Abstract
Rationale
The neural circuitry subserving cocaine-seeking after extinction vs abstinence alone requires different constituent brain structures. Spontaneous recovery of cocaine-seeking, a model, which incorporates both extinction and abstinence, depends on an unknown neural circuit.
Objectives
The present study examined the hypothesis that the spontaneous recovery of cocaine-seeking would require overlapping but distinct neural circuits compared to models that incorporate either extinction or abstinence alone.
Material and methods
Rats were trained to self-administer cocaine (0.2 mg/inf), then responding on the cocaine-paired lever was extinguished, followed by an additional period of abstinence in the home cage. Finally, rats were returned to the self-administration context for a test of spontaneous recovery (SR TEST). Just before the SR TEST, discrete brain regions were inactivated with a GABA agonist cocktail (1 mM baclofen + 0.1mM muscimol) to determine the relative importance of these brain regions in the spontaneous recovery of cocaine-seeking.
Results
The inactivation of the ventromedial prefrontal cortex (vPFC) enhanced cocaine-seeking, whereas the inactivation of the basolateral amygdala (BLA) attenuated spontaneous recovery. Inactivation of the nucleus accumbens core (Core) resembled the effects of BLA inactivation, but these results were confounded by an inhibitory effect of the vehicle treatment. Finally, the spontaneous recovery of cocaine-seeking was unaltered by manipulations of the dorsomedial prefrontal cortex (dPFC) and the nucleus accumbens shell (Shell).
Conclusions
The neural circuitry subserving cocaine-seeking behavior in a spontaneous recovery model requires the BLA and possibly the Core, like extinction models. In addition, this behavior is subject to regulation by vPFC, in a manner functionally opposite to that of the BLA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relapse is frequently modeled as the reinstatement of drug-seeking behavior in animals previously trained to self-administer addictive drugs (Shaham et al. 2003; de Wit and Stewart 1981). The reinstatement of drug-seeking typically follows extinction procedures wherein animals learn to reduce responding on the drug-paired lever once cocaine is withheld. In these extinction–reinstatement models, a key neurobiological substrate underlying this form of cocaine-seeking behavior is the activation of the dorsomedial prefrontal cortex (dPFC) projection to the nucleus accumbens core (Core) (Kalivas et al. 2005; McFarland et al. 2003; Park et al. 2002). Recently, however, cocaine-seeking after abstinence that occurs in the absence of any explicit extinction training was found to occur independently of this ‘final common pathway’ from dPFC to Core, instead relying solely on the dorsolateral caudate putamen, which presumably reflects the more habitual nature of drug-seeking that occurs in the abstinence-alone model (Fuchs et al. 2006; See et al. 2007; Jog et al. 1999). The extinction process induces neurobiological changes associated with learning-induced plasticity (Cammarota et al. 2005; Myers and Davis 2002) that may account for the recruitment of some of the neural circuitry mediating cocaine-seeking in extinction–reinstatement models, possibly including the dPFC to Core projection. Abstinence may also be an active process because increasing the duration of abstinence results in greater rates of responding in relapse tests, a phenomenon known as ‘incubation’ (Lu et al. 2004; Grimm et al. 2001). The present study sought to determine the neural circuitry responsible for cocaine-seeking in a model of spontaneous recovery, which shares elements of both extinction–reinstatement and abstinence-alone models.
Spontaneous recovery is a term used to describe the behavioral phenomenon whereby a conditioned response triggered by conditioned cues strengthens over time, although responding to the conditioned cues was previously extinguished (Rescorla 2004). Hence, the conditioned response is said to ‘spontaneously recover’ with time, or in this case, with a period of abstinence from the conditioned context. Spontaneous recovery can occur for a variety of conditioned behaviors and has been previously documented for conditioned cocaine-seeking behavior in rats trained on second-order schedules (Di Ciano and Everitt 2002). Still, other authors have examined the neural circuitry involved in cocaine-seeking behavior in models where a period of abstinence is interposed between extinction and the drug-seeking test, again in rats trained on second-order schedules (Kantak et al. 2002a,b). In these second-order models, cocaine-seeking is thought to be driven by a BLA to Core projection (Di Ciano and Everitt 2004) and does not appear to require the dPFC (Di Ciano et al. 2007). The present study sought to extend previous findings by first demonstrating spontaneous recovery in animals trained on simple, FR-1 schedules of cocaine reinforcement, followed by a subsequent analysis of the circuitry controlling this form of cocaine-seeking.
