Abstract
Rationale
Perphenazine, a classical antipsychotic drug, has the potential to induce extrapyramidal side effects (EPS). Dopaminergic and serotonergic pathways are involved in the therapeutic and adverse effects of the drug.
Objectives
To evaluate the impact of polymorphisms in the dopamine D2 and D3 and serotonin 2A and 2C receptor genes (DRD2, DRD3, HTR2A, and HTR2C) on short-term effects of perphenazine monotherapy in schizophrenic patients.
Materials and methods
Forty-seven Estonian inpatients were evaluated before and after 4–6 weeks of treatment by Simpson–Angus rating scale, Barnes scale, and Positive and Negative Symptom Scale. Genotyping was performed for common DRD2, DRD3, HTR2A, and HTR2C gene polymorphisms, previously reported to influence receptor expression and/or function.
Results
Most of the patients (n = 37) responded to the treatment and no significant association was observed between the polymorphisms and antipsychotic response. The 102C allele of HTR2A and the −697C and 23Ser alleles of HTR2C were more frequent among patients with EPS (n = 25) compared to patients without EPS (n = 22) (p = 0.02, 0.01, and 0.02, respectively). The difference between patients with and without EPS in variant allele frequencies remained significant after multiple model analyses including age, gender, and duration of antipsychotic treatment as covariants. There was no significant association between EPS occurrence and polymorphisms in the DRD2 and DRD3 genes.
Conclusions
An association was observed between polymorphisms in HTR2A and HTR2C genes and occurrence of acute EPS in schizophrenic patients treated with perphenazine monotherapy. Larger study populations are needed to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perphenazine is a classical antipsychotic drug with a potential of inducing extrapyramidal side effects (EPS). Although the newer, second-generation antipsychotic drugs have clinical advantages, such as lower incidence of EPS and high efficacy against negative symptoms, the metabolic side effects induced by these drugs are a common concern (Melkersson and Dahl 2004). It is interesting to note that the recently published CATIE trial (Lieberman et al. 2005) suggested that the overall treatment outcome may not be any better with some of the newer and more costly compounds compared to perphenazine. Thus, the classical antipsychotics still remain important in the treatment of schizophrenia.
Drug-induced EPS are categorized into acute (acute dystonia, parkinsonism, and akathisia) and delayed (tardive dyskinesia) syndromes based on the time of occurrence during antipsychotic treatment. It was shown that patients who experience acute EPS have higher risk in developing tardive dyskinesia (Muscettola et al. 1999). Older age, female sex, high drug doses and concentrations, and longer duration of antipsychotic treatment were shown to predispose for EPS (Casey 1991). More recently, genetic predisposition was also considered. While genetic factors influencing the kinetics of antipsychotics (i.e., polymorphisms in the cytochrome P450 enzyme-coding genes) were long recognized to contribute to the large variability in the clinical outcome of antipsychotic treatment (Scordo and Spina 2002), an increasing number of evidence now points toward a possible association between polymorphisms in receptor-coding genes and interindividual differences in therapeutic and adverse effects (Malhotra et al. 2004). Acute EPS may have special clinical importance due to their negative influence on patient adherence that is very crucial in the long-term treatment of schizophrenia. So far, only a few studies have evaluated genetic risk factors for acute EPS.
Both the antipsychotic effect and EPS of classical antipsychotic drugs were hypothesized to be caused by blockade of dopamine D2 receptors while the newer compounds have higher affinity for serotonin (5-HT)2 receptors than for dopamine D2 receptors (Casey 1991; Meltzer et al. 1989). It was also reported that the 5-HT system has regulatory effects on dopamine release in the nigrostriatal and mesolimbic dopamine pathways (Di Matteo et al. 2001). Several polymorphisms were identified in the dopamine D2 receptor gene (DRD2), located on chromosome 11. The Taq1A polymorphism was reported to affect dopamine D2 receptor expression (Pohjalainen et al. 1998). The Taq1A1 allele was found to be associated with reduced receptor binding (Thompson et al. 1997) and decreased receptor density in striatum of healthy volunteers (Jonsson et al. 1999). On the other hand, the deletion variant of the −141CIns/Del polymorphism in the DRD2 gene was suggested to associate with high dopamine receptor density (Jonsson et al. 1999). Another polymorphism in DRD2, the Ser311Cys polymorphism, was postulated to modulate receptor–G protein interaction and alter receptor function (Cravchik et al. 1996). Previous studies evaluating a possible association between dopamine receptor gene polymorphisms and EPS induced by antipsychotic drugs failed to discover any correlation, with the exception of the Ser9Gly polymorphism at position 9 of the first exon of dopamine D3 receptor gene (DRD3) (Segman et al. 2000). On the other hand, a recent report suggested a possible role for the Taq1A1 allele as a risk factor for EPS during treatment with selective 5-HT reuptake inhibitors (Hedenmalm et al. 2006).
