Abstract
Rationale
Reduced expression of a drug-induced conditioned place preference (CPP) may reflect a decrease in the drug’s conditioned rewarding effects. However, CPP is also open to disruption by processes unrelated to the underlying motivation. In unpublished studies, we previously observed that ethanol pretreatment before testing disrupted expression of ethanol-induced CPP in DBA/2J mice. We hypothesized that this interference effect was due to large ethanol-induced increases in activity.
Objective
The present studies were designed to examine the relationship between test activity and expression of ethanol-induced CPP both in the presence and absence of ethanol. To assess the generality of this relationship, we examined these effects both in DBA/2J (which are highly activated by ethanol) and in NZB/B1NJ mice (which show similar CPP, but less ethanol-induced activation).
Materials and methods
In separate experiments, inbred mice from each strain underwent ethanol (2 g/kg) place conditioning. Saline or ethanol was then administered immediately before the test.
Results
Ethanol, given immediately before the test, blocked the expression of ethanol CPP in DBA/2J, but not in NZB/B1NJ mice. Moreover, ethanol significantly increased test activity levels in DBA/2J and to a much lesser degree in NZB/B1NJ mice. Correlation analyses showed an inverse phenotypic relationship between preference and test activity, reflecting stronger preferences in less active mice.
Conclusions
Disruption of ethanol-CPP observed in DBA/2J mice may be a consequence of high ethanol-induced activity levels. More generally, these studies suggest that competing behaviors can affect expression of a drug-induced CPP independent of affecting the conditioned rewarding effects of the drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Conditioned place preference (CPP) has been used historically to investigate the rewarding effects of many different drugs in the absence of drug (Carr et al. 1989; Tzschentke 1998). Drug-induced CPP develops when a drug’s rewarding effects are paired with contextual cues. As a result of such pairings, subjects presumably form an association between these cues and the drug’s motivational effects. Acquisition of this association is then measured indirectly as an approach response to the drug-paired cue during the test session, which is typically drug-free. Thus, expression of CPP depends on the drug’s hedonic value and the memory of the previously learned association. Although there are limitations to using CPP as a measure of drug reward (e.g., difficulty showing systematic dose–effect relationships; Bardo et al. 1995), a general advantage is that expression of the context–drug association can be evaluated in the absence of drug (Bardo and Bevins 2000). That is, one can examine effects of drug (or other) treatments that are given only during acquisition, and interpretation of the expression test is not complicated by the possibility that the treatment produces sensory–motor or other effects that directly interfere with performance of the spatial choice response. Although this advantage is useful when studying experimental treatments given during the acquisition of CPP, it may disappear when such treatments are introduced during tests for expression of CPP.
Studies of treatments that alter expression of CPP may have important implications for understanding the motivational processes hypothesized to underlie drug-induced craving and relapse in humans. For example, opioid antagonists have been reported to reduce craving in human alcoholics (e.g., O’Malley et al. 1992; Volpicelli et al. 1992) and to interfere with the expression or maintenance of ethanol’s conditioned rewarding effects as indexed by CPP in mice (Cunningham et al. 1995, 1998; Kuzmin et al. 2003; Middaugh and Bandy 2000). Such findings suggest commonality in the mechanisms underlying ethanol-induced craving and expression of ethanol-induced CPP. However, despite the potential power of this technique to improve understanding of the motivational processes that underlie craving and drug seeking, expression of CPP is subject to disruption by processes unrelated to the presumed conditioned rewarding effect of the drug-paired cue.
Although the literature contains many studies in which the effects of various treatments on expression of drug-induced CPP have been examined (Tzschentke 1998), insufficient attention has been given to alternative interpretations that do not involve a presumed interaction with the conditioned motivational processes underlying CPP. It is possible, for example, that disturbances or deficits in sensory–motor processes during the expression test might directly interfere with a subject’s ability to detect or discriminate between cues or with the subject’s ability to approach and maintain contact with the stimulus. In such cases, the subject’s failure to express place preference could be misinterpreted as a decrease in the drug’s conditioned rewarding effects. The potential for extraneous influences on expression of CPP is well illustrated in a study by Vezina and Stewart (1987) who reported that increases in apparatus size at the time of testing interfered with expression of morphine-induced CPP in rats. This outcome was explained by arguing that the greater novelty of the large test space interfered with expression of CPP in groups trained in the smaller compartments, an interpretation that was supported by the finding of higher test session activity in those groups. Although this experiment did not involve a drug pretreatment, it raises the possibility that any manipulation, which affects locomotor activity during testing, might alter expression of CPP.