In FR-1 extinction–reinstatement models, the inactivation of the dPFC and the Core attenuates relapse (McFarland et al. 2004; McFarland and Kalivas 2001; Fuchs et al. 2004, 2005; Capriles et al. 2003) and inactivation of the BLA when relapse is triggered either by discrete or contextual conditioned cues (McLaughlin and See 2003; Fuchs et al. 2005). Because the present study did not pair any discrete cues with the delivery of cocaine, presumably spontaneous recovery in this model is driven by reexposure to the cocaine-paired environment on the spontaneous recovery test (SR TEST) and might be considered a form of cocaine-seeking triggered by contextual cues. Recently, an extinction–reinstatement model of cocaine-seeking where reinstatement was triggered by contextual cues was shown to require the dPFC and the BLA, similar to the reinstatement of cocaine-seeking elicited by discrete cues (Fuchs et al. 2005; McLaughlin and See 2003). The inactivation of the ventromedial prefrontal cortex (vPFC) and the nucleus accumbens shell (Shell), by contrast, often produces no measurable effects on cocaine-seeking behavior (McLaughlin and See 2003; McFarland and Kalivas 2001; Fuchs et al. 2004, 2005; except for the Shell in stress-induced reinstatement, McFarland et al. 2004). The present study examined the role of five brain structures on cocaine-seeking behavior in a spontaneous recovery model, including the dPFC, vPFC, Core, Shell, and BLA. Based on the previous findings outlined above, we hypothesized that the inactivation of the dPFC, BLA, and Core would inhibit cocaine-seeking on the SR TEST, while the inactivation of the vPFC and Shell would not alter cocaine-seeking on the SR TEST.
Materials and methods
Subjects
All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina Animal Care and Use Committee. Male Sprague–Dawley rats (Charles River Laboratories, Indianapolis, IN, USA) weighing 250–275 g on arrival were housed individually in a temperature- and humidity-controlled environment with a 12-h light/dark cycle (7:00 a.m. lights on). Rats received food ad libitum until 1 day before behavioral training; after which, food-restriction procedures (20 g of rat chow per day) were implemented and maintained throughout the duration of the experiment. Water was always available ad libitum. A total of 152 rats were used in this study, of which 62 animals were eliminated from data analysis for the following reasons: 24 for histology (e.g., bad injection sites), 9 for problems with clogged cannulae or headcap issues, 9 for health problems, 10 for failure to acquire self-administration behavior, 9 for failure to extinguish, and 4 for other miscellaneous reasons.
Drugs
Cocaine hydrochloride was generously provided by the National Institute on Drug Abuse (Bethesda, MD, USA) and dissolved in 0.9% sterile saline for systemic injection and intravenous infusion. A cocktail consisting of the GABA-B (baclofen, 1 mM) and GABA-A (muscimol, 0.1 mM) agonists (Bac–Mus) was prepared in phosphate-buffered saline (PBS) and used for intracranial delivery.
Surgery
Rats were allowed to acclimate to the vivarium between 2 and 7 days before performing surgery. Anesthesia was a combination of ketamine (K, 87.5 mg/kg; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (X, 5 mg/kg; Rompun, Bayer, Shawnee Mission, KS, USA) administered in 0.2/0.1 ml (K/X) into the vastus lateralis of alternating limbs. An intravenous jugular catheter was implanted as described previously (McFarland and Kalivas 2001), and immediately thereafter, stereotaxic surgery was performed to implant the intracranial guide cannulae aimed at one of five brain regions using the following coordinates: PFC dorsal or ventral: +3.2 anteroposterior (AP), ±0.6 mediolateral (ML), −2.8 dorsoventral (DV); Core: +1.6 mm AP, ±1.6 mm ML, −6.6 mm DV; Shell: +1.8 mm AP, ±0.8 mm ML, −6.8 mm DV; BLA: −2.5 mm AP, ±4.7 mm ML, −7.8 mm DV; all coordinates were relative to the bregma and skull surface. Guide cannulae were secured to the skull with dental cement and jeweler’s screws. To prevent obstruction by debris, cannulae were plugged with 33-gauge wire stylets that were identical in length to the cannulae, except for the vPFC group, whose stylets extended 2 mm beyond the tip of the cannulae. This was done to ensure the greatest integrity of dPFC at all times, while also preventing extensive damage on the test day by insertion of the microinjection needle. Rats were allowed to recover for approximately 1 week before food-restriction procedures were implemented.
