Abstract
Rationale
Systemic injections of 5-HT1B receptor agonists have been shown to have specific anti-aggressive effects in aggressive individuals. One site of action for these drugs is the 5-HT1B receptors in the ventral orbitofrontal cortex (VO PFC), an area that has been implicated in the inhibitory control of behavior and is a terminal region for 5-HT projections.
Objective
To assess the anti-aggressive effects of the 5-HT1B receptor agonist CP-94,253 when microinjected into the VO PFC (0.1, 0.56, and 1.0 μg/0.2 μl) or into the infralimbic prefrontal cortex (IL PFC; 1.0 μg/0.2 μl) in separate groups of aggressive resident male mice. To confirm the 5-HT1B receptor as the critical site of action for the anti-aggressive effects, the 5-HT1B/D antagonist GR-127,935 was microinjected at 10.0 μg/0.2 μl into the VO PFC. After recovery from surgery, the anti-aggressive effects of microinjected CP-94,253 were studied during 5-min resident–intruder confrontations that were recorded and analyzed.
Results
Microinjections of CP-94,253 (0.56 and 1.0 μg/0.2 μl) dose-dependently reduced the frequency of attack bites and sideways threats. This effect was behaviorally specific because non-aggressive motor activities were not significantly altered by the drug. In the IL vmPFC or in an area lateral to the VO PFC, CP-94,253 (1.0 μg/0.2 μl) did not have significant behavioral effects.
Conclusions
The results highlight the 5-HT1B receptors in the VO PFC as a particularly important site for the inhibition of species-typical aggressive behavior in male mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When activated systemically, 5-HT1B receptors exert an inhibitory role on several types of aggressive behavior in mice (for reviews, see Miczek et al. 2002; de Almeida et al. 2005). The 5-HT1B receptor agonists such as CP-94,253, anpirtoline, and zolmitriptan administered systemically have anti-aggressive effects in individuals with moderate as well as high levels of aggression without impairing non-aggressive activities (Fish et al. 1999; de Almeida et al. 2001b; de Almeida and Miczek 2002). Further support for the significant role of this receptor subtype derives from the finding of increased aggression in mutant 129Sv mice lacking the 5-HT1B receptor gene (Saudou et al. 1994; but see Bouwknecht et al. 2001 for a review).
The mouse and rat 5-HT1B receptor is functionally homologous to the human 5-HT1B receptor, differing by a single amino-acid (asparagine vs theonine) in the seventh transmembrane domain of the receptor (Schlicker et al. 1997; Sari 2004). The receptors are coupled to Gi/o proteins and are located both pre- and postsynaptically in several brain areas, with high density in areas such as the basal ganglia, striatum, and the frontal cortex, as well as in the raphe nuclei where they may act as somatodendritic autoreceptors (Boschert et al. 1994; Davidson and Stamford 1995; for a review, see Sari 2004). Activation of presynaptic 5-HT1B receptors inhibits 5-HT release and decreases extracellular concentrations of 5-HT in the cortex, ventral hippocampus, striatum, and diencephalon in rats and primates (Engel et al. 1986; Hoyer and Middlemiss 1989; Hjörth and Sharp 1991; Chopin et al. 1994; Martin and Humphrey 1994; Rollema et al. 1996; Bonaventure et al. 1997; Roberts et al. 1997; Knobelman et al. 2000, for a review, see Sari 2004). Recently, in vivo microdialysis data confirmed a 30–40% suppression of extracellular 5-HT in mouse medial PFC after systemic CP-94,253 administration of an anti-aggressive 10 mg/kg dose (Faccidomo et al. 2005). CP-94,253 exhibits significantly greater binding affinity for the 5-HT1B receptors (Ki=2.0±0.4 nM) than for other serotonergic receptor subtypes (Koe et al. 1992; Koe and Lebel 1995). This compound represents a useful tool for characterizing the role of 5-HT1B receptors in specific terminal regions in the regulation of aggressive behavior.
Administration of this agonist inhibits parental defense and predatory attacks, and it plays a specific inhibitory role in rodent aggression (Bell et al. 1995). Blockade of the CP-94,253 effects with the 5-HT1B/D receptor antagonist GR-127,935 points to the 5-HT1B receptors as the relevant site of action for anti-aggressive, antidepressant, locomotor stimulating, and anxiolytic-like effects (O’Neill et al. 1997; Parsons et al. 1998; Fletcher and Korth 1999; Knobelman et al. 2000; Millan et al. 2002). The 5-HT1B receptors appear to be particularly relevant to aggressive behavior, as agonists at this site reduce aggression without impairing locomotor behavior in mice and rats (Olivier et al. 1989a,b; Sijbesma et al. 1991; Sanchez et al. 1993; Fish et al. 1999; for a review, see Olivier and van Oorschot 2005). The selective agonist of the 5-HT1B receptor, CP-94,253, has proven to be very effective in reducing intensely high levels of aggression engendered by administration of alcohol or social instigation in mice (Fish et al. 1999; de Almeida et al. 2001a,b). Anpirtoline, another piperidine derivative with 5-HT1B agonist effects, also decreases alcohol-heightened aggression in mice without compromising motor functions (Miczek and de Almeida 2001).
