Abstract
Rationale
Unpleasant side effects of drugs of abuse often limit their repeated use; however, such effects may be attenuated in adolescents compared to adults.
Objectives
We investigated whether the anxiogenic, aversive, or locomotor effects of delta-9-tetrahydrocannabinol (THC) differ between adolescent and adult rats.
Methods
We used the elevated plus maze (EPM) and light-dark tests of anxiety, the conditioned taste aversion and conditioned place aversion (CPA) tests of generalized aversion, and measures of stress hormone levels in serum to examine effects of THC in adolescent and adult rats. Locomotor activity was also recorded in the EPM, light-dark task, and CPA association sessions.
Results
In the EPM and light-dark tasks, THC was anxiogenic in both age groups, but the drug was more anxiogenic in adults than in adolescents. In the place and taste aversion tasks, THC was aversive in both ages, and at 1.25 and 5 mg/kg, was more aversive in adults than in adolescents. The locomotor response to THC, as measured in the anxiety tasks and CPA, affected adults more than adolescents. Multiple measures revealed a locomotor-decreasing effect in adults, whereas some measures suggested a small locomotor-increasing effect in adolescent rats.
Conclusions
These results suggest that THC can have greater anxiogenic, aversive, and locomotor-reducing effects in adult rats than in adolescent rats. These findings suggest an explanation for reduced marijuana use in adult humans compared to teenagers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marijuana is a widely used drug, which can elicit many of the DSM-IV characteristics of drug dependence (APA 1994; Chen et al. 1997). Use of marijuana and other abused drugs typically begins in adolescence and peaks in the late teens and early 20s (Chen and Kandel 1995). This is a concern because younger users are more likely to develop dependence than older users at all levels of consumption (Chen et al. 1997). Initiation of drug use is largely dependent on opportunity and other social factors, but each individual’s decision to repeat drug use is largely dependent on the pleasantness of the first experience (Haertzen et al. 1983). Thus, the initial subjective effects of a drug can determine whether a user repeats drug use and therefore progresses to addiction.
The age of the user is an important determinant in the subjective effects of many drugs of abuse. Conditioned taste aversion (CTA) to amphetamine is smallest in adolescent rats compared to either juvenile or young adult rats (Infurna and Spear 1979). Similar observations have been made for nicotine (Shram et al. 2006; Wilmouth and Spear 2004). For ethanol, adolescent rats are less sensitive to social inhibitory effects (Varlinskaya and Spear 2004b), hangover-induced anxiety (Doremus et al. 2003; Varlinskaya and Spear 2004a), and sedative effects (Little et al. 1996). These and many other examples demonstrate that adolescents may be less sensitive to use-limiting, undesirable effects of drugs of abuse. This age effect may begin to explain the elevated prevalence of drug use and dependence during adolescence.
Marijuana has many positive and negative effects on its users. One common adverse reaction to cannabis use is acute anxiety or panic attack (Thomas 1996). An adverse reaction like this often leads to cessation of drug intake (Thomas 1996).
The purpose of the present study was to characterize undesirable effects of THC and to examine the effect of age on these properties. We hypothesized that acute THC might be less anxiogenic or aversive in adolescent rats. We tested the anxiety-related effects of THC using the elevated plus maze (EPM) and the light-dark task. We examined THC-induced aversion using CTA and conditioned place aversion (CPA) and measured stress hormone responses to an acute dose of THC. The results show that THC is anxiogenic, aversive, and can induce a stress hormone response in both adolescent and adult rats. The undesirable effects were stronger in adult than in adolescent rats.
Materials and methods
Subjects
Male CD rats were obtained from Charles River Laboratories (Raleigh, NC, USA) and allowed to habituate to our housing colony for 7 days before the start of each experiment. Naive rats were used in each experiment. “Adolescent” rats were 28 days of age; “adult” rats were 64–66 days of age at the beginning of each experiment (Spear 2000). All procedures were approved by Duke University’s Animal Care and Use Committee.