Different polymorphisms in the 5-HT2A-receptor-coding gene, HTR2A, located on chromosome 13, were evaluated in several association studies in relation to schizophrenia, antipsychotic response, and EPS. A silent polymorphism, 102T/C, within the coding region, was found to be in linkage disequilibrium with the −1438A/G polymorphism in the promoter region of HTR2A (Spurlock et al. 1998). A recent study suggested that the −1438A/G polymorphism may alter promoter activity and expression of 5-HT2A receptors (Parsons et al. 2004). Another polymorphism of HTR2A, His452Tyr, causing an amino acid substitution within the cytoplasmic C-terminal tail of the receptor, was shown to alter 5-HT-induced calcium mobilization in platelets (Ozaki et al. 1997).
The gene coding for the 5-HT2C receptor, HTR2C, is located on the long arm of the X chromosome (Milatovich et al. 1992). Two polymorphisms in the promoter region of HTR2C, −759C/T, and −697G/C were found to be closely linked (Yuan et al. 2000). The functional importance of −697G/C is not known, but an increased promoter activity related to a haplotype containing −697C and−759T was reported (Yuan et al. 2000). Another polymorphism, 68G/C, in the coding region of HTR2C, leads to a cysteine substitution by a serine at position 23 (Cys23Ser) (Sodhi et al. 1995).
The effect of polymorphisms in receptor-coding genes on the clinical outcome of perphenazine treatment was not reported previously. In the light of previous studies, it can be hypothesized that functional polymorphisms in the genes coding for dopamine and 5-HT receptors might influence the risk to develop EPS. Therefore, we evaluated the impact of polymorphisms in DRD2, DRD3, HTR2A, and HTR2C receptor genes on the short-term clinical effects of perphenazine monotherapy in schizophrenic patients.
Materials and methods
Patients and study design
The patient population consisted of 47 Estonian patients (22 women and 25 men, aged 17–68 years), diagnosed with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders IV criteria and a psychotic episode that required hospitalization. After a minimum of 1 week washout period, patients were prescribed oral perphenazine as monotherapy. The dose of perphenazine was decided by the psychiatrist in charge of the patient, based on clinical evaluation. Fifteen of the patients had no previous exposure to antipsychotic drugs. Only benzodiazepines as sedative/hypnotic (22 patients) and trihexylphenidyl (4 patients), in case the patient developed neurological side effects, were allowed as concomitant therapy. The drugs were given on the ward under the observation of a nurse. Psychotic symptoms were evaluated with PANSS (Positive and Negative Symptom Scale) before starting perphenazine treatment and after 4–6 weeks of therapy, after a minimum 1 week of stable perphenazine dose (4–48 mg/day). Patients who showed more than 20% reduction in the PANSS score were defined as responders. EPS were evaluated with SAS (Simpson Angus Score) and BAS (Barnes rating score). A total score higher than three on SAS scale was classified as parkinsonism, and a BAS score of four or higher was classified as akathisia. The evaluation of efficacy and side effects by PANSS, SAS, and BAS was performed by a psychiatrist blind to the dosage of perphenazine. Blood samples for genotyping were taken at the beginning of perphenazine treatment, and for plasma drug concentration analysis after 4–6 weeks of therapy, simultaneously with the clinical ratings as described above. The study was approved by the local ethics committee at the University of Tartu and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. A written informed consent was obtained from each patient before inclusion in the study.
