Abstract
Objective
To characterize in vivo the high-affinity cannabinoid CB1 receptor (CB1R) selective anandamide analog AM-1346 [alkoxyacid amide of N-eicosa-tetraenylamine] using drug discrimination procedures. d-Amphetamine and also morphine in the (R)-methanandamide-trained group (see below) were examined to assess pharmacological specificity.
Methods
Three groups of rats were trained to discriminate between vehicle and (1) 1.8 mg/kg Δ9-tetrahydrocannabinol (Δ9-THC); (2) 5.6 mg/kg Δ9-THC; and (3) 10 mg/kg (R)-methanandamide (AM-356; a metabolically stable analog of anandamide). Δ9-THC was given i.p. 30 min and (R)-methanandamide 15 min before training.
Results
AM-1346 generalized to all three training conditions, both at 15 and 30 min after administration. The rank order potency was: Δ9-THC > AM-1346 > (R)-methanandamide. AM-1346 appeared slightly more potent 30 min compared to 15 min postadministration. In the presence of 0.3 mg/kg of the CB1R antagonist/inverse agonist SR-141716A, the dose generalization curves of Δ9-THC and AM-1346 resulted in parallel shifts to the right in the 1.8 mg/kg Δ9-THC-trained group. A long duration of action for AM-1346 (relative to AM-356) was indicated in tests where AM-1346 was examined in the 5.6 mg/kg Δ9-THC-trained group. Neither d-amphetamine, nor morphine generalized in either of the groups, suggesting pharmacological specificity.
Conclusion
Unlike (R)-methanandamide, the surmountable antagonism between SR-141716A and AM-1346 shows that the structural features of anandamide can be modified in ways that reduce the dissociation between the discriminative stimulus and rate decreasing effects of CB1R agonists derived from an anandamide template.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endocannabinoid neuromodulatory system has emerged as a major component in regulatory physiological functions. To date, two cannabinergic recognition sites (CB1 and CB2) have been identified, both of which belong to the superfamily of G associated proteins. The CB1 receptor (CB1R) is the recognition site thought to be primarily responsible for the central (psychoactive) effects of anandamide and related endocannabinoid lipids. The subjective “high” after marijuana ingestion likely is mediated through this system. Although abundant in the central nervous system (CNS), CB1R’s are also present in the periphery, e.g., in the gut and the reproductive system. The CB2 receptor (CB2R) is considered linked primarily to the immune system (Howlett et al. 2002). However, contrary to earlier beliefs (e.g., Griffin et al. 1999), CB2R may be present in the CNS during normal as well as pathological conditions (van Sickle et al. 2005).
Several endogenous ligands have been discovered including anandamide (arachidonoyl ethanolamine) and 2-archidonoylglycerol (2-AG). Selective CB1R antagonists include SR141716A (rimonabant), AM-251, AM-281, and LY-320135. Other cannabinoid ligands such as SR-144528 as well as AM-630 selectively block the CB2R (Pertwee 2005; Thakur et al. 2005).
Although anandamide and exogenous cannabinoids such as (−)-Δ9-tetrahydrocannabinol (Δ9-THC) share some of their effects, the overlap is not complete. This is especially evident when the agonists are examined in the presence of the CB1R antagonist/inverse agonist SR-141716A. For example, operant responding and open-field effects of the anandamide analog (R)-methanandamide (AM-356; Abadji et al. 1994) in rats were not normalized by coadministration of SR-141716A; rather, the motor effects were augmented (Järbe et al. 2003a,b; see also Adams et al. 1998). Thus, behaviors were more suppressed when rats were examined after combinations of (R)-methanandamide and SR-141716 compared to when given the CB1R agonist alone. Furthermore, in tests for surmountable antagonism, sufficiently high doses of Δ9-THC overcame the blockade of the discriminative stimulus effects by SR-141716A (1 mg/kg), whereas in tests with combinations of SR-141716A and (R)-methanandamide, rats stopped responding (Järbe et al. 2001; see also Wiley et al. 2004).
Such data suggest differences in the mode of action between Δ9-THC and these anandamides. Two possibilities that are not mutually exclusive have been suggested for such differential interactions. Data by Howlett and colleagues (e.g., Houston and Howlett 1998; Mukhopadhyay and Howlett 2005), as well as by others (e.g., Bonhaus et al. 1998), suggested that cannabinoid ligands might interact with the CB1R differently, resulting in different cascades of events down stream. Others have pointed to the possibility that there might be more than one central cannabinoid receptor (e.g., Breivogel et al. 2001; Monory et al. 2002; for reviews see Hajos and Freund 2002; Wiley and Martin 2002).