Cocaine self-administration
Before cocaine self-administration, rats were shaped on the right lever in a single food-training session wherein each response on the right lever delivered a single 45 mg food pellet. Rats were allowed to lever press for food for up to 15 h until they were shaped on the right lever. It is important to note that using food training before cocaine training introduces a potential confound, and in the future, these studies should be extended in animals trained only to self-administer cocaine. The following day, rats began the acquisition of cocaine self-administration in daily 2 h sessions for 7–12 days. During this phase, each response on the right lever resulted in an infusion of cocaine (0.2 mg in 0.05 ml), followed by a timeout period of 20 s wherein responding was without consequence, which was intended to help prevent cocaine overdose. No discrete cues were paired with cocaine delivery during acquisition. Failure to self-administer at least 15 infusions per day over the last 2 successive maintenance days resulted in the elimination from the study. Thereafter, extinction procedures were implemented wherein cocaine was withheld. Rats extinguished in daily 2 h sessions for 14–19 days, and the final 2 days of extinction were averaged to obtain an extinction pretest baseline (EXT) for each subject. If this average was greater than 40 presses, the subject was eliminated from the study. Otherwise, abstinence procedures were implemented wherein the subject was handled daily but did not undergo behavioral sessions of any kind and instead remained in the home cage.
Spontaneous recovery testing
After 28 ± 4 days of abstinence, rats were returned to the operant chambers for the SR TEST, which was defined by a reinstatement of lever-pressing on the right lever relative to their extinction responding before abstinence. Immediately before their placement in the chamber, rats received a microinfusion of either PBS or Bac–Mus directly into one of the five brain structures studied. Stylets were removed and 33-gauge needles were inserted to extend 1 mm beyond the cannula (or 3 mm for the vPFC). Bilateral microinfusions were performed concomitantly at a rate of 0.3 μl/min in a total volume of 0.3 μl/side (or 0.5 μl/side for the BLA). Injectors were left in place 1–2 min to permit diffusion, then the subjects were placed in the experimental chamber and the test session began. The test session lasted for 2 h and was essentially an additional extinction session conducted after a period of abstinence. At the end of the session, the stylets were replaced, and the animals were returned to their home cage. The following day, another extinction session was conducted in the same manner, except no microinfusions were delivered. Responding during this session was analyzed as a post test baseline (POST).
Histology and statistics
After experimentation, rats were overdosed with pentobarbital (approximately 350 mg/kg, i.p.) and then perfused transcardially with 10% formalin. Brains were dissected out and anatomical placement was verified according to the atlas of Paxinos and Watson (2005). A two-way ANOVA with experimental phase (EXT, SR TEST, POST) as the within-subjects factor and drug (PBS, Bac–Mus) as the between-subjects factor was used to determine effects of Bac–Mus on spontaneous recovery (GraphPad Prism software). If a significant main effect of drug or phase × drug interaction was observed, a Bonferroni post hoc test was conducted to determine significant differences between PBS and Bac–Mus groups. If a significant main effect of phase was observed, separate one-way ANOVAs were conducted on the PBS and Bac–Mus groups, and when a significant F score was identified using the one-way ANOVA, a Tukey’s multiple comparison post hoc analysis was conducted to determine on which phase spontaneous recovery occurred.
