Abstract
Rationale
Previous work has shown that some mouse strains (e.g., DBA/2J [D2]) readily develop robust ethanol-induced conditioned place preference (CPP) while others (e.g., C57BL/6J [B6]) do not. Though commonly interpreted as a difference between strains in sensitivity to ethanol reward, other explanations for this finding are possible.
Objectives
To explore the hypothesis that variation in sensitivity to contextual cues underlies CPP differences, the present work investigated ethanol-induced CPP in D2 and B6 mice trained with a standard tactile (floor) cue procedure compared to mice trained with tactile plus visual-spatial cues.
Methods
In an unbiased CPP procedure, mice were assigned to either a single element cue (one-compartment apparatus with tactile cue presented in the dark) or multi-modal cues (two-compartment apparatus with distinct tactile floors and lights on). To track CPP development, mice received preference tests during training in addition to a final test.
Results
Adding visual-spatial cues accelerated CPP acquisition in both D2 and B6 mice. However, this enhancement was observed after just one ethanol-conditioning trial in D2 mice, but was observed only after four ethanol-conditioning trials in B6 mice. Differences between groups trained with single or multi-modal cues disappeared as conditioning reached asymptote, with D2 mice showing a more rapid loss of the effect and a higher maximum CPP.
Conclusions
Although multi-modal cues produce more rapid conditioning, their inability to reduce or eliminate strain differences in CPP supports the interpretation that these strains differ in their sensitivity to ethanol reward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous research has shown substantial genetic variation in conditioned place preference (CPP) induced by ethanol in mice (Cunningham et al. 1992; Cunningham 1995, 2014). Despite exposure to the same contextual cues, ethanol doses, and training procedures, some inbred strains (e.g., DBA/2J [D2]) consistently develop a robust CPP while others do not (e.g., C57BL/6J [B6]). The most common interpretation of such effects is a strain difference in sensitivity to ethanol’s rewarding effect. Nevertheless, other interpretations are possible. For example, since CPP depends on learning and memory processes, strains might differ in their ability to form context-drug associations or in their ability to retrieve memories based on those associations. That interpretation predicts a strong genetic correlation between strain performance in the CPP task and strain performance in other learning and memory tasks, including those that involve non-drug reinforcement. However, examination of several different learning phenotypes (e.g., visual discrimination, Morris water maze, fear conditioning) failed to show any consistent genetic correlation with ethanol-induced CPP, arguing against the idea that strain differences in CPP can be explained simply in terms of strain differences in learning and memory (see Table 11 in Cunningham 2014).
Another possible interpretation of strain differences in CPP is that strains differ in sensitivity to the contextual cues paired with drug exposure. That is, the relative salience or associability of a specific cue (or combination of cues) might differ across strains. This possibility is suggested by previous studies showing that D2 mice acquire CPP to tactile (floor) cues more readily than to visual cues in the absence of unique spatial cues (Cunningham et al. 2006b). The broader implication of this within-strain finding is that there could also be between-strain differences in the abilities of tactile, visual, and spatial cues (individually or in combination) to acquire associative control over ethanol-induced CPP. However, this possibility has received relatively little consideration in the interpretation of strain differences in CPP.
The present studies were designed to explore this possibility by examining the development of ethanol-induced CPP in two commonly studied inbred mouse strains (B6, D2) using several different contextual cue configurations. Previous studies showing stronger ethanol-induced CPP in D2 mice than in B6 mice have all been conducted using only one cue configuration in which tactile cues were presented in the absence of visual or spatial cues (i.e., a one-compartment [1C] training procedure conducted in the dark: Cunningham et al. 1992; Gabriel and Cunningham 2008; Cunningham 2014; Cunningham and Shields 2018). In the 1C conditioning procedure, which was adapted from a procedure originally described for rats by Vezina and Stewart (1987a), the apparatus is not subdivided into multiple compartments during drug or vehicle conditioning trials. Rather, one tactile floor cue is presented throughout the apparatus on drug trials while a different tactile cue is presented on vehicle trials. In both cases, the animal has access to the entire space. Thus, when training occurs in a darkened enclosure, there are no explicit visual or spatial cues during conditioning, only tactile cues. The alternative tactile cues are isolated to different spatial locations only at the time of preference testing. This procedure contrasts with the more commonly used two-compartment (2C) procedure that exposes animals to spatially distinct portions of the apparatus that typically differ in their cue properties during conditioning trials. More thorough theoretical descriptions of differences between the 1C and 2C procedures can be found elsewhere (Cunningham et al. 2006b; Cunningham and Zerizef 2014; Hitchcock et al. 2014).