Analytical methods
Genomic DNA was isolated from peripheral leukocytes with Qiagen Blood and Cell Culture kit (Qiagen, Hilden, Germany). Genotyping was performed by PCR-RFLP analysis according to the methods previously described with minor modifications. The Taq1A (Grandy et al. 1993), Ser311Cys (Arinami et al. 1994), and −141CIns/Del (Arinami et al. 1997) polymorphisms of DRD2; the Ser9Gly (Crocq et al. 1992) polymorphism of the DRD3 gene; the 102T/C (Warren et al. 1993) and His452Tyr (Ozaki et al. 1997) polymorphisms of the HTR2A gene; and the −759C/T, −697G/C (Yuan et al. 2000), and Cys23Ser (Lappalainen et al. 1995) polymorphisms of the HTR2C gene were analyzed. With respect to HTR2C, male patients were described as hemizygous because the HTR2C gene is located on the X chromosome.
Plasma perphenazine concentrations were determined at the Department of Clinical Pharmacology, Huddinge University Hospital, Stockholm, Sweden, by high-performance liquid chromatography, according to a previously published method (Mandal and Ace 1993), in samples taken 12 ± 1 h after the last perphenazine dose. The limit of quantitation was 0.1 nmol/l of perphenazine.
Statistical analysis
Kruskal–Wallis and Mann–Whitney tests were used to compare the characteristics (age, perphenazine dose and concentration, duration of illness and treatment, and BAS and SAS scores) of the patient groups (Prism 4 Software, GraphPad, San Diego, CA, USA). Fisher’s exact test was used for the comparisons of allele frequencies in patient groups and multiple model analyses were performed using Statistical Analyses Software (SAS), version 9.1. Statistical significance was defined as p < 0.05.
Results
Thirty-seven patients showed more than 20% reduction in PANSS score and were classified as responders, while the rest (n = 10) were classified as nonresponders. The age and perphenazine dose did not differ between responders and nonresponders (Table 1). The percentage of drug-naïve patients was similar in both groups. It is interesting to note that perphenazine concentration was higher in nonresponders compared to responders [2.2 (1.1–21.1) vs 1.0 (0.2–13.7) nmol/l; median (range); p = 0.01]. Duration of illness and of previous antipsychotic treatment were longer in nonresponders compared to responders, but the differences were not statistically significant (Table 1). The variant allele frequencies of dopamine or 5-HT receptor genes did not differ between responders and nonresponders (data not shown).
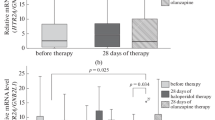
Twenty-five of the 47 patients experienced EPS, 12 patients having parkinsonism, 2 akathisia, and 11 both. Patients with EPS were older, had longer duration of illness and of antipsychotic treatment, and higher perphenazine dose and concentration than patients without EPS but the differences were not statistically significant (Table 1). The frequencies of DRD2 Taq1A1, 311Cys, −141CDel, and DRD3 9Gly variant alleles did not differ between patients with and without EPS (Fig. 1). On the other hand, patients with EPS had significantly higher frequency of the 102C allele of HTR2A [p = 0.02, odds ratio (OR) = 3.18, 95% confidence interval (CI) = 1.153–8.75] and the −697C (p = 0.01, OR = 4.30, 95% CI = 1.42–13.0) and the 23Ser alleles of HTR2C (p = 0.02) compared to patients without EPS (Fig. 2). After evaluation in multiple model analyses, including variant allele carriage, age, gender, and the duration of antipsychotic treatment as covariants, the differences in the frequencies of the HTR2A 102C and HTR2C −697C alleles remained significant between patients with and without EPS (p = 0.03 and 0.01, respectively).
The characteristics of patients with different genotypes with respect to HTR2A 102T/C, HTR2C −697G/C, and HTR2C Cys23Ser are given in Table 2. The SAS score was significantly higher in patients carrying the 23Ser variant compared to patients with Cys/Cys genotype (p =0.03). No other significant differences were found between the genotype groups.
Discussion
The mechanisms underlying the occurrence of EPS during antipsychotic treatment are still not fully understood. The blockade of D2 receptors in the nigrostriatal pathway was hypothesized to cause drug-induced EPS (Casey 1991). The Ser9Gly polymorphism in the DRD3 gene was previously reported to associate with antipsychotic-induced EPS, the 9Gly variant being more frequent among patients with tardive dyskinesia than patients without tardive dyskinesia (Lerer et al. 2002; Segman et al. 1999). In our study, no association was found between polymorphisms in DRD2 or DRD3 genes and antipsychotic response or the occurrence of acute EPS.