Results
Inactivation of the vPFC enhances spontaneous recovery of cocaine-seeking
A significant main effect of phase [F(2,36) = 3.790, p = 0.032] was observed in the vPFC subjects (Fig. 1). To determine if the effect of phase indicated spontaneous recovery, separate one-way repeated measures ANOVAs followed by Tukey’s multiple comparison post hoc analyses were conducted within the PBS and Bac–Mus groups, and revealed that in the PBS group [F(2,23) = 14.58, p < 0.001], EXT pressing was different from the SR TEST and POST test session (p < 0.05 for each). In the Bac–Mus group [F(2,32) = 3.93, p = 0.037], EXT pressing was also different from the SR TEST (p < 0.05), but not from pressing on the POST test session. A significant main effect of drug [F(1,18) = 4.693, p = 0.044] was also measured in vPFC-injected rats. The Bonferroni post hoc test revealed that this was due to significantly enhanced pressing in the Bac–Mus group compared to the PBS controls on the SR TEST (p < 0.01). None of these effects could be attributed to the differences in cocaine self-administration between PBS and Bac–Mus groups because active lever pressing during maintenance was not different between groups (92 ± 10 for PBS, 71 ± 21 for Bac–Mus).
Inactivation of the vPFC enhances the spontaneous recovery of cocaine-seeking. The mean ± SEM number of active lever presses for PBS (n = 9, open bars) and Bac–Mus (n = 11, closed bars) drug groups is shown on the y-axis. Microinfusions of drug were administered into vPFC immediately before the test for spontaneous recovery (SR TEST) compared to the baseline extinction responding (EXT) and responding during the next day’s POST test extinction session (POST). Both EXT and POST phases were conducted in a drug-free state. The asterisk indicates p < 0.05 compared to the respective EXT. The plus sign indicates p < 0.01 compared to the respective PBS
Inactivation of the BLA or Core inhibits spontaneous recovery of cocaine-seeking
A significant main effect of phase [F(2,22) = 8.783, p = 0.002] and a significant phase × drug interaction [F(2,22) = 3.569, p = 0.046] were observed with BLA inactivation. One-way repeated measures ANOVAs followed by Tukey’s post hoc analyses revealed that the PBS group [F(2,17) = 4.40, p = 0.043] showed a significant difference between EXT pressing and pressing on the SR TEST (p < 0.05), while the Bac–Mus group [F(2,20) = 7.80, p = 0.007] showed a significant increase in lever pressing between the POST test relative to EXT (p < 0.05, Fig. 2a). Furthermore, pressing on the POST test was significantly elevated compared to the SR TEST (p < 0.05) in the Bac–Mus group, suggesting that the POST test was the first session where significant spontaneous recovery was observed for this treatment group. The lack of significant spontaneous recovery on the SR TEST after Bac–Mus injection into the BLA and the significant rebound in lever pressing on the first drug-free session (i.e., the POST test session) supports the notion that inhibiting neuronal activity in the BLA suppressed spontaneous recovery. None of these effects could be attributed to the differences in cocaine self-administration between PBS and Bac–Mus groups because active lever pressing during maintenance was not different between groups (89 ± 17 for PBS, 88 ± 23 for Bac–Mus).
Inactivation of the BLA and the Core inhibits the spontaneous recovery of cocaine-seeking. The number of active lever presses for PBS (open bars) and Bac–Mus (closed bars) drug groups is shown on the y-axis. Microinfusions of drug were administered into either the BLA (n = 6 for PBS, n = 7 for Bac–Mus) (a) or the Core (n = 9 per group) (b) immediately before the test for spontaneous recovery (SR TEST) compared to the baseline extinction responding (EXT) and responding during the next day’s POST test extinction session (POST). Both EXT and POST phases were conducted in a drug-free state. The asterisk indicates p < 0.05 compared to the respective EXT. The plus sign indicates p < 0.05 compared to the respective SR TEST
A significant main effect of phase [F(2,32) = 27.13, p < 0.001] and a significant phase × drug interaction [F(2,32) = 4.449, p = 0.020] were also observed with Core inactivation. Both PBS [F(2,28) = 7.95, p = 0.004] and Bac–Mus [F(2,28) = 21.25, p < 0.001] groups showed significant spontaneous recovery, and in both groups, this occurred on the POST test compared to EXT pressing, (p < 0.05; Fig. 2b). Similar to the BLA, the SR TEST and POST test sessions were significantly different only in the Bac–Mus group (p < 0.05), supporting the notion that suppression of neuronal activity in the Core on the SR TEST may have contributed to a rebound of lever pressing on the POST test session. However, the fact that the PBS control animals did not exhibit significant spontaneous recovery on the SR TEST hinders the interpretation of these results. There were no significant preexisting differences in cocaine self-administration between PBS and Bac–Mus groups because active lever pressing during maintenance was not different between groups (73 ± 21 for PBS, 114 ± 52 for Bac–Mus).