Elevated plus maze
The EPM measures anxiety [time in the open vs closed arms (Pellow et al. 1985; Pellow and File 1986)] and activity [transitions between arms (Doremus et al. 2004)]. The maze is made of sealed wood arranged in a “+” shape which is elevated 90 cm above the floor. The north and south arms are open and are 50 × 10 cm. The east and west arms are enclosed with walls that are 36 cm high. The EPM was surrounded by white curtains on all four sides. The room was lit by incandescent light so that the brightness on the open arms was approximately 7 lx, and in the closed arms was approximately 3.5 lx. A total of 99 rats were used for this experiment. Rats were placed in an open field apparatus in the same room for 5 min before placement on the EPM. They were then moved immediately to the maze, where a video camera recorded their behavior from above for 5 min. Videos were scored for entries into and time spent in each arm. Percentage of time in the open arms is calculated as:
Light-dark task
The light-dark task was performed in Hamilton–Kinder activity monitors (40 × 40 × 40 cm) fitted with “dark” inserts (20 × 40 × 40 cm). The room was lit by incandescent lamps, resulting in 85–125 lx on the light side and 0–2 lx on the dark side. A small opening allowed free movement between the two sides. The floor of both halves of the box was covered with corncob bedding. Each rat was gently placed in the light side of the chamber and monitored for 15 min. Eighty rats were used for this experiment.
Three dependent measures from these sessions are reported in “Results”: (1) total distance traveled (cm) in both the light and dark sides, a measure of locomotor activity; (2) time spent in the light half of the chamber (seconds), an indicator of anxiety [one rat, which spent 900 s on the light side, was excluded from this analysis as a statistical outlier (Grubbs test)]; and (3) emergence from the dark (emergence interval), a second measure of anxiety. Each 15-min session was divided into 5-s intervals (180 intervals per session). The rat’s position during each interval was noted (light side, dark side, or transitioning). Most rats initially explored the light half of the chamber for 10–15 s and then went into the dark side, emerging again later. The “emergence interval” is the interval at which the rat first re-enters the light side. If a rat went into the dark and never emerged again, he was given a score of 180. One rat was excluded from this analysis because he spent the first 79 intervals in the light, then entered the dark, and never emerged again.
Conditioned taste aversion
For CTA experiments, rats were water-deprived for 24 h and then allowed 15-min access to tap water in cages containing only pre-weighed water bottles and bedding. They were then returned to the home cage with ad libitum access to food and water for 24 h. The next day, they were water deprived for 24 h and given 15-min access to 0.2% saccharin solution. Immediately after the 15-min saccharin access, they were injected intraperitoneally with the indicated dose of THC, vehicle, or saline and returned to their home cages with ad libitum access to drinking water for 24 h. On the final day, the rats were water-deprived for 24 h and then given 15-min access to two bottles—one containing water and the other containing 0.2% saccharin solution. The water bottle was always placed on the right side of the cage, saccharin on the left, to generate both a place and taste association. The intake of all fluids was measured by weighing the bottles before and after each session. The measure of interest is the “percent saccharin choice” on the test day, expressed as:
Rats which consumed less than 1 ml of total solution on either the water day, saccharin day, or test day were excluded from further analysis. Out of a total of 121 rats, 16 were excluded for drinking less than 1 ml.
Conditioned place aversion
CPA experiments were performed in Hamilton–Kinder activity monitors (40 × 40 × 40 cm) fitted with inserts to create a two-chamber apparatus (each chamber was 20 × 40 × 40 cm). One chamber had black walls and a smooth, transparent Plexiglas floor. Metal bars from the shelves supporting the equipment were visible below the chamber floor. The other half of the box contained white walls on three sides and a mesh floor made of Pet-D-fence screening (Lowe’s home improvement) held in place by screen framing. The central wall dividing the two chambers was black and contained an arch-shaped opening to allow movement between the two sides. This doorway was blocked with black Plexiglas for conditioning sessions (see below). Incandescent bulbs provided ambient lighting resulting in 6–12 lx on the black side of the chamber and 10–21 lx on the white side. Fecal matter was removed and the chambers wiped with water after each session. Time spent per side and distance traveled (inches) was recorded in each session.