The first experiment reported here was originally designed to examine the effect of pretreatment with ethanol itself on the expression of ethanol-induced CPP in DBA/2J inbred mice. This manipulation is of interest because of its potential implications for understanding whether exposure to ethanol during abstinence would suppress or exacerbate conditioned motivational responses (CRs) that might trigger relapse to ethanol-seeking behavior. For example, suppression of such CRs might be expected to protect the individual from relapse, whereas enhancement of these CRs might be expected to promote relapse. However, interpretation of this study was complicated by the fact that ethanol pretreatment produced substantial locomotor activation in DBA/2J mice, raising the possibility that high activity itself interfered with expression of CPP (Vezina and Stewart 1987). To address this hypothesis and to assess the generality of the findings from Experiment 1, our second experiment examined effects of the same pretreatment on expression of ethanol-induced CPP in a different inbred mouse strain (NZB/B1NJ). This strain was selected because it shows a monotonic dose-effect curve for CPP that is similar to that seen in DBA/2J mice, but is considerably less activated by ethanol (unpublished data). If the interference effect in DBA/2J mice is due to a general ability of ethanol pretreatment to reduce conditioned motivational effects elicited by an ethanol-paired cue, one might expect to see the same outcome in a different mouse strain tested at the same dose. However, if interference in DBA/2J mice is a byproduct of ethanol-induced activation, one might not expect to see interference in NZB/B1NJ mice, which show much less activation after ethanol injection.
Materials and methods
Animals
Male DBA/2J (n = 96) and NZB/B1NJ (n = 64) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) at 6–7 weeks of age. Animals were housed on a Thoren rack (Thoren Caging Systems, Hazleton, PA) in ventilated polycarbonate cages. DBA/2J mice were housed in groups of four, whereas NZB/BINJ mice were housed in pairs or singly due to observed aggressive behavior towards cage mates. Animals were kept at an ambient temperature of 21 ± 1°C on a 12-h light–dark cycle (lights on at 0700 hours). Experiments were carried out during the light portion of the cycle beginning at 1300 hours. “Labdiet” rodent chow (Richmond, IN) and bottled water were continuously available in the home cage. The National Institutes of Health (NIH) “Principles of Laboratory Animal Care” were followed in conducting these studies, and the protocol was approved by the Oregon Health & Science University IACUC.
Apparatus
The apparatus consisted of 12 identical acrylic and aluminum boxes \( {\left( {30 \times 5 \times 15\,cm} \right)} \) enclosed in individual ventilated, light- and sound-attenuating chambers (Coulbourn Instruments Model E10-20). Six sets of infrared light sources and photodetectors mounted 2.2 cm above the floor at 5-cm intervals along the long wall of the box detected general activity, location in the box, and time spent on each side of the chamber (10-ms resolution).
Conditioned stimuli (CS) consisted of two interchangeable distinctive floor halves placed beneath each chamber. The hole floor was made from perforated stainless steel sheet metal (16 gauge) containing 6.4-mm round holes on 9.5 mm staggered centers. The grid floor was constructed from 2.3-mm stainless steel rods mounted 6.4 mm apart in acrylic rails. This floor texture combination was selected on the basis of previous studies demonstrating that drug-naïve control mice from both strains spend about half their time on each floor type during choice tests (Cunningham et al. 2003). The inside and floors of the box were wiped with a damp sponge, and the litter paper underneath the flooring was changed between animals.
Drugs
Ethanol (95%) was diluted in 0.9% saline (20% v/v) and administered at a dose of 2 g/kg (12.5 ml/kg). In previous experiments, this ethanol dose has reliably induced a place preference of similar magnitude in DBA/2J (e.g., Cunningham et al. 2003) and NZB/B1NJ (unpublished data) mice without detrimental behavioral effects of repeated injections at this dose or concentration (Cunningham et al. 1997). Saline was administered in a volume of 12.5 ml/kg.
Procedure
Each experiment involved three phases: habituation (one session), conditioning (eight sessions), and testing (one session). Each animal was given an intraperitoneal (i.p.) injection immediately before being placed in the center of the apparatus for each session. Sessions were conducted 5 days per week. A more detailed description of our place conditioning procedure can be found elsewhere (Cunningham et al. 2006a).