Higher doses of CP-94,253 can also produce locomotor stimulating effects in different behavioral tests that do not involve aggression (O’Neill et al. 1997; Parsons et al. 1998; Fletcher and Korth 1999; Knobelman et al. 2000; Millan et al. 2002; Fish et al. 2000). The 5-HT1B receptors are thus of potential interest in the treatment of aggressive outbursts in the context of depression, schizophrenia, Parkinson’s disease, and impulsive disorders (Boulenguez et al. 1998; Moret and Briley 2000; Audinot et al. 2001; Millan et al. 2002).
The prefrontal cortex contains a high density of 5-HT1B receptors, and it is one of the most important sites of action for these drugs. The ventral orbitofrontal cortex (VO PFC) has been implicated in the inhibitory control of conditioned behavior (Chudasama and Robbins 2003) and is related to a range of affective and motivated behaviors in rodents, primates, and humans (Morgan and LeDoux 1995; Wall and Messier 2000; Wall et al. 2003). There is evidence showing regional differences between the dorsal and ventral [ventral prelimbic and infralimbic, ventromedial orbital] areas of the PFC in the control of cognitive, affective, and autonomic processing. For example, Chudasama and Robbins (2003) found that the VO and the IL PFC mediate dissociable but complementary processes in associative and inhibitory control over classically conditioned discriminative behavior. There is considerable further evidence that rodents possess well-defined subregions such as prelimbic, infralimbic, and orbitofrontal cortices (McAlonan and Brown 2003, Morgan et al. 2003), each with different cognitive, affective, and behavioral functions (Bechara et al. 2000; Kolb and Robbins 2003).
One strategy for evaluating the role of 5-HT1B receptors is to microinject a relatively selective agonist into specific VO PFC or IL PFC regions; another is to investigate differential contributions of these receptor pools to the control of aggressive behavior. To confirm the 5-HT1B receptor as a critical site of action for the anti-aggressive effects, a 5-HT1B/D antagonist GR-127,935 was microinjected into the ventral orbital prefrontal cortex.
Materials and methods
Animals
Adult male CF1 mice (FEPPS, Porto Alegre, RS, Brazil), weighing ca. 25 g on arrival, were housed in clear polycarbonate cages (28×17×14 cm) with wood chip bedding and wire lids through which rodent chow and water were available without restriction. Male mice (n=85) were housed as “residents” in pairs with females of the same strain. Additional male mice (n=90) were housed in groups of ten and served as “intruder” mice. The subjects were allowed to acclimate to the laboratory environment for 7 days with free access to food and water. All mice were housed in an environmentally controlled room maintained at 22±1°C on a 12:12 h photo cycle, with light on at 4 a.m. The animals were tested in the light phase of the photo cycle from 1 to 4 p.m. twice a week, each test separated by at least 72 h.
Surgery and histological analysis
After anesthesia (ketamine 100 mg/kg and xylazine 10 mg/kg body weight intramuscular), male mice (n=85) were placed in a stereotaxic apparatus with a mouse frame adaptor (David Kopf; Tujunga, CA, USA). The animals were implanted with a unilateral guide cannula (27 gauge) in the left hemisphere, fixed to the skull with dental cement. The coordinates for the ventral orbital prefrontal cortex were as follows: 2.2 mm anterior to bregma, 0.8 mm lateral to the midsagittal line, and 1.0 mm below dura mater; and for the infralimbic prefrontal cortex: 1.42 mm anterior to bregma, 0.6 mm lateral to the midsagittal line, and 1.0 mm below dura mater. The skull position was adjusted so that bregma and lambda were at the same level.
At the end of the experiment, all mice received an overdose of anesthetics. Brains were perfused with 0.9% saline solution and then with 10% formaldehyde. The brains were removed and fixed in 10% formaldehyde and later cut into 100 μm coronal sections on a vibratome. The slices were stained with Cresyl violet, and placements of the cannula tracks and tips were examined by microscopy to identify animals with an exact localization.
Drugs and injections
CP-94,253 (3-(1,2,5,6-tetrahydro-4-pyridyl)-5-propoxypirolo[3,2-b]pyridine; Charles Pfizer, Groton, CT, USA) was dissolved with the aid of sonication in a vehicle of 5% Tween 80, 5% dimethyl sulfoxide, and 90% distilled water. GR-127,935 (N-[4-methoxy-3-(4-methyl-1-piperazinyl)phenyl]-2-methyl-4′-(methyl-1,2,4-oxadiozol-3-yl)-[1,1-biphenyl]-4-carboxamide; Glaxo Research and Development, London, England) was suspended in 10% hydroxypropyl-betacyclodextrin and sonicated.
