Abstract
Rationale
Dopamine and glutamate are thought to interact in the ventral striatum and to play important roles there in the cocaine-seeking of cocaine-experienced animals.
Objectives
We sought to determine the relative roles of the two transmitters in the two major zones of the nucleus accumbens (NAS), the core and shell subregions.
Methods
We assessed the effects of dopamine and glutamate receptor blockade in the core and shell on intravenous cocaine self-administration in rats. Trained animals were allowed to self-administer cocaine for an initial hour, and then D1-type or D2-type dopamine receptor blockers or NMDA-type or AMPA-type glutamate receptor blockers were infused by reverse microdialysis into one of the two regions for an additional 3 h of testing.
Results
The D1-type antagonist SCH23390 and the D2-type antagonist raclopride each increased cocaine intake whereas the AMPA-type antagonist CNQX decreased responding when infused into the core. SCH23390 increased cocaine intake less strongly when infused into the shell, while raclopride and CNQX were each ineffective when infused into the shell. The NMDA-antagonist CPP failed to affect cocaine self-administration when infused into either site.
Conclusions
These findings implicate the core of NAS in the maintenance of established cocaine self-administration in trained animals, despite the fact that the reinforcement of responding in untrained animals appears to results from cocaine actions in the olfactory tubercle and medial shell and not the core of accumbens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rewarding effects of cocaine have been classically linked to dopaminergic actions (de Wit and Wise 1977) in nucleus accumbens (NAS: Pettit et al. 1990; Roberts et al. 1977), the underlying olfactory tubercle (OT: Ikemoto 2003) and other terminal fields (Goeders and Smith 1983; McGregor et al. 1996; McGregor and Roberts 1995) of the mesocorticolimbic dopamine system. Within NAS, the major target of dopaminergic afferents is the medium spiny neurons (MSNs) that also receive various glutamatergic inputs. Dopaminergic and glutamatergic axons synapse on the spines of MSNs often in close apposition (Sesack and Pickel 1990; Totterdell and Smith 1989), and dopamine and glutamate interact to regulate medium spiny output (O'Donnell and Grace 1994; Yim and Mogenson 1986). While dopaminergic input to NAS is thought to mediate reward function, glutamatergic input is thought to reflect contextual, emotional, and other cognitive input essential for goal-directed behavior (Grace 2000).
NAS comprises two subregions, a “core,” strongly immunoreactive for substance P and contiguous with the overlying caudate-putamen, and a “shell,” strongly immunoreactive for calcium binding protein and contiguous with the underlying OT (Voorn et al. 1989). The core and shell have different efferent targets (Heimer et al. 1991), and appear to play different roles in motivation and reward function (e.g., Kelley and Swanson 1997; Bassareo et al. 2007). Evidence from intracranial cocaine self-administration studies suggest that the site at which cocaine has its habit-forming (reinforcing) action is in the medial OT and shell, but not core, of NAS (Carlezon et al. 1995; Ikemoto 2003). Consistent with this evidence, rats with extensive lesions of either NAS core or shell learn normally to self-administer intravenous cocaine when trained on a continuous reinforcement schedule (Ito et al. 2004). On the other hand, evidence from both lesion (Ito et al. 2004) and pharmacological inactivation studies (Di Ciano and Everitt 2001; McFarland and Kalivas 2001; Fuchs et al. 2001; Di Ciano et al. 2008) suggests that the core, not shell, of NAS is important for the maintenance and instigation of cocaine-seeking by cocaine-associated environmental stimuli.
The present study was designed to advance our understanding of the roles of NAS dopamine and glutamate in cocaine self-administration. Specifically, we assessed the effects of local administration of dopamine or glutamate antagonists into the core or shell on regulation of cocaine intake in well-trained animals. In order to establish steady-state antagonism for prolonged periods in the targeted regions, the antagonists were infused continuously by reverse microdialysis. Reverse-dialysis minimizes the efflux up the cannula shaft frequently caused by hydraulic injection (Wise and Hoffman 1992) and avoids the fluctuations in concentration that accompany traditional drug treatments (Zaffaroni 1981). The microdialysis probes were angled across the midline to avoid ventricular penetration and to irrigate the NAS shell and adjacent OT where cocaine is most effective for intracranial cocaine self-administration, or angled through the dorsolateral striatum to irrigate the NAS core, where lesions and pharmacological disruption affect cue-maintained and cue-instigated cocaine-seeking in trained animals.