Habituation
This session was intended to reduce the novelty and stress associated with handling, injection, and exposure to the apparatus. Subjects were weighed and given an i.p. injection of saline just before placement in the apparatus on a smooth paper floor for 5 min. Animals were not exposed to the distinctive floor types to avoid latent inhibition.
Conditioning
In each experiment, mice were randomly assigned to one of two groups that differed in the pretreatment received on the test day. The Ethanol Group was pretreated with ethanol (2 g/kg), whereas the Saline Group was pretreated with saline before the test session. Within each test treatment group, mice were randomly assigned to one of two conditioning subgroups (Grid+ or Grid−). Thus, an unbiased subject assignment procedure was used (Cunningham et al. 2003). Both subgroups were exposed to a differential Pavlovian conditioning procedure in which they received four CS+ and four CS− trials. Mice in the Grid+ condition received ethanol paired with the grid floor (CS+) and saline paired with the hole floor (CS−). Mice in the Grid− condition received ethanol paired with the hole floor (CS+) and saline paired with the grid floor (CS−). Each animal received four 5-min conditioning trials of each type on alternating days over a period of 8 days, with the presentation order of CS+ and CS− trials counterbalanced within each group. A one-compartment training procedure was used on all trials (Bevins and Cunningham 2006; Cunningham et al. 2006b). That is, the assigned tactile cue was present on both sides of the apparatus, and the animal had access to the entire apparatus. Because the size of the compartment did not change between conditioning and testing, this procedure avoided complications that might be related to novelty-induced increases in activity during the test (Vezina and Stewart 1987).
Place preference test
The test (30 min in duration) began 24 h after the last conditioning trial. Experiment 1 examined the effect of injecting ethanol (or saline) before the test session on expression of place preference in DBA/2J mice, whereas Experiment 2 examined this effect in NZB/B1NJ mice. Immediately after injection, mice were placed in the center of the apparatus with both test floors (half grid/half hole). Position (i.e., left vs right) of each floor type was counterbalanced within subgroups.
Data analysis
The primary dependent variable was the amount of time spent on the grid floor during the test session. In this unbiased design, the magnitude of the difference in time spent on the grid floor between the Grid + and Grid− conditioning subgroups is indicative of conditioned preference (see Cunningham et al. 2003 for a more complete discussion of dependent variables used in place conditioning studies). As Experiment 2 was performed to test the generality of the findings in Experiment 1, data from both experiments were evaluated separately by analysis of variance (ANOVA) with the alpha level set at 0.05. To control overall alpha level within each experiment, p-values were Bonferroni-corrected for the number of post-hoc comparisons between group means. Test treatment (Ethanol vs Saline) and conditioning subgroup (Grid+ vs Grid−) were treated as between-group factors, whereas trial type (CS+ vs CS−) was treated as a within-subject factor.
Results
Data from two mice were completely removed from each experiment due to procedural errors. Also, data from 12 subjects were removed from test session analyses in Experiment 1 due to a data storage error. These subjects were equally distributed across the test treatment × conditioning subgroup conditions (n = 2/group). The final number of mice in each group is reported in the figure captions.
Place preference test
Experiment 1
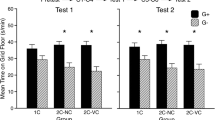
The left panel of Fig. 1 shows mean time spent by DBA/2J mice on the grid floor during the test. As expected, the saline treated group (saline group) showed a strong conditioned place preference. In contrast, ethanol pretreatment completely disrupted expression of conditioned place preference (i.e., no difference between the Grid+ and Grid− conditioning subgroups within the ethanol group). These observations were supported by a two-way ANOVA (conditioning subgroup × test treatment) that yielded a significant interaction [F(1,78) = 23.9, p < 0.0001], confirming that ethanol pretreatment altered expression of place preference. There was also a significant main effect of conditioning subgroup [F(1,78) = 54.9, p < 0.0001], but no main effect of test treatment. Follow-up pair-wise comparisons of the Grid+ and Grid− conditioning subgroups showed significant differences in saline treated (Bonferroni-corrected p < 0.0001), but not in ethanol-treated mice.