The dPFC and the Shell are not necessary for the spontaneous recovery of cocaine-seeking
No differences were observed in spontaneous recovery after the inactivation of either dPFC or Shell (Fig. 3). However, main effects of phase were measured for both the dPFC [F(2,36) = 6.812, p = 0.003] and the Shell [F(2,28) = 7.209, p = 0.003]. Furthermore, one-way repeated measures ANOVAs revealed that these effects could be attributed to significant spontaneous recovery on the SR TEST, but not the POST test session, relative to EXT in both the PBS [dPFC, F(2,29) = 3.58, p = 0.049; Shell, F(2,20) = 5.19, p = 0.024] and Bac–Mus [dPFC, F(2,29) = 3.82, p = 0.042; Shell, F(2,26) = 4.32, p = 0.032] groups. As with the other brain structures studied in this report, there were no preexisting differences in cocaine self-administration behavior between PBS and Bac–Mus groups because active lever pressing during maintenance was not different between groups for the dPFC (53 ± 8 for PBS, 54 ± 5 for Bac–Mus) or for the Shell (73 ± 21 for PBS, 61 ± 7 for Bac–Mus).
The dPFC and the Shell are not necessary for the spontaneous recovery of cocaine-seeking. The number of active lever presses for PBS (open bars) and Bac–Mus (closed bars) drug groups is shown on the y-axis. Microinfusions of drug were administered into either the dPFC (n = 10 per group) (a) or the Shell (n = 7 for PBS, n = 9 for Bac–Mus) (b) immediately before the test for spontaneous recovery (SR TEST) compared to the baseline extinction responding (EXT) and responding during the next day’s post test extinction session (POST). Both EXT and POST phases were conducted in a drug-free state. The asterisk indicates p ≤ 0.05 compared to the respective EXT
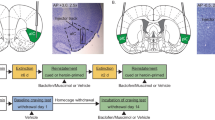
Histology
Figure 4 shows the location of the microinjection sites within the five brain regions studied for all subjects. Numbers to the left of each panel indicate millimeters from the bregma. The micrographs show representative infusion sites from individual animals.
Histological verification of the microinfusion sites. The most ventral sites of the microinfusions identified for each targeted brain structure are marked by closed circles for components of the ventral circuit and by open circles for components of the dorsal circuit. Numbers to the left of each panel indicate millimeters anterior to the bregma. Photomicrographs of Nissl-stained coronal sections showing the representative placements for each injection site (indicated by arrows) are shown below each structure
Discussion
Few studies have examined the neurobiological basis of spontaneous recovery in animals trained to self-administer cocaine. While spontaneous recovery is observed for a number of conditioned responses (Rescorla 2004) and has been more frequently studied in conditioned fear (Bouton et al. 2006; Quirk 2002), its expression in animal models of addiction has not been studied in great length (see Di Ciano and Everitt 2002). Hence, it was of interest to examine the neural circuitry subserving this behavioral phenomenon in cocaine self-administering animals. By inhibiting brain nuclei with a baclofen–muscimol GABA cocktail, it was found that inhibiting activity within the vPFC enhanced the spontaneous recovery of cocaine-seeking, whereas inhibition of the BLA and Core diminished the spontaneous recovery of cocaine-seeking.
