyl-5-HT, 1-phenylbiguanide, or methyl-chlorophenylbiguanide on DA uptake sites has been shown to contribute to the excitatory effects of these agonists on basal DA release in vitro (Benuck and Reith 1992; Schmidt and Black 1989; Zazpe et al. 1994) and in vivo (Santiago et al. 1995). In line with this possibility, selective 5-HT3 antagonists failed to reduce the effect of 5-HT on DA release in vitro and in vivo in several studies (Bonhomme et al. 1995; Jacocks and Cox 1992; Yi et al. 1991; Zazpe et al. 1994). Given that ICS205930 is able to block 5-HT4 receptors (Dumuis et al. 1989), it was already postulated in 1991 by Deutch and colleagues that the facilitatory effect of 5-HT reported by Blandina et al. (1989) could be attributable to 5-HT4 receptor stimulation (see below).
It is noteworthy to mention that the facilitatory effects of exogenous 5-HT or 2-methyl-5-HT described by Blandina et al. (1989), which were partially direct on striatal DA terminals, were studied in the continuous presence of the DA uptake blocker nomifensine. However, the excitatory effect of 5-HT on DA release in synaptosomes that persist in the presence of nomifensine is not affected by the co-administration of the 5-HT3/4 antagonist DAU 6285 (De Deurwaerdère et al. 1997). In addition, a persisting effect of 5-HT or phenylbiguanide in the presence of nomifensine or cocaine on striatal DA release has been observed in several studies (Andrews et al. 1978; Benuck and Reith 1992; De Deurwaerdère et al. 1997; Jacocks and Cox 1992; Zazpe et al. 1994). The remaining effects of 5-HT or 1-phenylbiguanide after blockade of the DA transporter, often representing a small contribution to their total effect, are insensitive to 5-HT3 antagonists and are likely dependent on the conditions of nomifensine perfusion (Benuck and Reith 1992).
The ability of striatal 5-HT3 receptors to affect striatal DA release has been reported in vivo but it has been associated with very restricted conditions (Porras et al. 2003; see the paragraph on the direct effects of 5-HT on DA terminals). Whether these conditions were met in the study of Blandina et al. (1989) remains an indefinable topic because of the concomitant loss of selectivity of 5-HT and 5-HT3 agents to DA uptake sites. For Fink and Göthert (2007), the in vitro preparation could have included part of the nucleus accumbens or olfactory tubercle in which the control exerted by 5-HT3 receptors might be more pronounced (Zazpe et al. 1994).
5-HT4 receptors
Some non-selective 5-HT4 agonists have been shown to sharply enhance DA release in vitro for a limited period of time (Steward et al. 1996). This effect occurs in rat striatal slices but not in rat striatal synaptosomes (De Deurwaerdère et al. 1997; Steward et al. 1996), suggesting that the effect exerted by some of these agonists is indirect with respect to the DA terminals. In vivo, striatal application of several 5-HT4 receptor agonists such as BIMU8, 5-MT, renzapride, cizapride, or (s)-zacopride enhanced striatal DA release (Bonhomme et al. 1995; De Deurwaerdère et al. 1997; Steward et al. 1996). The effect of BIMU8 and 5-MT is partially blocked by 5-HT3/4 antagonists (Benloucif et al. 1993; Bonhomme et al. 1995) while the effect elicited by (s)-zacopride is suppressed by the non-selective 5-HT4 antagonist SDZ205557 (Steward et al. 1996). Further, the excitatory effect of 5-HT on DA release has been partly reduced by a variety of 5-HT antagonists sharing the property of blocking 5-HT4 receptors such as DAU 6285, ICS 205930, GR 113808, or GR 125487 (Benloucif et al. 1993; Bonhomme et al. 1995; De Deurwaerdère et al. 1997; Suchowski and Galloway 1995; see Table 2).
The postsynaptic location of striatal 5-HT4 receptors with respect to DA terminals implies that the excitatory influence of 5-HT on DA release via 5-HT4 receptors is indirect. Tetrodotoxin (TTX), a blocker of fast sodium voltage-dependent channels, allows the determination of whether the effect elicited by 5-HT4 receptor stimulation is indirect. Indeed given that action potentials are blocked by TTX, most indirect actions involving another neuronal system are suppressed, so that mainly direct effects on striatal DA terminals persist in the presence of TTX. Using such an approach, it has been reported that the facilitatory effects on the striatal DA release of (s)-zacopride in vivo or renzapride and cisapride in vitro were suppressed in the presence of TTX (De Deurwaerdère et al. 1997; Steward et al. 1996). The data concerning 5-HT are more complex. Indeed in vivo 5-HT-evoked striatal DA release is reduced by almost 50% in the presence of TTX, suggesting the involvement of mechanisms both pre- and postsynaptic with respect to DA terminals (De Deurwaerdère et al. 1996; see Fig. 3). However, the ability of 5-HT4 antagonists to block the facilitatory effect of 5-HT on DA release was lost in the presence of TTX in vivo, suggesting that 5-HT4 antagonists are able to prevent only the indirect but not the direct excitatory effects of 5-HT (De Deurwaerdère et al. 1997).
Evidence for a carrier-mediated release of striatal DA induced by the local infusion of 1 μM 5-HT. Tetrodotoxin (TTX) and nomifensine (NOM) were applied at 1 μM as indicated by the gray and the black horizontal bars, respectively. 5-HT was infused at 1 μM during 15 min. These results illustrate that TTX, which inhibits striatal DA release, does not completely prevent the effect of 5-HT (p < 0.001, Tukey’s test after significant ANOVA). In these conditions, NOM induces a slight increasing effect on striatal DA release. When combining TTX+NOM, the TTX-insensitive effect of 5-HT on DA release is abolished. Notably, such an experiment could not be performed without TTX. Indeed 1 μM NOM enhanced DA release by about 660% of baseline, preventing an accurate study of the effect of 1 μM 5-HT (about 160% of baseline values). Results are expressed in percent (%) of baseline (adapted from De Deurwaerdère et al. 1996; courtesy of the International Society of Neurochemistry)
In conclusion, striatal 5-HT4 receptors would be able to facilitate striatal DA release through an indirect mechanism involving other striatal neurotransmitters.
Other 5-HT receptors
Due to the lack of agonists and antagonists at 5-HT1E, 5-HT6, and 5-HT7 receptors until recently (Barnes and Sharp 1999; Saudou and Hen 1994), no direct evidence is yet available concerning the influence of these receptors on DA release. The data showing that neither ritanserin nor methiotepine effect exogenous 5-HT-stimulated DA release in vivo (Benloucif and Galloway 1991; De Deurwaerdère et al. 1997) suggest indirectly that the 5-HT6/7 receptors are not involved in this effect. Indeed ritanserin and methiotepine display a moderate to high affinity for 5-HT6/7 receptors (Monsma et al. 1993). Whatever the situation concerning these 5-HT receptors, the available data suggest that several 5-HT receptors could contribute to the excitatory effects of exogenous 5-HT both in vivo and in vitro. It is noteworthy, however, that a complete blockade of the facilitatory effect of 5-HT has never been observed in vitro or in vivo, whatever the receptor subtypes considered. Another mechanism underlying the excitatory effect of 5-HT on striatal DA release has to be considered.
5-HT receptors involved in the inhibitory effects of 5-HT on DA release
The inhibitory effect of 5-HT appears sensitive to various 5-HT antagonists although a clear picture concerning the 5-HT receptor subtype(s) involved is also lacking.
5-HT1A receptors
Striatal 5-HT1A receptors have not been directly involved in the inhibitory effect elicited by 5-HT on striatal DA release, but they have been claimed to mediate the inhibitory effect of 5-HT on striatal DA synthesis. Indeed several 5-HT1A agonists including 8-OHDPAT are able to reduce DA synthesis in vitro on rat brain synaptosomes (Johnson et al. 1993, 1996). In addition, the inhibitory effect of 5-HT as well as some 5-HT1A agonists on tyrosine hydroxylase activity was blocked by methysergide (de Belleroche and Bradford 1980) or by non-selective 5-HT1A antagonists (Johnson et al. 1993, 1996). In this latter study, however, each non-selective 5-HT1A antagonist induced an inhibitory effect (Johnson et al. 1993). In addition, the EC50 of 5-HT1A agonists or 5-HT to inhibit tyrosine hydroxylation was almost a thousand times higher than their Kd for 5-HT1A receptors (Zifa and Fillion 1992). Finally, 5-HT1A receptors are not located on DA terminals and the inhibitory effect of 5-HT1A drugs on striatal DA synthesis may not involve a specific action at 5-HT1A receptors.
5-HT1B receptors
Non-selective 5-HT agonists such as 5-MT, 5-N,N-dimethyl-5-HT or tryptamine have been shown to mimic the inhibitory effect of exogenous 5-HT on [3H]-DA release in striatal slices (Ennis et al. 1981). More recently, the 5-HT1B agonist CP93129 and the non-selective 5-HT1 agonist 5-CT, but not the 5-HT1A agonist 8-OHDPAT, have been shown to decrease [3H]-DA released by 20 mM K+ in rat brain synaptosomes (Sarhan et al. 1999). Although the concentration of these compounds required to inhibit stimulated [3H]-DA release is very high (>300 nM) with respect to their respective Kd for 5-HT1B receptors (1–10 nM), it has been postulated that the efficacy of 5-HT1B agonists on postsynaptic 5-HT1B receptors is lower compared to presynaptic 5-HT1B receptors controlling 5-HT release (Sarhan and Fillion 1999). The inhibitory effect elicited by 5-HT, CP 93129, and 5-CT is also reduced by the 5-HT1B antagonist SB 224289 in rat striatal synaptosomes (Sarhan et al. 1999; Sarhan and Fillion 1999). Finally, the effect of CP93129 or 5-CT is reduced in mutant mice lacking 5-HT1B receptors (Sarhan et al. 2000). These data suggest the involvement of 5-HT1B receptors in the inhibitory effect of 5-HT.
As for the situation described for the 5-HT4 receptor-dependent excitatory control, one may expect that the inhibitory control exerted by 5-HT1B receptors on DA release is indirect because 5-HT1B receptors are not present on DA terminals. Nevertheless, the inhibitory effects of 5-HT on [3H]-DA release are direct on striatal DA terminals. Indeed the ability of 5-HT to inhibit electrical- or K+-stimulated striatal [3H]-DA and DA synthesis occurs on synaptosomes or on striatal slices in the presence of TTX (Sarhan et al. 1999; Westfall and Tittermary 1982). This finding has been reported with several 5-HT agonists including CP 93129 (Ennis et al. 1981; Sarhan et al. 1999). Therefore, due to the anatomical findings all supporting a postsynaptic location of 5-HT1B receptor with respect to striatal DA terminals, the involvement of 5-HT1B receptors in the inhibitory control of enhanced striatal DA release is not clear. It is possible that the loss of selectivity of these 5-HT1 ligands may dramatically interfere with DA terminals function (see below).
5-HT2 receptors
A role for 5-HT2 receptors was originally postulated to explain the inhibitory effect of 5-HT on striatal DA release. Indeed non-selective 5-HT antagonists such as methysergide or methiotepine (Ennis et al. 1981; Westfall and Tittermary 1982) and non-selective 5-HT2 antagonists such as ketanserin or mianserin (Ennis et al. 1981; Muramatsu et al. 1988) are able to reduce the inhibitory effect of 5-HT or 5-MT on K+-stimulated [3H]-DA release. These compounds are unfortunately not selective toward 5-HT2 receptors.
Pehek and colleagues (Alex and Pehek 2007; Alex et al. 2005) have shown that the intrastriatal infusion of the 5-HT2B/2C receptor antagonist SB 206553 enhances striatal DA release in vivo, suggesting that striatal 5-HT2C receptors may participate in the ability of peripheral 5-HT2C antagonists to unmask the tonic inhibitory control of 5-HT2C receptors on in vivo striatal DA release (De Deurwaerdère et al. 2004; Di Giovanni et al. 1999; Navailles and De Deurwaerdère 2011). An inhibitory action of 5-HT2C receptors is further supported by data reporting an excitatory effect of local infusion of the 5-HT2C antagonist RS 102221 into the nucleus accumbens (Dremencov et al. 2005).
Concluding remarks
Several receptors have been altogether proposed to mediate the effects of 5-HT on striatal DA function (Fig. 4). The absence of complete blockade of 5-HT-induced DA release with any of several 5-HT antagonists suggests that another mechanism is likely involved in the excitatory effects elicited by 5-HT on DA release. In addition, despite the biochemical evidence supporting a direct inhibitory role of 5-HT1B, 5-HT2, or 5-HT1A receptors on striatal DA function, anatomical data lessens this possibility.