The drug discrimination (DD) technique previously has been used to investigate the discriminative stimulus functions of Δ9-THC and (R)-methanandamide (Alici and Appel 2004; Burkey and Nation 1997; Järbe et al. 1998, 2000, 2001), a more stable chiral analog of anandamide (Abadji et al. 1994). In DD procedures, animals (or humans) are trained to discriminate between the presence and absence of the effects of a training or reference drug by emitting different responses using two or more choice manipulanda. The procedure characteristically exhibits a high degree of pharmacological specificity. In this study, the focus is on the anandamide analog AM-1346. In vitro receptor binding suggested a high degree of CB1R affinity and selectivity (see below) and, unlike Δ9-THC or (R)-methanandamide, AM-1346 is considered a full acting CB1R agonist (Makriyannis and Goutopoulos 2004). Given that onset of action of cannabinoids can vary (e.g., Järbe et al. 1981, 1986, 1989), two injection-to-test intervals with AM-1346 were explored in three groups of rats trained to discriminate between vehicle and Δ9-THC (1.8 and 5.6 mg/kg) or (R)-methanandamide (10 mg/kg). Two training doses of Δ9-THC were included to examine if the degree of generalization was influenced by the sensitivity of the procedure. In DD, lower training doses tend to be more inclusive, i.e., a propensity for increasing generalization compared to a higher training dose of the same drug (Bergman et al. 2000). Additionally, tests for surmountable antagonism (antagonist dose fixed and agonist dose varied) using SR-141716A together with Δ9-THC and AM-1346 were carried out in the group trained with 1.8 mg/kg Δ9-THC and estimates of the duration of effect of AM-1346 in the group trained with 5.6 mg/kg Δ9-THC.
All three discrimination groups additionally were tested with d-amphetamine, and also morphine in the (R)-methanandamide-trained group, to assess pharmacological specificity. Morphine was examined previously in Δ9-THC (1.8, 3, and 5.6 mg/kg)-trained groups and found not to generalize (Järbe et al. 1998); see also Solinas et al. (2004); Weissman (1978) (and with regard to the opioid agonist heroin, Solinas and Goldberg 2005).
Materials and methods
Apparatus
Training and testing occurred in eight chambers (ENV-001, Med. Associates, St Albans/Georgia, VT, USA) equipped with two response levers, house and lever lights, and a grid floor. Each chamber was enclosed within sound- and light-attenuating boxes with an exhaust fan and interfaced with a PC. Response contingencies were programmed using Med-PC software (Med. Associates).
Animals
Male Sprague–Dawley rats (Taconic Farms, Germantown, NY, USA) were individually housed in a colony room with an average temperature of 20°C and a 12-h light/dark cycle (rats were trained and tested during the light phase). Animals (90 days old at the beginning of the study) were experimentally naïve at the time of shaping the lever pressing response (see below). Some data collected from the Δ9-THC-trained animals have been presented separately (Järbe et al. 2000). Harlan Rat Chow® (# 2018) was restricted to approximately 12 g/day, maintaining body weights between 330 to 400 g. All procedures were approved by the Animal Care and Use Committee of Temple University, Philadelphia, PA, USA. The “Principles of animal laboratory care” (National Institutes of Health 1996) were followed.
Training
Rats were trained to eat food pellets (45 mg, formula A, Noyes® ) from a food receptacle located midway between the two response levers, and shaped to lever press for food reinforcement until they responded 10 times for each reinforcer (FR-10 schedule of reinforcement; FR-10). Under our conditions, when the house light was off, and the stimulus lights above the response levers lit, completion of 10 presses on the state appropriate lever resulted in the delivery of two 45-mg food pellets; followed by a 10-s timeout period with only the house light on. At the end of the 10-s time-out period, the stimulus lights above the levers were lit, the house light turned off, and the FR10 schedule of reinforcement reinstated. Termination of a session was indicated by all lights in the box being turned off.
Once daily, the rats were trained in this two-choice task to respond on drug- or vehicle-appropriate levers. The position of drug-appropriate levers was randomly assigned among subjects so that it was to the right of the food cup for half the subjects. (R)-Methanandamide (10 mg/kg) or vehicle was administered intraperitoneally (i.p.) in a volume of 3 ml/kg, 15 min before session onset (n=12). The animals discriminating between Δ9-THC (1.8 and 5.6 mg/kg) and vehicle (2 ml/kg) were administered the suspensions i.p. 30 min before sessions (n=12). Throughout the session, the aforementioned schedule was in effect. Presses on the incorrect lever were recorded, but had no programmed consequences. The schedule of drug (D) or vehicle (V) administrations was nonsystematic, with no more than two consecutive D or V sessions. Approximately an equal number of D and V training sessions occurred throughout the study. To avoid the influence of odor cues left in a chamber by a preceding subject, the order in which D and V training sessions were conducted for animals trained in the same chamber was randomized (Extance and Goudie 1981). Training took place Monday through Friday, and lasted for 20 min. Training continued until animals reached the acquisition criterion of selecting the lever appropriate for the training condition on at least 8 out of 10 consecutive training days. Correct selection was defined as total presses before the first reinforcement (FRF) being equal to, or less than 14 (i.e., the incorrect lever not pressed more than 4 times before completing 10 responses on the lever appropriate for the prevailing training condition; FRF ≤14).