Materials and methods
Subjects
Eighty-six male Long–Evans rats (Charles-River, Raleigh, NC), weighing 350–400 g at the time of the surgery, were used. They were housed individually under a reverse light–dark cycle with free access to food and water. Animals were always tested during the dark period. The guidelines of the National Institutes of Health (NIH publication 86-23 1996) were followed and all procedures were approved by the local Animal Care and Use Committee.
Drugs
(−)-Cocaine hydrochloride (cocaine) and the surgical anesthetics were obtained from the NIH. R(+)-SCH-23390 (SCH23390: D1 antagonist), S(−)-raclopride(+)-tartrate salt (raclopride: D2 antagonist), 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX: AMPA antagonist) and (±)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP: NMDA antagonist), were obtained from Sigma, Inc (Saint Louis, MO). Cocaine was dissolved in sterile saline (0.9% w/v). All antagonists were dissolved in artificial cerebrospinal fluid (aCSF, composition in mM: NaCl, 148; KCl, 2.7; CaCl2, 1.2; MgCl2, 0.8, pH 7.4). Each drug solution was adjusted to pH 7.4.
Apparatus
Ten operant conditioning chambers (Med Associates, Georgia, VT), each measuring 25 × 27 × 30 cm3, were used for cocaine self-administration. Attenuating noise was supplied in each chamber by a ventilating fan. Each chamber had two levers: presses on a retractable ‘active’ lever activated the infusion pump, while presses on a stationary ‘inactive’ lever did not activate the pump. Each chamber was equipped with a liquid swivel system that allowed free movement of animals, delivery of cocaine injections and collection of microdialysis samples.
Surgery
For all surgical procedures, rats were anesthetized with a mixture of pentobarbital (30 mg/kg, i.p.) and chloral hydrate (140 mg/kg, i.p.). Rats were implanted with bilateral microdialysis guide cannulae (CMA, N. Chelmsford, MA) and an intravenous microranathane catheter (Braintree Science, Braintree, MA). Rats received the bilateral guide cannulae aimed at shell or core. The shell cannulae were inserted from the opposite hemisphere through the midline, and angled at 160° toward the midline to minimize diffusion of drug solution to the core (Fig. 1). In order to avoid collision of the bilateral cannulae into the shell, two different coordinates were used: (1) +1.2 mm anterior, ±1.2 mm lateral to bregma, and −8.4 mm from skull; (2) +1.7 mm anterior, ±1.0 mm lateral to bregma, and −8.0 mm from skull. The two coordinates for the shell were counterbalanced between the left and right hemispheres. For consistency, two different coordinates were also utilized and counterbalanced between the left and right hemisphere of rats in the ‘core’ groups: (1) +1.2 mm anterior, ±1.6 mm lateral to bregma, and −7.8 mm from skull; (2) +1.7 mm anterior, ±1.6 mm lateral to bregma, and −7.4 mm from skull. The core cannulae were angled at 10° toward the midline. All cannulae were positioned at 1.0 mm above the tip of probes. Immediately following the cannulae implantation, rats were surgically implanted with an intravenous catheter which was inserted into the right external jugular vein. The catheters were flushed daily with 0.05 ml Gentocin™ (4 mg/ml in sterile saline) and 0.05 ml heparin solution (30 U/ml in sterile saline). Five rats were excluded from the experiment because their catheters became non-patent or developed leaks.
Probe placements. a Lines indicate the representative locations of the active portion (1.0 mm) of dialysis membrane in the core (solid bars) and the shell (hatched bars). Line drawings were adapted from Paxinos and Watson (1998). The numbers indicate the distance (mm) anterior to the bregma. b Low-power view of a core placement showing the tip of the guide cannula (wide portion of track) and the tip of the probe itself (narrow portion). c Higher magnification of the core placement showing relatively healthy tissue adjacent to the active membrane. d Low power showing the track from a shell placement crossing the midline just ventral to the corpus callosum and avoiding the central sulcus and the lateral ventricle. e Higher magnification of the shell placement. Tissue quality around the probe is best illustrated in c, which was typical of clean sections in both core and shell placements. The section in d and e, while not clean, was selected to show the entire track of the crossed placement in a section that was consequently almost completely bisected by the cannula track. Tissue quality is not well illustrated in this case, as e shows debris at the tip of the track that was a thin layer of tissue just caudal to the probe and shows some folded tissue (dark segments) along the edges of the track, particularly on the lateral edge and bottom of the track
Design and procedure
The present experiment comprised from two phases: cocaine self-administration training (10 days) and testing (3 days). Rats were randomly assigned to one of eight experimental groups defined by site of microdialysis and type of antagonists. These eight groups are followings: core-SCH23390, core-raclopride, core-CNQX, core-CPP, shell-SCH23390, shell-raclopride, shell-CNQX, and shell-CPP groups (N = 8–11 per group). Throughout the entire experiment, each rat was housed, trained and tested in a single operant conditioning chamber.