Drawing representing the influences of exogenous 5-HT on striatal DA release in vivo. Exogenous 5-HT, applied in the striatum, triggers both a Ca2+-independent/TTX-insensitive (directly on DA terminals) and Ca2+-dependent/TTX-sensitive mechanisms (indirect with respect to DA terminals). The direct effect elicits a non-exocytotic release of DA that is abolished by DA reuptake blockers, but not by 5-HT4 antagonists. The putative indirect effect is sensitive to 5-HT4 antagonists. Various 5-HT4 agonists are able to increase DA release in a TTX-sensitive manner via 5-HT4 receptor stimulation
Direct effects of 5-HT on DA terminals: involvement of DA reuptake sites
Involvement of DA transporters
The involvement of DA transporters, a protein located on the plasma membrane of DA terminals and involved in the high rate of extracellular DA clearance in normal conditions (Luthman et al. 1993), has been proposed to explain the excitatory effect of 5-HT on DA release. Briefly, such a mechanism is direct on striatal DA terminals and is non-exocytotic. Thus, as opposed to an exocytotic release, the output of DA from the cytosol to the extracellular space occurs independently of the extracellular Ca2+ ions via the reversal of the direction of DA transport (Levi and Raiteri 1993). The release of DA induced through DA transporters is sensitive to all DA uptake blockers. Amphetamine and tyramine are two well-described DA releasers (Seiden et al. 1993; Sulzer et al. 1995) (Fig. 5).
Drawing representing the influences by steps of 5-HT entry into striatal DA terminals. (1) Serotonin enters DA terminals by DA transporters. Other molecules such as amphetamine are known to enter DA terminals by DA transporters but may also enter by other mechanisms (small target). (2) Cytosolic 5-HT competes with DA to enter the exocytotic vesicles on VMAT. In this case, 5-HT can be released from DA terminals by exocytosis. (3) Cytosolic 5-HT is metabolized by monoamine oxydase A in the cytosol. (4) Cytosolic DA is dramatically increased by less metabolism and less entry in exocytotic vesicles. (5) The increase in cytosolic DA inhibits tyrosine hydroxylase activity. (6) Via a mechanism depending on the releaser, the functioning of the DA transporter is inverted and DA is released by a non-exocytotic process. 5-HT, 5-hydroxytryptamine; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; 5-HIAA, 5-hydroxyindolacetic acid
To be operational, a carrier-mediated release of DA requires the entry of the releaser inside DA terminals. It has been shown for many years that 5-HT is a substrate of DA uptake sites (Shaskan and Snyder 1970) and may be transported inside DA neurons (Feuerstein et al. 1986; Kelly et al. 1985; Ternaux et al. 1977; Zhou et al. 2005). The “false labeling” of DA terminals by 5-HT in in vitro caudate preparations of rodents occurs for concentrations of [3H]-5-HT likely lower than 0.1 μM in the presence of a 5-HT uptake blocker (Feuerstein et al. 1986; Kelly et al. 1985). It requires to some extent functional DA reuptake sites since the false labeling of DA neurons with 5-HT is dramatically reduced in the presence of nomifensine (Feuerstein et al. 1986; Lupp et al. 1992; Zhou et al. 2002, 2005 for functional aspects). These data indicate that 5-HT can enter DA terminals.
Presynaptic versus postsynaptic effect
Several studies have attempted to determine whether 5-HT acts directly on DA terminals (presynaptic) or indirectly, involving another neurotransmitter system (postsynaptic). The fact that 5-HT enhanced DA on striatal synaptosomes suggested the existence of mechanisms occurring directly at the level of DA terminals (Andrews et al. 1978; De Deurwaerdère et al. 1997; Yi et al. 1991). As mentioned above, it has been shown that 5-HT still enhances/inhibits DA release on rat striatal slices completely (Ennis et al. 1981; Jacocks and Cox 1992; Zazpe et al. 1994) or partly (Blandina et al. 1989) in the presence of TTX. In vivo, half of the 5-HT-evoked striatal DA release persists in the presence of TTX and this remaining effect is insensitive to 5-HT3/4 antagonists (De Deurwaerdère et al. 1996, 1997; see Table 2).
Exocytotic versus non-exocytotic mechanism
Studies have provided evidence for both an exocytotic and non-exocytotic mechanism of action mediated by 5-HT regarding its excitatory and inhibitory effects on striatal DA release. First, numerous in vitro and ex vivo data have suggested that 5-HT or its metabolic precursor 5-hydroxytryptophan (5-HTP) elicit a non-exocytotic release of DA (Andrews et al. 1978; Awazi and Gulberg 1978; Butcher et al. 1972; Ng et al. 1972). It has been shown that the release of [3H]-DA or endogenous DA elicited by 5-HT in rat striatal slices or synaptosomes did not depend on the presence of extracellular Ca2+ (Jacocks and Cox 1992; Yi et al. 1991; Zazpe et al. 1994). In vivo, the effect of 5-HT was reduced by half following the removal of Ca2+ ions from the perfusion medium, indicating that 5-HT is able to elicit a non-exocytotic release of DA (De Deurwaerdère et al. 1996). Given that a TTX-sensitive effect is also Ca2+ dependent (Westerink et al. 1987), the similar reduction observed with TTX and with the removal of Ca2+ ions indirectly suggested that the TTX/Ca2+-insensitive mechanism of 5-HT is entirely non-exocytotic in vivo (De Deurwaerdère et al. 1996, 1997). The contribution of this mechanism might be even higher in the total effect of 5-HT in vivo. Indeed the consequence of the entry of 5-HT into DA terminals is necessarily associated with the fact that DA reuptake is impeded. That part, which would be similar to a DA reuptake blocker, is a TTX-sensitive mechanism. Consequently, a small contribution of other mechanisms such as 5-HT4 receptors might have been magnified simply because DA reuptake sites were less efficient in the presence of 5-HT.
At variance, the inhibitory effect of 5-HT or 5-HT agonists on stimulated [3H]-DA release in striatal slices or synaptosomes is dependent on the extracellular presence of Ca2+ (Sarhan et al. 1999). This approach is, however, subject to caution given that the K+-stimulating effect on DA release is also suppressed in the absence of Ca2+ and reduced in the presence of Ca2+ channel blockers (El Ayadi et al. 2001). Therefore, the fact that 5-HT or 5-HT agonists are unable to affect [3H]-DA release in the absence of Ca2+ does not necessarily imply that their ability to reduce K+-induced [3H]-DA release is a receptor-mediated mechanism.
Influence of DA reuptake site blockers in the facilitatory effect of 5-HT
The evidence for a non-exocytotic process elicited by 5-HT on DA release is compatible with an effect mediated by DA transporters (Levi and Raiteri 1993). As discussed above, in vitro, the excitatory effect of 5-HT on basal or stimulated DA release is reduced by various DA uptake blockers (Andrews et al. 1978; Benuck and Reith 1992; De Deurwaerdère et al. 1997; Jacocks and Cox 1992; Kamal et al. 1983; Nurse et al. 1988; Zazpe et al. 1994; Zhou et al. 2005) (see Table 2). In vivo, a concentration of the DA uptake blocker nomifensine as low as 100 nM was able to reduce the release of DA induced by 3 μM 5-HT (De Deurwaerdère et al. 1996). To better address the role of DA reuptake sites, the effect of 5-HT was studied in the presence of TTX and nomifensine. This procedure further reduces the effect of nomifensine itself since, in the presence of TTX, the effect of DA reuptake inhibitors is strongly attenuated (Westerink et al. 1987, 1989). In these conditions, the TTX-resistant release of DA elicited by low 5-HT concentrations was suppressed in the presence of 1 μM nomifensine (Fig. 3).
Behavioral data also support the involvement of a DA carrier-mediated release of DA induced by 5-HT. Indeed it has been reported that the local administration of 5-HT (20 μg/μl) in the ventral striatum elicits orofacial stereotypies in rats (Yeghiayan and Kelly 1995). This effect, insensitive to various 5-HT antagonists, disappeared in rats with a lesion of DA neurons, administering the non-selective DA receptor antagonist haloperidol or the DA uptake blocker GBR12909 (Yeghiayan et al. 1997). These authors conclude that the behavioral effect elicited by the local administration of 5-HT in the striatum is primarily attributable to a carrier-mediated release of DA elicited by 5-HT.
In conclusion, 5-HT is capable of inducing a carrier-mediated release of striatal DA. This mechanism is consistent among all studies (in vitro or in vivo) reporting an excitatory effect of 5-HT on basal DA release.
Influence of DA reuptake site blockers in the inhibitory effects of 5-HT
The rationale for studying the influence of DA reuptake sites in the inhibitory effect of 5-HT is to prevent the entry of 5-HT inside DA terminals. Indeed it is known that amphetamine and tyramine may inhibit stimulated [3H]-DA release from [3H]-DA preloaded striatal slices and synaptosomes (Bowyer et al. 1987; de Langen et al. 1979; Kamal et al. 1983). This effect occurs at concentrations unable to induce a basal efflux of DA through DA transporters and is Ca2+ dependent. At higher concentrations of amphetamine or tyramine, this mechanism is concomitant to the DA carrier-mediated release of DA (Bowyer et al. 1987; Kamal et al. 1983). The inhibitory effect of amphetamine on stimulated [3H]-DA release from striatal slices is blocked by DA reuptake blockers (Kamal et al. 1983).
Concerning 5-HT, Westfall and Tittermary (1982) have described the inhibitory effect of 5-HT on the electrically stimulated release of [3H]-DA in the presence of the DA uptake blocker benztropine, but these authors have not studied the effect of 5-HT without benztropine. On the other hand, the inhibitory effect of 5-HT on K+-stimulated release of [3H]-DA was suppressed by nomifensine and benztropine (Nurse et al. 1988) (Table 2). For these authors, 5-HT is displacing preloaded [3H]-DA from the vesicular pool in the cytoplasm so that less [3H]-DA is available when inducing a depolarizing stimulation (Nurse et al. 1988). This hypothesis is supported by in vivo data showing that 5-HT can be released by DA neurons in rats bearing a lesion of 5-HT neurons and receiving an acute administration of 5-HTP (Jackson and Wightman 1995). Studies conducted by Ng et al. (1972) have shown by fluorometry that 5-HTP enters DA neurons and is converted to 5-HT inside DA terminals by endogenous l-aromatic acid decarboxylase. They showed that an inhibitor of decarboxylase blocked the facilitatory effect of 5-HTP on [3H]-DA in [3H]-DA preloaded striatal slices. These results indicated that the ability of 5-HTP to elicit an efflux of DA is dependent on its conversion to 5-HT inside DA terminals. More importantly, Feuerstein et al. (1986) have clearly shown in vitro that [3H]-5-HT, loaded in DA terminals, can be released by high K+ stimulation while Zhou et al. (2005) have reported that 5-HT can be co-released with DA provided that 5-HT is able to enter DA terminals via DA reuptake sites. These data are compatible with the fact that vesicular transporters (VMAT2), ATPase pumps coupling the co-transport of the monoamine with two protons, are identical for all monoamines (Fig. 5) (Adam et al. 2008). Similarly, brain 5-HT terminals are responsible for the release of DA induced by an injection of its metabolic precursor l-DOPA in hemiparkinsonian rats (Navailles et al. 2010).
This mechanism, proposing that a drug can dilute the quantity of DA available for release, has also been used to explain the inhibitory effects of amphetamine on DA release in vitro and in vivo (Jones et al. 1998; Kamal et al. 1983; Kuhr et al. 1985; Schmitz et al. 2001). In addition, the inhibitory effects of 5-HT on DA synthesis have also been blocked by cocaine (Andrews et al. 1978). For these authors, the displacement of DA from its storage vesicle enhances free DA in the cytosol and, consequently, inhibits tyrosine hydroxylase activity (Fig. 5; Table 2).
Influence of DA mechanisms in the inhibitory effects of 5-HT agents
The data are more complex with 5-HT agonists because their putative direct or indirect interaction with DA transporters is not evident and even less studied. Nonetheless, 5-HT-related compounds that are able to inhibit stimulated [3H]-DA release from [3H]-DA preloaded in rat brain preparations have their own stimulatory effects in the same studies or in others. In particular, CP 93129 produces a non-exocytotic release of [3H]-DA on striatal synaptosomes when applied at high concentrations (>1 μM) (Sarhan et al. 1999), implying that at lower concentrations (300 nM) CP 93129 has already reached the cytosol of the DA terminal. Similarly, 5-MT (De Deurwaerdère et al. 1997), 5-NN-MeOT (Berge et al. 1983; Yeghiayan et al. 1997), and 5-CT (Ng et al. 1999) have been shown to enhance DA transmission (Table 2). The data are not clear for tryptamine although this compound is a releaser of 5-HT and noradrenaline in the myenteric plexus while its affinity for 5-HT receptors is very low (Takaki et al. 1985). In addition, it enters DA terminals (Zhou et al. 2005). Therefore, the entry of 5-HT and 5-HT agonists into DA terminals has to be considered to explain their inhibitory effects on stimulated [3H]-DA release. Unfortunately, there is not one signature for a DA releaser to modify DA parameters from DA terminals. For instance, to the best of our knowledge, 5-HT3 agonists have never been reported to decrease K+-stimulated [3H]-DA release though they have clearly been involved in a carrier-mediated release of DA. Thus, the effects of all these agents are critically dependent on their pharmacological properties and the in vitro experimental procedures employed (see Herdon et al. 1985 and Kamal et al. 1983 for comparison between amphetamine and 5-HT; Fink and Göthert 2007).
Concluding remarks
The displacement of DA by 5-HT from its vesicle is a satisfactory alternative to explain the inhibitory effects of 5-HT in vitro. Indeed, as mentioned earlier, this effect is direct on DA terminals whereas 5-HT receptors thought to mediate these effects, namely, 5-HT1A, 5-HT1B, and 5-HT2A/2C receptors, are not present on DA terminals. Obviously, this hypothesis implies that 5-HT antagonists lose their selectivity for 5-HT receptors at the concentrations used and that they directly act inside DA terminals. Additional studies are warranted to further evaluate this pharmacological issue.