The results of the present study suggest an association between polymorphisms in HTR2A and HTR2C genes and EPS occurrence in schizophrenic patients treated with perphenazine monotherapy. The HTR2A 102C allele was more frequent among patients with EPS and all patients that developed EPS carried at least one 102C variant allele, while none of the patients with T/T genotype had EPS. In a postmortem study, the expression of 5-HT2A receptors was lower in temporal cortex of schizophrenic patients carrying the 102C allele (Polesskaya and Sokolov 2002). It is possible that this polymorphism, or the linked promoter −1438A/G polymorphism, is related to constitutive changes, which might cause susceptibility to schizophrenia (Abdolmaleky et al. 2004), poor response to antipsychotic treatment (Arranz et al. 2000), and EPS (Lerer et al. 2005). A significant association between tardive dyskinesia and the 102C–452Tyr HTR2A haplotype was reported in a meta-analysis that included 635 patients with different ethnic backgrounds (Lerer et al. 2005). In our study no linkage between these two polymorphisms was found.
The serotonergic system was reported to have an inhibitory control on dopaminergic activity in mesolimbic and nigrostriatal regions, via 5-HT2C receptors (Alex et al. 2005; Di Matteo et al. 2002). In addition, systemic and local (subthalamic nucleus) injections of 5-HT2C agonists induced orofacial dyskinesia, which was blocked by 5-HT2C antagonists, in rats (Eberle-Wang et al. 1996; Stewart et al. 1989). The higher frequency of the HTR2C −697C allele in patients with EPS compared to patients without EPS observed in the present study (p = 0.01, OR 4.30, 95% CI 1.42–13.0) is in concordance with the results of a previous study, reporting a significant association between this allele and tardive dyskinesia in Chinese chronic schizophrenic male patients (Zhang et al. 2002). Despite the close linkage between −697C and −759T alleles, the frequency of −759T allele did not differ between patients with and without EPS in our study, consistent with the results of Zhang et al. (2002).
The 23Ser allele of the HTR2C gene was also found to be more frequent among patients with tardive dyskinesia (Segman et al. 2000). The allele frequency of the 23Ser variant was reported to be 13% in healthy male Caucasians (Lappalainen et al. 1995). It is interesting to note that in our study the frequency of the 23Ser allele was 17% in patients with EPS while none of the patients without EPS carried this allele. It was suggested that the 23Ser allele might code for a constitutively more active receptor than 23Cys does (Okada et al. 2004). Considering the inhibitory effect of the serotonergic system on dopamine release in the nigrostriatal pathway, patients carrying a constitutively more active 5-HT2C receptor could be expected to have a predisposition to develop EPS.
Perphenazine, like other antipsychotic drugs, shows large interindividual variation in pharmacokinetics, mainly due to differences in the metabolic capacity of the genetically regulated CYP2D6 enzyme (Dahl 2002). The risk of EPS was reported to be concentration-related in perphenazine-treated patients (Hansen and Larsen 1985). In our study, the plasma concentration of perphenazine did not differ between patients with and without EPS.
Although the number of patients included in the present study is small, the fact that patients were treated with perphenazine in monotherapy provides the advantage of excluding drug–drug interactions. The results of the present study are in line with previous reports evaluating a possible association between tardive dyskinesia and receptor gene polymorphisms, and suggest that the 102T/C polymorphism of HTR2A and the −697G/C and Cys23Ser polymorphisms of HTR2C genes may influence the risk to develop EPS in patients treated with perphenazine. The associations remained significant even when evaluated in multiple model analyses with age, gender, and duration of previous antipsychotic treatment as covariants. This further supports the observed association between these polymorphisms and the risk to develop acute EPS. Larger study populations are needed to evaluate the predictive value of polymorphisms, especially in the HTR2C gene, on the risk of acute EPS.