The cue configurations used here were adapted from previous studies in D2 mice that examined effects of apparatus illumination on ethanol-induced conditioning to tactile cues in the 1C and 2C procedures (Cunningham and Zerizef 2014). Those studies showed no difference between procedures when training occurred in the dark, indicating no effect of adding spatial cues in the absence of visual cues. However, when training occurred in the light, the 2C procedure produced stronger CPP than the 1C procedure, suggesting either that adding visual cues in the absence of unique spatial locations for the tactile cues impaired conditioning (1C procedure) or that combining visual and tactile cues in distinct spatial locations enhanced conditioning (2C). In another study involving cocaine reward, B6 mice that were trained and tested in the light also acquired stronger CPP in a 2C procedure than in a 1C procedure (Hitchcock et al. 2014). Taken together, these studies raised the possibility of an interactive effect among visual, spatial, and tactile cues, but comparison between strains is complicated by the use of different drugs (i.e., ethanol vs. cocaine). The question of primary interest in the present studies was whether the previously observed genetic difference in ethanol-induced CPP would be reduced or eliminated when tactile cues are combined with visual-spatial cues. That outcome would suggest that the previously observed strain difference in CPP was mediated by a strain difference in the salience or associability of the multi-modal cue configuration in addition to or instead of a strain difference in sensitivity to ethanol reward.
A total of four experiments are reported here, two for each strain. The design and procedure for the first experiment with each strain was identical. In that experiment, one group was exposed to a standard 1C procedure using only tactile cues presented in the dark. A second group was conditioned in a 2C procedure in the light, thus exposing mice to tactile cues in combination with visual-spatial cues. The third group also received a 2C procedure in the light, but in this case, tactile cues were combined with explicit visual cues (stripes or dots) placed on the wall of the apparatus. The procedure for the second experiment with each strain was tailored differently for each strain in order to replicate or extend the findings from the first experiment. In general, we expected the addition of visual-spatial cues to enhance CPP in both strains, and possibly to reduce or eliminate the previously observed strain difference in sensitivity to ethanol reward.
Method
Subjects
B6 and D2 male mice were shipped from the Jackson Laboratory at 6 weeks of age. Mice were group housed (4/cage) in a Thoren rack and allowed to acclimate for 2 weeks before training. Tap water and standard rodent chow were continuously available. The housing room was maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.) at a temperature of 21 ± 1 °C. All procedures were conducted during the light portion of the cycle in accordance with the National Institutes of Health Principles of Laboratory Animal Care, and the OHSU Institutional Animal Use and Care Committee approved the protocol.
Apparatus
Place conditioning was conducted in 12 clear acrylic boxes (30 × 15 × 15 cm) with aluminum end panels that were placed inside ventilated, light- and sound-reducing enclosures (Model E10-20; Coulbourn Instruments, Allentown, PA). For some groups, a 4-in. (10-cm) Mini Moon Lite (Model 73060; AmerTac, Saddle River, New Jersey; 3 VDC) was placed on the floor behind the conditioning box to illuminate the enclosure. Six sets of infrared emitter/detector pairs were mounted 5 cm apart, 2.2 cm above the floor along the walls of each box. A computer recorded activity (beam breaks/min) and side preference (seconds spent on each side) during all sessions. A detailed description of the apparatus has been published (Cunningham et al. 2006a).
Each box was placed on top of interchangeable floor halves that served as the tactile conditioned stimuli (CSs). The grid floor consisted of 2.3-mm stainless steel rods mounted 6.4 mm apart on acrylic rails. The hole floor was stainless steel (16 gauge) perforated with 6.4-mm holes on 9.5-mm staggered centers. Previous studies have shown that drug-naive control groups from the B6 and D2 strains show no appreciable preference for either floor type (Cunningham 2014). For some groups, explicit visual cues (VC) were added to the walls and end panels by attaching laminated sheets of white paper (92 brightness, 20 lb. [75 g/m2]) printed with an irregular pattern of black dots (2.4-cm-diameter dots staggered 4.4 cm apart center to center) or vertical black stripes (1.9-cm-wide stripes separated by a 1.9-cm white background).
General procedure
A total of four separate experiments were conducted. B6 mice were used in experiments 1A and 1B whereas D2 mice were used in experiments 2A and 2B. Experiments for B6 and D2 mice were not run concurrently, but the same experimenter conducted all experiments at the same time of day in the same apparatus. All experiments included three types of sessions: (a) a single pretest or habituation session, (b) multiple (1–6) conditioning sessions of each trial type, and (c) one or more post-conditioning test sessions. A preconditioning preference test (pretest) was conducted in experiments 1A and 2A to determine whether these strains had any initial biases for the specific cue combinations used here. Mice were handled, weighed, and given an intraperitoneal (IP) injection of saline (12.5 ml/kg) immediately before being placed in the center of the apparatus for a 30-min session. The center divider was removed, and mice were free to enter either side of the apparatus, which was configured and illuminated according to group assignment (see below). Experiments 1B and 2B began with a single 5-min habituation session. After being weighed and injected with saline, mice were placed in the center of the apparatus in the absence of the center divider or any conditioned stimuli. The floors on both sides were covered with white paper, the walls were covered with laminated white paper, and illumination was consistent with group assignment.