Conclusion
Although the involvement of 5-HT receptors has been proposed, the inhibitory effect of 5-HT on striatal DA release has been described mainly in one type of in vitro study (stimulated [3H]-DA release from [3H]-DA preloaded preparations) that relies on the integrity of the releasable pool (Herdon et al. 1985). In any case, due to the concentration of 5-HT used (>100 nM), the presence of 5-HT inside DA terminals and vesicles has to be considered. Thus, both the excitatory and inhibitory effects of 5-HT on striatal DA release and synthesis might have the same origin: the entry of 5-HT into DA terminals.
The concomitant action of 5-HT inside DA terminals warns further studies concerning the striatal 5-HT mechanisms elicited by exogenous 5-HT in the control of DA release. For instance, the data accumulated lately concerning the contribution of 5-HT4 receptors in the control of striatal DA release does not match the findings obtained with 5-HT4 agonists and antagonists. Indeed, it has been hypothesized that 5-HT4 receptors stimulate only the impulse-dependent striatal DA release (Porras et al. 2003; Pozzi et al. 1995). In their recent review, Fink and Göthert (2007) discuss the possibility that the conditions to highlight presynaptic influence of 5-HT4 receptors on striatal DA release have not been completely addressed, in particular in the presence of concomitant and depolarizing triggering stimuli. Indeed, the excitatory effect of exogenous 5-HT on DA release is not an impulse-dependent release of DA. Thus, one may question the extent to which the mechanisms described in vitro or in vivo by directly applying 5-HT or 5-HT agonists occur in physiological conditions (Gallager et. al 1975). An important point seems to address the putative ability of endogenous 5-HT to enter DA neurons.
Effect of Endogenous 5-HT on striatal DA function: Significance and physiological relevance of a carrier-mediated release of DA elicited by 5-HT
The important question coming from these studies is to determine the extent to which 5-HT may enter DA terminals as a basic physiological process regulating DA release and synthesis. In the following section, the ability of endogenous 5-HT to induce a carrier-mediated release of DA will be mainly considered although, as illustrated before, inhibitory effects of 5-HT on stimulated striatal DA release in vitro, even if they are independent of 5-HT receptors, occur at lower 5-HT concentrations.
It has been shown in mice lacking monoamine oxidase that 5-HT may be found in DA terminals, suggesting that 5-HT may be accumulated over a long period of time in DA terminals. However, such an accumulation is not necessarily associated with acute effects such as the above-mentioned inhibitory or excitatory effects on DA release and synthesis. The limiting factor for eliciting such mechanisms in vivo should be the concentration of endogenous 5-HT reached in the vicinity of DA terminals. This concentration could be close to 100 nM (probably lower) to enter DA terminals through DA transporters (Feuerstein et al. 1986; Zhou et al. 2005). It remains to be determined whether endogenous 5-HT, released from striatal 5-HT terminals, can reach approximately a 100 nM concentration at nearby DA terminals.
Extracellular concentration of 5-HT
The basal extracellular concentration of 5-HT, determined in microdialysis experiments in vivo, is extremely low in the striatum, ranging from 0.5 to 2 nM (Ferré et al. 1994; Kalén et al. 1988; Kreiss et al. 1993). Moreover, given that 5-HT terminals are rarely in close apposition to DA terminals in this region (Soghomonian et al. 1987), it seems difficult for 5-HT to enter striatal DA terminals and, a fortiori, trigger a carrier-mediated mechanism in resting conditions.
Mobilization of endogenous 5-HT
Methods to enhance endogenous 5-HT release have been tried by increasing 5-HT nerve activity or impairing 5-HT reuptake with pharmacological manipulations (Table 3).
Dorsal raphe electrical stimulation
DRN electrical stimulation has been shown to increase endogenous 5-HT release from 5-HT nerve terminals (Sharp et al. 1989). This effect is of small magnitude with respect to other pharmacological-based treatments (see below) since it elicits an increase of 2 to 5 times basal extracellular levels of 5-HT. In these conditions, DRN electrical stimulation failed to enhance DA release in the striatum (De Deurwaerdère et al. 1998; De Simoni et al. 1987). These data suggest that a carrier-mediated release of DA elicited by endogenous 5-HT cannot be induced by simply increasing 5-HT nerve activity. It is not likely that a concentration of 100 nM is obtained following such a procedure. This hypothesis is consistent with in vitro data performed at the level of the SN, a region that displays a dense 5-HT innervation (Moukhles et al. 1997), in which repeated pulse stimulation enhanced extracellular 5-HT concentration to about 65 nM (Bunin and Wightman 1998).
Pharmacological manipulations
Pharmacological manipulations of 5-HT nerve terminal activity in the striatum represent another way to elicit an increase of endogenous 5-HT extracellular levels. It is usually achieved by infusing selective 5-HT reuptake inhibitors (SSRI) through the dialysis probe of the microdialysis system. The major drawback of such an approach is that these molecules may display a non-negligible affinity for DA reuptake sites and may directly act inside DA terminals (Lupp et al. 1992). Most of them display an affinity ranging from 5 to 50 nM for 5-HT reuptake sites while their affinity for DA uptake sites is close to micromolar concentrations (Thomas et al. 1987). Considering a relative probe membrane recovery of 10% in vivo, most of these compounds should not be infused above 10 μM to keep a relative selectivity for 5-HT uptake sites with respect to DA uptake sites. Each time a concentration higher than 10 μM has been used, 5-HT uptake inhibitors such as clomipramine (Santiago et al. 1998), fluvoxamine (Koch and Galloway 1997), citalopram (De Deurwaerdère et al. 1995), fluoxetine (Benloucif and Galloway 1991) or alaproclate (Yadid et al. 1994), enhanced striatal DA release. Confirming the loss of selectivity of high concentrations of these compounds, the excitatory effects elicited by 25 μM citalopram or 100 μM chlomipramine on striatal DA release were not affected by a lesion of 5-HT neurons (Lucas et al. 2000a; Santiago et al. 1998). At lower concentrations (1 μM), citalopram or fluoxetine failed to modify striatal DA release. At higher concentrations in vitro, fluoxetine behaved as DA transporter blocker (Izenwasser et al. 1990). Again, the enhancement of extracellular 5-HT in these conditions is relatively small and should not potentiate more than 5 times basal extracellular levels of 5-HT (Kreiss et al. 1993). Of note, the stimulatory effect of alaproclate has been suppressed by a 5,7-DHT lesion of dorsal raphe nucleus 5-HT neurons (Yadid et al. 1994). Given the poor selectivity of this compound that behaves as a voltage-gated potassium channels blocker or a noncompetitive antagonist of the NMDA receptor (see Wilkinson et al. 1994) and considering the very high concentrations used by Yadid et al. (400 μM), one may foresee methodological problems in this experiment. As a further comment, the DA effects elicited by high concentrations of these drugs may come from multiple sources including the blockade of DA uptake sites, their affinity for sigma receptors (Narita et al. 1996), known to affect striatal DA release (Moison et al. 2003), or their numerous other targets in the brain (for review see Millan 2006).
A strong enhancement of endogenous 5-HT release can be obtained by directly infusing 5-HT releasers such as fenfluramine (Kirby et al. 1995; Schwartz et al. 1989). Such an increase may reach over 1000% of basal levels of 5-HT (Schwartz et al. 1989). However, the facilitatory effect of 25 μM d-fenfluramine, a concentration known to enhance 5-HT release (Berger et al. 1992), was not reduced in rats bearing a quasi-total destruction of 5-HT neurons (De Deurwaerdère et al. 1995). These data indicated that intrastriatal infusion d-fenfluramine is not a good pharmacological approach to selectively address the role of striatal endogenous 5-HT. As discussed previously, the ability of the 5-HT releaser MDMA to affect striatal DA release via 5-HT-dependent mechanisms is obtained when it is peripherally administered (Nash, 1990), but not when it is directly applied in the striatum (Koch and Galloway 1997; Schmidt et al. 1994).
Altogether, these data suggest that intrastriatal manipulations of endogenous 5-HT barely affect striatal DA release in resting conditions (Table 3).
Endogenous 5-HT and inhibitory effect on striatal DA release
The in vitro data indicated that the inhibitory effects of 5-HT on stimulated striatal DA release are observed at lower concentrations of 5-HT. Some inhibitory effects on striatal DA release have been reported when central 5-HT transmission is increased (De Deurwaerdère et al. 1998; Dewey et al. 1995). It is unlikely, however, that this inhibitory effect corresponds to the in vitro data because it has been observed in resting, but not in stimulated DA conditions. Rather, in conditions in which exocytotic release of DA is enhanced by haloperidol or electrical stimulation of the medial forebrain bundle, an enhancement of 5-HT extracellular levels either potentiated or did not affect stimulated striatal DA release (De Simoni et al. 1987; Dugast et al. 1994; Lucas et al. 2000a). Therefore, a direct effect of 5-HT in inhibiting stimulated striatal DA release seems unlikely.
The inhibitory effects induced by a global enhancement of endogenous 5-HT elicited by DRN stimulation or SSRI on striatal DA release could be due to the entry of 5-HT at the level of DA cell bodies. Indeed, 5-HT terminals are concentrated in the SN (Moukhles et al. 1997), and it has been reported that genetic or pharmacological impairment of 5-HT transporters induced 5-HT-immunoreactivity in DA cell bodies but not in the striatum (Zhou et al. 2002). Consequently, it cannot be excluded that the inhibitory effects elicited by DRN electrical stimulation or high doses of SSRI promotes an efflux of DA in the SN, indirectly inhibiting DA neuron impulse-flow via D2 autoreceptors.
Concluding remarks
Although a carrier-mediated release of DA induced by 5-HT can be elicited in vivo (De Deurwaerdère et al. 1996; Yeghiayan and Kelly 1995; Yeghiayan et al. 1997), it should probably not occur in physiological conditions when considering the anatomy and functional relationship between DA and 5-HT terminals in the striatum. The difference between exogenous and endogenous 5-HT is explainable by the fact that, when 5-HT is directly applied in the tissue, it spreads around striatal DA terminals independently of the anatomical substrate linking 5-HT and DA terminals. In contrast, the highest increase in 5-HT extracellular levels induced by SSRI, d-fenfluramine and DRN stimulation might be restricted to a limited area. This increase would not be sufficient to elicit a carrier-mediated release of DA.
The situation could be different in other brain areas where the density of 5-HT terminals is higher such as in the cortex. Numerous recent data have suggested that such an entry of monoamines in heterologous monoamine systems may participate in the mechanism of action of antidepressant or psychostimulant drugs (Di Chiara et al. 1992; Di Matteo et al. 2008; Rothman and Baumann 2006; Zhou et al. 2005) have brought up recent elements suggesting that, after a long-term treatment with fluoxetine and tryptophan to enhance 5-HT release, 5-HT and DA may be co-released from DA terminals. However, the concentrations of 5-HT were extremely low, probably because monoamine oxidase A, which is highly concentrated in DA terminals (Lupp et al. 1992; Westlund et al. 1988), has a better affinity for 5-HT than for DA (Youdim et al. 1986).
The state-dependent involvement of striatal 5-HT in the control of striatal DA release and metabolism
Although all these studies minimize the influence of endogenous 5-HT and 5-HT receptors on striatal DA release, new elements have been discovered allowing us to better understand the subtleties of this interaction. Indeed, whereas the involvement of most 5-HT receptors seems silenced in resting conditions, various pharmacological treatments known to alter the activity of DA neurons lead to the involvement of striatal 5-HT receptors in the control of striatal DA release. The purpose of the following section is to present some of the conditional factors allowing striatal 5-HT receptors to control DA terminals nerve activity.
Alteration of DA neuron activity
Several agents such as amphetamine, MDMA, haloperidol, morphine, apomorphine or cocaine affect nigrostriatal DA nerve activity and striatal DA transmission. Interestingly, these agents trigger specific cellular mechanisms that recruit discrete 5-HT controls on striatal DA release. Of note, the intrastriatal administration of an SSRI, without effect by itself (see above), enhanced the output of striatal DA release induced by haloperidol (Lucas et al. 2000a). Given that 5-HT receptors are located on striatal neurons, it might be suggested that a modification of central DA transmission (not restricted to striatal cells) would increase the influence exerted by some 5-HT receptors on striatal cells.
Another key factor is that the different drugs able to modulate DA release trigger different 5-HT receptors in the control of DA neuron activity that may be localized in the striatum or elsewhere. In different studies, it has been proposed that 5-HT receptors (and the cells carrying these receptors) would preferentially affect one type of DA release. Indeed, extracellular level of DA is an integrated parameter relying on various processes occurring in the regulation of DA terminal activity (firing, depolarization, synthesis, reuptake, etc.) (Fig. 6). Because the triggering drugs have to be peripherally administered, the control exerted by one 5-HT receptor may occur outside the striatal system. Yet, numerous data are available now to demonstrate the role of striatal 5-HT receptors in the state-dependent control of striatal DA release.