Cocaine self-administration training
Daily 4-h training for cocaine self-administration began 3 days after the surgeries and lasted for 10 days. At the beginning of each training session, the ‘active’ lever was inserted into operant conditioning chamber and rats received a priming infusion of cocaine (1.0 mg/kg/injection, i.v.). Rats were then allowed to self-administer cocaine (1.0 mg/kg/injection, i.v.) under a fixed ratio 1 (FR1) schedule of reinforcement for 4 h. Reinforced lever presses on active lever delivered cocaine injections (1.0 mg/kg/injection, i.v.). For a 20-s “time-out” period immediately following a reinforced lever press, a stimulus light was lit and active lever presses did not lead to further injections. During this phase, rats were required to attain two consecutive days of stable responding (less than 10% day-to-day variability) with minimum of 30 cocaine injections per session. Five rats failed to achieve this criterion and were excluded. Total numbers per session of active and inactive lever presses as well as cocaine injections were recorded and used for statistical analyses.
Cocaine self-administration testing
Upon completion of the last training session, microdialysis probes with 1.0 mm active membrane (CMA/12 14/01, CMA, North Chelmsford, MA) were bilaterally inserted into core or shell. A three-channel swivel (Scipro, Sanborn, NY) was used to connect the two probes and intravenous catheter to microdialysis (CMA/100) and injection pumps, respectively. The rat was infused with aCSF at the rates of 0.4 µl/min. On the following day, the infusion was increased to the testing flow rate of 2.0 µl/min and was equilibrated for a minimum of 30 min before testing. As in the case of training, each testing session was started with insertion of the active lever and a priming injection of cocaine (1.0 mg/kg/i.v.). During each testing session, rats were allowed to self-administer cocaine (1.0 mg/kg/i.v.) under FR1 for 4 h as described for the training sessions. During the first hour of testing, designated as ‘loading phase’, animals received only aCSF. During the next 3 h of testing, designated as ‘maintenance phase’, each animal received one type of receptor antagonist into either core or shell. The antagonist was continuously infused through the dialysis probes at 2.0 µl/min. Three doses (0.0, 0.1, 1.0 mM) of each antagonist were tested in each animal in a counterbalanced design, one dose per day in random sequence. Because there was no previous study examined the effects on cocaine self-administration of dopamine and glutamate antagonists infused into NAS via reverse-dialysis, we selected the current doses based on our previous study (You et al. 2007). In this study, infusion via reverse-dialysis of the broad-spectrum antagonist for ionotropic glutamate receptors, kynurenic acid (0.1, 1.0 and 5.0 mM), into the VTA dose-dependently increased lever pressing for cocaine. Kynurenic acid at 1.0 and 5.0 mM were similarly effective. Total numbers per session as well as numbers per 20 min interval of active and inactive lever presses were recorded and used for statistical analyses. Total numbers of cocaine injections obtained were also recorded and used for statistical analyses.
Histology
After completion of the experiments, rats were anesthetized with sodium pentobarbital. Brains were removed and post-fixed in 10% formalin. Coronal sections (50 µm) were mounted onto slides and stained with cresyl violet for verification of probe placements. Only data obtained from animals with both probes placed within core or shell were retained for statistical analyses. Twelve animals were excluded because one or both probes were found to be outside of core or shell. Final numbers of subjects in each experimental group retained for statistical analyses were the following: core-SCH23390 (11), core-raclopride (8), core-CNQX (9), core-CPP (8), shell-SCH23390 (8), shell-raclopride (9), shell-CNQX (9) and shell-CPP (8). Figure 1 depicts representative probe placements in these groups.
Data analyses
Total numbers of cocaine injections obtained during the last training session were analyzed with two-way between analyses of variance (ANOVA) with infusion Site (two levels) and Antagonist (four levels) as the between factors. Similarly, active and inactive lever presses during the last training were analyzed with two-way between and one-way within ANOVA with infusion Site (two levels) and Antagonist (four levels) as the between factors and Lever (2 levels) as the within factor. Total numbers of cocaine injections obtained during testing were analyzed by two-way between and one-way within ANOVA with infusion Site (two levels) and Antagonist (four levels) as the between factors and Dose (three levels) as the within factor. Total numbers of cocaine injections obtained by each experimental group during testing were analyzed by one-way within ANOVA with Dose (three levels) as the within factor. Numbers per 20 min of active and inactive lever presses by each experimental group during testing were analyzed by two-way within ANOVA with Dose (three levels) and Time (12 levels) as the within factor. Post-hoc Scheffe comparisons were made according to Kirk (1968). All analyses together with post-hoc Scheffe comparisons were conducted with Statistica (1999 edition) statistical software.