Mice in all groups received an unbiased place conditioning procedure, i.e., cues were assigned as the CS+ (ethanol-paired cue) or CS− (saline-paired cue) in a counterbalanced manner without regard to pretest preference levels. Within each of the main treatment conditions (described for each experiment in a later section), mice were assigned to one of two conditioning subgroups: GRID+ or GRID− (n = 11–16/subgroup). Mice in the GRID+ subgroups were given an IP injection of 2 g/kg ethanol (20% v/v in saline, 12.5 ml/kg) immediately before placement on the grid floor on CS+ trials, but received an IP injection of saline (12.5 ml/kg) just before placement on the hole floor on CS− trials. These contingencies were reversed for mice in the GRID− subgroups. Each conditioning trial lasted 5 min (Cunningham and Prather 1992), and the order of CS+ and CS− conditioning trials was counterbalanced within each subgroup.
Post-conditioning test sessions were identical to the pretest sessions. These 30-min tests were always given about 24 h after the most recent place conditioning session. The conditioning box, floors, and laminated visual cues were wiped with a damp sponge, and the litter paper was replaced after each animal.
Experiments 1A and 1B: cue effects on CPP in B6 mice
Experiment 1A
B6 mice (N = 96) were randomly assigned to one of three large groups that differed in terms of apparatus illumination and cue configuration. Mice assigned to the 1C group were exposed to a 1C training procedure with tactile floor cues in a darkened sound enclosure. Mice had unrestricted access to both sides of the apparatus on conditioning trials (i.e., no center divider), and each side contained the same floor cue. Thus, CSs included a tactile component, but no explicit visual or spatial elements. In contrast, mice assigned to both of the other groups received a 2C training procedure with tactile cues in an illuminated sound enclosure. A different tactile cue served as the floor on each side, and mice were confined to one side of the apparatus on each conditioning trial using a clear acrylic barrier. Thus, CSs for these groups included tactile, visual, and spatial elements. For mice in the 2C-NC group, there were no explicit visual cues beyond those already provided by the floors and illuminated sound enclosure. However, for mice in the 2C-VC group, laminated panels printed with black dots or stripes were attached to walls to enhance visual cues on each side. Visual-tactile cue combinations and side assignments were counterbalanced within each group. All mice received two preference tests. The first test was given after four conditioning trials of each type, and the second test was given after six conditioning trials of each type.
Experiment 1B
This experiment was designed as a partial replication of Exp. 1A. B6 mice (N = 64) were randomly assigned to two large groups: 1C or 2C-VC. Group 2C-NC was not included since it did not differ from group 2C-VC in Exp. 1A. The only other difference between experiments was that Exp. 1B included an additional preference test after the first two conditioning trials of each type.
Experiments 2A and 2B: cue effects on CPP in D2 mice
Experiment 2A
D2 mice (N = 96) were randomly assigned to three large groups (1C, 2C-NC, or 2C-VC) using an experimental design and procedure identical to Exp. 1A.
Experiment 2B
Since Exp. 2A failed to show any group differences in CPP after four or six conditioning trials of each type, Exp. 2B was designed to test whether group differences could be detected at an earlier point in conditioning, before groups had reached a performance asymptote. D2 mice (N = 94) were randomly assigned to two large groups that were conditioned as described earlier: 1C or 2C-VC. Half of each group was tested after only one trial of each type, while the other half was tested after two trials of each type.
Data analyses
Preference test performance was indexed by time spent on the grid floor (sec/min) during each 30-min test session. Grid time and activity data were analyzed separately for each test by factorial analysis of variance (ANOVA) using cue configuration group, number of conditioning trials (Exp. 2A only), and conditioning subgroup (G+ vs. G−) as between-group variables. In the counterbalanced unbiased place conditioning procedure used here, significant differences between the G+ and G− conditioning subgroups indicate development of CPP (Cunningham et al. 2003, 2006a, 2011). Significant main effects or interactions in the overall ANOVAs were interpreted as needed by making appropriate Bonferroni-corrected post hoc comparisons. The alpha level was set at 0.05 for all analyses.