Testing
After animals reached acquisition criterion, test sessions were conducted on average three times every 2 weeks; on interim days, regular drug or vehicle training sessions of 20 min duration took place. Approximately 2 weeks before initial testing, animals began receiving two i.p. injections before the training sessions (i.e., drug plus vehicle, or vehicle plus vehicle) to accustom the animals to a double injection procedure such as that used for antagonism testing. Typically, the order of sessions was: D, V, T, V, D (week 1); V, T, V, D, T (week 2); V, D, T, D, V (week 3); and D, T, D, V, T (week 4), where T stands for test. A drug training session preceded half the test sessions; the other half was preceded by a vehicle session. Tests were conducted only if responding during the preceding training sessions had been correct (FRF ≤14) during the initial six reinforcement cycles of the session. If incorrect, animals were retrained for at least three sessions where FRF ≤14 before additional testing. During test sessions reinforcers were delivered for 10 presses on either lever for six reinforcers or until 20 min had elapsed, whichever occurred first. There was one session per test day. Doses were examined in a mixed order. For each dose tested, the percentage of responding on the drug-appropriate lever was calculated from the ratio of the number of presses on the (R)-methanandamide- or Δ9-THC-associated lever to the total number of lever presses in a test session (excluding responding during the time-out periods). Only data for animals receiving at least one reinforcer during the test session were considered for this measure, i.e., animals must have made a minimum of 10 presses on one of the two levers. Additionally, response rate (responses per second) across all subjects was calculated. This measure was based on the performance of all animals, including nonresponders. Responding during time-out periods was not included in the rate data.
Statistics
Analyses of response rate data were performed with one-way repeated ANOVA using the software package SigmaStat (v. 3.10; Systat Software, Point Richmond, CA; http://www@systat.com). When ANOVA was significant, post hoc analyses were carried out with the Holm–Sidak method involving a reference or control mean (vehicle vs the corresponding other conditions) with alpha, two-tailed set at 0.05 (i.e., for the collection of comparisons). Linear regression analyses of dose-generalization and antagonism data after log-dose transformation were performed using Prism 4 software (v. 4.02, GraphPad Software, San Diego, CA; http://www.graphpad.com) to provide ED50 estimates ±95% confidence limits (±95% C.L.). All data points shown in the figures (see “Results”) were included in the regressions. Using the F-test, the Prism program also estimates if slopes are equal (parallel) and if the elevations or intercepts are equal (a measure of potency). All data shown in the “Results” were obtained from test sessions.
Drugs
(R)-Methanandamide [(R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide; AM-356; Ki (CB1)=28 nM; Ki (CB2)=867 nM], synthesized according to Abadji et al. (1994) and AM-1346 [alkoxyacid amide of N-eicosa-(5Z, 8Z, 11Z, 14Z)-tetraenylamide; Ki (CB1)=1.5 nM; Ki (CB2)=152 nM], were sent to the site of behavioral evaluation in argon capped vials on a monthly basis. This shipment schedule was implemented to minimize the likelihood of drug decomposition over time. Upon arrival, (R)-methanandamide and AM-1346 were dissolved in ethanol, appropriate amounts withdrawn, the ethanol evaporated under a stream of nitrogen, the residue then dissolved in a solution of propylene glycol (PG) and Tween-80 (T-80), and stored at −20°C. Shortly before being used, the solute was diluted with normal (0.9%) saline after the solute had been sonicated for 20–30 min. This procedure was followed for preparing suspensions of Δ9-THC as well. The levo isomer of Δ9-THC, dissolved in ethanol (200 mg/ml), was kindly provided by the National Institute on Drug Abuse (NIDA; Bethesda, Maryland, USA) and also stored at −20°C until used. SR-141716A (N-(piperidin-1-yl)-5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl; provided by Sanofi-Recherche, France) was stored and refrigerated at 4°C and dissolved in the PG/T-80 mixture (final suspension 5/3% for all drugs) before being diluted with saline (92%). AM-356 and AM-1346 were synthesized in the Department of Pharmaceutical Sciences, University of Connecticut at Storrs. All training drug doses were administered i.p. in a volume of 2 ml/kg (Δ9-THC), or 3 ml/kg [(R)-methanandamide and AM-1346]. Suspensions were prepared fresh daily. Morphine SO4 (NIDA) and d-amphetamine SO4 (Sigma, St Louis, MO) were dissolved in physiological saline and administered i.p. 1 ml/kg, 20 min before testing.