Influence of 5-HT receptors on striatal DA release. Striatal DA release is supported by various cellular mechanisms including DA synthesis, firing activity of the neuron, or depolarization of the terminals. The different origin of release is important to consider as basal DA release is not critically dependent on DA synthesis in contrast to MDMA-induced DA release. 5-HT receptors affect DA release under certain restricted conditions. 5-HT2A receptors stimulate DA release in a phasic manner and act on DA firing rate and/or DA synthesis. 5-HT4 receptors stimulate DA release in a phasic manner on an impulse-dependent release of DA. 5-HT2C receptors tonically inhibit the impulse-dependent release of DA. 5-HT3 receptors exert a phasic excitatory control restricted to the impulse-dependent and/or depolarization-induced DA release. It is important to note that only 5-HT3 receptors would require an increase in 5-HT levels
Nature of DA released
The most studied drugs to date are morphine, haloperidol, cocaine, amphetamine, MDMA. These drugs differ in their mechanism of action to release DA. Haloperidol and morphine are known to exert an impulse-dependent release of DA (Carboni et al. 1989; Di Chiara and Imperato 1988; Mereu et al. 1984). Cocaine, by blocking DA transporters, induces an increase in extracellular levels of DA associated with a decrease in DA neuron impulse-flow (Benwell et al. 1993; Bunney et al. 2000; Di Chiara and Imperato 1988; White 1997). Amphetamine and MDMA induce mainly a non-exocytotic release of DA (Di Chiara and Imperato 1988; Gudelsky and Yamamoto 2008; Pitts and Marwah 1988; Yamamoto et al. 1995). Moreover, the enhancement of striatal DA induced by haloperidol, amphetamine and MDMA relies critically on DA synthesis (Lucas et al. 2000b; Millan et al. 1999; Patterson and Schenk 1991; Porras et al. 2003; Schmitz et al. 2001). Finally, a last criterion to consider is the ability or not of these drugs to enhance endogenous 5-HT tone at the same time (Andrews and Lucki 2001; Gudelsky and Yamamoto 2008; Lucas et al. 2001; Navailles et al. 2004; Porras et al. 2003; Tao and Auerbach 1994; 1995).
In these conditions, modifications of endogenous 5-HT tone have been shown to alter the enhancement of DA release and/or metabolism induced by these drugs. Thus, the preferential 5-HT1A agonist 8-OHDPAT reduced DA release induced by amphetamine (Ichikawa and Meltzer 1995) and haloperidol, depending on the dose administered (Lucas et al. 1997; 2000b). The administration of SSRI citalopram and fluoxetine have been shown to enhance DA release induced by haloperidol (Lucas et al. 2000a,b).
Involvement of striatal 5-HT receptors
5-HT2A receptors
As indicated above, intrastriatal administration of the 5-HT2A antagonists M100907 or SR46349B significantly reduced the increase in DA release induced by systemic administration of MDMA or haloperidol, respectively (Lucas and Spampinato 2000; Schmidt et al. 1994). Intrastriatal injection of the mixed agonist DOI enhanced striatal DA release induced by haloperidol (Lucas and Spampinato 2000). Additionally, the increase in DA release induced by amphetamine was reduced by the systemic administration of SR46349B (Egerton et al. 2008; Porras et al. 2002a) while that of the mixed agonist DOI further enhanced the effect of amphetamine (Ichikawa and Meltzer 1995). These data indicate that 5-HT2A receptors play a permissive role on striatal DA function. Given that systemic administration of these antagonists did not affect DA release stimulated by morphine or cocaine, and that 5-HT2A receptors exert a state-dependent increase in DA synthesis (Sorensen et al. 1993; Waldmeier and Delini-Stula 1979), it has been proposed that 5-HT2A receptors stimulate the release of DA that relies on DA synthesis (Lucas et al. 2000b; Schmidt et al. 1993).
5-HT2C receptors
Among 5-HT receptors, the 5-HT2C subtype is the only receptor able to affect striatal DA release in resting conditions but its involvement on activated DA transmission also requires specific conditions. 5-HT2C antagonists are able to increase striatal DA release induced by morphine, haloperidol, cocaine, but not amphetamine while 5-HT2C agonists decrease striatal DA release induced by haloperidol, morphine, nicotine but not cocaine and amphetamine (Di Matteo et al. 2004; Lucas et al. 2000b; Navailles et al. 2004, 2006; Porras et al. 2002a; Willins and Meltzer 1998). These data suggest that 5-HT2C receptors exert an inhibitory control of exocytotic DA release, and preferentially on the impulse-dependent release of DA by acting at the level of nigrostriatal DA neuron activity (Navailles et al. 2004; 2006). Nevertheless, the site of action of 5-HT2C receptors has not been elucidated.
5-HT3 receptors
Against the proposal that 5-HT3 receptors are not able to modulate striatal DA release, it seems that, in very restricted conditions, 5-HT3 receptors exert a state-dependent excitatory control of striatal DA release. Thus, the stimulated release of DA induced by haloperidol, low-dose morphine, amphetamine, or cocaine is not affected by 5-HT3 antagonism. However, 5-HT3 antagonism reduced the increase in DA release induced by a high dose of morphine and by the cocktail haloperidol plus citalopram (Porras et al. 2003). More interestingly, local infusion of ondansetron mimicked the effect of systemic ondansetron, confirming previous data showing that the involvement of 5-HT3 receptors may occur under very particular conditions (De Deurwaerdère et al. 2005). These results indicate that 5-HT3 receptors can stimulate DA release provided that the stimulated release of DA relies on depolarized terminals and that the endogenous 5-HT tone is increased.
Thus, 5-HT3 receptors are not selectively involved in the control of neurons along the mesocorticolimbic DA pathways as previously shown (Bachy et al. 1993; Frankel et al. 1998). The preferential influence in the mesoaccumbal DA pathway is likely related to another population of 5-HT3 receptors located at the level of the VTA and able to initiate an increase in the impulse-dependent DA release in the nucleus accumbens (De Deurwaerdère et al. 2005; Imperato and Angelucci 1989).
5-HT4 receptors
The 5-HT4 receptors exert a state-dependent facilitatory control of DA release stimulated by morphine and haloperidol, but not cocaine or amphetamine (Lucas et al. 2001; Porras et al. 2002b; Pozzi et al. 1995). The role of striatal 5-HT4 receptors is not clear. On the one hand, intrastriatal infusion of GR113808 did not modify the effect of morphine (Pozzi et al. 1995). On the other hand, intrastriatal infusion of GR 125487 reduced the effect of haloperidol (Lucas et al. 2001). In this latter case, however, it is not determined whether this effect is related to the local decrease in endogenous 5-HT tone induced by 5-HT4 antagonists (Ge and Barnes 1996; Lucas et al. 2001) or to a more direct mechanism toward DA neuron activity.
Concluding remarks
These data indicate that the 5-HT system may affect striatal DA function in conditions in which central DA transmission is modified. The conditional involvement of the numerous 5-HT receptors in the control of striatal DA function depends on the nature of the DA released and the level of DA transmission. Among the receptors described in the striatum, 5-HT2A, 5-HT3, and 5-HT4 receptors may enhance striatal DA release in restricted conditions (Fig. 6). Complementary studies are needed to determine the status of 5-HT1B and 5-HT6 receptors.
In this context, it is important to mention that numerous 5-HT receptors are able to control the firing activity of nigrostriatal DA neurons, conditioning the activity of DA terminals and participating in the recruitment of striatal 5-HT receptors in the control of striatal DA release. Several 5-HT receptors are known to affect the electrical activity of DA neurons by acting in the SNc or centrally. For instance, DA neuron activity is indirectly decreased by 5-HT2C receptors (Di Giovanni et al. 1999, 2008), thereby acting against the ability of striatal 5-HT receptors to enhance the impulse-dependent DA release. In contrast, DA neuron activity is activated by 5-HT4 receptors in specific conditions (Porras et al. 2002a). The control of DA nerve activity by 5-HT receptors has been the purpose of a well-documented review (Di Giovanni et al. 2008).
Concluding remarks
The study of the influence of exogenous 5-HT on DA terminal activity has led to conflicting results. The origin of these discrepancies is primarily attributable to the ability of 5-HT and several related compounds to enter DA terminals. Inside DA terminals and depending on the activity of the molecules in the cytosol, various pathways involved in the synthesis or release of DA might be affected. At high concentrations of 5-HT, a carrier-mediated release of DA predominates. In these conditions, it appears difficult to determine the influence of concomitant 5-HT receptors involved in the control of striatal DA release in vivo and in vitro. These actions have to be taken into account when using these approaches to study the influence of the 5-HT system on other neurotransmitter systems in the vicinity of DA neurons.
The striatum participates in sensorimotor integration, procedural memory, formation of habits, and behavioral automatisms (Graybiel 2005; Mink 1996). DA plays a fundamental role in all these functions. Physiologically, several 5-HT receptors can participate in the control of the activity of striatal DA terminals. It seems that striatal 5-HT, through various 5-HT receptors, exerts excitatory effects on striatal DA release with the exception of the 5-HT2C receptor. Most of the excitatory 5-HT controls occur in a state-dependent manner. These findings are not incompatible with the functional “inhibitory” role of 5-HT on DA-mediated behavior (Spoont 1992; Soubrié 1986). Indeed by acting on the effector neurons of DA, the 5-HT system limits the efficacy of striatal DA transmission (Giambalvo and Snodgrass 1978; Waldmeier and Delini-Stula 1979). This would indirectly lead to adjust the release of DA via the modulatory influence of 5-HT on striatal neurons. In this context, DA release might not be an accurate index of the changes of DA-dependent behaviors triggered by 5-HT drugs. For instance, the reversal of haloperidol-induced catalepsy by 5-HT2 antagonists occurs independently from changes in DA release (Lucas et al. 1997). Conversely, while 5-HT4 antagonists reduce morphine- or haloperidol-induced striatal DA release, 5-HT4 antagonists do not affect haloperidol-induced catalepsy and morphine-induced catatonia (De Deurwaerdère et al. 2002; Ward and Dorsa 1999). Thus, although various striatal 5-HT receptors are able to affect behaviors related to striatal DA dysfunction such as dyskinesia, catalepsy, stereotypies, locomotor activity, or even addiction, the picture is still unclear (Di Matteo et al. 2008; Soubrié et al. 1984). In light of the biochemical and anatomical data, it is becoming evident that each 5-HT receptor interacts in a specific manner in the striatum, either by acting indirectly on striatal DA function or even independently from DA function, as suggested by the available literature concerning striatal 5-HT6 receptors (Pouzet et al. 2002; Svenningsson et al. 2002). It is likely that, functionally, each 5-HT receptor will affect a specific component of behaviors associated with the striatum. The availability of selective pharmacological agents will permit the determination of the role of the various 5-HT receptors in the control of striatal neuron activity and function.
Overall, the studies available to date represent a major endeavor to better understand the various modalities of control exerted by the numerous 5-HT receptors on DA neuron activity within the striatum and provide critical data to envision 5-HT drugs as potential therapeutic tools to improve treatments of numerous disorders associated with chronic alteration of DA transmission, such as Parkinson’s disease, schizophrenia, and addiction.