Left Panel Mean time spent by DBA/2J mice on the grid floor (s/min + SEM) during the 30-min test in Experiment 1. Solid bars depict the Grid+ conditioning subgroups; hatched bars depict the Grid− conditioning subgroups. Place preference was indexed by comparing the Grid+ and Grid− subgroups. One pair of subgroups was injected with saline before the preference test (n = 13 per conditioning subgroup), whereas the other pair of subgroups was injected with 2 g/kg ethanol (n = 28 per conditioning subgroup). Right Panel Mean activity rates (counts per min + SEM) during the 30-min test (saline: n = 26; ethanol: n = 56)
Consistent with previous studies in DBA/2 mice (e.g., Cunningham et al. 1992; Phillips et al. 1994), 2 g/kg ethanol had a substantial activating effect during the test (right panel of Fig. 1). One-way ANOVA confirmed a significant effect of test treatment [F(1,80) = 385.3, p < 0.0001].
Experiment 2
The left panel of Fig. 2 depicts mean time spent on the grid floor during the test for NZB/B1NJ mice. As indicated by differences between Grid+ and Grid− subgroups, conditioned place preference was observed in both pretreatment groups. Moreover, ethanol pretreatment did not affect expression of conditioned preference. These observations were supported by a two-way ANOVA (conditioning subgroup × test treatment) that revealed a significant main effect of conditioning subgroup [F(1,58) = 37.6, p < 0.0001], but no interaction with test treatment. The absence of a significant interaction confirms that ethanol pretreatment did not alter expression of CPP in this strain. However, the two-way ANOVA also yielded a significant main effect of test treatment [F(1,58) = 8.4, p < 0.01], reflecting the fact that ethanol-intoxicated mice generally spent less time on the grid floor during testing regardless of conditioning subgroup. This nonspecific effect of ethanol pretreatment may indicate a preference for the better footing provided by the hole floor during intoxication. For a similar finding in rats, see Cunningham (1979).
Left Panel Mean time spent by NZB/B1NJ mice on the grid floor (s/min + SEM) during the 30-min test in Experiment 2. Solid bars depict the Grid+ conditioning subgroups; hatched bars depict the Grid− conditioning subgroups. Place preference was indexed by comparing the Grid+ and Grid− subgroups. One pair of subgroups was injected with saline before the preference test (n = 16 per conditioning subgroup), whereas the other pair of subgroups was injected with 2 g/kg ethanol (n = 14–16 per conditioning subgroup). Right Panel Mean activity rates (counts per min + SEM) during the 30-min test (saline: n = 32; ethanol: n = 30)
As shown in the right panel of Fig. 2, ethanol pretreatment also had an activating effect in NZB/B1NJ mice, although to a much smaller degree than in DBA/2J mice. One-way ANOVA yielded a significant Test Treatment effect [F(1,60) = 50.4, p < 0.0001], confirming that activity was higher after ethanol injection than after saline injection.
Test session activity × preference correlations
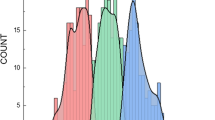
To further assess the apparent inverse relationship between conditioned preference and test session activity, Pearson correlations were calculated using test session data from each experiment (Fig. 3). Initial analyses, which ignored test treatment group, showed a significant negative correlation in DBA/2J mice [r = −0.72, n = 58, p < 0.0001], but not in NZB/B1NJ mice [r = −0.23, n = 47, p = 0.12]. When the same analyses were applied separately to groups pretreated with saline (saline group) or ethanol (ethanol group), significant negative correlations were found in DBA/2J mice under both conditions [saline group: r = −0.53, n = 18, p < 0.05; ethanol group: r = −0.44, n = 40, p < 0.01] and in NZB/B1NJ mice pretreated with ethanol [saline group: r = −0.18, n = 23, p = 0.40; ethanol group: r = −0.41, n = 24, p < 0.05].