References
Abdolmaleky HM, Faraone SV, Glatt SJ, Tsuang MT (2004) Meta-analysis of association between the T102C polymorphism of the 5HT2a receptor gene and schizophrenia. Schizophr Res 67:53–62
Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA (2005) Modulation of dopamine release by striatal 5-HT2C receptors. Synapse 55:242–251
Arinami T, Itokawa M, Enguchi H, Tagaya H, Yano S, Shimizu H, Hamaguchi H, Toru M (1994) Association of dopamine D2 receptor molecular variant with schizophrenia. Lancet 343:703–704
Arinami T, Gao M, Hamaguchi H, Toru M (1997) A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet 6:577–582
Arranz MJ, Munro J, Birkett J, Bolonna A, Mancama D, Sodhi M, Lesch KP, Meyer JF, Sham P, Collier DA, Murray RM, Kerwin RW (2000) Pharmacogenetic prediction of clozapine response. Lancet 355:1615–1616
Casey DE (1991) Neuroleptic drug-induced extrapyramidal syndromes and tardive dyskinesia. Schizophr Res 4:109–120
Cravchik A, Sibley DR, Gejman PV (1996) Functional analysis of the human D2 dopamine receptor missense variants. J Biol Chem 271:26013–26017
Crocq MA, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, Collier D, Lannfelt L, Sokoloff P, Schwartz JC et al (1992) Association between schizophrenia and homozygosity at the dopamine D3 receptor gene. J Med Genet 29:858–860
Dahl ML (2002) Cytochrome p450 phenotyping/genotyping in patients receiving antipsychotics: useful aid to prescribing? Clin Pharmacokinet 41:453–470
Di Matteo V, De Blasi A, Di Giulio C, Esposito E (2001) Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci 22:229–232
Di Matteo V, Cacchio M, Di Giulio C, Esposito E (2002) Role of serotonin(2C) receptors in the control of brain dopaminergic function. Pharmacol Biochem Behav 71:727–734
Eberle-Wang K, Lucki I, Chesselet MF (1996) A role for the subthalamic nucleus in 5-HT2C-induced oral dyskinesia. Neuroscience 72:117–128
Grandy DK, Zhang Y, Civelli O (1993) PCR detection of the TaqA RFLP at the DRD2 locus. Hum Mol Genet 2:2197
Hansen LB, Larsen NE (1985) Therapeutic advantages of monitoring plasma concentrations of perphenazine in clinical practice. Psychopharmacology (Berl) 87:16–19
Hedenmalm K, Guzey C, Dahl ML, Yue QY, Spigset O (2006) Risk factors for extrapyramidal symptoms during treatment with selective serotonin reuptake inhibitors, including cytochrome P-450 enzyme, and serotonin and dopamine transporter and receptor polymorphisms. J Clin Psychopharmacol 26:192–197
Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC (1999) Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 4:290–296
Lappalainen J, Zhang L, Dean M, Oz M, Ozaki N, Yu DH, Virkkunen M, Weight F, Linnoila M, Goldman D (1995) Identification, expression, and pharmacology of a Cys23–Ser23 substitution in the human 5-HT2c receptor gene (HTR2C). Genomics 27:274–279
Lerer B, Segman RH, Fangerau H, Daly AK, Basile VS, Cavallaro R, Aschauer HN, McCreadie RG, Ohlraun S, Ferrier N, Masellis M, Verga M, Scharfetter J, Rietschel M, Lovlie R, Levy UH, Meltzer HY, Kennedy JL, Steen VM, Macciardi F (2002) Pharmacogenetics of tardive dyskinesia: combined analysis of 780 patients supports association with dopamine D3 receptor gene Ser9Gly polymorphism. Neuropsychopharmacology 27:105–119
Lerer B, Segman RH, Tan EC, Basile VS, Cavallaro R, Aschauer HN, Strous R, Chong SA, Heresco-Levy U, Verga M, Scharfetter J, Meltzer HY, Kennedy JL, Macciardi F (2005) Combined analysis of 635 patients confirms an age-related association of the serotonin 2A receptor gene with tardive dyskinesia and specificity for the non-orofacial subtype. Int J Neuropsychopharmacol 8:411–425
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223
Malhotra AK, Murphy GM, Jr., Kennedy JL (2004) Pharmacogenetics of psychotropic drug response. Am J Psychiatry 161:780–796
Mandal TK, Ace LN (1993) High-performance liquid chromatographic determination of perphenazine in plasma. J Clin Pharm Ther 18:205–208
Melkersson K, Dahl ML (2004) Adverse metabolic effects associated with atypical antipsychotics: literature review and clinical implications. Drugs 64:701–723
Meltzer HY, Matsubara S, Lee JC (1989) Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther 251:238–246