Results
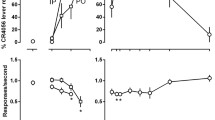
Figure 1 shows the generalization test results of three cannabinoid ligands for animals trained to discriminate between vehicle and (1) 1.8 mg/kg Δ9-THC (top left graph); (2) 5.6 mg/kg Δ9-THC (top middle graph); and (3) 10 mg/kg (R)-methanandamide (AM-356; top right graph). The ED50 estimates (±95% C.L.) for these generalization curves are summarized in Table 1. Clearly AM-1346 generalized to both Δ9-THC and (R)-methanandamide. There is a trend for lower ED50 values at the 30-min compared to the 15-min postinjection test intervals, significantly so in the (R)-methanandamide-trained group [F(1, 89)=14.93; p=0.0002]. The linear regressions suggested no deviations from parallelism (p>0.05). Hence, the following rank order potency was established: Δ9-THC > AM-1346 > AM-356 [(R)-methanandamide]. d-Amphetamine, and also morphine in the (R)-methanandamide-trained group, did not generalize to Δ9-THC or (R)-methanandamide. That effective dose ranges were examined is indicated by marked rate suppression (see below). Thus, these cannabinoid agonist discriminations exhibited pharmacological specificity.
Generalization test results (top) and corresponding response rate data (bottom) for the (1) 1.8 mg/kg Δ9-THC (left; n=9–11); (2) 5.6 mg/kg Δ9-THC (middle; n=10–12); and (3) (R)-methanandamide (AM-356, right; n=10–12) vs vehicle-trained rats. Training occurred 30 and 15 min (t=30′ and 15′) for the two CB1R agonists, respectively. The generalization results represent the mean percentage of lever presses on the drug appropriate lever out of the total number of lever presses emitted during a test session; injection-to-test intervals being 15 min and/or 30 min (15′ and 30′) post administration. (Y-axis); doses examined in milligram per kilogram (X-axis). Rate refers to the mean (±SEM) number of lever presses per second emitted during a test session (Y-axis); doses in milligram per kilogram (X-axis). Dotted lines represent the average test vehicle rates for the three groups. Data points are based on one observation for each rat and were obtained on separate test days. Amph. d-Amphetamine SO4; Morph. morphine SO4. Numbers within brackets indicate the number of rats responding (i.e., obtaining at least one reinforcement) out of the total number used for the test. All data based on a test session of a maximum of six reinforcers. One animal in the low-dose Δ9-THC (1.8 mg/kg) group died during the training phase; hence, only 11 rats were available for testing in this group. Asterisk Significantly (p≤0.05) different from vehicle training rate as indicated by horizontal dotted lines (Holm–Sidak procedure). Due to a technical error, the mean response rate depicted for 0.1 mg/kg Δ9-THC in the right lower graph is based on only four animals (this data point was not included in the rate analysis)
The lower graphs in Fig. 1 show the rate of responding during the generalization tests. The dotted horizontal lines represent the vehicle rate for the three discrimination conditions and were obtained on separate test occasions. ANOVA suggested significance for AM-1346 in the low-dose Δ9-THC (1.8 mg/kg)-trained group [F(5, 39)=10.12; p≤0.001; F(5, 39)=4.49; p=0.003; 15 and 30 min, respectively], but only the contrast at 15 min after administration was significant using the Holm–Sidak post hoc comparison involving a control mean (left bottom graph). Rates were also reduced in tests with d-amphetamine [F(4, 32)=16.03; p<0.001] in the low-dose Δ9-THC group.
In the high-dose Δ9-THC-trained group (bottom middle graph), response rates were reduced in tests with 1.0 and 5.6 mg/kg Δ9-THC [F(6, 64)=2.43; p=0.036]. Reduced rates also occurred in tests with 3.0 and 5.6 mg/kg AM-1346 conducted 15 min after administration [F(4, 40)=6.78; p<0.001], as well as in the test with 10 mg/kg AM-1346 30 min after administration [F(4, 39)=4.21; p=0.006]. All doses of d-amphetamine reduced the rate of responding [F(4, 36)=4.95; p=0.003].
Tests in the (R)-methanandamide-trained group (right bottom graph) suggested reduced rates of responding in the tests with d-amphetamine [F(4, 43)=25.75; p<0.001], as well as in the tests with morphine [F(4, 40)=24.48; p<0.001]. The other ANOVAs were not significant.
Figure 2 (top graph) shows that the generalization gradients (dose–response curves) for both Δ9-THC and AM-1346 were shifted to the right in the 1.8 mg/kg Δ9-THC-trained group when these two CB1R agonists were tested in the presence of 0.3 mg/kg SR-141716A, suggesting surmountable antagonism. The relative order of potency for the two drugs was similar to that observed when the two cannabinoid agonists were examined singly (see Table 1). Thus, the potency of Δ9-THC continued to be about twice that of AM-1346 also when examined in the presence of 0.3 mg/kg SR-141716A [F(1, 49)=16.36; p=0.0002] in the low-dose Δ9-THC group. Rate of responding was reduced in the 10 mg/kg AM-1346 plus 0.3 mg/kg SR-141716A condition [F(3, 24)=6.99; p=0.002]. There was a significant rate increase (compared to vehicle, 3 ml/kg) in the test with 1.8 mg/kg AM-1346 [F(5, 39)=4.22; p=0.004].