Results
Experiments 1A and 1B: cue effects on CPP in B6 mice
Experiment 1A
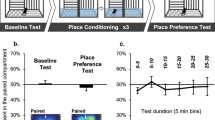
One mouse (group 2C-VC) was removed from the study after the first post-conditioning test due to an injection injury. The pretest data showed no significant differences among groups in initial preference as indicated by the absence of significant main effects or interaction in a two-way ANOVA (group × conditioning subgroup). Overall, B6 mice spent 31.3 ± 0.8 s/min on the grid floor during the pretest (i.e., about 52% of the session).
Figure 1 depicts the time spent on the grid floor during the first (left panel) and second (right panel) preference tests, which were conducted after the first four and after all six pairs of conditioning trials, respectively. CPP was somewhat weaker on both tests in the 1C group than in the 2C groups, which were similar. Two-way ANOVAs (group × conditioning subgroup) applied separately to the data from each test showed significant main effects of the conditioning subgroup on both tests, confirming development of CPP [T1: F(1, 90) = 35.2, p < .001; T2: F(1, 89) = 35.1, p < .001]. However, there was no main effect [T1: F(2, 90) = 0.5, p = 0.62; T2: F(2, 90) = 0.5, p = 0.62] or interaction [T1: F(2, 90) = 1.8, p = 0.17; T2: F(2, 90) = 1.2, p = 0.29] with group on either test. Nevertheless, based on our a priori hypotheses, we conducted Bonferroni-corrected post hoc tests comparing the GRID+ and GRID− subgroups to determine whether place conditioning was significant within each treatment group. These tests indicated development of significant CPP for both of the 2C groups on both tests (p < .001), but only during the second test for the 1C group (p < .05).
Mean time spent on the grid floor (s/min ± SEM) by C57BL/6J mice after four (left panel) and six (right panel) pairs of conditioning trials in Exp. 1A. Each bar represents data from 15 to 16 mice. The timeline at the top depicts the sequence of conditioning (C) trials and tests. 1C = one-compartment procedure in the dark; 2C-NC = two-compartment procedure in the light with no added visual cues; 2C-VC = two-compartment procedure in the light with additional visual cues on walls. *Significant difference between G+ (GRID+) and G− (GRID−) conditioning subgroups (post hoc Bonferroni-corrected p < .05)
Locomotor activity rates during each test are shown in Table 1. Although mice had access to both sides of the apparatus during these tests, mice that had previously been confined to one side on conditioning trials (i.e., 2C groups) were generally less active than mice that had previously had access to both sides during conditioning (group 1C). One-way ANOVA confirmed this, yielding a significant main effect of group on both tests [T1: F(2, 93) = 7.2, p < .001; T2: F(2, 92) = 4.6, p < .05]. For both tests, Bonferroni-corrected post hoc comparisons indicated that group 1C was significantly more active than either of the other groups (p ≤ .05), which did not differ.
Experiment 1B
Due to an equipment problem, data from one mouse (group 2C-VC) were lost from the final CPP test. Figure 2 depicts the CPP test behavior for all groups on all three tests, which were given after each exposure to two conditioning trials of each type. As expected, the CPP magnitude was positively related to the total number of conditioning trials. Consistent with the pattern of results in Exp. 1A, group 2C-VC showed a stronger CPP than group 1C during the second and third tests. Two-way ANOVAs (group × conditioning subgroup) applied separately to the data from each test showed no significant effects on test 1, but significant main effects of conditioning subgroup and significant group × conditioning subgroup interactions on tests 2 [conditioning: F(1, 60) = 19.8, p < .001; interaction: F(1, 60) = 6.2, p < .05] and 3 [conditioning: F(1, 59) = 26.9, p < .001; interaction: F(1, 59) = 8.1, p < .01]. Bonferroni-corrected post hoc tests comparing the GRID+ and GRID− subgroups confirmed development of CPP in group 2C-VC on tests 2 and 3 (p < .001). However, group 1C failed to show significant CPP on any test.
Mean time spent on the grid floor (s/min ± SEM) by C57BL/6J mice after two (test 1), four (test 2), and six (test 3) pairs of conditioning trials in Exp. 1B. Each bar represents data from 15 to 16 mice. The timeline at the top depicts the sequence of conditioning (C) trials and tests. 1C = one-compartment procedure in the dark; 2C-VC = two-compartment procedure in the light with additional visual cues on walls. *Significant difference between G+ (GRID+) and G− (GRID−) conditioning subgroups (post hoc Bonferroni-corrected p < .05)
Table 1 lists mean activity rates on each test. One-way ANOVAs showed a significant group difference in activity only on test 3 [F(1, 61) = 7.8, p < .01], with group 1C showing a higher level of activity than group 2C-VC.