Results
Continuous bilateral infusion of antagonists via reverse-dialysis differentially affected the maintenance of cocaine self-administration depending on the type, dose, and site of infusion (Antagonist [F 3,61 = 15.89, p < 0.001], Dose [F 2,122 = 7.09, p < 0.01], Site × Antagonist interaction [F 3,61 = 5.25, p < 0.01], Antagonist × Dose interaction [F 6,122 = 16.85, p < 0.001] and Site × Antagonist × Dose interaction [F 6,122 = 4.55, p < 0.001]). Prior to the testing, there was no pre-existing difference in cocaine intake or active lever pressing. Under all testing conditions, there was no significant effect of the antagonists on inactive lever pressing.
Dopamine antagonists
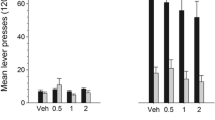
The effects of dopamine antagonists on cocaine self-administration (depicted as cumulative numbers of active and inactive lever presses) are summarized in Fig. 2. When infused into the core, the D1-type antagonist, SCH23390 (Fig. 2a), and the D2-type antagonist, raclopride (Fig. 2b), each increased lever press for cocaine. ANOVAs conducted on the numbers per 20-min interval of active lever press confirmed significant effects of SCH23390 (Dose [F 2,27 = 40.91, p < 0.001], Time [F 11,297 = 14.38, p < 0.001] and Dose × Time interaction [F 22,297 = 4.84, p < 0.001]) and raclopride (Dose [F 2,21 = 11.61, p < 0.001], Time [F 11,231 = 4.90, p < 0.001] and Dose × Time interaction [F 22,231 = 2.94, p < 0.001]). Post-hoc Scheffe comparisons confirmed that active lever presses under 0.1 and 1.0 mM of intra-core SCH23390 were significantly greater than those under aCSF starting, respectively, at 40 min and 140 min after the onset of antagonist infusion (p < 0.05–0.001). Post-hoc Scheffe comparisons confirmed that active lever press under 1.0 mM of intra-core raclopride were significantly greater than those under aCSF starting at 40 min after the onset of antagonist infusion (p < 0.05–0.01). The total numbers of cocaine injections (±SEM) obtained under intra-core SCH23390 at 0.0, 0.1, and 1.0 mM doses were 37.12 (±1.89), 46.21 (±2.52), and 56.80 (±2.33), respectively. The total numbers of cocaine injections (±SEM) obtained under continuous infusion of raclopride into core at 0.0, 0.1 and 1.0 mM doses were 37.13 (±2.19), 43.25 (±2.51), and 48.88 (±2.74), respectively. ANOVAs conducted on cocaine infusions confirmed significant effects of SCH23390 (Dose [F 2,18 = 21.25, p < 0.001]) and raclopride (Dose [F 2,14 = 19.86, p < 0.001]). Post-hoc Scheffe comparisons confirmed that cocaine intake was increased by intra-shell SCH23390 at 0.1 and 1.0 mM as well as raclopride at 1.0 mM (p < 0.05–0.001).