The injection needle (30 gauge) was 1.0 mm longer than the guide cannula, which was fixed 1.0 mm dorsal to the target areas. Therefore, only the inner cannula penetrated into the tissue. The solution was slowly infused over a 60-s period, using a Hamilton syringe connected by polyethylene tubing to the injection needle which stayed in situ for a further minute after the injection to allow drug diffusion. Animals were randomly assigned to be injected into the different brain regions with CP-94,253, GR-127,935, or vehicle.
One dose of CP-94,253 (0.1, 0.3, or 1.0 μg/kg for VO PFC and 1.0 μg/kg for IL PFC) or vehicle was administered in a counterbalanced sequence 10 min before the confrontation with the intruder. In the agonist dose–effect experiment, each animal received one drug treatment and one corresponding vehicle.
GR-127,935 (10.0 μg/kg) was administered 30 min before CP-94,253 (1, 3.0, 10.0 μg/kg), and all tests occurred 10 min after the administration of CP-94,253. Each dose combination was administered to separate groups of animals, and each drug test was accompanied by a vehicle test. In the antagonism experiment, the animals received maximally two microinjections, consisting of CP-94,253 and GR-127,935 on one occasion and the corresponding vehicles on the other.
Four cohorts of mice were studied as follows:
-
1.
CP-94,253 dose–effect determination into VO PFC. Four groups of seven mice each (n=28) were used to determine the dose-dependent effects of CP-94,253 (0.1, 0.56, or 1.0 μg/0.2 μl) or vehicle on aggressive behavior into the VO PFC.
-
2.
CP 94,253 dose–effect determination and antagonism by GR-127,935 into VO PFC. Fourty-five additional mice were used to determine the effects of GR-127,935 (10.0 μg/0.2 μl) on the dose–effect curve of CP-94,253 (1.0, 3.0, or 10.0 μg/0.2 μl) on aggressive behavior into the VO PFC. Specifically, the mice were assigned as follows:; for vehicle, n=8; GR-127,935 at the dose of 10.0 μg/0.2 μl, n=8; Vehicle + vehicle, n=8; GR-127,935 10.0 μg/0.2 μl + CP-94,253 1.0 μg/0.2 μl, n=7; GR-127,935 10.0 μg/0.2 μl + CP-94,253 3.0 μg/0.2 μl, n=6; and GR-127,935 10.0 μg/0.2 μl + CP-94,253 10.0 μg/0.2 μl, n=8.
-
3.
CP-94,253 into the IL PFC. Six more mice were used to assess the effects of CP-94,253 (1.0 μg/0.2 μl) and the corresponding vehicle on aggressive behavior into the IL PFC.
-
4.
For the microinjection experiment with CP-94,253 (1.0 μg/0.2 μl) into an area, more lateral to the VO PFC 6 mice were used.
Behavioral analysis
After 72 h of recovery from surgery, the effects of microinjected CP-94,253 and GR-127,935 were studied in male CF1 mice during resident–intruder confrontations. The behavioral recording began 30 min after the microinjection of GR127935 and 10 min after vehicle or CP-94,253 microinjections. The male intruder was placed in the resident’s cage, and their behaviors were videotaped (Miczek and O’Donnell 1978). An investigator with proven reliability analyzed the videotapes at a later time. Each behavioral sample lasted for exactly 5 min commencing with the resident’s first attack bite (i.e., the pre-attack data were not included).
The analysis of aggressive behavior comprised the measurement of salient acts and postures such as anogenital contact and sniffing the intruder, pursuit, sideways threat, bite, and tail rattle, as well as non-aggressive elements such as grooming, walking, and rearing as previously defined and illustrated (Miczek and O’Donnell 1978). Frequency and duration of all behaviors were recorded. Inter- and intra-observer reliability for encoding these behaviors was calculated using the Spearman correlation coefficient and ranged from 0.87 for the duration of walking to 0.95 for the frequency of attack bites.
Data analysis
All data for the experiments on the dose–effect determination of CP-94,253 and on the antagonism with GR-127,935 on aggression were analyzed using a one-way analysis of variance (One-Way ANOVA), and when appropriate (α<0.05), Bonferroni post hoc t tests were used with vehicle as the common control. The effect of GR-127,935 alone as compared with vehicle was analyzed using a paired t test. ED50s were calculated based on linear regression of the dose–effect curve, and 95% confidence intervals were calculated based on linear regression of the confidence intervals of the individual doses.