Antagonism test results (top right) and corresponding response rate data (bottom right) for combinations of 0.3 mg/kg SR-141716 and (1) Δ9-THC (open circles; n=9) and (2) AM-1346 (open diamonds; n=9) 30 min postadministration in the 1.8 mg/kg Δ9-THC vs vehicle-trained rats [for comparison, also the generalization data from Figure 2 are shown; (1) filled circles Δ9-THC (n=11); and (2) filled diamonds AM-1346 (n=9)]. Data in condition V (vehicle) shown at top left indicate the percentage of drug responding when the animals were tested with vehicle (1) 2 ml/kg alone (filled circle; n=11); (2) 3 ml/kg (filled diamond; n=10); and (3) 2 plus 3 ml/kg (embedded open circle and diamond; n=9). The corresponding rate data for these conditions are shown at bottom left. Drug lever responding results (top) represent the mean percentage of lever presses on the Δ9-THC (1.8 mg/kg) appropriate lever out of the total number of lever presses emitted during a test session (Y-axis); doses examined in milligram per kilogram (X-axis). Rate (bottom) refers to the mean (±SEM) number of lever presses per second emitted during a test session (Y-axis); doses in milligram per kilogram (X-axis). Data points are based on one observation for each rat and were obtained on separate days. All data based on a test session of a maximum of six reinforcers. Asterisk Significantly (p≤0.05) different from the respective vehicle response rate (Holm–Sidak procedure)
Figure 3 shows the duration of effect when AM-1346 was examined singly at different postinjection intervals (i.e., 15, 30, 60, and 120 min; open symbols) in the group discriminating between vehicle and 5.6 mg/kg Δ9-THC. Also, the AM-1346 vehicle (3 ml/kg) was examined singly (circles, light gray); these tests resulted in a low degree of drug (Δ9-THC) appropriate responding (≤10%). A dose of Δ9-THC (1.8 mg/kg) that had resulted in response generalization to a degree comparable to that of 5.6 mg/kg of AM-1346 at 30 min postadministration in the high-dose Δ9-THC-trained group was evaluated also at 60 and 120 min postadministration (filled circles). Dose generalizations for AM-1346 were similar during the earlier injection-to-test intervals (ED50’s ≅4.2, 3.8, and 3.3 mg/kg, respectively) with a trend for beginning to taper off at 120 min post administration (ED50 ≈7.0 mg/kg). The time course for Δ9-THC (1.8 mg/kg) was similar to that of 5.6 mg/kg AM-1346.
Time course of different doses of AM-1346 (open symbols; n=11) and of 1.8 mg/kg Δ9-THC (filled circles; n=11) in the group trained to discriminate between vehicle and 5.6 mg/kg Δ9-THC. Drug lever responding results (top) represent the mean percentage of lever presses on the Δ9-THC (5.6 mg/kg) appropriate lever out of the total number of lever presses emitted during a test session (Y-axis); doses examined in milligram per kilogram (X-axis). Rate (bottom) refers to the mean (±SEM) number of lever presses per second emitted during a test session (Y-axis); doses in milligram per kilogram (X-axis). Data points are based on one observation for each rat and were obtained on separate days. All data based on a test session of a maximum of six reinforcers. Vehicle (gray shading), 3 ml/kg (n=11). Asterisk Significantly (p≤0.05) different from the respective vehicle response rate (Holm–Sidak procedure)
Rates of responding increased over the corresponding control values (vehicle, 3 ml/kg) with the higher doses of AM-1346 when tested 30 and 60 min after administration [F(4, 39)=6.16 and 6.43; p<0.001, respectively]. There were no significant changes regarding response rate in the tests with 1.8 mg/kg Δ9-THC (included in above rate analyses).
Discussion
The current study was undertaken to characterize the discriminative stimulus functions of the full-acting CB1R selective high-affinity anandamide analog AM-1346 in vivo. To that end, three groups of rats were trained to discriminate between vehicle and the two CB1R agonists (R)-methanandamide (AM-356; 10 mg/kg) and Δ9-THC (two groups, one with 1.8 mg/kg and the other group with 5.6 mg/kg). AM-1346 generalized to all three training conditions. The rank order of potencies was: Δ9-THC > AM-1346 > (R)-methanandamide. The antagonism by 0.3 mg/kg SR-141716A of the AM-1346 response generalization to Δ9-THC (1.8 mg/kg) was surmountable with increasing doses of AM-1346. Such surmountable antagonism was also evident for Δ9-THC itself. In both cases, there was approximately a fivefold parallel shift to the right of these CB1R agonist dose generalization curves in the presence of SR-141716A compared to the agonists alone. The duration of effect for AM-1346 appeared to be comparatively long.