Experiments 2A and 2B: cue effects on CPP in D2 mice
Experiment 2A
Mice in the 2C groups tended to spend less time on the grid floor during the pretest than mice in group 1C, suggesting that the addition of visual-spatial cues altered the initial bias for the tactile floor cues. Mean times spent on the grid floor (sec/min ± SEM) were 28.5 (1.0), 23.1 (0.9), and 25.6 (0.9) for groups 1C, 2C-NC, and 2C-VC, respectively. Two-way ANOVA (group × conditioning subgroup) revealed a significant main effect of group [F(2, 90) = 9.1, p < .001], but no main effect or interaction with conditioning subgroup. Bonferroni-corrected post hoc comparisons indicated that group 1C spent significantly less time on grid than group 2C-NC (p < .001). None of the other pairwise comparisons were significant. The absence of a significant conditioning subgroup main effect or interaction indicates that the GRID+ and GRID− subgroups within each treatment group were matched for initial bias.
As can be seen in Fig. 3, all of the D2 groups showed a strong CPP on both tests, with no differences among the cue configurations. Two-way ANOVAs on each test confirmed this conclusion, yielding only significant main effects of conditioning subgroup [T1: F(1, 90) = 640.2, p < .001; T2: F(1, 90) = 391.2, p < .001], but no significant main effect of group or interaction. Group 1C mice were more active during both CPP tests than either of the 2C groups (see Table 1). One-way ANOVA supported this conclusion [T1: F(2, 93) = 19.8, p < .001; T2: F(2, 93) = 10.4, p < .001]. For both tests, Bonferroni-corrected post hoc comparisons showed that group 1C was significantly more active than either of the other groups (p < .001), which did not differ.
Mean time spent on the grid floor (s/min ± SEM) by DBA/2J mice after four (left panel) and six (right panel) pairs of conditioning trials in Exp. 2A. Each bar represents data from 16 mice. A main effect of conditioning subgroup (G+ vs. G−) indicated significant conditioned place preference regardless of group assignment (p < .001). The timeline at the top depicts the sequence of conditioning (C) trials and tests. 1C = one-compartment procedure in the dark; 2C-NC = two-compartment procedure in the light with no added visual cues; 2C-VC = two-compartment procedure in the light with additional visual cues on walls
Experiment 2B
Figure 4 shows performance during the CPP test in mice given only one trial of each type (1-trial groups) or two trials of each type (2-trial groups) before testing. Both of the groups given two trials before testing developed a similar magnitude CPP, albeit weaker than that shown by D2 mice tested after four trials in Exp. 2A (left panel, Fig. 3). Of greater interest, in D2 mice given only one trial of each type before testing, group 2C-VC showed stronger CPP than group 1C. An overall three-way ANOVA (number of trials × group × conditioning subgroup) indicated a significant main effect of conditioning subgroup [F(1, 86) = 87.0, p < .001] and a significant number of trials × conditioning subgroup interaction [F(1, 86) = 13.6, p < .001]. The group × conditioning subgroup interaction fell just short of the criterion for significance [F(1, 86) = 3.5, p = .065]. Given our a priori hypotheses, we conducted Bonferroni-corrected post hoc tests comparing GRID+ and GRID− subgroups to determine whether place conditioning was significant within each treatment group. These comparisons confirmed the development of significant CPP in all groups (p < .001) except the 1-trial 1C group (p > .3).
Mean time spent on the grid floor (s/min ± SEM) by DBA/2J mice after one (1-trial groups) or two (2-trial groups) pairs of conditioning trials in Exp. 2B. Each bar represents data from 11 to 12 mice. The timeline at the top depicts the sequence of conditioning (C) trial(s) and test 1. 1C = one-compartment procedure in the dark; 2C-VC = two-compartment procedure in the light with additional visual cues on walls. *Significant difference between G+ (GRID+) and G- (GRID−) conditioning subgroups (post hoc Bonferroni-corrected p < .05)
As shown in Table 1, mice in groups tested after only one pair of conditioning trials were generally less active during the test than mice tested after two trials of each type. Moreover, as seen in Exp. 2A, mice in group 1C were more active than mice in group 2C-VC. These observations were supported by a two-way ANOVA (number of trials × group) that showed significant main effects of number of trials [F(1, 90) = 6.7, p = .01] and group [F(1, 90) = 38.9, p < .001], but no significant interaction.