Milatovich A, Hsieh CL, Bonaminio G, Tecott L, Julius D, Francke U (1992) Serotonin receptor 1c gene assigned to X chromosome in human (band q24) and mouse (bands D-F4). Hum Mol Genet 1:681–684
Muscettola G, Barbato G, Pampallona S, Casiello M, Bollini P (1999) Extrapyramidal syndromes in neuroleptic-treated patients: prevalence, risk factors, and association with tardive dyskinesia. J Clin Psychopharmacol 19:203–208
Okada M, Northup JK, Ozaki N, Russell JT, Linnoila M, Goldman D (2004) Modification of human 5-HT(2C) receptor function by Cys23Ser, an abundant, naturally occurring amino-acid substitution. Mol Psychiatry 9:55–64
Ozaki N, Manji H, Lubierman V, Lu SJ, Lappalainen J, Rosenthal NE, Goldman D (1997) A naturally occurring amino acid substitution of the human serotonin 5-HT2A receptor influences amplitude and timing of intracellular calcium mobilization. J Neurochem 68:2186–2193
Parsons MJ, D’Souza UM, Arranz MJ, Kerwin RW, Makoff AJ (2004) The −1438A/G polymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol Psychiatry 56:406–410
Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J (1998) The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry 3:256–260
Polesskaya OO, Sokolov BP (2002) Differential expression of the “C” and “T” alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. J Neurosci Res 67:812–822
Scordo MG, Spina E (2002) Cytochrome P450 polymorphisms and response to antipsychotic therapy. Pharmacogenomics 3:201–218
Segman R, Neeman T, Heresco-Levy U, Finkel B, Karagichev L, Schlafman M, Dorevitch A, Yakir A, Lerner A, Shelevoy A, Lerer B (1999) Genotypic association between the dopamine D3 receptor and tardive dyskinesia in chronic schizophrenia. Mol Psychiatry 4:247–253
Segman RH, Heresco-Levy U, Finkel B, Inbar R, Neeman T, Schlafman M, Dorevitch A, Yakir A, Lerner A, Goltser T, Shelevoy A, Lerer B (2000) Association between the serotonin 2C receptor gene and tardive dyskinesia in chronic schizophrenia: additive contribution of 5-HT2Cser and DRD3gly alleles to susceptibility. Psychopharmacology (Berl) 152:408–413
Sodhi MS, Arranz MJ, Curtis D, Ball DM, Sham P, Roberts GW, Price J, Collier DA, Kerwin RW (1995) Association between clozapine response and allelic variation in the 5-HT2C receptor gene. Neuroreport 7:169–172
Spurlock G, Heils A, Holmans P, Williams J, D’Souza UM, Cardno A, Murphy KC, Jones L, Buckland PR, McGuffin P, Lesch KP, Owen MJ (1998) A family based association study of T102C polymorphism in 5HT2A and schizophrenia plus identification of new polymorphisms in the promoter. Mol Psychiatry 3:42–49
Stewart BR, Jenner P, Marsden CD (1989) Induction of purposeless chewing behaviour in rats by 5-HT agonist drugs. Eur J Pharmacol 162:101–107
Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN, Court JA (1997) D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 7:479–484
Warren JT Jr., Peacock ML, Rodriguez LC, Fink JK (1993) An MspI polymorphism in the human serotonin receptor gene (HTR2): detection by DGGE and RFLP analysis. Hum Mol Genet 2:338
Yuan X, Yamada K, Ishiyama-Shigemoto S, Koyama W, Nonaka K (2000) Identification of polymorphic loci in the promoter region of the serotonin 5-HT2C receptor gene and their association with obesity and type II diabetes. Diabetologia 43:373–376
Zhang ZJ, Zhang XB, Sha WW, Zhang XB, Reynolds GP (2002) Association of a polymorphism in the promoter region of the serotonin 5-HT2C receptor gene with tardive dyskinesia in patients with schizophrenia. Mol Psychiatry 7:670–671
Acknowledgements
This study was supported by grants from the Swedish Research Council, the Swedish Society of Medicine, Svenska Lundbeckstiftelsen, Thurings Stiftelse, and the Karolinska International Research and Training Program. Niclas Eriksson, MSc., Uppsala Clinical Research Center, is gratefully acknowledged for assistance in the statistical analysis. The study was performed in accordance with the laws of the countries in which it was performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunes, A., Scordo, M.G., Jaanson, P. et al. Serotonin and dopamine receptor gene polymorphisms and the risk of extrapyramidal side effects in perphenazine-treated schizophrenic patients. Psychopharmacology 190, 479–484 (2007). https://doi.org/10.1007/s00213-006-0622-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0622-x