Naive rats were first given a choice test in which they were placed on the white side of the chamber and allowed to move freely between the two sides for 20 min. For the following 3 days, rats were exposed to conditioning sessions. Each morning (between 0800 and 1100 hours), they were given an injection of ethanol/emulphor vehicle (see “Drug Injections” below) 10 min before being confined to one side of the chamber for 30 min. Each afternoon (between 1300 and 1500 hours), they were given an injection of drug (vehicle, 0.5 mg/kg THC or 5 mg/kg THC as indicated) 10 min before being confined to the opposite side of the chamber for 30 min. These methods were adapted from several published reports (Braida et al. 2004; Cheer et al. 2000; Sanudo-Pena et al. 1997; Schramm-Sapyta et al. 2004). Association of the drug and vehicle with the black and white sides of the chamber was counterbalanced across the group, and there was no effect of side on place aversion or locomotion. After 3 days of association training, the rats were again given a choice test as on day 1. The data from the final choice test is reported in Fig. 6 as the total time spent, in seconds, on the drug-associated side of the chamber. A reduction in time spent on the drug-associated side compared to vehicle treatment is considered evidence of a place aversion. A total of 63 rats were used for these experiments.
Stress hormones
For measurement of hypothalamic pituitary adrenal (HPA) axis activation, rats were weighed, injected intraperitoneally with the indicated dose of THC, and returned to their home cage. After the indicated time period, they were removed from the cage and were killed by decapitation under Isoflurane anesthesia for collection of trunk blood. Rats in the same cage were injected and killed within 2 min of each other to minimize the effect of disturbance on hormonal activation. Anesthesia was achieved in less than 60 s, and previous experiments have demonstrated that this duration of Isoflurane anesthesia does not affect adrenocorticotropic hormone (ACTH) or corticosterone levels (unpublished observations). The blood was kept on ice until it was centrifuged at 3,000×g for 15 min. Serum was frozen at −80°C until analysis of corticosterone and ACTH levels. Corticosterone levels were determined using the rat corticosterone coat-a-count kit from Diagnostic Products Corporation (Los Angeles, CA, USA), and ACTH levels were determined using the ACTH kit from Diasorin (Stillwater, MN, USA). Inter- and intra-assay coefficients of variation varied less than 10 and 5% respectively. A total of 172 rats were used for these experiments.
Drug injections
THC was dissolved in a solution containing 10% ethanol:emulphor (1:1) and 90% normal saline. Rats were injected intraperitoneally with the indicated doses in a volume of 1 ml/kg. Controls received the 10% ethanol/emulphor/90% saline vehicle or normal saline where indicated. For light-dark and EPM experiments, rats were injected 30 min before the start of the test. For stress hormone measurements, rats were injected at the times indicated before decapitation. For CTA experiments, rats were injected immediately after the saccharin drinking session. For CPA association training, rats were injected 10 min before being placed in the chamber.
Statistics
Effects of age, dose, and time were assessed using ANOVA, repeated measures ANOVA, and the indicated post-hoc tests where appropriate. Data were analyzed using Microsoft Excel and Statview statistical software.
Results
Elevated plus maze
Results obtained from the EPM are shown in Fig. 1. THC was anxiogenic in rats of both ages. There was a significant effect of drug dose on the percentage of time spent in the open arms (p < 0.05), but no significant age effect and no age × drug interaction (Fig. 1a). Vehicle-treated adolescent and adult rats spent equal amounts of time in the open arms. After 0.5 mg/kg THC, adults spent less time in the open arms than adolescents (Fisher’s protected least significant difference, p < 0.05). After 2.5 mg/kg THC, adolescents and adults spent equal amounts of time in the open arms, which was significantly less than the time spent after saline injection (Fisher’s PLSD, p < 0.05). Although there was not an overall effect of age, THC was less anxiogenic in adolescents than in adults at 0.5 mg/kg.
Behavior in the EPM. Rats were placed in the center of the EPM and videotaped for 5 minutes (see methods) and their % open arm time (a) and entries into both open and closed arms (b) are presented. *, significantly different from adolescents at same dose (p < 0.05). **, significantly different from vehicle treated group (p < 0.05). N = 16–17 per group
THC also had a stronger locomotor-reducing effect in adults than in adolescents in the EPM. Total arm entries, a measure of locomotor activity, showed an age-dependent effect of THC (Fig. 1b). There was a significant effect of age (p < 0.001) and a significant age × dose interaction (p < 0.05). Adults exhibited a significant decrease in total entries with increasing doses of THC (ANOVA, p < 0.05). Adolescents, in contrast, did not decrease their arm entries after THC and showed a trend toward an increase in total entries (ANOVA, p = 0.08). Paired comparisons at each dose revealed that adults made fewer entries than adolescents at 0.5 mg/kg (p < 0.01) and 2.5 mg/kg (p < 0.01). By this measure, THC reduced locomotor activity in adults but not in adolescents.