Results
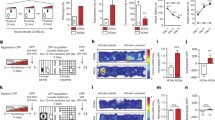
The histological analysis showed that 73 mice out of the total 85 animals had their cannula correctly placed in the VO PFC (Fig. 1), and six mice had their cannula correctly placed in the IL PFC (Fig. 2). An anatomical control group (n=6) was included in this experiment, comprised of animals with placements in a region more lateral to the VO PFC.
a Schematic representation of successive coronal sections (rostral to caudal) of the mouse brain (n=73) showing the histological verification of cannula placements in the ventral orbital prefrontal cortex. * represents the site of the cannula tip. VO (ventro orbital cortex): 2.68; 2.58; 2.46; 2.34, and 2.22 mm anterior to the bregma. Cg1 Cingulate cortex, area 1, PrL prelimbic cortex, MO medial orbital cortex, LO lateral orbital cortex. Images from Paxinos and Franklin 2001. b Photomicrograph of histological placement in the ventral orbital frontal cortex
a Schematic representation of successive coronal sections (rostral to caudal) of the mouse brain (n=6) showing the histological verification of cannula placements in infralimbic PFC. * represents the site of the cannula tip. IL: (infralimbic cortex): 1.78, 1.70, 1.54, and 1.42 mm anterior to the bregma. Cg1 cingulate cortex, area 1, M2 secondary motor cortex; PrL prelimbic cortex. Images from Paxinos and Franklin 2001. b Photomicrograph of histological placement in the infralimbic prefrontal cortex
Behavioral effects of CP-94,253 into ventral orbitofrontal prefrontal cortex
CP-94,253 exerted significant anti-aggressive effects when injected into the VO PFC. Specifically, CP-94,253 (0.56 and 1.0 μg/0.2 μl) reduced the frequency of attack bites [F(3,24)=9.889, p<0.001; Fig. 3a) and sideways threats [F(3,24)=16.877, p<0.001; Fig. 3b). The ED50 for the anti-aggressive effects of CP-94,253 (0.1–1.0 μg/0.2 μl) was 0.42 μg (CI95=0.21, 0.91). Microinjections of 1 μg CP-94,253 into the far lateral VO PFC (n=6) did not alter any of the salient elements of aggressive behavior (Table 2).
Effects of CP-94,253 on the frequency of attack bites (a) and lateral threat postures (b) by resident male mice. CP-94,253 was microinjected into VO PFC either alone (circles) or 30 min after microinjection of 10.0 μg GR-127,935 (diamonds). Data points are means±SEM, with *p<0.05 and **p<0.01 compared to vehicle. Correlation coefficients (r 2) for the linear regression lines ranged from 0.839 to 0.998
Non-aggressive motor activities such as walking, rearing, and grooming were not significantly altered by CP-94,253 in the presently studied dose range in either the VO PFC (Table 1) or the far lateral VO PFC (Table 2).
Behavioral effects of CP-94,253 into infralimbic prefrontal cortex
No significant effects on aggressive or non-aggressive behaviors were found after the microinjection of 1.0 μg CP-94,253 into the IL PFC (Fig. 4) as compared to the vehicle group (Table 2).
Effects of GR-127935 on aggression
No significant effects on aggressive and non-aggressive behaviors were found after microinjection of GR-127,935 (10.0 μg/0.2 μl) or after vehicle injections.
Effects of CP-94,253 on aggression: antagonism by GR-127,935
Pretreatment with GR-127,935 antagonized the reduction of aggressive behaviors such as frequency of bites and sideways threats (Fig. 3). After pretreatment with GR-127,935, the ED50 for the anti-aggressive effects of CP-94,253 was shifted from 0.42 to 13.33 μg (CI95=1.40–31.33).
Discussion
The key finding of the present studies is that the 5-HT1B receptor agonist CP-94,253 microinjected directly into the prefrontal cortex, particularly into the ventral orbital area, decreases aggression in male mice in a pharmacologically and behaviorally specific manner. When injected into the VO PFC, CP-94,253 (0.56 and 1.0 μg/0.2 μl) significantly reduced the frequency of attack bites and sideways threats and did not have significant effects on the non-aggressive behavioral elements. The decrease in aggression can be considered as behaviorally specific because the non-aggressive motor activities were not significantly altered by the drug. By contrast, when injected into the IL PFC, CP-94,253 (1.0 μg/0.2 μl) did not have significant behavioral effects on either species-typical aggression or on the non-aggressive behavioral elements. Similarly, microinjections into the region lateral of the VO PFC at the same 1.0 μg dose remained without significant behavioral effects.
Evidence for the 5-HT1B receptor as an important site of action for CP-94,253 originates from the observation that administration of GR-127,935, an antagonist with high affinity (ca. 10 nM) for 5-HT1B/D receptors (Skingle et al. 1996), produced a rightward shift of the dose–effect curve for CP-94,253. Systemic injections of GR-127,935 have also been shown to antagonize the effects of 5-HT1B agonists on various behaviors such as locomotor activity, alcohol intake, drug reinforcement, and aggressive behavior (Maurel et al. 1998; Parsons et al. 1998; Fish et al. 1999; Castanon et al. 2000; Tomkins and O’Neill 2000; de Almeida et al. 2001b; Miczek and de Almeida 2001; Fletcher et al. 2002).