References
Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M (1995) Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology 34:1635–1645
Adam Y, Edwards RH, Schuldiner S (2008) Expression and function of the rat vesicular monoamine transporter 2. Am J Physiol Cell Physiol 294:C1004–C1008
Alex KD, Pehek EA (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113:296–320
Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA (2005) Modulation of dopamine release by striatal 5-HT2C receptors. Synapse 55:242–251
Andersson JL, Nomikos GG, Marcus M, Hertel P, Mathé JM, Svensson TH (1995) Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectively in the mesolimbic dopaminergic system. Naunyn-Schmiedeberg’s Arch Pharmacol 352:374–385
Andrews CM, Lucki I (2001) Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology (Berl) 155:221–229
Andrews DW, Patrick RL, Barchas J (1978) The effects of 5-hydroxytryptophan and 5-hydroxytryptamine on dopamine synthesis and release in rat brain striatal synaptosomes. J Neurochem 30:465–470
Antonelli T, Fuxe K, Tomasini MC, Bartoszyk GD, Seyfried CA, Tanganelli S, Ferraro L (2005) Effects of sarizotan on the corticostriatal glutamate pathways. Synapse 58:193–199
Awazi N, Guldberg HC (1978) On the interaction of 5-hydroxytryptophan and 5-hydroxytryptamine with dopamine metabolism in the rat striatum. Naunyn-Schmiedeberg’s Arch Pharmacol 303:63–72
Azmitia EC, Segal M (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179:641–668
Bachy A, Héaulme M, Giudice A, Michaud JC, Lefevre IA, Souilhac J, Manara L, Emerit MB, Gozlan H, Hamon M (1993) SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eur J Pharmacol 237:299–309
Balcioglu A, Wurtman RJ (1998) Dexfenfluramine enhances striatal dopamine release in conscious rats via a serotoninergic mechanism. J Pharmacol Exp Ther 284:991–997
Barnes JM, Barnes NM, Costall B, Ironside JW, Naylor RJ (1989) Identification and characterisation of 5-hydroxytryptamine 3 recognition sites in human brain tissue. J Neurochem 53:1787–1793
Barnes JM, Barnes NM, Champaneria S, Costall B, Naylor RJ (1990) Characterization and autoradiographic localisation of 5-HT3 receptor recognition sites identified with [3H]-(S)-zacopride in the forebrain of the rat. Neuropharmacology 29:1037–1045
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38:1083–1152
Barnes NM, Hales TG, Lummis SC, Peters JA (2009) The 5-HT3 receptor—the relationship between structure and function. Neuropharmacology 56:273–284
Baxter G, Kennett G, Blaney F, Blackburn T (1995) 5-HT2 receptor subtypes: a family re-united? Trends Pharmacol Sci 16:105–110
Beart PM, McDonald D (1982) 5-Hydroxytryptamine and 5-hydroxytryptaminergic-dopaminergic interactions in the ventral tegmental area of rat brain. J Pharm Pharmacol 34:591–593
Bender E, Pindon A, van Oers I, Zhang YB, Gommeren W, Verhasselt P, Jurzak M, Leysen J, Luyten W (2000) Structure of the human serotonin 5-HT4 receptor gene and cloning of a novel 5-HT4 splice variant. J Neurochem 74:478–489
Benloucif S, Galloway MP (1991) Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur J Pharmacol 200:1–8
Benloucif S, Keegan MJ, Galloway MP (1993) Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J Pharmacol Exp Ther 265:373–377
Bentué-Ferrer D, Reymann JM, Rousselle JC, Massot O, Bourin M, Allain H, Fillion G (1998) 5-HT-moduline, a 5-HT(1B/1D) receptor endogenous modulator, interacts with dopamine release measured in vivo by microdialysis. Eur J Pharmacol 358:129–137
Benuck M, Reith MEA (1992) Dopamine releasing effect of phenylbiguanide in rat striatal slices. Naunyn-Schmiedeberg’s Arch Pharmacol 345:666–672
Benwell ME, Balfour DJ, Lucchi HM (1993) Influence of tetrodotoxin and calcium on changes in extracellular dopamine levels evoked by systemic nicotine. Psychopharmacology (Berl) 112:467–474
Berge OG, Chacho D, Hole K (1983) Inhibitory effect of 5-methoxy-N,N-dimethyltryptamine on the synaptosomal uptake of 5-hydroxytryptamine. Eur J Pharmacol 90:293–296
Berger UV, Gu XF, Azmitia EC (1992) The substituted amphetamines 3,4-methylenedioxymethamphetamine, methamphetamine, p-chloroamphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. Eur J Pharmacol 215:153–160
Besson MJ, Chéramy A, Feltz P, Glowinski J (1969) Release of the newly synthesized dopamine from dopamine-containing terminals in the striatum of the rat. Proc Natl Acad Sci USA 62:741–748
Blandina P, Goldfarb J, Craddock-Royal B, Green JP (1989) Release of endogenous dopamine by stimulation of 5-HT3 receptors in rat striatum. J Pharmacol Exp Ther 251:803–809
Blondel O, Gastineau M, Dahmoune Y, Langlois M, Fischmeister RJ (1998) Cloning, expression, and pharmacology of four human 5-hydroxytryptamine 4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J Neurochem 70:2252–2261
Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M (1976) The raphe nuclei of the cat brain-stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res 113:449–486
Bockaert J, Sebben M, Dumuis A (1990) Pharmacological characterization of 5-HT4 receptors positively coupled to adenylate cyclase in adult guinea pig hippocampal membranes: effect of substituted benzamide derivatives. Mol Pharmacol 37:408–411
Bonaventure P, Voorn P, Luyten WH, Jurzak M, Schotte A, Leysen JE (1998) Detailed mapping of serotonin 5-HT1B and 5-HT1D receptor messenger RNA and ligand binding sites in guinea-pig brain and trigeminal ganglion: clues for function. Neuroscience 82:469–484
Bonaventure P, Hall H, Gommeren W, Cras P, Langlois X, Jurzak M, Leysen JE (2000) Mapping of serotonin 5-HT4 receptor mRNA and ligand binding sites in the post-mortem human brain. Synapse 36:35–46
Bonhomme N, De Deurwaerdère P, Le Moal M, Spampinato U (1995) Evidence for 5-HT4 receptor subtype involvement in the enhancement of striatal dopamine release induced by serotonin: a microdialysis study in the halothane-anesthetized rat. Neuropharmacology 34:269–279
Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A (2007) Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32:1840–1854
Boschert U, Amara DA, Segu L, Hen R (1994) The mouse 5-hydroxytryptamine 1B receptor is localized predominantly on axon terminals. Neuroscience 58:167–182
Bourson A, Boess FG, Bös M, Sleight AJ (1998) Involvement of 5-HT6 receptors in nigro-striatal function in rodents. Br J Pharmacol 125:1562–1566
Bowyer JF, Masserano JM, Weiner N (1987) Inhibitory effects of amphetamine on potassium-stimulated release of [3H]dopamine from striatal slices and synaptosomes. J Pharmacol Exp Ther 240:177–186
Bruinvels AT, Palacios JM, Hoyer D (1993) 5-Hydroxytryptamine1 recognition sites in rat brain: heterogeneity of non-5-hydroxytryptamine1A/1C binding sites revealed by quantitative receptor autoradiography. Neuroscience 53:465–473
Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM (1994) Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 33:367–386
Bubar MJ, Cunningham KA (2007) Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience 146:286–297
Bubser M, Backstrom JR, Sanders-Bush E, Roth BL, Deutch AY (2001) Distribution of serotonin 5-HT2A receptors in afferents of the rat striatum. Synapse 39:297–304
Bunin MA, Wightman RM (1998) Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci 18:4854–4860
Bunney EB, Appel SB, Brodie MS (2000) Cocaine potentiates ethanol-induced excitation of dopaminergic reward neurons in the ventral tegmental area. J Pharmacol Exp Ther 293:383–389
Burnet PWJ, Eastwood SL, Lacey K, Harrison PJ (1995) The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res 676:157–168
Butcher LL, Engel J, Fuxe K (1972) Behavioral, biochemical, and histochemical analyses of the central effects of monoamine precursors after peripheral decarboxylase inhibition. Brain Res 41:387–411
Carboni E, Acquas E, Frau R, Di Chiara G (1989) Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur J Pharmacol 164:515–519
Carta M, Carlsson T, Kirik D, Bjorklund A (2007) Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Cheramy A, Leviel V, Glowinski J (1981) Dendritic release of dopamine in the substantia nigra. Nature 289:537–542
Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC (2000) Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39:123–132
Compan V, Daszuta A, Salin P, Sebben M, Bockaert J, Dumuis A (1996) Lesion study of the distribution of serotonin 5-HT4 receptors in rat basal ganglia and hippocampus. Eur J Neurosci 8:2591–2598
Compan V, Segu L, Buhot MC, Daszuta A (1998) Selective increases in serotonin 5-HT1B/1D and 5-HT2A/2C binding sites in adult rat basal ganglia following lesions of serotonergic neurons. Brain Res 793:103–111
Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L (1999) Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409:187–209
D’Amato RJ, Largent BL, Snowman AM, Snyder SH (1987) Selective labeling of serotonin uptake sites in rat brain by [3H]citalopram contrasted to labeling of multiple sites by [3H]imipramine. J Pharmacol Exp Ther 242:364–371
Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF (1999) The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397:359–363
De Belleroche JS, Bradford HF (1980) Presynaptic control of the synthesis and release of dopamine from striatal synaptosomes: a comparison between the effects of 5-hydroxytryptamine, acetylcholine and glutamate. J Neurochem 35:1227–1234
De Deurwaerdère P, Bonhomme N, Le Moal M, Spampinato U (1995) d-Fenfluramine enhances striatal dopamine release in vivo by a mechanism independent of serotonergic nerve endings and dopaminergic uptake sites. J Neurochem 65:1100–1108
De Deurwaerdère P, Bonhomme N, Lucas G, Le Moal M, Spampinato U (1996) Serotonin enhances striatal dopamine outflow in vivo through dopamine uptake sites. J Neurochem 66:210–215
De Deurwaerdère P, L’hirondel M, Bonhomme N, Lucas G, Chéramy A, Spampinato U (1997) Serotonin stimulation of 5-HT4 receptors indirectly enhances dopamine release in the rat striatum. J Neurochem 68:195–203
De Deurwaerdère P, Stinus L, Spampinato U (1998) Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: role of 5-HT3 receptors. J Neurosci 18:6528–6538
De Deurwaerdère P, Cervo L, Stinus L, Spampinato U (2002) Central 5-HT4 receptors and dopamine-dependent motor behaviors: searching for a functional role. Pharmacol Biochem Behav 71:627–633
De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U (2004) Constitutive activity of serotonin2C receptor inhibits in vivo dopamine release in the rat nucleus accumbens and striatum. J Neurosci 24:3235–3241
De Deurwaerdère P, Moison D, Navailles S, Porras G, Spampinato U (2005) Regionally and functionally distinct serotonin3 receptors control in vivo dopamine outflow in the rat nucleus accumbens. J Neurochem 94:140–149
De Deurwaerdère P, Le Moine C, Chesselet MF (2010) Selective blockade of serotonin2C receptor enhances Fos expression specifically in the striatum and the subthalamic nucleus within the basal ganglia. Neurosci Lett 469:251–255
De Groote L, Olivier B, Westenberg HG (2003) Role of 5-HT1B receptors in the regulation of extracellular serotonin and dopamine in the dorsal striatum of mice. Eur J Pharmacol 476:71–77
de Langen CD, Stoof JC, Mulder AH (1979) Studies on the nature of the releasable pool of dopamine in synaptosomes from rat corpus striatum: depolarization-induced release of 3H-dopamine from superfused synaptosomes labelled under various conditions. Naunyn-Schmiedeberg’s Arch Pharmacol 308:41–49
De Simoni MG, Dal Toso G, Froditto F, Sokola A, Algeri S (1987) Modulation of striatal metabolism by the activity of dorsal raphe serotonergic afferences. Brain Res 411:81–88
Descarries L, Mechawar N (2000) Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog Brain Res 125:27–47
Descarries L, Seguela P, Watkins KC (1991) Nonjunctional relationships of monoamine axon terminals in the cerebral cortex of adult rat. In: Fuxe K, Agnati LF (eds) Volume transmission in the brain: novel mechanisms for neural transmission. Raven, New York, pp 53–62
Deutch AY, Moghaddam B, Innis RB, Krystal JH, Aghajanian GK, Bunney BS, Charney DS (1991) Mechanisms of action of atypical antipsychotic drugs: implications for novel therapeutic strategies for schizophrenia. Schizophr Res 4:21–156
Dewar KM, Reader TA, Grondin L, Descarries L (1991) [3H]paroxetine binding and serotonin content of rat and rabbit cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region. Synapse 9:14–26
Dewey SL, Smith GS, Logan J, Alexof D, Ding Y, King P, Pappas N, Brodie JD, Ashby CR (1995) Serotonergic modulation of striatal dopamine measured with positon emission tomography (PET) and in vivo microdialysis. J Neurosci 15:821–829
Di Chiara G (1990) In vivo brain dialysis of neurotransmitters. Trends Pharmacol Sci 11:116–121
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278
Di Chiara G, Tanda GL, Frau R, Carboni E (1992) Heterologous monoamine reuptake: lack of transmitter specificity of neuron-specific carriers. Neurochem Int 20:231S–235S
Di Giovanni G, De Deurwaerdère P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U (1999) Selective blockade of serotonin2B/2C receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience 91:587–597
Di Giovanni G, Di Matteo V, Pierucci M, Esposito E (2008) Serotonin–dopamine interaction: electrophysiological evidence. Prog Brain Res 172:45–71