Discussion
These studies show that combining tactile with visual-spatial cues yields stronger ethanol-induced CPP than using tactile cues alone in both B6 and D2 mice, although the conditioning parameters required to show this effect were quite different between the two strains. More specifically, while D2 mice showed the enhancing effect of visual-spatial cues after only one ethanol-conditioning trial (Exp. 2A, Fig. 4), four ethanol-conditioning trials were necessary for B6 mice (Exp. 1A, Fig. 1). Thus, the addition of visual-spatial cues did not appear to reduce or eliminate the strain difference in ethanol-induced CPP reported in previous studies that involved only tactile cues (Cunningham et al. 1992; Gabriel and Cunningham 2008; Cunningham 2014; Cunningham and Shields 2018). Moreover, the groups exposed to tactile cues alone (i.e., the 1C groups) replicated previous findings of a genetic difference by showing a more rapid development of a larger magnitude CPP in D2 mice (i.e., CPP after only two trials: Exp. 2A, Fig. 4) than in B6 mice (i.e., CPP only after 6 trials: Exp. 1A, Fig. 1). Overall, these data suggest that the strain difference in ethanol CPP is better explained as a genetic difference in ethanol reward sensitivity rather than a genetic difference in the salience or associability of the ethanol-paired cues.
The finding of stronger CPP in mice exposed to a combination of tactile and visual-spatial cues compared to tactile cues alone builds on previous work comparing the 1C and 2C procedures in mice (Cunningham and Zerizef 2014; Hitchcock et al. 2014). Neither of those studies directly compared the specific cue configurations used here with ethanol, but a cross-experiment visual comparison of data reported by Cunningham and Zerizef (2014: Exp. 1 vs. 2) suggested no cue-configuration effect in D2 mice after four conditioning trials, an observation consistent with the results of Exp. 2A. Moreover, the current studies extend the findings from previous studies in several important ways. First, they show that D2 mice are nevertheless sensitive to the enhancing effects of visual-spatial cues early in training (i.e., after only one ethanol conditioning trial) before CPP has reached its maximum magnitude. Second, these studies show that B6 mice are also sensitive to the enhancing effects of visual-spatial cues when trained with ethanol, extending the finding previously reported with cocaine (Hitchcock et al. 2014), although this effect requires more ethanol conditioning trials in B6 mice than in D2 mice. Finally, Exps. 1A and 2A showed no difference in either strain between groups given explicit visual cues in each compartment (2C-VC) and groups only given the visual cues provided by apparatus illumination (2C-NC). That finding suggests that systematically adding black stripes or dots to the walls of each compartment did not enhance the overall salience of the visual cues or that spatial location cues simply exerted greater behavioral control over (i.e., “overshadowed”) the visual cues.
There are several possible explanations of why CPP to tactile cues was enhanced by the addition of visual-spatial cues. One simple explanation is that increasing the number of relevant cue dimensions increased the likelihood that mice sampled stimulus elements that differed on drug and vehicle trials, thereby establishing a stronger association between the drug-paired cue configuration and ethanol’s rewarding effect. This explanation is generally consistent with early suggestions that using multiple cues is better based on anecdotal reports of difficulties in establishing CPP when only one modality cue was used (Mucha et al. 1982; Barr et al. 1985; Carr et al. 1989, p. 289). Stimulus-element models of Pavlovian conditioning such as the Rescorla-Wagner model (Rescorla and Wagner 1972) more formally incorporate the notion that using multiple stimulus elements can produce greater increments in associative strength, at least early in training. That model states that the different elements of a multi-modal compound stimulus will compete for changes in associative strength in proportion to their relative salience. According to the model, before conditioning has achieved asymptote, the net associative strength of a compound CS containing two or more stimulus elements (e.g., 2C procedure) will increase more rapidly over trials than the associative strength of a single element CS (e.g., 1C procedure). Thus, this prediction is consistent with the results of the first test for B6 mice in experiment 1A and with the results of the 1-trial groups for D2 mice in experiment 2B, both of which showed stronger conditioning in the 2C groups. Furthermore, the model predicts that, as the total associative strength of all stimuli present on each trial reaches asymptote, the differences between groups given one element and groups given multiple elements will disappear with continued training, which is exactly what happened in the experiments reported here. Strain differences in the rate at which group differences were detected and disappeared, which we attribute to an underlying strain difference in ethanol reward sensitivity, can be handled within the model by assuming that the asymptote of conditioning supported by the ethanol US (λ) and the US-associated learning rate parameter (β) are lower for B6 mice. Simulations of these predictions based on the Rescorla-Wagner model are provided in the supplementary material (Fig. S1).