The 5-HT1B receptors have been implicated in several physiological functions and behavioral and psychiatric disturbances including migraine, locomotor hyperactivity, drug reinforcement, depression and anxiety states, in addition to aggressive behavior (for a review, see Sari 2004). For example, stimulation of 5-HT1B receptors can result in a suppression of migraine (Humphrey et al. 1991), an increase in locomotor activity (Rempel et al. 1993; Scearce-Levie et al. 1999), increased satiety (Lee and Simansky 1997), a decrease of anxiety-like behavior, increased antidepressant effects (Lucas et al. 1997; Matzen et al. 2000) as well as enhancement of some of the reinforcing effects of cocaine (Parsons et al. 1996;1999) but a decrease in alcohol intake (Maurel et al. 1999; Tomkins and O’Neill 2000). It appears that this receptor subtype represents an important target for several potential therapeutic interventions, and it remains to be discovered whether the mechanisms for the anti-aggressive effects can be dissociated from those on other behavioral and physiological functions.
Several pharmacological studies have shown that the activation of 5-HT1B receptors reduces aggression in male mice and rats after systemic injections (Olivier et al. 1989a,b; Sanchez et al. 1993; Mos et al. 1992, 1993; Fish et al. 1999; de Almeida et al. 2001b; Bannai et al. 2006). A nonselective agonist eltoprazine, acting on 5-HT1A/1B/2C receptors, decreases aggression by resident male rats toward an intruder (Schipper et al. 1990). CGS 12066B, CP-94,253, and other 5-HT1B receptor agonists such as anpirtoline and zolmitriptan also reduce aggression in male mice (Bell et al. 1995; Fish et al. 1999; Miczek and de Almeida 2001; de Almeida et al. 2001a,b).
CP-94,253 is one of the most selective agonists for 5-HT1B receptors (Ki=2.0±0.4 nM; Koe et al. 1992; Koe and Lebel 1995). When microinjected into neural areas which contain a high density of 5-HT1B receptors such as the frontal cortex (Bruinvels et al. 1993; Sari et al. 1999; Millan et al. 2002), CP-94,253 decreases aggressive behavior by modulating serotonergic transmission either at presynaptic or postsynaptic receptors.
Increased levels of serotonin (5-HT) are often related to a decrease in aggression in individuals with a high aggressive trait (Chiavegatto and Nelson 2003; Mehlman et al. 1994; Higley et al. 1996; Ferrari et al. 2005; de Almeida et al. 2005). For example, SSRIs increase brain 5-HT and exert anti-aggressive effects (Olivier et al. 1989a; Ferris and Delville 1994). These data are interesting and suggestive but at the same time limited because they do not provide sufficient information on the time course and anatomical specificity of the precise serotonergic changes that are linked to aggressive behavior. Contrary to the serotonin deficiency hypothesis, it remains to be resolved how agonists at 5-HT1A and 5-HT1B receptors decrease serotonin in corticolimbic terminals via action at somatodendritic or presynaptic sites, at the same time significantly decrease aggressive behavior (de Almeida and Lucion 1997; Miczek et al. 1998; Bannai et al. 2006; de Boer and Koolhaas 2005). There are only a few studies that try to establish a parallel between specific brain areas rich in 5-HT1B receptors and changes in aggressive behavior upon activation of a discrete receptor pool (e.g., Faccidomo and Miczek 2004; Faccidomo et al. 2005).
5,7-DHT neurotoxic lesions of the raphe nuclei do not appear to prevent the anti-aggressive effects of CP-94,253 when injected systemically (de Almeida et al. 2001b). These data suggest that the effects of CP-94,253 originate from action on 5-HT1B receptors that are localized postsynaptically because destruction of the 5-HT containing neurons in the dorsal raphe did not alter the effects of CP-94,253. However, because the neurotoxic lesions were only partial, a presynaptic site of action for CP-94,253 cannot be excluded.
Recently, the prefrontal cortex, more specifically the orbitofrontal region, has been assigned an important role in the inhibitory control of behavior, mainly impulsive and aggressive behavior (Blair 2001; Seguin 2004; Cardinal et al. 2004; Spinella 2004; Kheramin et al. 2005). The infralimbic area has been involved more in anxiety-like effects and cognitive functions such as attention and memory than in aggressive behavior (Wall and Messier 2002; Dalley et al. 2004). Faccidomo et al. (2005) found a 30% decrease of 5-HT in the infralimbic area after intraperitoneal CP-94,253 in male mice. Moreover, it is important to point out that the samples were collected from single mice in the absence of aggressive behavior. Fighting itself can lower levels of 5-HT in prefrontal cortex of resident rats (van Erp and Miczek 2000), and the lowered tonic activity of 5-HT may influence how 5-HT1B agonists achieve their effects.