Light-dark task
To corroborate our findings from the EPM, we examined behavior in another measure of anxiety, the light-dark task. Results from these experiments are shown in Fig. 2. Again, we found dose-specific age differences in THC’s anxiogenic effects and significant age differences in locomotor activity. After vehicle injection, adolescent and adult rats spent similar amounts of time in the light half of the chamber (Fig. 2a). The time in the light was reduced dose-dependently by THC in both adolescents and adults (dose effect: adult group, p < 0.01; adolescent group, p < 0.02; age and interaction effects were not significant). However, when we examined the time in the light in 5-min blocks, as shown in Fig. 2b, we observed a baseline difference between the two ages. There was also a significant overall effect of THC dose (p < 0.01) and a significant age × time interaction (p < 0.05). Adolescents spent more time in the light during the first 5 min than adults, and this was reduced by THC in a dose-dependent manner. Adults spent so little time in the light during the first 5 min that no further decrease could be detected. The overall anxiogenic effect in adults became apparent at later time points. In short, during the first 5 min of the light-dark task, THC was anxiogenic in adolescents but not in adults due to a floor effect in the adults.
Position in the light-dark task. Rats were treated with the indicated doses of THC (see methods) and behavior in the light-dark box was recorded for 15 minutes. (a) Time in light out of 15 minutes. (b) Time in light in sessions divided into 5-minute time blocks (c) Emergence from the dark after initial entry. N = 9–10 per group
Emergence from the dark (Fig. 2c) showed age differences at baseline and in response to THC. Upon vehicle treatment, adolescents emerged from the dark more rapidly than adults [F(1,17) = 5.563; p = 0.0306]. With increasing doses of THC, adults exhibited an increase in time to emerge, but adolescents did not (adult group, dose effect, p = 0.0110; adolescent group, dose effect, NS). Thus, by this measure, THC is more anxiogenic in adults than in adolescents.
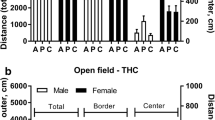
The light-dark task also revealed that THC was more locomotor-reducing in adults than in adolescents. Total distance traveled in both the light and the dark is shown in Fig. 3a. For this measure, there was an overall effect of age (p < 0.05) and an overall effect of THC (p < 0.01). Although there was no age × drug interaction effect, there was a significant effect of drug dose in the adult group (p < 0.05) but not in the adolescent group. When we divided the 15-min session into 5-min blocks, as shown in Fig. 3b, there were main effects of age, drug, and time (p < 0.01 or less) but no interaction effects. In the young rats, there was no effect of THC dose. In the adult rats, the dose effect was significant (p < 0.01). Both age groups exhibited a decrease in distance traveled across the three time blocks (p < 0.01), which is characteristic of habituation in this task. Overall, THC reduced locomotion in the light-dark task more in adults than in adolescents.
Locomotion in the light-dark apparatus. Distance traveled was measured in both the light and dark sides simultaneously with position measurements presented in Fig. 2. (a) Total distance traveled in 15 minutes. *, significantly different from adults at same dose, p < 0.05. (b and c) distance traveled in session divided into 5-minute time blocks. B, adults; C, adolescents
Stress hormone levels
To assess the HPA axis response to THC, we examined corticosterone and ACTH levels in the serum at various time points after intraperitoneal injection. Levels of stress hormones in the serum obtained from trunk blood after decapitation are shown in Fig. 4. Five-mg/kg THC induced a prolonged elevation of both ACTH and corticosterone in the serum (significantly different from vehicle treatment, p < 0.01). ACTH level was significantly affected by age (p < 0.05), and there was a significant age × drug interaction effect (p < 0.05). Adults demonstrated higher ACTH levels after THC treatment than adolescents. For corticosterone, there was no significant effect of age and no age × drug interaction effect, although there was a significant drug effect (p < 0.01) and a significant drug × time interaction (p < 0.01). Corticosterone was elevated by the injection of either vehicle or THC at early time points and remained elevated for a prolonged period (more than 90 min) with THC.