Although anandamide by itself has been reported to generalize to Δ9-THC, albeit only at doses markedly affecting rate of responding (Wiley et al. 1995), other studies reported negative effects (Alici and Appel 2004; Burkey and Nation 1997; Järbe et al. 2001; Wiley et al. 1997). However, anandamide analogs such as (R)-methanandamide (AM-356), O-1812 [(R)-(20-cyano-16,16-dimethyl docosa-cis-5,8,11,14-tetraenoyl)-1′-hydroxy-2′-propylamine] and the methylated fluoroanandamide compound 2-methylarachidonyl-2′-fluoroethylamide generalized to Δ9-THC (Alici and Appel 2004; Burkey and Nation 1997; Järbe et al. 1998, 2000, 2001; Wiley et al. 1997, 2004; see also Wiley et al. 1998). Presumably, these analogs are more resistant to degradation by the enzyme fatty acid amide hydrolase (FAAH) compared to anandamide (Cravatt et al. 1996). AM-1346 is a poor substrate for FAAH (Makriyannis et al., in preparation), presumably contributing to its long duration of action.
The duration of action of AM-1346 is at least as long as that for Δ9-THC (Järbe et al. 1981, 1986) and arguably longer than that of (R)-methanandamide (Järbe et al. 2001). More recent data (Järbe et al., unpublished) suggest that the duration of action of AM-1346 may even surpass that of Δ9-THC. The current estimate on duration of action for AM-1346 is less than ideal given that the limited drug availability precluded testing doses of AM-1346 that had produced close to 100% generalization in the high-dose Δ9-THC-trained group. The highest dose of AM-1346 examined for the time course evaluation was 5.6 mg/kg producing ≈70% Δ9-THC (5.6 mg/kg) appropriate responding. A dose of 1.8 mg/kg Δ9-THC produced a degree of generalization comparable to that of the highest dose of AM-1346 examined for time course in the high-dose Δ9-THC-trained group. The time course of the 1.8 mg/kg dose of Δ9-THC mirrored the outcome seen with 5.6 mg/kg AM-1346.
While SR-141716A produced complete antagonism of the Δ9-THC-like discriminative stimulus effects of (R)-methanandamide (Järbe et al. 2006) or O-1812 (Wiley et al. 2004), when increasing doses of antagonist were examined, previous attempts to demonstrate surmountable antagonism of the Δ9-THC-like discriminative effects of (R)-methanandamide by SR-141716A (antagonist dose fixed, agonist dose varied) were not completely successful due to marked rate-decreasing effects of high doses of (R)-methanandamide. At the highest dose of (R)-methanandamide (30 mg/kg) that could be tested together with SR-141716 without eliminating responding, only 50% Δ9-THC-appropriate responding was found (Järbe et al. 2001). In contrast, no antagonism of the rate-decreasing effects of (R)-methanandamide (Baskfield et al. 2004; Järbe et al. 2003b) or the open-field activity-depressant effects of (R)-methanandamide (Järbe et al. 2003a) were found with increasing doses of SR-141716A up to 3 or 5.6 mg/kg. In addition, (R)-methanandamide decreased rates of responding in CB1-receptor deficient mice (Baskfield et al. 2004), clearly indicating that these behavioral depressant effects of (R)-methanandamide are not mediated by CB1 receptors.
In the current study, surmountable antagonism was evaluated in the low-dose (1.8 mg/kg) Δ9-THC-trained group. Increasing doses of Δ9-THC and AM-1346 overcame the blockade of 0.3 mg/kg SR-141716A, suggesting a similar mechanism of action (CB1R activation) for the discriminative stimulus effects of these two CB1R agonists. The shift of the dose–response generalization curves was approximately fivefold without marked suppression of response rate (see Fig. 2 for ED50 values). This fivefold agonist ratio between the absence and presence of the antagonist is consistent with previously reported response generalization data on Δ9-THC/SR-141716A interactions (Järbe et al. 2001). We would have preferred to have examined antagonism across all three training conditions but were limited by the amounts of AM-1346 available. Thus, it still remains to be determined if surmountable antagonism would have occurred in the high-dose (5.6 mg/kg) Δ9-THC or in the (R)-methanandamide-trained groups. In our previous study (Järbe et al. 2001), the reference condition was 3.0 mg/kg Δ9-THC.