Involvement of the prefrontal cortex in spontaneous recovery
This study documents, for the first time, a role for the vPFC in a unique form of cocaine-seeking, namely, the spontaneous recovery of cocaine-seeking triggered by previously extinguished contextual cues. This observation is consistent with studies on spontaneous recovery of conditioned fear (Sierra-Mercado et al. 2006; Quirk 2002; Milad and Quirk 2002) and appetitive Pavlovian conditioning (Rhodes and Killcross 2004). In studies of conditioned fear, lesions of the ventral portion of the medial prefrontal cortex resulted in normal within-session extinction, but abnormal recovery of the conditioned response relative to the control subjects after a substantial delay and protein synthesis inhibitors injected locally within the vPFC produced similar effects (Santini et al. 2004; Quirk et al. 2000). Hence, the vPFC may be one site where extinction memory for conditioned fear is consolidated or stored. If the vPFC is playing a similar role in the extinction of conditioned cocaine-seeking responses, the enhancement in spontaneous recovery observed with vPFC inactivation reported in this study might reflect the release of some representation of the extinction memory stored within vPFC and would further suggest that an extinction memory remains even after long periods of abstinence. Indeed, the likelihood that extinction memory remains at least partly intact in these animals is supported by the observation that cocaine-seeking in this spontaneous recovery model is relatively modest (35–45 presses) relative to context-induced cocaine-seeking after abstinence without prior extinction training in rats trained on a similar regimen of cocaine self-administration (approximately 130 lever presses; Fuchs et al. 2006).
Also striking was the apparent lack of involvement of the neighboring dPFC in this unique form of contextual cue-driven cocaine-seeking. In this manner, the spontaneous recovery of cocaine-seeking to previously extinguished contextual cues resembles the abstinence-alone model (Fuchs et al. 2006). In extinction–reinstatement models, however, the dPFC has a well-documented role in promoting relapse (McFarland and Kalivas 2001; McLaughlin and See 2003; Capriles et al. 2003), including relapse triggered by contextual cues (Fuchs et al. 2005). Because the relapse test is typically conducted during late extinction, amid daily extinction training sessions, the apparent lack of dPFC control over cocaine-seeking in this spontaneous recovery model suggests that the abstinence period interposed before the SR TEST may fundamentally alter the role of the dPFC, such that continuous extinction training may be necessary to maintain a dPFC-regulated circuit. Alternatively, this apparent lack of dPFC involvement in the spontaneous recovery of cocaine-seeking resembles cocaine-seeking triggered by second-order conditioned cues (Di Ciano et al. 2007). The spontaneous recovery observed in the model reported in this study was modest, perhaps due to the use of an AAA design. Because extinction was conducted in the same environment where conditioning occurred, if the extinction memory were strong enough, only a modest increase in the conditioned response might be predicted. This small but significant increase in cocaine-seeking after abstinence could be triggered entirely by the conditioned contextual cues of the operant chamber, which would presumably acquire all conditioned reinforcing properties normally attributed to discrete cues paired with cocaine, which were absent in our model of spontaneous recovery. If this form of cocaine-seeking were dependent solely on the conditioned contextual cues, it might be expected to rely on the BLA as reported previously for cue-controlled drug-seeking (McLaughlin and See 2003; Di Ciano and Everitt 2004; Fuchs et al. 2005).
Role of the BLA and nucleus accumbens in spontaneous recovery
Inactivation of the BLA blocked the spontaneous recovery of cocaine-seeking after abstinence. Given the similarity in the magnitude of spontaneous recovery observed in the current study with that of cue-induced reinstatement studies conducted under extinction conditions, these results are consistent with the notion that the BLA is necessary for the ability of cues, be they discrete or contextual in nature, to facilitate cocaine-seeking behavior (Fuchs et al. 2005; See 2005). As previously proposed by Di Ciano and Everitt (2004) for cocaine-seeking triggered by second-order conditioned cues, the BLA projection to the Core may provide the neurobiological basis for spontaneous recovery in this model as well. Although the results with Core inactivations must be interpreted in the light of the nonspecific inhibitory effects of the vehicle injection for that group, the fact that the inactivation of the Core appeared to inhibit spontaneous recovery is consistent with a role for a BLA–Core circuit. This hypothesis needs to be investigated further using pharmacological disconnection procedures (Di Ciano and Everitt 2004; McFarland and Kalivas 2001) and combining the inactivation of the BLA with microdialysis for glutamate in the Core to disrupt the BLA–Core projection. Although the results on the Core were confounded by inhibitory effects of PBS within this structure, critical involvement of the Core in cocaine-seeking is consistent with numerous other accounts (McFarland and Kalivas 2001; Di Ciano and Everitt 2004; Fuchs et al. 2004). Indeed, it is specifically the Core, and not the Shell of the accumbens, that has been shown to be critical for cocaine-seeking using similar inactivation methods (McFarland and Kalivas 2001; Fuchs et al., 2004), and the lack of involvement of the Shell observed in this model of spontaneous recovery is consistent with these reports.