Di Matteo V, Pierucci M, Esposito E (2004) Selective stimulation of serotonin2c receptors blocks the enhancement of striatal and accumbal dopamine release induced by nicotine administration. J Neurochem 89:418–429
Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G (2008) Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson’s disease and other motor disorders. Prog Brain Res 172:423–463
Doménech T, Beleta J, Palacios JM (1997) Characterization of human serotonin 1D and 1B receptors using [3H]-GR-125743, a novel radiolabelled serotonin 5HT1D/1B receptor antagonist. Naunyn-Schmiedeberg’s Arch Pharmacol 356:328–334
Doucet E, Miquel MC, Nosjean A, Vergé D, Hamon M, Emerit MB (2000) Immunolabeling of the rat central nervous system with antibodies partially selective of the short form of the 5-HT3 receptor. Neuroscience 95:881–892
Dremencov E, Newman ME, Kinor N, Blatman-Jan G, Schindler CJ, Overstreet DH, Yadid G (2005) Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology 48:34–42
Dugast C, Suaud-Chagny MF, Gonon F (1994) Continuous in vivo monitoring of evoked dopamine release in the rat nucleus accumbens by amperometry. Neuroscience 62:647–654
Dumuis A, Bouhehal R, Sebben M, Cory R, Bockaert J (1988) A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol Pharmacol 34:880–887
Dumuis A, Sebben M, Bockaert BRL (1989) BRL 24924: a potent agonist at a non-classical 5-HT receptor positively coupled with adenylate cyclase in colliculi neurons. J Eur Pharmacol 162:381–384
Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KC (1997) Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience 76:323–329
Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF (1997) Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol 384:233–247
Egerton A, Ahmad R, Hirani E, Grasby PM (2008) Modulation of striatal dopamine release by 5-HT2A and 5-HT2C receptor antagonists: [11C]raclopride PET studies in the rat. Psychopharmacology (Berl) 200:487–496
El Ayadi A, Afailal I, Errami M (2001) Effects of voltage-sensitive calcium channel blockers on extracellular dopamine levels in rat striatum. Metab Brain Dis 16:121–131
Engel G, Göthert M, Hoyer D, Schlicker E, Hillenbrand K (1986) Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn-Schmiedeberg’s Arch Pharmacol 332:1–7
Ennis C, Kemp JD, Cox B (1981) Characterization of inhibitory 5-hydroxytryptamine receptors that modulate dopamine release in the striatum. J Neurochem 36:1515–1520
Erlander MG, Lovenberg TW, Baron BM, de Lecea L, Danielson PE, Racke M, Slone AL, Siegel BW, Foye PE, Cannon K, Burns JE, Sutcliffe JG (1993) Two members of a distinct subfamily of 5-hydroxytryptamine receptors differentially expressed in rat brain. Proc Natl Acad Sci USA 90:3452–3456
Ferré S, Cortés R, Artigas F (1994) Dopaminergic regulation of the serotonergic raphe-striatal pathway: microdialysis studies in freely moving rats. J Neurosci 14:4839–4846
Feuerstein TJ, Hertting G, Lupp A, Neufang B (1986) False labelling of dopaminergic terminals in the rabbit caudate nucleus: uptake and release of [3H]-5-hydroxytryptamine. Br J Pharmacol 88:677–684
Fink KB, Göthert M (2007) 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev 59:360–417
Fox SH, Brotchie JM (2000) 5-HT2C receptor binding is increased in the substantia nigra pars reticulata in Parkinson’s disease. Mov Disord 15:1064–1069
Frankel PS, Harlan RE, Garcia MM (1998) The 5-HT3 receptor antagonist, MDL 72222, dose dependently potentiates morphine-induced immediate-early gene expression in the caudate putamen. Brain Res 814:186–193
Frechilla D, Cobreros A, Saldise L, Moratalla R, Insausti R, Luquin M, Del Río J (2001) Serotonin 5-HT1A receptor expression is selectively enhanced in the striosomal compartment of chronic parkinsonian monkeys. Synapse 39:288–296
Fujita S, Kiguchi M, Lee J, Terakado M, Suga K, Hatanaka H, Koshikawa N (2008) 5-HT1A and 5-HT1B receptors in the ventrolateral striatum differentially modulate apomorphine-induced jaw movements in rats. J Oral Sci 50:387–395
Gallager DW, Sanders-Bush E, Aghajanian GK, Sulser F (1975) An evaluation of the use of intraventricularly administered [3H]-5-hydroxytryptamine as a marker for endogenous brain 5-hydroxytryptamine. Brain Res 93:111–122
Galloway MP, Suchowski C, Keegan MJ, Hjorth S (1993) Local infusion of the selective 5-HT1B agonist CP-93,129 facilitates striatal dopamine release in vivo. Synapse 15:90–92
Ge J, Barnes NM (1996) 5-HT4 receptor mediated modulation of 5-HT release in the rat hippocampus in vivo. Brain J Pharmacol 117:1475–1480
Gerald C, Adham N, Kao H-T, Olsen MA, Laz TM, Schechter LE, Bard JA, Vaysse PJ-J, Hartig PR, Branchek TA, Weinshank LW (1995) The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO J 14:2806–2815
Gérard C, el Mestikawy SE, Lebrand C, Adrien J, Ruat M, Traiffort E, Hamon M, Martres M-P (1996) Quantitative RT-PCR distribution of serotonin 5-HT6 receptor mRNA in the central nervous system of control or 5,7-dihydroxytryptamine-treated rats. Synapse 23:164–173
Gérard C, Martres MP, Lefèvre K, Miquel MC, Vergé D, Lanfumey L, Doucet E, Hamon M, el Mestikawy SE (1997) Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res 746:207–219
Gerfen CR (1984) The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311:461–464
Gerfen CR (1985) The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol 236:454–476
Gerfen CR (1987) The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci 7:3915–3934
Giambalvo CT, Snodgrass SR (1978) Biochemical and behavioral effects of serotonin neurotoxins on the nigrostriatal dopamine system: comparison of injection sites. Brain Res 152:555-566
Gozlan H, El Mestikawi S, Pichat L, Glowinski J, Hamon M (1983) Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature (Lond) 305:40–142
Graybiel AM (1991) Basal ganglia—input, neural activity, and relation to the cortex. Curr Opin Neurobiol 1:644–651
Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 15:638–644
Grossman CJ, Kilpatrick GJ, Bunce KT (1993) Development of a radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br J Pharmacol 109:618–624
Gudelsky GA, Yamamoto BK (2008) Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav 90:198–207
Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikstrom H, Sedvall G (1997) Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res 745:96–108
Hållbus M, Magnusson T, Magnusson O (1997) Influence of 5-HT1B/1D receptors on dopamine release in the guinea pig nucleus accumbens: a microdialysis study. Neurosci Lett 225:57–60
Hamon M, Lanfumey L, el Mestikawy S, Boni C, Miquel MC, Bolaños F, Schechter L, Gozlan H (1990) The main features of central 5-HT1 receptors. Neuropsychopharmacology 3:349–360
Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Verge D (1999) Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology 21:68S–76S
Heidmann DEA, Metcalf MA, Kohen R, Hamblin MW (1997) Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rats produced by alternative splicing: species differences due to altered intron–exon organization. J Neurochem 68:1372–1381
Hen R (1992) Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci 13:160–165
Herdon H, Strupish J, Nahorski SR (1985) Differences between the release of radiolabelled and endogenous dopamine from superfused rat brain slices: effect of depolarizing stimuli, amphetamine and synthesis inhibition. Brain Res 348:309–320
Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA (1994) VII. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev 46:157–203
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Hrdina PD, Foy B, Hepner A, Summers RJ (1990) Antidepressant binding sites in brain: autoradiographic comparison of [3H]paroxetine and [3H]imipramine localization and relationship to serotonin transporter. J Pharmacol Exp Ther 252:410–418
Ichikawa J, Meltzer HY (1995) Effect of antidepressants on striatal and accumbens extracellular dopamine levels. Eur J Pharmaco 281:255–261
Imperato A, Angelucci L (1989) 5-HT3 receptors control dopamine release in the nucleus accumbens of freely-moving rats. Neurosci Lett 101:214–217
Iyer RN, Bradberry CW (1996) Serotonin-mediated increase in prefrontal cortex dopamine release: pharmacological characterization. J Pharmacol Exp Ther 277:40–47
Izenwasser S, Werling LL, Rosenberger JG, Cox BM (1990) Characterization of binding of [3H]GBR 12935 (1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-piperazine) to membranes and to solubilized membrane extracts from terminal field regions of mesolimbic, mesocortical and nigrostriatal dopamine pathways. Neuropharmacology 29:1017–1024
Jackson BP, Wightman RM (1995) Dynamics of 5-hydroxytryptamine released from dopamine neurons in the caudate putamen of the rat. Brain Res 674:163–166
Jacocks HM, Cox BM (1992) Serotonin-stimulated release of (3H)-dopamine via reversal of the dopamine transporter in rat striatum and nucleus accumbens: a comparison with release elicited by potassium, N-methyl-d-aspartic acid, glutamic acid and d-amphetamine. J Pharmacol Exp Ther 262:356–364
Jakeman LB, To ZP, Eglen RM, Wong EHF, Bonhaus DW (1994) Quantitative autoradiography of 5-HT4 receptors in brains of three species using two structurally distinct radioligands, [3H]GR113808 and [3H]BIMU-1. Neuropharmacology 33:1027–1038
Jenner P, Sheehy M, Marsden CD (1983) Noradrenaline and 5-hydroxytryptamine modulation of brain dopamine function: implications for the treatment of Parkinson’s disease. Br J Clin Pharmacol 15:277S–289S
Johnson EA, Tsai CE, Shahan YH, Azzaro AJ (1993) Serotonin 5-HT1A receptors mediate inhibition of tyrosine hydroxylation in rat striatum. J Pharmacol Exp Ther 266:133–141
Johnson EA, Fox JL, Azzaro AJ (1996) The anxiolytic serotonin 5-HT1A receptor agonists buspirone, ipsapirone and gepirone are inhibitors of tyrosine hydroxylation in rat striatum. Behav Brain Res 73:331–335
Jones SR, Gainetdinov RR, Wightman RM, Caron MG (1998) Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci 18:1979–1986
Kalén P, Strecker RE, Rosengren E, Björklund A (1988) Endogenous release of neuronal serotonin and 5-hydroxyindoleacetic acid in the caudate-putamen of the rat as revealed by intracerebral dialysis coupled to high-performance liquid chromatography with fluorimetric detection. J Neurochem 51:1422–1435
Kamal LA, Arbilla S, Galzin AM, Langer SZ (1983) Amphetamine inhibits the electrically evoked release of [3H]dopamine from slices of the rabbit caudate. J Pharmacol Exp Ther 227:446–458
Kelly E, Jenner P, Marsden CD (1985) Evidence that [3H]dopamine is taken up and released from nondopaminergic nerve terminals in the rat substantia nigra in vitro. J Neurochem 45:137–144
Kidd EJ, Laporte AM, Langlois X, Fattaccini C-M, Doyen C, Lombard MC, Gozlan H, Hamon M (1993) 5-HT3 receptors in the rat central nervous system are mainly located on nerve fibres and terminals. Brain Res 612:289–298
Kilpatrick G, Jones BJ, Tyers MB (1989) Binding of the 5-HT3 ligand, 3H GR 65630, to rat area postrema, vagus nerve and the brains of several species. Eur J Pharmacol 159:157–164
Kirby LG, Kreiss DS, Singh A, Lucki I (1995) Effect of destruction of serotonin neurons on basal and fenfluramine-induced serotonin release in striatum. Synapse 20:99–105
Koch S, Galloway MP (1997) MDMA induced dopamine release in vivo: role of endogenous serotonin. J Neural Transm 104:135–146
Kreiss DF, Wieland S, Lucki I (1993) The presence of a serotonin uptake inhibitor alters pharmacological manipulations of serotonin release. Neuroscience 52:295–301
Kuhr WG, Ewing AG, Near JA, Wightman RM (1985) Amphetamine attenuates the stimulated release of dopamine in vivo. J Pharmacol Exp Ther 232:388–394
Laporte AM, Lima L, Gozlan H, Hamon M (1994) Selective in vivo labelling of brain 5-HT1A receptors by [3H]WAY 100635 in the mouse. Eur J Pharmacol 271:505–514
Laprade N, Radja F, Reader TA, Soghomonian JJ (1996) Dopamine receptor agonists regulate levels of the serotonin 5-HT2A receptor and its mRNA in a subpopulation of rat striatal neurons. J Neurosci 16:3727–3736
Levi G, Raiteri M (1993) Carrier-mediated release of neurotransmitters. Trends Neurosci 16:415–419
Leysen JE, Eens A, Gommeren W, van Gompel P, Wynants J (1988) Identification of nonserotonergic [3H]ketanserin binding sites associated with nerve terminals in rat brain and with platelets; relation with release of biogenic amine metabolites induced by ketanserin- and tetrabenazine-like drugs. J Pharmacol Exp Ther 244:310–321
Lindvall O, Björklund A (1978) Anatomy of the dopaminergic neuron systems in the rat brain. Adv Biochem Psychopharmacol 19:1–23
López-Giménez JF, Vilaró MT, Palacios JM, Mengod G (1998) [3H]MDL 100,907 labels 5-HT2A serotonin receptors selectively in primate brain. Neuropharmacology 37:1147–1158
Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT (1999) Human striosomes are enriched in 5-HT2A receptors: autoradiographical visualization with [3H]MDL 100,907, [125I](±)DOI and [3H]ketanserin. Eur J Neurosci 11:3761–3765
López-Giménez JF, Vilaró MT, Palacios JM, Mengod G (2001) Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL 100,907 autoradiography and in situ hybridization studies. J Comp Neurol 429:571–589
Loric S, Launay JM, Colas JF, Maroteaux L (1992) New mouse 5-HT2-like receptor. Expression in brain, heart and intestine. FEBS Lett 312:203–207