Effects on cocaine self-administration of bilateral infusion of dopamine receptor antagonists into the core versus shell. During the maintenance phase of cocaine self-administration, animals received continuous bilateral infusion of core-SCH23390 (a), core-raclopride (b), shell-SCH23390 (c), or shell-raclopride (d). For all panels, solid and open symbols, respectively, depict the mean (±SEM) numbers of active and inactive lever presses as a function of aCSF (circles) or antagonist infusion at 0.1 mM (squares) or 1.0 mM (triangles). Arrows depict the starting point (1 h into the cocaine self-administration sessions) of each antagonist infusion. *p < 0.05–0.001 vs. aCSF infusion as revealed by post-hoc Scheffe comparisons following ANOVA conducted on the rates per 20-min interval of lever press. n = 8–11/group
When infused into the shell, SCH23390 increased cocaine self-administration (Fig. 2c). However, intra-shell infusion of raclopride failed to affect cocaine self-administration (Fig. 2d). ANOVAs conducted on the numbers per 20-min interval of active lever press confirmed significant effects of SCH23390 (Dose [F 2,21 = 14.52, p < 0.001], Time [F 11,231 = 4.54, p < 0.001], Dose × Time interaction [F 22,231 = 2.78, p < 0.001]), but not of raclopride (Dose [F 2,24 = 0.34, ns] and Dose x Time interaction [F 22,264 = 0.73, ns]). Post-hoc Scheffe comparisons confirmed that active lever press under 1.0 mM of intra-core SCH23390 were significantly greater than those under aCSF starting at 100 min after the onset of antagonist infusion (p < 0.05–0.001). The total numbers of cocaine injections (±SEM) obtained under intra-shell SCH23390 at 0.0, 0.1 and 1.0 mM doses were 37.25 (±1.85), 41.23 (±2.18) and 49.51 (±2.01), respectively. The total numbers of cocaine injections (±SEM) obtained under intra-shell raclopride at 0.0, 0.1, and 1.0 mM doses were 36.11 (±1.86), 38.03 (±2.32), and 39.06 (±1.73), respectively. ANOVAs conducted on cocaine injections confirmed significant effects of SCH23390 (Dose [F 2,14 = 17.93, p < 0.001]), but not of raclopride. Post-hoc Scheffe comparisons confirmed that cocaine intake was increased by intra-shell SCH23390 at 1.0 mM (p < 0.05).
In the present study, infusion of SCH23390 into NAS, regardless of core or shell, significantly affected cocaine self-administration. This is consistent with previously reported findings that microinjections of SCH23390 into NAS, regardless of core or shell, reduced lever pressing for cocaine under a progressive ratio schedule (Bari and Pierce 2005). However, post-hoc Scheffe comparisons on the current data confirmed that intra-core, relative to intra-shell, infusion of SCH23390 more readily affected cocaine self-administration: compared to aCSF infusion, SCH23390 infusion into core and shell increased active lever presses starting, respectively, at 40 min and 100 min after the onset of antagonist infusion (p < 0.05–0.001). To further analyze the effects of SCH23390 in core versus shell, numbers per 20-min interval of active lever presses from the two SCH23390 groups were analyzed by one-way between and two-way within ANOVA with perfusion Site (two levels) as the between factors and Dose (three levels) and Time (12 levels) as the within factor. ANOVAs confirmed significant effects of Site (F 1,48 = 7.05, p < 0.05), Dose (F 1,48 = 50.27, p < 0.001), Time (F 1,528 = 14.76, p < 0.001), Site × Dose interaction (F 2,48 = 3.70, p < 0.05), Site × Time interaction (F 11,528 = 2.52, p < 0.01), Dose × Time interaction (F 11,528 = 4.48, p < 0.001) and Site × Dose × Time interaction (F 22,528 = 2.80, p < 0.001). Post-hoc Scheffe comparisons confirmed that active lever presses were significantly greater in core-SCH23390 relative to shell-SCH23390 group (p < 0.01).
Glutamate antagonist
The effects of glutamate antagonists on cocaine self-administration (depicted as cumulative numbers of active and inactive lever presses) are summarized in Fig. 3. When infused into the core, the AMPA receptor antagonist, CNQX, decreased lever pressing for cocaine (Fig. 3a). However, infusion of the NMDA receptor antagonist, CPP, into the core failed to affect cocaine self-administration (Fig. 3b). ANOVAs conducted on the numbers per 20-min interval of active lever presses under intra-core CNQX (Fig. 3a) confirmed a significant effect of Dose (F 2,24 = 41.31, p < 0.001), Time (F 11,264 = 21.71, p < 0.001) and a Dose × Time interaction (F 22,264 = 1.61, p < 0.05). Post-hoc Scheffe comparisons confirmed that active lever presses under 1.0 Mm of intra-core CNQX were significantly less than those under aCSF starting at 40 min after the onset of antagonist infusion (p < 0.05–0.01). ANOVAs conducted on the numbers per 20-min interval of active lever presses under intra-core CPP (Fig. 3b) revealed no significant effect. Post-hoc Scheffe comparisons confirmed that operant responding for cocaine was decreased under intra-core CNQX at 1.0 mM (p < 0.05–0.001). The total numbers of cocaine injections (±SEM) obtained under intra-core infusion of CNQX at 0.0, 0.1, and 1.0 mM doses were 38.67 (±1.87), 35.56 (±2.26) and 18.65 (±2.81), respectively. The total numbers of cocaine injections (±SEM) obtained under intra-core infusion of NMDA at 0.0, 0.1, and 1.0 mM doses were 36.87 (±2.03), 38.88 (±2.21), and 37.25 (±2.05), respectively. ANOVAs conducted on cocaine injections obtained confirmed significant effects of CNQX (Dose [F 2,16 = 22.11, p < 0.001]), but not of CPP. Post-hoc Scheffe comparisons confirmed that cocaine intake was decreased by intra-core CNQX at 1.0 mM (p < 0.001).