It is also worth noting that the ED50 ratio for the AM-1346 generalization (ED50 AM-1346/ED50 Δ9-THC) differed by a factor of 4, i.e., ≈4 mg/kg (high-dose group) compared to a factor of 2, i.e., ≈1 mg/kg (low-dose group) in the Δ9-THC-trained animals (see Table 1 and Fig. 1). Yet, in spite of this apparent sensitivity to training drug dose manipulation AM-1346 generalized fully in both Δ9-THC-trained groups. This was not the case in generalization tests with (R)-methanandamide in previous studies (Järbe et al. 1998, 2000). A maximum of ≈50% high-dose Δ9-THC (5.6 mg/kg) appropriate responding was observed at the highest test dose of 30 mg/kg (R)-methanandamide examined; this dose was accompanied by a markedly reduced response rate.
The noncannabinoid d-amphetamine, and also morphine in the (R)-methanandamide-trained group, produced very limited CB1R agonist-like responding. This is congruent with previous reports evaluating these agents in rats discriminating between 3.0 to 3.2 mg/kg Δ9-THC and vehicle (Barrett et al. 1995; for overviews, see Balster and Prescott 1992; Browne and Weissman 1981; Järbe and Mathis 1992; Weissman 1978). An exception is morphine which was also examined in rats trained with 1.8 and 5.6 mg/kg Δ9-THC (Järbe et al. 1998). Additionally, the absence of generalization by morphine in the current (R)-methanandamide-trained group is replicated by unpublished data obtained in a separate batch of (R)-methanandamide-trained rats (Järbe et al. 2006). Thus, with 3 mg/kg morphine, we obtained 15.5% (R)-methanandamide-associated responding (8 out of the 10 rats obtained at least one reinforcer). With 10 mg/kg, there was 25.2% (R)-methanandamide responding (4 out of 10 rats responded). With 18 mg/kg there was 0% (R)-methanandamide responding (only 1 out of the 10 rats responded enough to obtain at least one reinforcer). Although supporting pharmacological specificity for the current discrimination assays, lack of generalization does not necessarily mean that there is no potential interaction among the pathways subserving their effects. For example, a link between the endocannabinoid and opioid signaling systems is evident in DD such that coadministration of morphine or heroin augments the effects of Δ9-THC by shifting the dose-generalization curve to the left in rats discriminating between the effects of Δ9-THC and vehicle. Conversely, the opioid antagonists, naloxone and naltrexone, shifted the Δ9-THC dose-generalization curve to the right (Solinas et al. 2004; Solinas and Goldberg 2005).
In conclusion, there appears to be a dissociation between the discriminative stimuli and the rate decreasing effects (as well as open-field behaviors) for CB1R agonists; this dissociation being more marked for (R)-methanandamide (and by inference also anandamide) compared to Δ9-THC and AM-1346. The results with AM-1346 suggest that the anandamide structure can be modified in a manner that leads to a reduced dissociation between the discriminative stimulus effects and response rate.
References
Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A (1994) (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem 37:1889–1893
Adams IB, Compton DR, Martin BR (1998) Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther 284:1209–1217
Alici T, Appel JB (2004) Increasing the selectivity of the discriminative stimulus effects of Δ9-tetrahydrocannabinol: complete generalization with methanandamide. Pharmacol Biochem Behav 79:431–437
Balster RL, Prescott WR (1992) Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev 16:55–62
Barrett RL, Wiley JL, Balster RL, Martin BR (1995) Pharmacological specificity of delta 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology 118:419–424
Baskfield CY, Martin BR, Wiley JL (2004) Differential effects of Δ9-tetrahydrocannabinol and methanandamide in CB1 knockout and wild-type mice. J Pharmacol Exp Ther 309:86–91
Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN (2000) Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology 153:67–84
Bonhaus DW, Chang LK, Kwan J, Martin GR (1998) Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther 287:884–888
Breivogel CS, Griffin G, Di Marzo V, Martin BR (2001) Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60:155–163
Browne RG, Weissman A (1981) Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol 21(Suppl):227S–234S
Burkey RT, Nation JR (1997) (R)-Methanandamide, but not anandamide, generalizes to Δ9-THC in a drug-discrimination procedure. Exp Clin Psychopharm 5:195–202
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87
Extance K, Goudie AJ (1981) Inter-animal olfactory cues in operant drug discrimination procedures in rats. Psychopharmacology 73:363–371
Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung M, Martin BR, Abood ME (1999) Evaluation of the cannabinoid receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol 377:117–125
Hajos N, Freund TF (2002) Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids 121:73–82
Houston DB, Howlett AC (1998) Differential receptor-G-protein coupling evoked by dissimilar cannabinoid receptor agonists. Cell Signal9:667–674