Lovenberg TW, Erlander MG, Baron BM, Racke M, Slone AL, Siegel BW, Craft CM, Burns JE, Danielson PE, Sutcliffe JG (1993) Molecular cloning and functional expression of 5-HT1E-like rat and human 5-hydroxytryptamine receptor genes. Proc Natl Acad Sci USA 90:2184–2188
Lucas G, Spampinato U (2000) Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J Neurochem 74:693–701
Lucas G, Bonhomme N, De Deurwaerdère P, Le Moal M, Spampinato U (1997) 8-OH-DPAT, a 5-HT1A agonist and ritanserin, a 5-HT2A/C antagonist, reverse haloperidol-induced catalepsy in rats independently of striatal dopamine release. Psychopharmacology 131:57–63
Lucas G, De Deurwaerdère P, Porras G, Spampinato U (2000a) Endogenous serotonin enhances the release of dopamine in the striatum only when nigro-striatal dopaminergic transmission is activated. Neuropharmacology 39:1984–1995
Lucas G, De Deurwaerdère P, Caccia S, Spampinato U (2000b) The effect of serotonergic agents on haloperidol-induced striatal dopamine release in vivo: opposite role of 5-HT2A and 5-HT2C receptor subtypes and significance of the haloperidol dose used. Neuropharmacology 39:1053–1063
Lucas G, Di Matteo V, De Deurwaerdère P, Porras G, Martin-Ruiz R, Artigas F, Esposito E, Spampinato U (2001) Neurochemical and electrophysiological evidence that 5-HT4 receptors exert a state-dependent facilitatory control in vivo on nigrostriatal, but not mesoaccumbal dopaminergic function. Eur J Neurosci 13:889–898
Lupp A, Bär KI, Lücking CH, Feuerstein TJ (1992) Different effects of serotonin (5-HT) uptake blockers in caudate nucleus and hippocampus of the rabbit: role of monoamine oxidase in dopaminergic terminals. Psychopharmacology (Berl) 106:118–126
Luthman J, Friedemann MN, Hoffer BJ, Gerhardt GA (1993) In vivo electrochemical measurements of exogenous dopamine clearance in normal and neonatal 6-hydroxydopamine-treated rat striatum. Exp Neurol 122:273–282
Molineaux SM, Jessell TM, Axel R, Julius D (1989) 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA 86:6793–6797
Malgouris C, Flamand F, Doble A (1993) Autoradiographic studies of RP 62203, a potent 5-HT2 receptor antagonist. Pharmacological characterization of [3H]RP 62203 binding in the rat brain. Eur J Pharmacol 233:37–45
Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D (1991) Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated ion channel. Science 254:432–437
Martín-Cora FJ, Pazos A (2004) Autoradiographic distribution of 5-HT7 receptors in the human brain using [3H]mesulergine: comparison to other mammalian species. Br J Pharmacol 141:92–104
Melamed E, Zoldan J, Friedberg G, Ziv I, Weizmann A (1996) Involvement of serotonin in clinical features of Parkinson’s disease and complications of l-DOPA therapy. Adv Neurol 69:545–550
Meltzer HY (1999) Treatment of schizophrenia and spectrum disorders: pharmacotherapy, psychosocial treatments, and neurotransmitter interactions. Biol Psychiatry 46:1321–1327
Meltzer HY, Nash JF (1991) Effects of antipsychotic drugs on serotonin receptors. Pharmacol Rev 43:587–604
Mengod G, Nguyen H, Le H, Waeber C, Lubbert H, Palacios JM (1990) The distribution and cellular localisation of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization hystochemistry. Comparison with receptor binding distribution. Neuroscience 35:577–591
Mengod G, Vilaró MT, Raurich A, López-Giménez JF, Cortés R, Palacios JM (1996) 5-HT receptors in mammalian brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochem J 28:747–758
Mereu G, Fanni B, Gessa GL (1984) General anesthetics prevent dopaminergic neuron stimulation by neuroleptics. In: Usdin E, Carlsson A, Dahlstrom A, Engel J (eds) Catecholamines: neuropharmacology and central nervous system—theoretical aspects. Liss, New York, pp 353–358
Mignon L, Wolf WA (2007) Postsynaptic 5-HT1A receptor stimulation increases motor activity in the 6-hydroxydopamine-lesioned rat: implications for treating Parkinson’s disease. Psychopharmacology (Berl) 192:49–59
Mikics E, Vas J, Aliczki M, Halasz J, Haller J (2009) Interactions between the anxiogenic effects of CB1 gene disruption and 5-HT3 neurotransmission. Behav Pharmacol 20:265–272
Millan MJ (2006) Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 110:135–370
Millan MJ, Brocco M, Gobert A, Joly F, Bervoets K, Rivet JM, Newman-Tancredi A, Audinot V, Maurel S (1999) Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci 11:4419–4432
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425
Miyake A, Mochizuki S, Takemoto Y, Akuzawa S (1995) Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol 48:407–416
Moison D, De Deurwaerdère P, Cagnotto A, Marrazzo A, Prezzavento O, Ronsisvalle G, Mennini T, Spampinato U (2003) Intrastriatal administration of sigma ligands inhibits striatal dopamine release in vivo. Neuropharmacology 45:945–953
Monsma FJ Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR (1993) Cloning and expression of a novel serotonin receptor whith high affinity for tricyclic psychotropic drugs. Mol Pharmacol 43:320–327
Morales M, Bloom FE (1997) The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci 17:3157–3167
Morales M, Wang S-D (2002) Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci 22:6732–6741
Morales M, Battenberg E, De Lecea L, Sanna PP, Bloom FE (1996) Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Mol Brain Res 36:251–260
Morilak DA, Garlow SJ, Ciaranello RD (1993) Immunocytochemical localization and description of neurons expressing serotonin2 receptors in the rat brain. Neuroscience 54:701–717
Moukhles H, Bosler O, Bolam JP, Vallée A, Umbriaco D, Geffard M, Doucet G (1997) Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 76:1159–1171
Muneoka KT, Takigawa M (2003) 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int J Dev Neurosci 21:133–143
Muramatsu M, Tamaki-Ohashi J, Usuki C, Araki H, Chaki S, Aihara H (1988) 5-HT2 antagonists and minaprine block the 5-HT-induced inhibition of dopamine release from rat striatal slices. Eur J Pharmacol 153:89–95
Nash F (1990) Ketanserin pretreatment attenuates MDMA-induced dopamine release in the striatum as measured by in vivo microdialysis. Life Sci 47:2401-2408
Nash F, Brodkin J (1991) Microdialysis studies on 3,4-methylenedioxymethamphetamine-induced DA release: effect of DA uptake inhibitors. J Pharmacol Exp Ther 259:820–825
Navailles S, De Deurwaerdère P (2011) The constitutive activity of 5-HT2C receptors as an additional modality of interaction of the serotonergic system. In 5-HT2C Receptors in the Pathophysiology of CNS Disease. Di Giovanni G, Esposito E, Vincenzo M (eds) Series: The Receptors, vol 22, first eds. Springer, Humana Press
Navailles S, De Deurwaerdère P, Porras G, Spampinato U (2004) In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology 29:319–326
Navailles S, Moison D, Ryczko D, Spampinato U (2006) Region-dependent regulation of mesoaccumbens dopamine neurons in vivo by the constitutive activity of central serotonin2C receptors. J Neurochem 99:1311–1319
Navailles S, Bioulac B, Gross C, De Deurwaerdère P (2010) Serotonergic neurons mediate an ectopic release of dopamine in a rat model of Parkinson’s disease. Neurobiol Dis 38:136–143
Narita N, Hashimoto K, Tomitaka S-I, Minabe Y (1996) Interaction of selective serotonin reuptake inhibitors with subtypes of σ receptors in the rat brain. Eur J Pharmacol 307:117–119
Neumaier JF, Sexton TJ, Yracheta J, Diaz AM, Brownfield M (2001) Localization of 5-HT7 receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J Chem Neuroanat 21:63–73
Ng LK, Chase TN, Colburn RW, Kopin IJ (1972) l-DOPA in parkinsonism. A possible mechanism of action. Neurology 22:688–696
Ng NK, Lee HS, Wong PT (1999) Regulation of striatal dopamine release through 5-HT1 and 5-HT2 receptors. J Neurosci Res 55:600–607
Numan S, Lundgren KH, Wright DE, Herman JP, Seroogy KB (1995) Increased expression of 5HT2 receptor mRNA in rat striatum following 6-OHDA lesions of the adult nigrostriatal pathway. Brain Res Mol Brain Res 29:391–396
Nurse B, Russell VA, Taljaard JJF (1988) Characterization of the effects of serotonin on the release of [3H]dopamine from rat nucleus accumbens and striatal slices. Neurochem Res 13:403–407
Oliver KR, Kinsey AM, Wainwright A, Sirinathsinghji DJ (2000) Localization of 5-ht5A receptor-like immunoreactivity in the rat brain. Brain Res 867:131–142
Pact V, Giduz T (1999) Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology 53:1154
Parsons LH, Justice JB (1993) Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res 606:195–199
Pasqualetti M, Nardi I, Ladinsky H, Marazziti D, Cassano GB (1996) Comparative anatomical distribution of serotonin 1A, 1D and 2A receptor mRNAs in human brain postmortem. Mol Brain Res 39:223–233
Pasqualetti M, Ori M, Castagna M, Marazziti D, Cassano GB, Nardi I (1999) Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience 92:601–611
Patel S, Roberts J, Moorman J, Reavill C (1995) Localization of serotonin-4 receptors in the striatonigral pathway in the rat brain. Neuroscience 69:1159–1167
Patterson TA, Schenk JO (1991) Effects of acute and chronic systemic administration of some typical antipsychotic drugs on turnover of dopamine and potassium ion-induced release of dopamine in the striatum of the rat in vivo. Neuropharmacology 30:943–952
Pazos A, Cortés R, Palacios JM (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res 346:231–249
Perry DC (1990) Autoradiography of [3H]quipazine in rodent brain. Eur J Pharmacol 187:75–85
Pitts DK, Marwah J (1988) Cocaine and central monoaminergic neurotransmission: a review of electrophysiological studies and comparison to amphetamine and antidepressants. Life Sci 42:949–968
Pompeiano M, Palacios JM, Mengod G (1992) Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci 12:440–453
Pompeiano M, Palacios JM, Mengod G (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2B receptors. Mol Brain Res 23:163–178
Porras G, Di Matteo V, De Deurwaerdère P, Esposito E, Spampinato U (2002a) Central 5-HT4 receptors selectively regulate the impulse-dependent exocytosis of dopamine in the rat striatum: in vivo studies with morphine amphetamine and cocaine. Neuropharmacology 43:1099–1109
Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdère P, Caccia S, Esposito E, Spampinato U (2002b) 5-HT2A and 5-HT2C/2B receptor subtypes respectively modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology 26:311–324
Porras G, De Deurwaerdère P, Moison D, Spampinato U (2003) Conditional involvement of striatal serotonin3 receptors in the control of in vivo dopamine outflow in the rat striatum. Eur J Neurosci 17:771–781
Pouzet B, Didriksen M, Arnt J (2002) Effects of the 5-HT6 receptor antagonist, SB-271046, in animal models for schizophrenia. Pharmacol Biochem Behav 71:635–643
Pozzi L, Trabace L, Invernizzi R, Samanin R (1995) Intranigral GR 113808, a selective 5-HT4 receptor antagonist, attenuates morphine-stimulated dopamine release in the rat striatum. Brain Res 692:265–268
Quirion R, Richard J (1987) Differential effects of selective lesions of cholinergic and dopaminergic neurons on serotonin-type 1 receptors in rat brain. Synapse 1:124–130
Radja F, Laporte A-M, Daval G, Vergé D, Gozlan H, Hamon M (1991) Autoradiography of serotonin receptor subtypes in the central nervous system. Neurochem Int 18:1–15
Radja F, Descarries L, Dewar KM, Reader TA (1993) Serotonin 5-HT1 and 5-HT2 receptors in adult rat brain after neonatal destruction of nigrostriatal dopamine neurons: a quantitative autoradiographic study. Brain Res 606:273–285
Rees S, den Daas I, Foord S, Goodson S, Bull D, Kilpatrick G, Lee M (1994) Cloning and characterisation of the human 5-HT5A serotonin receptor. FEBS Lett 355:242–246
Reynolds GP, Mason SL, Meldrun A, De Keczer S, Parnes H, Eglen RM, Wong EHF (1995) 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol 114:993–998
Roberts JC, Reavill C, East SZ, Harrison PJ, Patel S, Routledge C, Leslie RA (2002) The distribution of 5-HT6 receptors in rat brain: an autoradiographic binding study using the radiolabelled 5-HT6 receptor antagonist [125I]SB-258585. Brain Res 934:49–57
Rothman RB, Baumann MH (2006) Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann NY Acad Sci 1074:245–260
Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC (1993a) A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun 193:268–276
Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang J-M, Schwartz J-C (1993b) Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci 90:8547–8551
Saavedra JM (1977) Distribution of serotonin and synthesizing enzymes in discrete areas of the brain. Fed Proc 36:2134–2141
Santiago M, Machado A, Cano J (1995) 5-HT3 receptor agonist induced carrier-mediated release of dopamine in rat striatum in vivo. Br J Pharmacol 116:1545–1550
Santiago M, Matarredona ER, Machado A, Cano J (1998) Influence of serotoninergic drugs on in vivo dopamine extracellular output in rat striatum. J Neurosci Res 52:591–598
Sarhan H, Fillion G (1999) Differential sensitivity of 5-HT1B auto and heteroreceptors. Naunyn-Schmiedeberg’s Arch Pharmacol 360:382–390
Sarhan H, Cloëz-Tayarani I, Massot O, Fillion MP, Fillion G (1999) 5-HT1B receptors modulate release of [3H]dopamine from rat striatal synaptosomes. Naunyn-Schmiedeberg’s Arch Pharmacol 359:40–47