Conditioned taste aversion
We used two tests to assess whether THC is aversive to rats in an age-dependent manner. In the CTA task, as shown in Fig. 5, there was no main effect of age on acquisition of taste aversion. Both ages demonstrated a decrease in saccharin consumption after it was paired with THC (dose effect, p < 0.01), and there was no age × dose interaction. At the lowest dose examined (1.25 mg/kg), adults exhibited significantly more aversion than adolescents (p < 0.05). The ethanol/emulphor vehicle was not aversive in either age group compared to saline. Thus, similar to our results with anxiety, THC may be slightly more aversive to adults than it is to adolescents.
Conditioned place aversion
To corroborate our results from taste aversion, we performed CPA in adolescent vs adult rats at a high and low dose of THC. Results from these experiments are shown in Figs. 6 and 7. As with taste aversion, THC generated a place aversion in both age groups (dose effect, p < 0.0001 overall; p < 0.0001 in adults; p < 0.05 in adolescents). In addition, we observed a significant age × dose interaction effect [F(2,57) = 4.633; p = 0.0137). Inspection of the results revealed that at 5 mg/kg THC, adult rats spent less time on the drug-associated side of the chamber than adolescent rats (Fisher’s PLSD, p = 0.0168). With the lower dose, 0.5 mg/kg, adults and adolescents spent approximately equal amounts of time on the drug-associated side, which did not differ significantly from vehicle in either age group. Therefore, adults exhibited a stronger place aversion than adolescents to the higher dose of THC.
Locomotor data obtained during the three drug-association sessions are shown in Fig. 7. These data revealed significant main effects of drug, age, and session but no interaction effects. Adults were overall less active than adolescents (p < 0.01), and repeated THC caused a slight reduction in activity in both age groups (p < 0.05). Both age groups exhibited a drug-independent reduction in locomotor activity from days 1 to 3 of association training (p < 0.0001), typical of habituation to the apparatus and experimental regimen.
Discussion
These results show that THC is less anxiogenic, less aversive, and less locomotor reducing in adolescent rats than in adult rats. Whereas some of these observations were dose- and task-specific, the findings were apparent in multiple measures of each outcome. This overall reduced sensitivity to the undesirable effects of THC in adolescents may contribute to elevated levels of use during this developmental stage.
Anxiety-related measures
Results from two rodent models of anxiety-related behavior revealed reduced anxiogenic effects of THC in adolescents. In the EPM, the low dose decreased open arm time only in adult rats. In the light-dark task, THC affected emergence into the light only in adults. This differential effect could reflect either an age-specific response to THC or more general age differences in these behaviors. Our previous findings of more impaired learning in adolescents treated with THC (Cha et al. 2006) suggest that adolescents are not globally less sensitive to THC. An abundant literature supports an alternative explanation that adolescents in general are less anxious, more risk-taking, and less sensitive to aversive consequences (Spear 2000). The greater activity of adolescents on the plus maze, their faster emergence into the light during the light/dark task, and greater locomotor activity during CPA indicate that adolescent rats are less behaviorally inhibited in novel environments. It is also possible that CB-1 receptor mechanisms responsible for the anxiogenic effects of THC may mature later than those regulating memory. Studies have shown that CB-1 receptor mechanisms in the hippocampus that are responsible for effects on learning are generally at adult levels by adolescence (Belue et al. 1995; Morozov and Freund 2003; Romero et al. 1997). However, sites in the amygdala, which are thought to mediate anxiogenic effects, have not been examined. Neural circuits from the amygdala to the cortex are among the latest in the mammalian brain to mature (Cunningham et al. 2002), raising the possibility that the processing of THC-evoked states as aversive may be different in early adolescence vs adulthood. The findings of the conditioned aversion studies discussed below support this interpretation.
Locomotion
As for the anxiety findings, adults’ locomotor activity was more strongly affected than adolescents. In the EPM and light-dark tasks, THC dose-dependently reduced measures of activity in adults but not in adolescents. These acute locomotor effects were observed in both tasks in which the rats experienced the drug in a novel environment. In contrast, in the CPA task, in which the rats had experienced two exposures to the apparatus before its association with THC, age-specific locomotor effects did not emerge until day 3 of treatment with the drug. This may be evidence of differential tolerance to the drug (as observed in our previous water maze study (Cha et al. 2006) or effects of other experimental variables (differences in lighting, prior handling, morning vs afternoon treatment, etc.). Although locomotor activity can be a confounding factor in the determination of anxiety in both the EPM and light-dark tasks, it did not affect our interpretation of the age and dose-specific effects in the present study. In both tests, the THC dose that elicited the greatest age difference in anxiety outcome showed the least different locomotor effects between the two ages.