Effects on cocaine self-administration of bilateral infusion of ionotropic glutamate receptor antagonists into the core versus shell. During the maintenance phase of cocaine self-administration, animals received continuous bilateral infusion of core-CNQX (a), core-CPP (b), shell-CNQX (c), or shell-CPP (d). For all panels, solid and open symbols, respectively, depict the mean (±SEM) numbers of active and inactive lever presses as a function of aCSF (circles) or antagonist infusion at 0.1 mM (squares) or 1.0 mM (triangles). Arrows depict the starting point (1 h into the cocaine self-administration sessions) of each antagonist infusion. *p < 0.05–0.01 vs. aCSF infusion as revealed by post-hoc Scheffe comparisons following ANOVA conducted on the rates per 20 min interval of lever press. n = 8–9/group
When infused into the shell, CNQX (Fig. 3c) and CPP (Fig. 3d) failed to affect cocaine self-administration. ANOVAs conducted on the numbers of active lever presses per 20 min interval confirmed no significant effect of CNQX and CPP. The total numbers of cocaine injections (±SEM) obtained under continuous intra-shell infusion of CNQX at 0.0, 0.1, and 1.0 mM doses were 39.11 (±1.78), 41.56 (±1.67), and 37.33 (±1.46), respectively. The total numbers of cocaine injections (±SEM) obtained under continuous intra-shell infusion of NMDA at 0.0, 0.1, and 1.0 mM doses were 39.75 (±2.50), 36.81 (±1.71), and 39.72 (±2.28), respectively. ANOVAs conducted on cocaine infusions confirmed no significant effect of CNQX or CPP infusion into the shell.
Discussion
The present findings are consistent with several reports suggesting different motivational roles for the core and shell subregions of NAS (e.g., Bari and Pierce 2005; Bassareo et al. 2007; Bassareo and Di Chiara 1999; Carlezon et al. 1995; Ikemoto 2003; Ito et al. 2004). In the present study, both dopamine and glutamate antagonists were differentially effective in core and shell: while core infusions were effective, shell infusions were ineffective or markedly less effective. The angled cannula placements in the present study reliably differentiate between the effects of drugs infused into core and shell, and because the shell infusions were between the core and OT, their ineffectiveness rules out the possibility that the core infusions were effective because they diffused to sites of action in OT, an adjacent structure where cocaine is known to have rewarding actions (Ikemoto 2003).
While the present data do not speak to the issue of reinforcement per se, they suggest that cocaine-induced dopamine actions in the core, but not shell, control the stable rate of cocaine self-administration that is typical of within-session binge (“maintenance phase”) responding in well-trained animals (Pickens and Thompson 1968; Tsibulsky and Norman 1999). While the D1 antagonist had a small effect when infused into the shell, the latency for this effect was long and the effect was weak when compared to the intra-core infusion. Thus, it is possible and perhaps likely that the intra-shell infusions of SCH23390 were marginally effective late in the session because they diffused to targets in the adjacent and much more strongly implicated core region. Because the degree of diffusion cannot be easily determined—concentration decreases as a power function of the distance from the probe and is affected by binding and metabolism—the only firm conclusion from the present findings is that receptor-mediated glutamate and dopamine actions in NAS core play important roles in controlling successive within-session lever presses in cocaine-intoxicated animals.