Conclusions
The inactivation of the BLA was found to inhibit the spontaneous recovery of cocaine-seeking after abstinence, as predicted based on previous studies implicating the importance of the BLA in cocaine-seeking triggered by conditioned cues. While the Core appeared to show the same effect, the results must be interpreted cautiously in light of nonspecific effects in the vehicle condition. It is surprising to note that the inactivation of the vPFC was found to enhance cocaine-seeking in this model, highlighting, for the first time, a role for this region of the medial prefrontal cortex in this unique form of cocaine-seeking. Also surprising was the finding that the inactivation of the neighboring dPFC did not alter cocaine-seeking in this model, resembling a form of relapse that follows abstinence without extinction. Hence, the circuitry mediating the spontaneous recovery of cocaine-seeking exhibits commonalities with extinction–reinstatement models (involvement of the BLA and Core), different commonalities with abstinence-alone models (lack of involvement by the dPFC and Shell), and demonstrates a unique role for the vPFC in regulating this form of cocaine-seeking.
References
Bouton ME, Westbrook RF, Corcoran KA, Maren S (2006) Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 60:352–360
Cammarota M, Bevilaqua LR, Barros DM, Vianna MR, Izquierdo LA, Medina JH, Izquierdo I (2005) Retrieval and the extinction of memory. Cell Mol Neurobiol 25:465–474
Capriles N, Rodaros D, Sorge RE, Stewart J (2003) A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 168:66–74
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 75:134–143
Di Ciano P, Everitt BJ (2002) Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol 13:397–405
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24:7167–7173
Di Ciano P, Benham-Hermetz J, Fogg AP, Osborne GE (2007) Role of the prelimbic cortex in the acquisition, re-acquisition or persistence of responding for a drug-paired conditioned reinforcer. Neuroscience (in press)
Fuchs RA, Evans KA, Parker MP, See RE (2004) Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 176:459–465
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309
Fuchs RA, Branham RK, See RE (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26:3584–3588
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142
Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM (1999) Building neural representations of habits. Science 286:1745–1749
Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650
Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002a) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22:1126–1136
Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002b) Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacology (Berl) 161:278–287
Lu L, Grimm JW, Hope BT, Shaham Y (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacolog 47(Suppl 1):214–226
McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663
McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537
McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–1560
McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 168:57–65
Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74
Myers KM, Davis M (2002) Behavioral and neural analysis of extinction. Neuron 36:567–584
Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC (2002) Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci 22:2916–2925
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, 5th edn. Academic, New York
Quirk GJ (2002) Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem 9:402–407
Quirk GJ, Russo GK, Barron JL, Lebron K (2000) The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20:6225–6231
Rescorla RA (2004) Spontaneous recovery. Learn Mem 11:501–509
Rhodes SE, Killcross S (2004) Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem 11:611–616
Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ (2004) Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 24:5704–5710
See RE (2005) Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol 526:140–146
See RE, Elliot JC, Feltenstein MW (2007) The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior following prolonged abstinence in rats. Psychopharmacology (Berl) 195:455
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3–20
Sierra-Mercado D Jr, Corcoran KA, Lebron-Milad K, Quirk GJ (2006) Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci 24:1751–1758
Acknowledgements
This work was supported by NIDA grants DA-12513 (PWK), DA-18486 (JP), and program project DA-05369.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, J., Vallone, J., Laurendi, K. et al. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology 197, 319–326 (2008). https://doi.org/10.1007/s00213-007-1034-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-1034-2