The age-specificity of these locomotor effects of THC is similar to previously-reported results with ethanol (Little et al. 1996). This behavioral difference might reflect the greater activity of adolescents in general (Spear 2000; Spear and Brake 1983). It is tempting to speculate that the greater locomotor impairment in adults may make both THC and ethanol less attractive to adults, and thereby help explain the reduction in marijuana and alcohol use in adulthood in humans (Chen and Kandel 1995; York et al. 2005).
Aversion
Results from the two aversion-related tasks suggest that THC is aversive to both adolescent and adult rats but more so in adults. This aversion is most likely related to the anxiogenic effects of the drug (Ettenberg and Geist 1991; Guitton and Dudai 2004). It is consistent that anxiety and aversion were both increased in adults compared to adolescents. Our results demonstrating increased CTA in adults compared to adolescents is similar to results obtained with other addictive substances. Amphetamine (Infurna and Spear 1979), nicotine (Shram et al. 2006; Wilmouth and Spear 2004), and cocaine (Schramm-Sapyta et al. 2006) are all less aversive to adolescents than to adults.
There is some debate about whether taste aversion to addictive substances is actually a measure of aversion (Parker 1995); some researchers have argued that it is an index of reward [(Gomez 2002), but see (Freeman et al. 2005; Freeman and Riley 2005; Phillips et al. 2005)]. As this debate remains unsettled, we included another aversion-related task, place aversion, in our analysis. We observed aversive responses at a similar range of doses in both tasks, which suggests that THC is, in fact, aversive. The age specificity of these effects, in combination with our EPM results, supports the interpretation that THC is less aversive in adolescents because it is less anxiogenic, likely because neural mechanisms responsible for this component of its action are immature.
Stress hormones
ACTH and corticosterone were both elevated for a prolonged time period after THC treatment at both ages, but ACTH was elevated more in adults than in adolescents. This is consistent with our behavioral observations that adults were also more anxious after THC treatment and found THC more aversive. The fact that we observed an age difference in ACTH levels but not in corticosterone levels is most likely due to the slower time course of onset of the corticosterone responses, coupled with the slow onset and offset of THC’s actions (McGilveray 2005). This result contrasts with more typical findings that adolescent rats exhibit exaggerated HPA axis responses to stressors and some drugs of abuse (Adriani and Laviola 2000; Laviola et al. 2002). This unique response to THC further supports the conclusion that THC is more aversive in adults than in adolescents.
The relationship of the stress hormones to anxiety, aversion, and responses to addictive substances is complex. HPA axis activation typically reflects stress and/or arousal, and its activation by drugs of abuse is thought to reflect both (Heinrichs and Joppa 2001; Heinrichs and Koob 2004). Corticosterone may facilitate the acquisition of self-administration of psychostimulants (Campbell and Carroll 2001; Deroche et al. 1997; Goeders and Guerin 1996; Piazza et al. 1991). Corticotropin-releasing factor can protect against cocaine-induced taste aversion (Heinrichs et al. 1998), yet can also contribute to withdrawal-induced anxiety (Baldwin et al. 1991; Basso et al. 1999; Lu et al. 2001; Menzaghi et al. 1994; Sarnyai et al. 1995). Thus, the stress hormones play a role in both the reinforcing and undesirable effects of drugs of abuse. The age-specific activation of ACTH that we observed is most likely a manifestation of the undesirable effects.
Summary
In this examination of several potentially undesirable effects of THC, adult rats were more affected than adolescents. Although THC was anxiogenic and aversive for both age groups at high doses, adolescents were less affected in every test we conducted (two anxiety-related tasks and two tests of aversiveness) at mid-range doses. In addition, although THC inhibited spontaneous locomotion in adults, it generally had no effect on locomotion in adolescents. This overall response profile is that of a more reinforcing, less aversive drug experience for adolescents. Finally, THC produced a greater elevation of ACTH in adults, perhaps suggesting a more “stressful” drug experience. These age-specific differences in some potentially undesirable effects of acute THC administration contrast with our report that showed greater impairment of performance in spatial learning by THC in adolescent than in adult rats (Cha et al. 2006). This disparity emphasizes that maturation of brain circuits involved in affective behavior, learning, and arousal contribute significantly to differences in how adolescents and adults respond to drugs of abuse.