While the finding that dopamine receptor blockade has similar effects to those caused by reducing cocaine dose per earned injection has traditionally been attributed to attenuation of the “reinforcing efficacy” of cocaine (Bari and Pierce 2005; de Wit and Wise 1977), this interpretation is problematic in the case of the present data. The concept of instrumental reinforcement has been used historically in the context of the learning of stimulus-response habits (Skinner 1933; Thorndike 1933) of instrumental response acquisition, and evidence from intracranial cocaine self-administration studies indicates that it is OT and NAS shell, but not NAS core where cocaine reinforces the learning of response habits (Carlezon et al. 1995; Ikemoto 2003: but see Ito et al. 2004). The present study deals with the rate of performance of a learned habit, rather than with the rate of habit acquisition, and it is the satiating effect of cocaine (Tsibulsky and Norman 1999) that readily explains the rate of performance of a self-administration habit in well-trained animals. Several findings point to negative feedback from the last injection—the objective definition of satiation—as the controlling factor in rate of performance. First, if the rate of cocaine metabolism is accelerated or decelerated, cocaine self-administration accelerates or decelerates accordingly (Dougherty and Pickens 1974). Second, if rats are given a supplemental intravenous drip of cocaine during a self-administration session, the animals wait longer between injections, compensating closely for the non-contingent drug supplement (Gerber and Wise 1989). Third, if an unpredictable sequence of doses is given to the animal, inter-response times are inversely proportional to the size of the last earned dose (Gerber and Wise 1989). Finally, the time of the next earned injection can, in well-trained animals, be predicted from the falling rate of cocaine in the blood; rate of responding can be readily predicted from the half-life of the already-injected drug (Tsibulsky and Norman 1999) or dopamine in NAS (Wise et al. 1995). The facts that cocaine in the shell but not the core is reinforcing (Carlezon et al. 1995; Ikemoto 2003: c.f. Ito et al. 2004) and that dopamine antagonists in the core but not the shell decrease the satiating effects of cocaine (present study) suggest independent mechanisms of reinforcement and learned performance. This suggestion fits well with the hypothesis that the conditioned stimuli that control instrumental habits do so through input to the core rather than the shell (Di Ciano and Everitt 2001; McFarland and Kalivas 2001; Fuchs et al. 2004; Ito et al. 2004; Di Ciano et al. 2008).
If it is difficult to identify the nature of the deficit from the neuroleptic-induced response acceleration (Wise 1987), it is even more difficult to identify the nature of the deficit from the diminished responding caused by any experimental manipulation (e.g., Bari and Pierce 2005; Di Ciano and Everitt 2001; Ito et al. 2004). In the present study, the diminished responding was caused by the AMPA antagonist, CNQX. The CNQX-induced decrease in lever pressing for cocaine could reflect a motor deficit, a memory deficit, disorientation, or any of a host of other performance problems not addressed by our experimental design. The behavioral disruption could also reflect a motivational deficit. The findings that priming injections of intra-NAS AMPA reinstate extinguished lever pressing for cocaine (Cornish et al. 1999; Cornish and Kalivas 2000; Suto et al. 2004) are consistent with this interpretation. In the present study, the NMDA antagonist, CPP, failed to affect cocaine self-administration. While we cannot rule out the possibility that the failure of CPP to affect lever pressing for cocaine is due to ineffective doses of the NMDA antagonist tested in the present study, the K i (nM) values for CNQX (3.4 nM and 65 nM for high and low affinity sites, respectively) and CPP (35 nM) are comparable with each other. Furthermore, the current findings are consistent with previously reported findings that microinjection of CPP into NAS, regardless of core or shell, failed to affect lever pressing for cocaine (Di Ciano and Everitt 2001) and cocaine-associated stimuli (Cornish and Kalivas 2000; Di Ciano and Everitt 2001: also see Cornish et al. 1999).
Glutamatergic input to NAS arises primarily from the prefrontal and limbic cortices (Groenewegen et al. 1999) and figures strongly in current theories of drug-seeking (Di Ciano and Everitt 2004; Kalivas 2004; Wise 1999). During cocaine self-administration, extracellular glutamate is elevated in the core (Miguens et al. 2008), but comparisons of glutamate input to core and shell have not yet been measured in self-administration studies. Thus far, microdialysis studies of the effects of reward-associated stimuli in core and shell only confirm differences between dopamine fluctuations in these regions (Ito et al. 2004). The present experiments were part of a larger microdialysis study and the data to date reveal that glutamate does fluctuate during cocaine self-administration under our conditions. Our microdialysis study will include comparisons between core and shell, between the effects of unearned (yoked) injections and earned (self-administered) injections, between cocaine injections in cocaine-trained and cocaine-naïve animals, and between various forms of motivational stimuli (discriminative stimuli, conditioned reinforcers) that are possible sources of glutamate input to these regions.
Reference
Bari AA, Pierce RC (2005) D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 135:959–68
Bassareo V, De Luca MA, Di Chiara G (2007) Differential impact of Pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 191:689–703
Bassareo V, Di Chiara G (1999) Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89:637–641
Carlezon WA Jr, Devine DP, Wise RA (1995) Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology 122:194–197
Cornish JL, Duffy P, Kalivas PW (1999) A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93:1359–1367
Cornish JL, Kalivas PW (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89
de Wit H, Wise RA (1977) Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol 31:195–203
Di Ciano P, Everitt BJ (2001) Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharm 25:341–60
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24:7167–7173
Di Ciano P, Robbins TW, Everitt BJ (2008) Differential Effects of Nucleus Accumbens Core, Shell, or Dorsal Striatal Inactivations on the Persistence, Reacquisition, or Reinstatement of Responding for a Drug-Paired Conditioned Reinforcer. Neuropsychopharmacology.
