Abstract
Rationale
The brainstem pedunculopontine tegmental nucleus (PPTg) is proposed to mediate hypothalamic self-stimulation reward via cholinergic activation of the ventral tegmental area (VTA). However, to date there is little direct evidence to support this hypothesis.
Objectives
To further study the role of PPTg in hypothalamic self-stimulation reward.
Methods
By using in vivo microdialysis, the levels of extracellular acetylcholine (ACh) in the PPTg and VTA were detected during lateral hypothalamic (LH) self-stimulation in rats. Rate–frequency curve shift procedure was used to evaluate the effects of nonselective muscarinic antagonist scopolamine (1∼100 μg/μl) and nicotinic antagonist mecamylamine (5∼100 μg/μl) microinjected into the PPTg on the rewarding efficacy of LH self-stimulation. Subsequently, the drugs were injected into the PPTg, and the extracellular ACh in the VTA was measured.
Results
LH self-stimulation produced a concurrent ACh release in the PPTg and VTA. Intra-PPTg injection of scopolamine (100 μg/μl) significantly reduced the frequency threshold for LH self-stimulation reward, but nicotinic antagonist mecamylamine did not shift the threshold. However, mecamylamine (10, 25 μg/μl) injected into the PPTg robustly diminished the nicotine-potentiated LH self-stimulation reward. The extracellular ACh in the VTA was dramatically increased by intra-PPTg scopolamine (10, 100 μg/μl), but not by mecamylamine.
Conclusions
Results confirm that PPTg plays an important role in brain stimulation reward by modulating the cholinergic activity of the VTA. The PPTg muscarinic receptors contribute to an inhibitory modulation of reward effects by self-stimulation, whereas nicotinic receptors seem to be more involved in nicotine potentiation of brain stimulation reward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pedunculopontine tegmental nucleus (PPTg) is a rostral midbrain structure at the junction of the pons and midbrain that contains a major group of cholinergic neurons. PPTg is suggested as a limbic–motor interface involved in the mediation of mesencephalic locomotor activities, sleep–wakefulness cycle, sensorimotor gating, and reward-related behaviors as well (Pahapill and Lozano 2000; Mena-Segovia et al. 2004; Diederich and Koch 2005). Of these, the involvement of PPTg in reward-related behaviors has been demonstrated in conditioned place preference (Tzschentke 1998; Kippin and van der Kooy 2003), intravenous drug self-administration (Olmstead et al. 1998; Lanca et al. 1999; Corrigall et al. 1999, 2001, 2002; Picciotto and Corrigall 2002; Alderson et al. 2004), and intracranial electrical self-stimulation (Yeomans et al. 1993; Nakahara et al. 2001).
The intracranial self-stimulation (ICSS) is most effective (rewarding) when applied to the medial forebrain bundle (MFB) extending from the lateral hypothalamus (LH) to the ventral tegmental area (VTA). Mesolimbic dopamine (DA) system, particularly its mesoaccumbens link, is critical to the ICSS (Wise 2002). It has been proposed that MFB stimulation may exert its effects not by directly stimulating the dopaminergic neurons but by descending nondopaminergic pathways that transsynaptically activate the ascending mesolimbic DA system. One component of these descending pathways seems to be cholinergic (Yeomans 1989; Wise et al. 1998). The PPTg receives dense afferents arising from the LH (Steininger et al. 1992; Semba and Fibiger 1992) and projects significant proportions of cholinergic innervations to the major mesencephalic dopaminergic nuclei, the VTA and substantia nigra pars compacta (SNc) (Oakman et al. 1995). Recent data suggested that PPTg might be one excitatory element in afferent modulation of mesolimbic and nigrostriatal dopamine release (Floresco et al. 2003; Forster and Blaha 2003). In the early 1990s, Yeomans et al. (1993) found that muscarinic drugs injected into the PPTg dramatically shifted hypothalamic stimulation thresholds and hypothesized that first-stage descending MFB fibers may activate cholinergic neurons within PPTg, which serve as the second stage, synaptically activating the third-stage mesolimbic dopaminergic neurons in the VTA. However, to date there is little direct evidence to support this hypothesis. Results from a lesion study even appear to rule out the possibility that PPTg plays an essential role in MFB stimulation of ascending cholinergic input to the VTA (Waraczynski and Perkins 1998).
Therefore, the present study aims to further explore the involvement of cholinergic PPTg in MFB self-stimulation reward, specially its functional modulation of cholinergic activation of VTA via acetylcholine receptors (AChRs). For this purpose, we tested the effects of (1) LH self-stimulation on the extracellular ACh in the VTA and PPTg, (2) intra-PPTg cholinergic antagonisms on the frequency threshold for LH stimulation reward, and (3) cholinergic antagonisms applied to PPTg on the extracellular ACh in the VTA. While the exact roles of nicotinic receptors in brain stimulation reward (BSR) and nicotine addiction still remain not fully understood, the present study for the first time shows the differential effects of nicotinic AChRs, at the level of PPTg, on the effectiveness of MFB self-stimulation when in the presence or absence of nicotine.