These differences may reflect variation in the maturation of either specific cannabinoid receptor signaling pathways in the brain or other downstream pathways.
The maturation of cannabinoid receptors is unlikely to explain our results. Cannabinoid receptors reach maximal levels in the limbic forebrain, striatum, and mesencephalon between 30 and 40 days of age and then decline slightly to adult levels (Rodriguez de Fonseca et al. 1993). Receptor patterns in the hippocampus attain adult levels at about the same time (Belue et al. 1995; Morozov and Freund 2003; Romero et al. 1997). The endocannabinoid, anandamide, is at near-adult levels by postnatal day 25 (Wenger et al. 2002). However, more anatomically localized analysis of areas related to stress and anxiety like the amygdala have not been conducted, and a number of studies suggest that these neural circuits continue to mature through adolescence (Cunningham et al. 2002). Neurotransmitter function of CB-1 target neurons, such as glutamate and GABA, are still developing during this period (Crair and Malenka 1995; Izumi and Zorumski 1995; Kirkwood et al. 1995; Liao and Malinow 1996; Partridge et al. 2000; Schramm et al. 2002) and likely contribute to the observed effects. Careful study of more anatomically defined areas is needed to answer this question.
Adolescents may be more susceptible than adults to some effects of chronic THC use. For example, in humans, reduced verbal IQ (Pope et al. 2003), and deficits in visual attention (Ehrenreich et al. 1999) are associated with early but not late onset of marijuana use, but other cognitive abilities are unaffected. Chronic THC use in teenagers can also increase later incidence of emotional problems (Patton et al. 2002). Younger users are also more likely to progress to drug dependence than older users (Chen et al. 1997). Thus, some consequences of chronic cannabinoid treatment more adversely affect adolescents than adults. One possible explanation for these findings suggested by the present study is that human adolescents may smoke more marijuana because it is less aversive and so experience greater effects of chronic exposure.
References
Adriani W, Laviola G (2000) A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology 39:334–346
APA (1994) Diagnostic and statistical manual for mental disorders. American Psychiatric Association
Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT (1991) CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 103:227–232
Basso AM, Spina M, Rivier J, Vale W, Koob GF (1999) Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 145:21–30
Belue RC, Howlett AC, Westlake TM, Hutchings DE (1995) The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol 17:25–30
Braida D, Iosue S, Pegorini S, Sala M (2004) Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol 506:63–69
Campbell UC, Carroll ME (2001) Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacology (Berl) 154:311–318
Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS (2006) Differential effects of delta(9)-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav Environ Exp Bot 83:448–455
Cheer JF, Kendall DA, Marsden CA (2000) Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 151:25–30
Chen K, Kandel DB (1995) The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health 85:41–47
Chen K, Kandel DB, Davies M (1997) Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug Alcohol Depend 46:53–67
Crair MC, Malenka RC (1995) A critical period for long-term potentiation at thalamocortical synapses. Nature 375:325–328
Cunningham MG, Bhattacharyya S, Benes FM (2002) Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol 453:116–130
Deroche V, Marinelli M, Le Moal M, Piazza PV (1997) Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther 281:1401–1407
Doremus TL, Brunell SC, Varlinskaya EI, Spear LP (2003) Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav 75:411–418
Doremus TL, Varlinskaya EI, Spear LP (2004) Age-related differences in elevated plus maze behavior between adolescent and adult rats. Ann NY Acad Sci 1021:427–430
Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR (1999) Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 142:295–301
Ettenberg A, Geist TD (1991) Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology 103:455–461
Freeman KB, Riley AL (2005) Cocaine-induced conditioned taste avoidance over extended conditioned stimulus-unconditioned stimulus intervals. Behav Pharmacol 16:591–595
Freeman KB, Konaklieva MI, Riley AL (2005) Assessment of the contributions of Na+ channel inhibition and general peripheral action in cocaine-induced conditioned taste aversion. Pharmacol Biochem Behav 80:281–288
Goeders NE, Guerin GF (1996) Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology 64:337–348
Gomez F (2002) Conditioned saccharin aversion induced by self-administered cocaine negatively correlates with the rate of cocaine self-administration in rats. Brain Res 946:214–220
Guitton MJ, Dudai Y (2004) Anxiety-like state associates with taste to produce conditioned taste aversion. Biol Psychiatry 56:901–904