The present results highlight the 5-HT1B receptors in the ventral orbital prefrontal cortex as a particularly important site for the inhibition of species-typical aggressive behavior. Future studies will have to determine whether this effect is achieved via pre- or postsynaptic 5-HT1B receptor pools. Ongoing work assesses the role of more specific 5-HT1B agonists and antagonists microinjected into amygdaloid nuclei, striatum, hypothalamic nuclei, dorsal periaqueductal area, and raphe nuclei to delineate the serotonergic circuit mediating male aggression.
References
Audinot V, Newman-Tancredi A, Millan MJ (2001) Constitutive activity at serotonin 5-HT1D receptors: detection by homologous GTPγS versus [35S]-GTPγS binding isotherms. Neuropharmacology 40:57–64
Bannai M, Fish EW, Faccidomo S, Miczek KA (2006) Escalated aggressive behavior and medial prefrontal (infralimbic) cortex 5-HT: reduction by the 5-HT1B receptor agonist CP-94,253 in mice. Neuropsychopharmacology (in press)
Bechara A, Damasio H, Damasio AR (2000) Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10:295–307
Bell R, Donaldson C, Gracey D (1995) Differential effects of CGS 12066B and CP-94,253 on murine social and agonistic behavior. Pharmacol Biochem Behav 52:7–16
Blair RJ (2001) Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J Neurol Neurosurg Psychiatry 71:727–731
Bonaventure P, Schotte A, Cras P, Leysen JE (1997) Autoradiographic mapping of 5-HT1B and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Receptors Channels 5:225–230
Boschert U, Amara DA, Segu L, Hen R (1994) The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience 58:167–182
Boulenguez P, Peters SL, Mitchell SN, Chauveau J, Gray JA, Joseph MH (1998) Dopamine release in the nucleus accumbens and latent inhibition in the rat following microinjections of a 5-HT1B agonist into the dorsal subiculum: implications for schizophrenia. J Psychopharmacol 12:258–267
Bouwknecht JA, Hijzen TH, van der GJ, Maes RA, Hen R, Olivier B (2001) Absence of 5-HT1B receptors is associated with impaired impulse control in male 5-HT1B knockout mice. Biol Psychiatry 49:557–568
Bruinvels AT, Palacios JM, Hoyer D (1993) Autoradiographic characterization and localization of 5-HT1D compared with 5-HT1B binding sites in rat brain. Naunyn-Schmiedeberg’s Arch Pharmacol 347:569–582
Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ (2004) Limbic corticostriatal systems and delayed reinforcement. Ann NY Acad Sci 1021:33–50
Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R (2000) Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol Biochem Behav 67:559–566
Chiavegatto S, Nelson RJ (2003) Interaction of nitric oxide and serotonin in aggressive behavior. Horm Behav 44:233–241
Chopin P, Moret C, Briley M (1994) Neuropharmacology of 5-hydroxytryptamine1B/D receptor ligands. Pharmacol Ther 62:385–405
Chudasama Y, Robbins TW (2003) Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci 23:8771–8780
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
Davidson C, Stamford JA (1995) Evidence that 5-hydroxytryptamine release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5HT1B and 5-HT1D autoreceptors. Br J Pharmacol 114:1107–1109
de Almeida RMM, Lucion AB (1997) 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology 134:392–400
de Almeida RMM, Miczek KA (2002) Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: inhibition by anpirtoline—a 5-HT1B receptor agonist. Neuropsychopharmacology 27:171–181
de Almeida RMM, Faccidomo S, Fish E, Miczek KA (2001a) Inhibition of alcohol-heightened aggression by action at post-synaptic 5-HT1B receptors in male mice. Aggress Behav 3:234–235
de Almeida RMM, Nikulina EM, Faccidomo S, Fish EW, Miczek KA (2001b) Zolmitriptan—a 5-HT1B/D agonist, alcohol, and aggression in mice. Psychopharmacology 157:131–141
de Almeida RMM, Ferrari PF, Parmigiani S, Miczek KA (2005) Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol 526:51–64
de Boer SF, Koolhaas JM (2005) 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526:125–139
Engel G, Gothert M, Hoyer D, Schlicker E, Hillenbrand K (1986) Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT binding sites. Naunyn-Schmiedeberg’s Arch Pharmacol 322:1–7
Faccidomo S, Miczek KA (2004) Cortical modulation of alcohol-heightened aggression in mice: effect of the 5-HT1B agonist, CP-94,253. Online Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC
Faccidomo S, Bannai M, van Trigt RL, DeBold JF, Miczek KA (2005) Alcohol-heightened aggression and corticolimbic 5-HT in mice: infusion and reverse microdialysis of 5-HT1B agonists into the infralimbic and orbitofrontal cortex. Online Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC
Ferrari PF, Palanza P, Parmigiani S, de Almeida RMM, Miczek KA (2005) Serotonin and aggressive behavior in rodents and nonhuman primates: predispositions and plasticity. Eur J Pharmacol 526:259–273
Ferris CF, Delville Y (1994) Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology 19:593–601
Fish EW, Faccidomo S, Miczek KA (1999) Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology 146:391–399
Fish EW, Sekinda M, Ferrari PF, Dirks A, Miczek, KA (2000) Distress vocalizations in maternally-separated mouse pups: modulation via 5-HT1A, 5-HT1B and GABAA receptors. Psychopharmacology 149:277–285
Fletcher PJ, Korth KM (1999) RU-24969 disrupts d-amphetamine self-administration and responding for conditioned reward via stimulation of 5-HT1B receptors. Behav Pharmacol 10:183–193
Fletcher PJ, Azampanah A, Korth KM (2002) Activation of 5-HT1B receptors in the nucleus accumbens reduces self-administration of amphetamine on a progressive ratio schedule. Pharmacol Biochem Behav 71:717–725
Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M (1996) CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry 40:1067–1082
Hjörth S, Sharp T (1991) Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci 48:1779–1786
Hoyer D, Middlemiss DN (1989) Species-differences in the pharmacology of terminal 5-HT autoreceptors in mammalian brain. Trends Pharmacol Sci 10:130–132
Humphrey PP, Feniuk W, Marriott AS, Tanner RJ, Jackson MR, Tucker ML (1991) Preclinical studies on the anti-migraine drug, sumatriptan. Eur Neurol 31:282–290
Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM (2005) The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav Brain Res 156:145–152
Knobelman DA, Kung HF, Lucki I (2000) Regulation of extracellular concentrations of 5-hydroxytryptamine (5-HT) in mouse striatum by 5-HT1A and 5-HT1B receptors. J Pharmacol Exp Ther 292:1111–1117
Koe BK, Lebel LA (1995) Effects of serotoninergic agents on downregulation of beta-adrenoceptors by the selective serotonin reuptake inhibitor sertraline. Arch Int Pharmacodyn Ther 329:231–244
Koe BK, Nielsen JA, Macor JE, Heym J (1992) Biochemical and behavioral studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res 26:241–250
Kolb B, Robbins T (2003) The rodent prefrontal cortex. Behav Brain Res 146:1–2
Lee MD, Simansky KJ (1997) CP-94,253: A selective serotonin (1B) (5-HT1B) agonist that promotes satiety. Psychopharmacology 131:264–270
Lucas JJ, Seguin L, Hen R (1997) 5-hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol Pharmacol 51:755–763
Martin GR, Humphrey PPA (1994) Classification review: receptors for 5-hydroxytryptamine: current perspectives on classification and nomenclature. Neuropharmacology 33:261–273
Matzen L, van Amsterdam C, Rautenberg W, Greiner HE, Harting J, Seyfried CA, Bottcher H (2000) 5-HT reuptake inhibitors with 5-HT1B/1D antagonistic activity: a new approach toward efficient antidepressants. J Med Chem 43:1149–1157
Maurel S, Schreiber R, De Vry J (1998) Role of 5-HT1B, 5-HT2A and 5-HT2C receptors in the generalization of 5-HT receptor agonists to the ethanol cue in the rat. Behav Pharmacol 9:337–343
Maurel S, De Vry J, Schreiber R (1999) 5-HT receptor ligands differentially affect operant oral self-administration of ethanol in the rat. Eur J Pharmacol 370:217–223
McAlonan K, Brown VJ (2003) Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146:97–103
Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M (1994) Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry 151:1485–1491
Miczek KA, O’Donnell JM (1978) Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and l-dopa. Psychopharmacology 57:47–55
Miczek KA, de Almeida RMM (2001) Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology 57:421–429
Miczek KA, Hussain S, Faccidomo S (1998) Alcohol-heightened aggression in mice: Attenuation by 5-HT1A receptor agonists. Psychopharmacology 139:160–168
Miczek KA, Fish EW, DeBold JF, de Almeida RMM (2002) Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology 163:434–458
Millan MJ, Newman-Tancredi A, Lochon S, Touzard M, Aubry S, Audinot V (2002) Specific labelling of serotonin 5-HT1B receptors in rat frontal cortex with the novel, phenylpiperazine derivative, [3H]GR125,743—a pharmacological characterization. Pharmacol Biochem Behav 71:589–598
Moret C, Briley M (2000) The possible role of 5-HT1B/D receptors in psychiatric disorders and their potential as a target for therapy. Eur J Pharmacol 404:1–12
Morgan MA, LeDoux JE (1995) Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109:681–688
Morgan MA, Schulkin J, LeDoux JE (2003) Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res 146:121–130
Mos J, Olivier B, Poth M, Aken H (1992) The effects of intraventricular administration of eltoprazine, 1-(3-trifluoromethylphenyl) piperazine hydrochloride and 8-OH-DPAT on resident intruder aggression in the rat. Eur J Pharmacol 212:295–298
Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H (1993) The effects of dorsal raphe administration of eltoprazine, TFMPP and 8-OH-DPAT on resident intruder aggression in the rat. Eur J Pharmacol 238:411–415
Olivier B, van Oorschot R (2005) 5-HT1B receptors and aggression: a review. Eur J Pharmacol 526:207–217
Olivier B, Mos J, Tulp M, Schipper J (1989a) Modulatory action of serotonin in aggressive behaviour. In: Archer T (ed) Behavioral pharmacology of 5-HT. Lawrence Erlbaum, Hillsdale, NJ
Olivier B, Mos J, Van der Heyden J, Hartog J (1989b) Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology 97:154–156
O’Neill MF, Fernández AG, Palacios JM (1997) Activation of central 5HT1B receptors increases locomotor activity in mice. Hum Psychopharmacol 12:431–435
Parsons LH, Weiss F, Koob GF (1996) Serotonin-1B receptor stimulation enhances domanime-mediated reinforcement. Psychopharmacology 128:150–160
Parsons LH, Weiss F, Koob GF (1998) Serotonin-1B receptor stimulation enhances cocaine reinforcement. J Neurosci 18:10078–10089
Parsons LH, Koob GF, Weiss F (1999) RU 24969, 5-HT1B/1A receptor agonist, potentiates cocaine-induced increases in nucleus accumbens dopamine. Synapse 32:132–135
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic, San Diego, CA
Rempel NL, Callaway CW, Geyer MA (1993) Serotonin-1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology 8:201–211
Roberts C, Price GW, Jones BJ (1997) The role of 5-HT1B/1D receptors in the modulation of 5-hydroxytryptamine levels in the frontal cortex of the conscious guinea pig. Eur J Pharmacol 326:23–30
Rollema H, Clarke T, Sprouse JS, Schulz DW (1996) Combined administration of a 5-hydroxytryptamine (5-HT) 1D antagonist and a 5-HT reuptake inhibitor synergistically increases 5-HT release in guinea pig hypothalamus in vivo. J Neurochem 67:2204–2207
Sanchez C, Arnt J, Hyttel J, Moltzen EK (1993) The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology 110:53–59
Sari Y (2004) Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28:565–582
Sari Y, Miguel M-C, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D (1999) Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88:899–915
Saudou F, Amara DA, Dierich A, Lemeur M, Ramboz S, Segu L, Buhot MC, Hen R (1994) Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265:1875–1878
Scearce-Levie K, Viswanathan SS, Hen R (1999) Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology 141:154–161
Schipper J, Tulp MT, Sijbesma H (1990) Neurochemical profile of eltoprazine. Drug Metabol Drug Interact 8:85–114
Schlicker E, Fink K, Molderings GJ, Price GW, Duckworth M, Gaster L, Middlemiss DN, Zentner J, Likungu J, Gothert M (1997) Effects of selective h5-HT1B (SB-216641) and h5-HT1D (BRL-15572) receptor ligands on guinea-pig and human 5-HT auto- and heteroreceptors. Naunyn-Schmiedeberg’s Arch Pharmacol 356:321–327
Seguin JR (2004) Neurocognitive elements of antisocial behavior: relevance of an orbitofrontal cortex account. Brain Cogn 55:185–197
Sijbesma H, Schipper J, de Kloet ER, Mos J, van Aken H, Olivier B (1991) Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Beh 38:447–458
Skingle M, Beattie DT, Scopes DI, Starkey SJ, Connor HE, Feniuk W, Tyers MB (1996) GR127935: a potent and selective 5-HT1D receptor antagonist. Behav Brain Res 73:157–161
Spinella M (2004) Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Int J Neurosci 114:95–104
Tomkins DM, O’Neill MF (2000) Effect of 5-HT1B receptor ligands on self-administration of ethanol in an operant procedure in rats. Pharmacol Biochem Behav 66:129–136
Van Erp AMM, Miczek KA (2000) Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci 20:9320–9325
Wall PM, Messier C (2000) Concurrent modulation of anxiety and memory. Behav Brain Res 109:229–241
Wall PM, Messier C (2002) Infralimbic kappa opioid and muscarinic M1 receptor interactions in the concurrent modulation of anxiety and memory. Psychopharmacology 160:233–244
Wall PM, Blanchard RJ, Yang M, Blanchard DC (2003) Infralimbic D2 receptor influences on anxiety-like behavior and active memory/attention in CD-1 mice. Prog Neuropsychopharmacol Biol Psychiatry 27:395–410
Acknowledgements
The authors would like to thank Mr. J. Thomas Sopko and Mr. Dirson João Stein for their outstanding technical support. This research was supported in part by UNISINOS, Universidade do Vale do Rio dos Sinos, RS, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Almeida, R.M.M., Rosa, M.M., Santos, D.M. et al. 5-HT1B receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology 185, 441–450 (2006). https://doi.org/10.1007/s00213-006-0333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0333-3