Since previous studies have shown inverse genetic (Cunningham 1995, 2014) and phenotypic (Vezina and Stewart 1987b; Gremel and Cunningham 2007) relationships between test session activity and CPP magnitude in mice, consideration must also be given to the possibility that differences between the 1C and 2C groups in test session activity contributed to cue configuration effects on CPP performance. Although both groups had free access to the entire apparatus during preference tests, test session activity rates were generally lower in the 2C groups than in the 1C groups (Table 1), possibly due to the group difference in apparatus illumination (e.g., Nagy and Forrest 1970; Cunningham and Zerizef 2014) or to the novelty of the change to a larger test space for 2C groups. This difference raises the possibility that the stronger CPP observed in the 2C groups was due, at least in part, to their lower activity levels. Conversely, one might argue that the higher activity levels in 1C groups somehow interfered with their expression of CPP. However, several aspects of the data suggest that the CPP differences cannot be explained entirely by activity differences. For example, mice in the 1C and 2C groups differed in CPP on test 2 in Exp. 1B, but did not differ in test session activity rates. In several other cases, mice in the 2C groups were significantly less active than mice in the 1C groups but showed no group difference in CPP magnitude (Exp. 1A, test 2; Exp. 2A, tests 1 and 2; Exp. 2B, 2 trial groups). While a possible contribution of activity levels to CPP performance cannot be entirely dismissed, the overall pattern of findings offers stronger support for concluding that CPP magnitude was higher in the 2C groups because increments in associative strength were greater when a multi-modal cue was used.
The present findings are of particular interest in the context of Vezina and Stewart’s (1987a) initial rationale for development of the 1C procedure, which was motivated by a general desire to enhance CPP magnitude in rats. Contrary to conventional wisdom at the time (i.e., more cues are better), they designed a procedure involving only tactile (floor) cues in the absence of visual, spatial, or olfactory cues. They hypothesized that CPP magnitude in conventional 2C procedures involving multi-modal cues might be limited because “animals do not actually have to enter the drug-paired side to be in commerce with the drug-paired stimuli” (p. 376). When tactile cues are used in a dark environment, they reasoned that rats would be required to enter the drug-paired compartment in order to contact the CS. Although they did not provide a direct comparison between 1C and 2C procedures, their 1C procedure induced a higher preference for a morphine-paired floor cue (73–85%) compared to levels noted in previous studies with conventional 2C procedures (rarely > 60%). A subsequent paper from their lab provided an additional rationale for the superiority of 1C procedures based on how changes in compartment size between training and testing affected CPP magnitude (Vezina and Stewart 1987b). More specifically, they found that rats exposed to a conditioning compartment that was smaller than the test compartment (as in 2C procedures) showed weaker morphine-induced CPP to tactile cues than rats exposed to the larger compartment size during both training and testing (as in 1C procedures). They attributed this outcome to the locomotor-activating effect of exposure to the novel larger compartment at the time of testing in rats that experienced a change in compartment size. Thus, the 1C procedure was thought to be superior to a 2C procedure on both theoretical and empirical grounds.
Mice, however, do not appear be affected by number of CPP training compartments in the same way as rats. First, in contrast to Vezina and Stewart’s (1987a) conclusion that training in a 1C procedure produced stronger CPP in rats than a 2C procedure, our mouse data (in two different strains) showed just the opposite outcome early in training and no difference later in training. Second, contrary to expectations based on Vezina and Stewart’s (1987b) data, mice trained in our 2C procedure did not show elevated activity relative to mice trained in the 1C procedure. In fact, mice trained in the 2C procedure actually showed lower test activity (Table 1). That lower level of activity is most likely due to the group difference in apparatus illumination rather than the change to a larger space for testing because a previous study showed no test activity differences between 1C and 2C groups when illumination levels during training and testing were identical in both groups (Cunningham and Zerizef 2014). This overall pattern of differences between rats and mice encourages greater caution when attempting to extrapolate CPP findings from one species to the other.
The present studies also demonstrate the value of conducting CPP tests after different amounts of conditioning since they clearly show that the cue configuration effect depended on the amount of training. At the same time, they illustrate that repeated within-group testing has the potential to impair CPP development. More specifically, although six conditioning trials were sufficient to induce a significant CPP in the B6 1C group in Exp. 1A (Fig. 1), six trials did not produce CPP in the B6 1C group in Exp. 1B (Fig. 2). The most likely reason for this discrepancy is the difference between experiments in the number of preference tests experienced before the final test (i.e., two tests in Exp. 1B vs. only one test in Exp. 1A). Since every preference test serves as a 30-min extinction trial, the failure to induce CPP in the 1C group after six trials in Exp. 2 can be explained by interference from the intervening tests. The best way to avoid this problem is to use a between-group procedure to examine effects of amount of training, as was done in Exp. 2B.