Scatter plots showing the relationship between place preference (expressed as percent time on the ethanol-paired floor) and test activity during the 30-min test in Experiments 1 (left panel) and 2 (right panel). Data include only subjects who expressed preference for the ethanol-paired floor (i.e., preference scores > 50%). Open circles depict mice pretreated with saline (DBA/2J: n = 18; NZB/B1NJ: n = 23); closed diamonds depict mice in the groups pretreated with ethanol (DBA/2J: n = 40; NZB/B1NJ: n = 24). Regression lines are based on analysis of all data within each panel. See text for more detail
Conditioning trial activity
Experiment 1
Data for 12 mice were lost on the second conditioning trial due to a data storage error, requiring that these mice be excluded from the overall conditioning trial analysis. These subjects were equally distributed across test treatment × conditioning subgroup conditions (n = 1/group). As expected, DBA/2J mice were substantially more active after ethanol injection than after saline injection, producing mean activity rates of 184.8 ± 3.7 and 60.7 ± 1.5 counts per minute, respectively (averaged across all four conditioning trials and both test treatment groups). Two-way ANOVA (test treatment × trial type) confirmed the significant main effect of trial type [F(1,80) = 1,277.1, p < 0.0001]. However, there was no effect or interaction with test treatment, indicating that the test groups did not differ in activity during the conditioning phase.
Experiment 2
In contrast to its strong activating effect in DBA/2J mice, ethanol produced a modest decrease in the conditioning trial activity of NZB/B1NJ mice. Mean activity rates were 34.1 ± 2.0 and 39.0 ± 1.8 counts per minute on ethanol and saline trials, respectively (averaged across all four conditioning trials and both test treatment groups). Two-way ANOVA (test treatment × trial type) yielded a significant main effect of trial type [F(1,60) = 8.7, p < 0.005], but no effect of test treatment or interaction.
Discussion
The most important findings from these studies are illustrated in the scatter plots shown in Fig. 3. More specifically, in studies with two different inbred strains, we found an inverse phenotypic relationship between expression of ethanol-induced conditioned place preference and test session activity, i.e., preference was generally stronger in mice that were less active during the test session. In the absence of ethanol, this relationship was significant only in DBA/2J mice (open symbols in left panel of Fig. 3). However, the relationship was significant in both strains in the presence of ethanol, which generally increased activity (closed symbols in both panels of Fig. 3). Because all mice within an inbred strain are genetically identical, differences among individuals must be attributed to the influence of environmental variables that are explicitly manipulated (e.g., drug pretreatment) or uncontrolled (e.g., early rearing conditions). The effect of ethanol pretreatment on expression of CPP in DBA/2J mice illustrates an extreme outcome in which the expression of place preference was completely obscured by a high level of test activity. Although ethanol pretreatment did not produce a significant decrease in the average preference expressed by NZB/B1NJ mice, a significant negative correlation between test activity and preference was nevertheless observed. A more general implication of these findings is that any manipulation that alters activity during testing may affect the expression of CPP (Vezina and Stewart 1987).
The most straightforward interpretation of the interference effect seen in DBA/2J mice is one that appeals to competing responses. That is, expression of CPP in this strain may have been reduced because large ethanol-induced increases in locomotor activity interfered with the ability of mice from this strain to approach and maintain contact with the ethanol-paired floor. According to this analysis, ethanol’s failure to disrupt expression of preference in NZB/B1NJ mice reflects the fact that ethanol had a much weaker effect on test activity in this strain. However, given that the inverse relationship between preference and activity was still apparent in NZB/B1NJ mice (right panel of Fig. 3), the implication is that other manipulations or variables that produce larger increases in test activity might produce significant decreases in preference in this strain as well.
Possible alternative interpretations fall short in explaining the current findings. For example, state dependent learning theory (Overton 1972) predicts that ethanol given during the test session should enhance retrieval of the ethanol-cue association. Instead, ethanol disrupted expression of ethanol CPP in DBA/2J mice, whereas not affecting the magnitude of preference expression in the NZB/B1NJ mice. Thus, our data clearly indicate that retrieval of the previously learned ethanol-cue association was not facilitated by ethanol treatment in either strain.
Another possibility is that test administration of ethanol reduced the ability of the CS+ to elicit a conditioned motivational response through a satiation-like process. In other words, much like a large meal reduces one’s appetite for a favorite food, ethanol intoxication may have interfered with the conditioned motivational response normally evoked by the ethanol-paired stimulus. However, to explain the strain difference in the pretreatment effect, this interpretation requires the assumption that these two genotypes differ substantially in the ability of the same dose of ethanol to produce this satiation-like effect. Although we cannot completely dismiss this possibility, the finding of similar magnitude CPP in vehicle-tested mice from both strains conditioned using either a 2 or 4 g/kg dose of ethanol (unpublished data) seems to argue against this interpretation.