Dougherty JD, Pickens R (1974) Effects of phenobarbital and SKF 525A on cocaine self-administration in rats. Drug Addiction 3:135–143
Fuchs RA, Evans KA, Parker MC, See RE (2004) Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 176:459–465
Gerber GJ, Wise RA (1989) Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol Biochem Behav 32:527–31
Goeders NE, Smith JE (1983) Cortical dopaminergic involvement in cocaine reinforcement. Science 221:773–775
Grace AA (2000) The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 95(Suppl 2):S119–128
Groenewegen HJ, Wright CI, Beijer AV, Voorn P (1999) Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877:49–63
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C (1991) Specificity in the projection patterns of the accumbal core and shell in the rat. Neuroscience 41:89–125
Ikemoto S (2003) Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci 23:9305–9511
Ito R, Robbins TW, Everitt BJ (2004) Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7:389–397
Kalivas PW (2004) Glutamate systems in cocaine addiction. Curr Opin Pharmacol 4:23–29
Kelley AE, Swanson CJ (1997) Feeding induced by blockade of AMPA and kainite receptors within the ventral striatum: a microinfusion mapping study. Behavioral Brain Research 89:107–13
Kirk RE (1968) Experimental design: procedures for the behavioral sciences. Brooks/Cole, Pacific Grove
McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663
McGregor A, Roberts DC (1995) Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behavioral Brain Research 67:75–80
McGregor A, Baker G, Roberts DC (1996) Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav 53:5–9
Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E (2008) Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 196:303–13
National Institutes of Health, Public Health Service (1996) Guide for the care and use of laboratory animals. NIH publication No. 86-23. Bethesda: NIH
O'Donnell P, Grace AA (1994) Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of profrontal cortical input. J Neurosci 15:3622–3639
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, San Diego, CA
Pettit HO, Pan HT, Parsons LH, Justice JB Jr (1990) Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem 55:798–804
Pickens R, Thompson T (1968) Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther 161:122–129
Roberts DCS, Corcoran ME, Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6:615–620
Sesack SR, Pickel VM (1990) In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res 527:266–279
Skinner BF (1933) On the rate of extinction of a conditioned reflex. J Gen Psychol 8:114–129
Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P (2004) Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology 29:2149–2159
Thorndike EL (1933) A theory of the action of the after-effects of a connection upon it. Psychol Rev 40:434–439
Totterdell S, Smith AD (1989) Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat 2:285–298
Tsibulsky VL, Norman AB (1999) Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res 839:85–93
Voorn P, Gerfen CR, Groenewegen HJ (1989) Compartmental organization of the ventral striatum of the rat; immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium binding protein. J Comp Neurol 289:189–201
Wise RA (1987) Methods of Assessing the Reinforcing Properties of Abused Drugs. In: Bozarth MA (ed) Intravenous drug self-administration: a special case of positive reinforcement. Springer, New York, pp 117–141
Wise RA (1999) Cognitive factors in addiction and nucleus accumbens function: some hints from rodent models. Psychobiology 27:300–310
Wise RA, Hoffman DC (1992) Localization of drug reward mechanisms by intracranial injections. Synapse 10:247–263
Wise RA, Newton P, Leeb K, Burnette B, Pocock P, Justice JB (1995) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 120:10–20
Yim CY, Mogenson GJ (1986) Mesolimbic dopamine projection modulates amygdala-evoked EPSP in nucleus accumbens neurons: an in vivo study. Brain Res 369:347–352
You ZB, Wang B, Zitzman D, Azari S, Wise RA (2007) A role for conditioned entral tegmental glutamate release in cocaine seeking. J Neurosci 27:10546–10555
Zaffaroni A (1981) Systems for controlled drug delivery. Med Res Rev 1:373–386
Acknowledgments
Supported by funding from the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. We thank Dr. Zhi-Bing You for technical assistance with the reverse-dialysis technique, Drs. Marisela Morales and Hui-Ling Wang for technical assistance with histology, and Eric Thorndike for technical assistance with analyses of self-administration data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suto, N., Ecke, L.E. & Wise, R.A. Control of within-binge cocaine-seeking by dopamine and glutamate in the core of nucleus accumbens. Psychopharmacology 205, 431–439 (2009). https://doi.org/10.1007/s00213-009-1553-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1553-0