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202
Järbe TUC, Mathis DA (1992) Dissociative and discriminative stimulus functions of cannabinoids and cannabinergics. In: A Bartke, L Murphy (eds) Marijuana/cannabinoids: neurobiology and neurophysiology. CRC Press, Boca Raton, FL, pp 425–459
Järbe TUC, Swedberg MDB, Mechoulam R (1981) A repeated tests procedure to assess onset and duration of the cue properties of (−)-delta-9-THC, (−)-delta-8-THC-DMH and (+)-delta-8-THC. Psychopharmacology 75:152–157
Järbe TUC, Hiltunen AJ, Lander N, Mechoulam R (1986) Cannabinergic activity (delta-1-THC cue) of cannabidiol monomethyl ether and two stereoisomeric hexahydrocannabinols in rats and pigeons. Pharmacol Biochem Behav 25:393–399
Järbe TUC, Hiltunen AJ, Mechoulam R (1989) Stereospecificity of the discriminative stimulus functions of the dimethylheptyl homologs of 11-OH-delta-8-tetrahydrocannabinol in rats and pigeons. J Pharmacol Exp Ther 250:1000–1005
Järbe TUC, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A (1998) Δ9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology 140:519–522
Järbe TUC, Lamb RJ, Lin S, Makriyannis A (2000) Δ9-THC training dose as a determinant for (R)-methanandamide generalization in rats: a systematic replication. Behav Pharmacol 11:81–86
Järbe TUC, Lamb RJ, Lin S, Makriyannis A (2001) (R)-Methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology 156:369–380
Järbe TUC, DiPatrizio NV, Li C, Makriyannis A (2003a) The cannabinoid receptor antagonist SR-141716 does not readily antagonize open-field effects induced by the cannabinoid receptor agonist (R)-methanandamide in rats. Pharmacol Biochem Behav 75:809–821
Järbe TUC, Lamb RJ, Liu Q, Makriyannis A (2003b) (R)-Methanandamide and Δ9-THC induced operant rate decreases in rats are not readily antagonized by SR-141716A. Eur J Pharmacol 466:121–127
Järbe TUC, Liu Q, Makriyannis A (2006) Antagonism of discriminative stimulus effects of Δ9-THC and (R)-methanandamide in rats. Psychopharmacology 184:36–45
Makriyannis A, Goutopoulos A (2004) Cannabinergics: old and new therapeutic possibilities. In: Makriyannis A, Biegel D (eds) Drug discovery strategies and methods 1. Marcel Dekker, New York, pp 89–128
Monory K, Tzavara ET, Lexime J, Ledent C, Parmentier M, Borsodi A, Hanoune J (2002) Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun 292:231–235
Mukhopadhyay S, Howlett AC (2005) Chemically distinct ligands promote differential CB1 cannabinoid receptor–Gi protein interactions. Mol Pharmacol 67:2016–2024
National Institutes of Health (1996) Principles of animal laboratory care. National Academy Press, Washington, DC
Pertwee RG (2005) Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76:1307–1324
Solinas M, Goldberg SR (2005) Involvement of mu-, delta- and kappa-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology 179:804–812
Solinas M, Zangen A, Thiriet N, Goldberg SR (2004) β-Endorphin elevations in the ventral tegmental area regulate the discriminative effects of delta-9-tetrahydrocannabinol. Eur J Neurosci 19:183–192
Thakur GA, Nikas SP, Li C, Makriyannis A (2005) Structural requirements for cannabinoid receptor probes. In: Pertwee R (ed) Handbook of experimental pharmacology, volume 168. Springer, Berlin, Heidelberg, New York, pp 209–246
van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332
Weissman A (1978) Generalization of the discriminative stimulus properties of delta-9-tetrahydrocannabinol to cannabinoids with therapeutic potential. In: Colpaert FC, Rosecrans JA (eds) Stimulus properties of drugs: ten years of progress. Elsevier/North Holland Biomed Press, Amsterdam, pp 99–122
Wiley JL, Martin BR (2002) Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem Phys Lipids 121:57–63
Wiley J, Balster R, Martin B (1995) Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol 276:49–54
Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin B (1997) Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav 58:1139–1144
Wiley JL, Ryan WJ, Razdan RK, Martin BR (1998) Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol 355:113–118
Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR (2004) A comparison of the discriminative stimulus effects of Δ9 tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol 12:173–179
Acknowledgements
United States Public Health Service Grants DA 09064, 00253 and 13429 (Philadelphia) and DA 03801, 9158, 7215, and 00152 (Boston) from the National Institute on Drug Abuse (NIDA) supported this work. We thank Michelle Harris for technical assistance. Additionally, three anonymous reviewers made suggestions for improving the manuscript. We also thank NIDA for supplies of (−)-Δ9-THC and morphine SO4. SR-141716A (as the salt) was a generous gift from Sanofi Recherchė, Montpellier, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Järbe, T.U.C., Lamb, R.J., Liu, Q. et al. Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog in rats trained with Δ9-THC or (R)-methanandamide (AM-356). Psychopharmacology 188, 315–323 (2006). https://doi.org/10.1007/s00213-006-0517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0517-x