Another alternative interpretation is that ethanol may have disrupted memory or recall of the CS–ethanol association (Ryabinin 1998). However, two considerations argue against this suggestion. First, data from human (e.g., Hutchison et al. 1964; Ryback 1970) and animal (e.g., Bammer and Chesher 1982) studies support the idea that acute ethanol intoxication has a relatively weak effect on the retrieval or recall of learned associations compared to its greater effect on the acquisition of associations. Second, this interpretation requires the assumption that ethanol-induced impairment in recall or retrieval differs substantially between the two genotypes, an assumption for which there is no independent support.
Previous studies have also supported the inverse relationship, showing that low activity levels are often associated with stronger expression of place preference (Vezina and Stewart 1987; Neisewander et al. 1990; Cunningham 1995; Cunningham et al. 1999). In some cases, this relationship has been based on the correlation of strain means for test session activity and ethanol CPP. That is, the basis for the relationship is presumably genetic, based on some overlap in the genes that influence each of these behavioral traits (Cunningham 1995). In other cases, the relationship depends on environmental influences such as pharmacological pretreatments (e.g., present studies; Neisewander et al. 1990) or changes in the spatial configuration of the apparatus at the time of testing (Vezina and Stewart 1987). The present studies join those reported by Cunningham et al. (1999) in showing an inverse phenotypic relationship between place preference and test activity within inbred strains. In fact, the magnitude of the negative correlation (r = −0.48, n = 136, p < 0.001) reported in cocaine conditioned DBA/2J mice (tested with saline) was generally similar to that found in Experiment 1, indicating that the inverse phenotypic relationship does not depend on the use of ethanol to induce place preference.
Whereas both strains demonstrated some level of activation during testing, only DBA/2J mice were activated by ethanol during conditioning (Cunningham et al. 1998, 2002). Consistent with previous findings (unpublished data), NZB/B1NJ mice did not reveal any ethanol-induced activation within the 5-min conditioning trial window. During testing, however, NZB/B1NJ mice showed activation when activity was measured over a longer time course. Thus, these results suggest strain differences in the time course of ethanol activation. The mechanisms underlying the strain difference in the temporal pattern of ethanol’s activity effects are unknown. Finally, contrary to predictions based on psychomotor stimulant theory (Wise and Bozarth 1987), our findings are also consistent with previous reports suggesting that the level of activation during conditioning is not correlated with the magnitude of preference expressed during testing (e.g., Cunningham 1995).
It is important to note that the present studies were not designed to provide a quantitative comparison between the DBA/2J and NZB/B1NJ strains, and there are several limitations on possible conclusions about strain differences. For example, one limitation is that it was sometimes necessary to house NZB/B1NJ mice in pairs or singly due to aggressive behavior displayed towards cage mates. In contrast, DBA/2J mice were consistently housed in groups of four. Thus, it is possible that the activity differences that we have attributed to strain may be due, in part, to differences in housing conditions. Although recent findings would suggest that different housing conditions might not affect expression of place preference (Fitchett et al. 2006), we cannot entirely dismiss this possibility. Another limitation is that each strain was conditioned and tested at only one ethanol dose. It is possible that the use of different ethanol doses might have produced a significant interference effect in NZB/B1NJ mice or failed to produce interference in DBA/2J mice. Nevertheless, whether the present findings are attributed to genotype, housing conditions, or a dose × genotype interaction, they strongly suggest that high levels of activity can have a detrimental effect on expression of CPP.
In general, these studies clearly illustrate the need to consider any behaviors that might disrupt approach responses when assessing the expression of a drug-induced CPP. The place conditioning literature has not been consistent in considering such behaviors in the interpretation of findings. For example, it was recently reported that induction of genetic mutations that eliminated both the dopamine (DAT) and serotonin (SERT) transporters also eliminated cocaine place preference (Sora et al. 2001). Of particular relevance in the present context, these knockout mice also demonstrated significantly higher basal activity levels. Thus, it is possible that the effect of the double knockout on CPP was not due to the hypothesized roles of DAT and SERT in the rewarding effects of cocaine, but was due instead to competition between the expression of preference and gene-deletion-induced hyperactivity. As the present results demonstrate, consideration of competing behaviors in the interpretation of place preference results is critical for understanding the mechanisms underlying drug reward as indexed by place preference. The hypothesis that competing behaviors can disrupt expression of a place preference would be strengthened by additional data showing that other stimulant treatments have similar effects on the expression of preference. Because the current study examined only one drug at one dose in two inbred strains, future studies must address the generality of the findings reported here. In light of the present and previous findings, however, investigators would be well advised to measure test session activity in all place-conditioning studies.