A possible limitation on our conclusions is the unexpected finding that D2 mice (but not B6 mice) assigned to the 2C groups showed a pretest bias against the grid floor compared to the 1C group (Exp. 2A). That bias appears to be related to apparatus illumination in the 2C groups because the 1C and 2C groups had otherwise been treated identically before the pretest. Moreover, many previous studies have shown no such pretest bias in D2 mice tested in the dark (e.g., Cunningham et al. 2003). A possible implication of this pretest difference is that it could complicate interpretation of the CPP tests. However, both D2 experiments suggest that the pretest bias did not prevent or interfere with development of CPP in the 2C groups. Indeed, Exp. 2A showed a robust CPP in all groups with no differences between the 1C and 2C groups. Moreover, the 2C groups showed a more rapid development of CPP than the 1C groups in Exp. 2B. Thus, it would appear that the formation of an association between the CS+ and ethanol reward outweighed the influence of the pretest bias in these studies.
In summary, the present studies provide compelling evidence that a multi-modal cue will produce more rapid development of CPP than a single element cue, but that the advantage of a multi-modal cue diminishes with continued training (as predicted by the Rescorla-Wagner model). The generalizability of this finding is supported by demonstration of this effect in two commonly used mouse strains, B6 and D2. These studies also support previous studies indicating that D2 mice are more sensitive to ethanol reward than B6 mice. Furthermore, these studies suggest that apparatus cue configurations can have different effects on mice and rats. Finally, they illustrate the value of testing CPP after different amounts of training.
References
Barr GA, Paredes W, Bridger WH (1985) Place conditioning with morphine and phencyclidine: dose dependent effects. Life Sci 36:363–368
Carr GD, Fibiger HC, Phillips AG (1989) Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ (eds) The neuropharmacological basis of reward. Clarendon, Oxford, pp 264–319
Cunningham CL (1995) Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharm 120:28–41
Cunningham CL (2014) Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav Neurosci 128:430–445. https://doi.org/10.1037/a0036459
Cunningham CL, Ferree NK, Howard MA (2003) Apparatus bias and place conditioning with ethanol in mice. Psychopharm 170:409–422. https://doi.org/10.1007/s00213-003-1559-y
Cunningham CL, Gremel CM, Groblewski PA (2006a) Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1:1662–1670. https://doi.org/10.1038/nprot.2006.279
Cunningham CL, Groblewski PA, Voorhees CM (2011) Place conditioning. In: Olmstead MC (ed) Animal models of drug addiction, Neuromethods, vol 53. Humana Press, New York, pp 167–189. https://doi.org/10.1007/978-1-60761-934-5_6
Cunningham CL, Niehus DR, Malott DH, Prather LK (1992) Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharm 107:385–393
Cunningham CL, Patel P, Milner L (2006b) Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci 120:1115–1132. https://doi.org/10.1037/0735-7044.120.5.1115
Cunningham CL, Prather LK (1992) Conditioning trial duration affects ethanol-induced conditioned place preference in mice. Anim Learn Behav 20:187–194
Cunningham CL, Shields CN (2018) Effects of sex on ethanol conditioned place preference, activity and variability in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav 173:84–89. https://doi.org/10.1016/j.pbb.2018.07.008
Cunningham CL, Zerizef CL (2014) Effects of combining tactile with visual and spatial cues in conditioned place preference. Pharmacol Biochem Behav 124:443–450. https://doi.org/10.1016/j.pbb.2014.07.012
Gabriel KI, Cunningham CL (2008) Effects of maternal strain on ethanol responses in reciprocal F1 C57BL/6J and DBA/2J hybrid mice. Genes Brain Behav 7:276–287. https://doi.org/10.1111/j.1601-183X.2007.00349.x
Gremel CM, Cunningham CL (2007) Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharm 191:195–202. https://doi.org/10.1007/s00213-006-0651-5
Hitchcock LN, Cunningham CL, Lattal KM (2014) Cue configuration effects in acquisition and extinction of a cocaine-induced place preference. Behav Neurosci 128:217–227. https://doi.org/10.1037/a0036287
Mucha RF, van der Kooy D, O'Shaughnessy M, Bucenieks P (1982) Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243:91–105
Nagy ZM, Forrest EJ (1970) Open-field behavior of C3H mice: effect of size and illumination of field. Psychon Sci 20:19–21. https://doi.org/10.3758/BF03335578
Rescorla RA, Wagner AR (1972) A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF (eds) Classical conditioning II: current research and theory. Appleton-Century-Crofts, New York, pp 64–99
Vezina P, Stewart J (1987a) Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharm 91:375–380
Vezina P, Stewart J (1987b) Morphine conditioned place preference and locomotion: the effect of confinement during training. Psychopharm 93:257–260
Funding
Research reported in this paper was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA007702.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1091 kb)
Rights and permissions
About this article
Cite this article
Cunningham, C.L., Shields, C.N. Effects of multi-modal cues on conditioned place preference in C57BL/6J and DBA/2J mice. Psychopharmacology 235, 3535–3543 (2018). https://doi.org/10.1007/s00213-018-5078-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5078-2