Haertzen CA, Kocher TR, Miyasato K (1983) Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend 11:147–165
Heinrichs SC, Joppa M (2001) Dissociation of arousal-like from anxiogenic-like actions of brain corticotropin-releasing factor receptor ligands in rats. Behav Brain Res 122:43–50
Heinrichs SC, Koob GF (2004) Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311:427–440
Heinrichs SC, Klaassen A, Koob GF, Schulteis G, Ahmed S, De Souza EB (1998) Corticotropin-releasing factor receptor blockade enhances conditioned aversive properties of cocaine in rats. Psychopharmacology (Berl) 136:247–255
Infurna RN, Spear LP (1979) Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav 11:31–35
Izumi Y, Zorumski CF (1995) Developmental changes in long-term potentiation in CA1 of rat hippocampal slices. Synapse 20:19–23
Kirkwood A, Lee HK, Bear MF (1995) Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature 375:328–331
Laviola G, Adriani W, Morley-Fletcher S, Terranova ML (2002) Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res 130:117–125
Liao D, Malinow R (1996) Deficiency in induction but not expression of LTP in hippocampal slices from young rats. Learn Mem 3:138–149
Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS (1996) Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res 20:1346–1351
Lu L, Liu D, Ceng X (2001) Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol 415:203–208
McGilveray IJ (2005) Pharmacokinetics of cannabinoids. Pain Res Manag 10:15A–22A
Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, Koob GF (1994) The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann NY Acad Sci 739:176–184
Morozov YM, Freund TF (2003) Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci 18:1213–1222
Parker LA (1995) Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev 19:143–157
Partridge JG, Tang KC, Lovinger DM (2000) Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol 84:1422–1429
Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W (2002) Cannabis use and mental health in young people: cohort study. BMJ 325:1195–1198
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24:525–529
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, McKinnon CS, Cunningham CL (2005) Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behav Neurosci 119:892–910
Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H (1991) Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA 88:2088–2092
Pope HG Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D (2003) Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 69:303–310
Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ (1993) Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4:135–138
Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ (1997) Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse 26:317–323
Sanudo-Pena MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM (1997) Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett 223:125–128
Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G (1995) Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res 675:89–97
Schramm-Sapyta NL, Pratt AR, Winder DG (2004) Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology (Berl) 84:344–352
Schramm-Sapyta NL, Morris RW, Kuhn CM (2006) Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav 173:41–48
Schramm NL, Egli RE, Winder DG (2002) LTP in the mouse nucleus accumbens is developmentally regulated. Synapse 45:213–219
Shram MJ, Funk D, Li Z, Le AD (2006) Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 186:201–208
Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463
Spear LP, Brake SC (1983) Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol 16:83–109
Thomas H (1996) A community survey of adverse effects of cannabis use. Drug Alcohol Depend 42:201–207
Varlinskaya EI, Spear LP (2004a) Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague–Dawley rats. Alcohol Clin Exp Res 28:40–50
Varlinskaya EI, Spear LP (2004b) Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann NY Acad Sci 1021:459–461
Wenger T, Gerendai I, Fezza F, Gonzalez S, Bisogno T, Fernandez-Ruiz J, Di Marzo V (2002) The hypothalamic levels of the endocannabinoid, anandamide, peak immediately before the onset of puberty in female rats. Life Sci 70:1407–1414
Wilmouth CE, Spear LP (2004) Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann NY Acad Sci 1021:462–464
York JL, Welte J, Hirsch J (2005) Regulation of alcohol intake with advancing age. Alcohol 36:41–46
Acknowledgment
The authors wish to thank Reynold Francis for expert technical assistance. This work was funded by DA019346 to Dr. Swartzwelder and by VA Senior Research Career Scientist awards to Drs. Swartzwelder and Wilson.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schramm-Sapyta, N.L., Cha, Y.M., Chaudhry, S. et al. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology 191, 867–877 (2007). https://doi.org/10.1007/s00213-006-0676-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0676-9