References
Bammer G, Chesher GB (1982) An analysis of some effects of ethanol on performance in a passive avoidance task. Psychopharmacology (Berl) 77:66–73
Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153:31–43
Bardo MT, Rowlett JK, Harris MJ (1995) Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19:39–51
Bevins RA, Cunningham CL (2006) Place conditioning: a methodological analysis. In: Anderson MJ (ed) Tasks and techniques: a sampling of methodologies for the investigation of animal learning, behavior, and cognition. Nova Science Publishers, Hauppauge, NY (in press)
Carr GD, Fibiger HC, Phillips AG (1989) Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ (eds) The neuropharmacological basis of reward. Clarendon, Oxford, pp 264–319
Cunningham CL (1979) Flavor and location aversions produced by ethanol. Behav Neural Biol 27:362–322
Cunningham CL (1995) Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology (Berl) 120:28–41
Cunningham CL, Niehus DR, Malott DH, Prather LK (1992) Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 107:385–393
Cunningham CL, Dickinson SD, Okorn DB (1995) Naloxone facilitates extinction but does not affect acquisition or expression of ethanol-induced conditioned place preference. Exp Clin Psychopharmacol 3:330–343
Cunningham CL, Okorn DA, Howard CE (1997) Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Anim Learn Behav 25:31–42
Cunningham CL, Henderson CM, Bormann NM (1998) Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 139:62–70
Cunningham CL, Dickinson SD, Grahame NJ, Okorn DM, McMullin CS (1999) Genetic differences in cocaine-induced conditioned place preference in mice depend on conditioning trial duration. Psychopharmacology (Berl) 146:73–80
Cunningham CL, Tull LE, Rindal KE, Meyer PJ (2002) Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 160:414–424
Cunningham CL, Ferree NK, Howard MA (2003) Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 170:409–422
Cunningham CL, Gremel CM, Groblewski PA (2006a) Drug-induced conditioned place preference and aversion in mice. Nature Protocols 1:1662–1670
Cunningham CL, Patel P, Milner L (2006b) Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci 120:1115–1132
Fitchett AE, Barnard CJ, Cassaday HJ (2006) There’s no place like home: cage odours and place preference in subordinate CD-1 male mice. Physiol Behav 87:955–962
Hutchison HC, Tuchtie M, Gray KG, Steinberg D (1964) A study of the effects of alcohol on mental functions. Can Psychiatr Assoc J 9:33–42
Kuzmin A, Sandin J, Terenius L, Ogren SO (2003) Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther 304:310–318
Middaugh LD, Bandy AL (2000) Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacology (Berl) 151:321–327
Neisewander JL, Pierce RC, Bardo MT (1990) Naloxone enhances the expression of morphine-induced conditioned place preference. Psychopharmacology (Berl) 100:201–205
O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B (1992) Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49:881–887
Overton DA (1972) State-dependent learning produced by alcohol and its relevance to alcoholism. In: Kissen B, Begleiter H (eds) The biology of alcoholism, vol 2. Plenum, New York
Phillips TJ, Dickinson S, Burkhart-Kasch S (1994) Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci 108:789–803
Ryabinin AE (1998) Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology (Berl) 139:34–43
Ryback RS (1970) Alcohol amnesia: observations on seven inpatient alcoholics. Q J Stud Alcohol 31:616–632
Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR (2001) Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA 98:5300–5305
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Vezina P, Stewart J (1987) Morphine conditioned place preference and locomotion: the effect of confinement during training. Psychopharmacology (Berl) 98:257–260
Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP (1992) Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49:876–880
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Acknowledgment
This research was supported by NIH-NIAAA grants AA007468, AA016041 and AA007702. We thank Tamara J. Phillips, Tara L. Fidler, and Laura Summers Bax for comments on earlier drafts of this paper. Experiments within this manuscript comply with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gremel, C.M., Cunningham, C.L. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology 191, 195–202 (2007). https://doi.org/10.1007/s00213-006-0651-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0651-5