Sarhan H, Grimaldi B, Hen R, Fillion G (2000) 5-HT1B receptors modulate release of [3H]dopamine from rat striatal synaptosomes: further evidence using 5-HT moduline, polyclonal 5-HT1B receptor antibodies and 5-HT1B receptor knock-out mice. Naunyn-Schmiedeberg’s Arch Pharmacol 361:12–18
Sari Y, Lefèvre K, Bancila M, Quignon M, Miquel MC, Langlois X, Hamon M, Vergé D (1997) Light and electron microscopic imunocytochemical visualisation of 5-HT1B receptors in the rat brain. Brain Res 760:281–286
Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D (1999) Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88:899–915
Saudou F, Hen R (1994) 5-HT receptor subtypes: molecular and functional diversity. Med Chem Res 4:16–84
Schiavi GB, Brunet S, Rizzi CA, Ladinski H (1994) Identification of serotonin 5-HT4 recognition sites in the porcine caudate nucleus by radioligand binding. Neuropharmacology 33:543–549
Schmidt CJ, Black CK (1989) The putative 5-HT3 agonist phenylbiguanide induces carrier-mediated release of [3H]dopamine. Eur J Pharmacol 167:309–310
Schmidt JC, Fadayel GM, Sullivan CK, Taylor VL (1992) 5-HT2 receptors exert a state dependent regulation of dopaminergic function: studies with MDL 100,907 and the amphetamine analogue, 3,4-methylenedioxymethamphetamine. Eur J Pharmacol 223:65–74
Schmidt CJ, Kehne JH, Carr AA, Fadayel GM, Humphreys TM, Ketteler HJ, McCloskey TC, Padich RA, Taylor VL, Sorensen SM (1993) Contribution of serotonin neurotoxins to understanding psychiatric disorders: the role of 5-HT2 receptors in schizophrenia and antipsychotic activity. Int Clin Psychopharmacol 8:25–32
Schmidt CJ, Sullivan CK, Fadayel GM (1994) Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J Neurochem 62:1382–1389
Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D (2001) Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci 21:5916–5924
Schoeffter P, Waeber C (1994) 5-Hydroxytryptamine receptors with a 5-HT6 receptor-like profile stimulating adenylyl cyclase activity in pig caudate membranes. Naunyn-Schmiedeberg’s Arch Pharmacol 350:356–360
Schwartz D, Hernandez L, Hoebel BG (1989) Fenfluramine administered systemically or locally increases extracellular serotonin in the lateral hypothalamus as measured by microdialysis. Brain Res 482:261–270
Sebben M, Ansanay H, Bockaert J, Dumuis A (1994) 5-HT6 receptors positively coupled to adenylyl cyclase in striatal neurones in culture. NeuroReport 5:2553–2557
Seiden LS, Sabol KE, Ricaurte GA (1993) Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol 33:639–677
Sharp T, Bramwell SR, Clark D, Grahame-Smith DG (1989) In vivo measurement of extracellular 5-hydroxytryptamine in hippocampus of the anaesthetized rat using microdialysis: changes in relation to 5-hydroxytryptaminergic neuronal activity. J Neurochem 53:234–240
Shaskan EG, Snyder SH (1970) Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther 175:404–418
Soghomonian JJ, Doucet G, Descarries L (1987) Serotonin innervation in adult rat neostriatum. I. Quantified regional distribution. Brain Res 425:85–100
Soghomonian JJ, Descarries L, Watkins K (1989) Serotonin innervation in adult rat neostriatum. II. Ultrastructural features: a radioautographic and immunocytochemical study. Brain Res 481:67–86
Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ (1993) Characterization of the 5-HT2 receptor antagonist MDL 100,907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther 266:684–691
Soubrié P (1986) Reconciling the role of central serotonin neurons in human and animal behavior. Behav Brain Sci 9:319–364
Soubrié P, Reisine TD, Glowinski J (1984) Functional aspects of serotonin transmission in the basal ganglia: a review and in vivo approach using the push–pull cannula technique. Neuroscience 13:605–625
Spoont MR (1992) Modulatory role of serotonin in neural information processing: implications for human psychopathology. Psychol Bull 112:330–350
Steinbusch HW (1984) Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Handbook of chemical neuroanatomy, vol 3, Classical transmitters and transmitter receptors in the CNS, part II. Elsevier, Amsterdam, pp 68–125
Steinbusch HW, Nieuwenhuys R, Verhoftad AA, Van der Kooy D (1981) The nucleus raphe dorsalis of the rat and its projections upon the caudo-putamen. A combined cytoarchitectonic, immunocytochemical and retrograde transport study. J Physiol Paris 77:157–174
Steward LJ, Bufton KE, Hopkins PC, Davies WE, Barnes NM (1993) Reduced levels of 5-HT3 receptor recognition sites in the putamen of patients with Huntington’s disease. Eur J Pharmacol 242:137–143
Steward LJ, Ge J, Stowe RL, Brown DC, Bufton RK, Stokes PRA, Barnes NM (1996) Ability of 5-HT4 receptor ligands to modulate rat striatal dopamine release in vitro or in vivo. Br J Pharmacol 117:55–62
Suchowski CS, Galloway MP (1995) Serotonin receptor subtypes involved in the 5-HT facilitation of dopamine release in the striatum: microdialysis study in vivo. 25th Annual Meeting Society for Neurosciences, San Diego, California, abstract #152.9
Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci 15:4102–4108
Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P (2002) DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci USA 99:3188–3193
Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD (1985) Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience 16:223–240
Tao R, Auerbach SB (1994) Increased extracellular serotonin in rat brain after systemic or intraraphe administration of morphine. J Neurochem 63:517–524
Tao R, Auerbach SB (1995) Involvement of the dorsal raphe but not median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neuroscience 68:553–561
Tecott LH, Maricq AV, Julius D (1993) Nervous system distribution of the serotonin 3 receptor RNAm. Proc Natl Acad Sci USA 90:1430–1434
Ternaux JP, Héry F, Bourgoin J, Adrien J, Glowinski J, Hamon M (1977) The topographical distribution of serotonergic terminals in the neostriatum of the rat and the caudate nucleus of the cat. Brain Res 121:311–326
Thomas DR (2006) 5-HT5A receptors as a therapeutic target. Pharmacol Ther 111:707–714
Thomas DR, Nelson DR, Johnson AM (1987) Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology 93:193–200
Thorré K, Ebinger G, Michotte Y (1998) 5-HT4 receptor involvement in the serotonin-enhanced dopamine efflux from the substantia nigra of the freely moving rat: a microdialysis study. Brain Res 796:117–124
To ZP, Bonhaus DW, Eglen RM, Jakeman JB (1995) Characterization and distribution of putative 5-HT7 receptors in guinea pig brain. Br J Pharmacol 115:107–116
Umbriaco D, Garcia S, Beaulieu C, Descarries L (1995) Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus 5:605–620
Van Bockstaele EJ, Pickel VM (1993) Ultrastructural of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol 334:603–617
Varnas K, Hall H, Bonaventure P, Sedvall G (2001) Autoradiographic mapping of 5-HT1B and 5-HT1D receptors in the post mortem human brain using [3H]GR 125743. Brain Res 915:47–57
Varnas K, Thomas DR, Tupala E, Tiihonen J, Hall H (2004) Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci Lett 367:313–316
Varnas K, Hurd YL, Hall H (2005) Regional expression of 5-HT1B receptor mRNA in the human brain. Synapse 56:21–28
Vergé D, Daval G, Marcinkiewicz M, Patey A, El Mestikawy S, Gozlan H, Hamon M (1986) Quantitative autoradiography of multiple 5-HT1 receptors subtypes in the brain of control and 5,7-DHT treated rats. J Neurosci 6:3474–3482
Vilaró MT, Cortes R, Gerald C, Branchek TA, Palacios JM, Mengod G (1996) Localization of 5-HT4 receptor mRNA in rat brain by in situ hybridization histochemistry. Mol Brain Res 43:356–360
Vilaro MT, Cortes R, Mengod G (2005) Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: distribution and effects of neurotoxic lesions. J Comp Neurol 484:418–439
Waeber C, Palacios JM (1989) Serotonin-1 receptor binding sites in the human basal ganglia are decreased in Huntington’s chorea but not in Parkinson’s disease: a quantitative in vitro autoradiography study. Neuroscience 32:337–347
Waeber C, Palacios JM (1994) Binding sites for 5-hydroxytryptamine-2 receptor agonists are predominantly located in striosomes in the human basal ganglia. Mol Brain Res 24:199–209
Waeber C, Moskowitz MA (1995) [3H]sumatriptan labels both 5-HT1D and 5-HT1F receptor binding sites in the guinea pig brain: an autoradiographic study. Naunyn-Schmiedeberg’s Arch Pharmacol 352:263–275
Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A (1994) Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology 33:527–541
Waldmeier PC, Delini-Stula AA (1979) Serotonin–dopamine interactions in the nigrostriatal system. Eur J Pharmacol 55:363–373
Ward RP, Dorsa DM (1996) Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol 370:405–414
Ward RP, Dorsa DM (1999) Molecular and behavioral effects mediated by Gs-coupled adenosine A2a, but not serotonin 5-HT4 or 5-HT6 receptors following antipsychotic administration. Neuroscience 89:927–938
Ward RP, Hamblin MW, Lachowitcz JE, Hoffman BJ, Sibley DR, Dorsa DM (1995) Localization of serotonin subtype 6 receptor messenger RNA in the rat brain by in situ hybridization histochemistry. Neuroscience 64:1105–1111
Wesolowska A (2002) In the search for selective ligands of 5-HT5, 5-HT6 and 5-HT7 serotonin receptors. Pol J Pharmacol 54:327–341
West AR, Galloway MP (1996) Desensitization of 5-hydroxytryptamine-facilitated dopamine release in vivo. Eur J Pharmacol 298:241–245
Westerink BHC, Tuntler J, Damsma G, Rollema H, De Vries JB (1987) The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain microdialysis. Naunyn-Schmiedeberg’s Arch Pharmacol 336:502–507
Westerink BH, Hofsteede HM, Tuntler J, de Vries JB (1989) Use of calcium antagonism for the characterization of drug-evoked dopamine release from the brain of conscious rats determined by microdialysis. J Neurochem 52:722–729
Westfall C, Tittermary V (1982) Inhibition of the electrically induced release of [3H]dopamine by serotonin from superfused rat striatal slices. Neurosci Lett 28:205–209
Westlund KN, Denney RM, Rose RM, Abell CW (1988) Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 25:439–456
Whitaker-Azmitia PM, Clarke C, Azmitia EC (1993) Localization of 5-HT1A receptors to astroglial cells in adult rats: implications for neuronal–glial interactions and psychoactive drug mechanism of action. Synapse 14:201–205
Williams J, Davies JA (1983) The involvement of 5-hydroxytryptamine in the release of dendritic dopamine from slices of rat substantia nigra. J Pharm Pharmacol 35:734–737
Willins DL, Meltzer HY (1998) Serotonin 5-HT2C agonists selectively inhibit morphine-induced dopamine efflux in the nucleus accumbens. Brain Res 781:291–299
Wilkinson A, Courtney M, Westlind-Danielsson A, Hallnemo G, Akerman KE (1994) Alaproclate acts as a potent, reversible and noncompetitive antagonist of the NMDA receptor coupled ion flow. J Pharmacol Exp Ther 271:1314–1319
White NM (1997) Mnemonic functions of the basal ganglia. Curr Opin Neurobiol 7:164–169
Yadid G, Karel P, Kopin IJ, Goldstein DS (1994) Endogenous serotonin stimulates striatal dopamine release in conscious rats. J Pharmacol Exp Ther 270:1158–1165
Yamamoto BK, Nash JF, Gudelsky GA (1995) Modulation of methylenedioxymethamphetamine-induced striatal dopamine release by the interaction between serotonin and gamma-aminobutyric acid in the substantia nigra. J Pharmacol Exp Ther 273:1063–1070
Yeghiayan SK, Kelley AE (1995) Serotonergic stimulation of the ventrolateral striatum induces orofacial stereotypy. Pharmacol Biochem Behav 52:493–501
Yeghiayan SK, Kelley AE, Kula NS, Campbell A, Baldessarini RJ (1997) Role of dopamine in behavioral effects of serotonin microinjected into rat striatum. Pharmacol Biochem Behav 56:251–259
Yi SJ, Gifford AN, Johnson KM (1991) Effect of cocaine and 5-HT3 receptor antagonists on 5-HT induce [3H] dopamine release from rat striatal synaptosomes. Eur J Pharmacol 199:185–189
Youdim MB, Feldman SC, Pappas GD, Pollard HB (1986) Serotonin metabolism and the nature of monoamine oxidase in squid central nervous system. Brain Res 381:300–304
Zangen A, Nakash R, Overstreet DH, Yadid G (2001) Association between depressive behavior and absence of serotonin–dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 155:434–439
Zazpe A, Artaiz I, Del Rio J (1994) Role of 5-HT3 receptors in basal K+-evoked dopamine release from rat olfactory tubercule and striatal slices. Br J Pharmacol 113:968–972
Zifa E, Fillion G (1992) 5-Hydroxytryptamine receptors. Pharmacol Rev 44:401–458
Zhou FC, Lesch KP, Murphy DL (2002) Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res 942:109–119
Zhou FM, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA (2005) Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron 46:65–74
Zoldan J, Friedberg G, Livneh M, Melamed E (1995) Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology 45:1305–1308
Acknowledgments
This work was supported by grants from “Centre National de la Recherche Scientifique” and Bordeaux 2 University. The authors report no biomedical financial interest or potential conflicts of interest. The authors thank Dr. Martin Guthrie for linguistic assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navailles, S., De Deurwaerdère, P. Presynaptic control of serotonin on striatal dopamine function. Psychopharmacology 213, 213–242 (2011). https://doi.org/10.1007/s00213-010-2029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2029-y