Materials and methods
Animals and surgery
Male Wistar rats (Japan SLC Inc., Shizuoka, Japan) weighing 330–350 g at the time of surgery were used. Rats were housed individually under a 12-h light/dark cycle (lights on at 7 a.m.), with food and water ad libitum. All procedures were approved by the institutional review committee and were in accordance with the Guide for the Care and Use of Laboratory Animals at Hamamatsu University School of Medicine.
The rats were anesthetized for surgery with pentobarbital (50 mg/kg, i.p.) and positioned in a stereotaxic frame. A monopolar electrode (stainless steel wire, 0.2 mm in diameter) was inserted into the right LH [coordinates: anteroposterior (AP) −4.0 mm, mediolateral (ML) 1.6 mm, and dorsoventral (DV) 8.0 mm; Paxinos and Watson 1998). In experiment I, a guide cannula for microdialysis was implanted ipsilaterally 1 mm above either the VTA (AP −5.5 mm, ML 1.0 mm, DV 7.5 mm) or PPTg (AP −8.3 mm, ML 1.0 mm, DV 6.5 mm). Here the cannula above the VTA was implanted at an angle 12° caudal to the dorsal/ventral axis for ease of implantation. In experiment IV, a guide cannula for microdialysis was implanted at a 20°-angle above the VTA, whereas a guide cannula (22-gauge, Plastics One) for drug microinjection was implanted ipsilaterally into the PPTg (coordinates: AP −8.0 mm, ML 2.0 mm, DV 7.0 mm) (Fig. 5a). Rats were allowed 12–14 days to recover from surgery.
Systemic and intracranial injections
Nicotine di-d-tartrate (Sigma) was dissolved in saline at a dose of 0.5 mg/ml (pH 7.2–7.4) and subcutaneously administered in a volume dose of 1 ml/kg 10 min before ICSS testing. Muscarinic acetylcholine receptor (mAChR) antagonist (−) scopolamine hydrochloride (Sigma) and nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine hydrochloride (Sigma) were dissolved in Ringer solution (1.2 mM CaCl2, 4 mM KCl, 147 mM NaCl, pH 6.7), which also served as the control injection. Intracranial microinjections were made using 28-gauge injectors (Plastics One). Drugs were injected in a volume of 1 μl over the course of 10 min, and the injectors were left in place for 10 min to allow sufficient diffusion of the drug.
In vivo microdialysis and high-performance liquid chromatography
Collection and analysis of extracellular ACh by in vivo microdialysis and high-performance liquid chromatography (HPLC) were conducted as described previously (Nakahara et al. 2001). Briefly, rats were placed in the ICSS chamber, and the dialysis probe with 1.0 mm of active membrane (Cuprophan, Nikkiso, Japan; molecular weight cutoff <35,000 Da) was inserted into the guide cannula. The dialysis probe was infused with Ringer solution containing 10 μM eserine (Wako Pure Chemical Industries, Osaka, Japan), an ACh esterase inhibitor, at a flow rate of 2 μl/min. After a 180-min stabilization period, three consecutive samples were collected at 20-min intervals to determine the steady-state (baseline) level. Rats were subsequently allowed to press the lever for self-stimulation for 1 h. Sample collections were continued during and for 2 h after the ICSS. ACh concentration in dialysates was analyzed by reverse-phase HPLC system (BAC-300 system, EICOM, Kyoto, Japan).
Procedures
Experiment I: effects of LH self-stimulation on extracellular ACh in VTA and PPTg
In this experiment, each rat had an electrode placed in the right LH and a guide cannula placed in the VTA (or PPTg) ipsilateral to the electrode. They were placed in a transparent acrylic chamber (30.0 × 25.0 × 26.5 cm) equipped with a lever and were trained to press the lever for LH self-stimulation. A continuous reinforcement schedule was used. Each lever press delivered a 0.3-s train of monophasic cathodal rectangular pulses of constant duration (0.1 ms) and frequency (100 Hz) and variable intensity (300–500 μA). The animals were trained for 1–3 times (1 h daily) until an established stable lever pressing at rate of no less than 250 responses per 5 min was achieved. Animals that would not meet this criterion were excluded. Microdialysis collections started 3 days after the last ICSS training, and the dialysates were instantly analyzed by reverse-phase HPLC as described above.
Experiment II: effects of intra-PPTg muscarinic antagonists on the frequency thresholds for LH stimulation reward
Rats were first trained to poke their nose into a hole equipped on the wall of a transparent acrylic chamber (30.0×30.0×35.0 cm) for self-stimulation. A fixed-interval (1 s) reinforcement schedule was used for this experiment. Each nose poke delivered a 0.3-s train of monophasic cathodal rectangular pulses with 0.1-ms duration. During the preliminary session, the frequency was held constant at 65 Hz and the current intensity was progressively increased until the subjects showed vigorous self-stimulation. Rats were then tested using two alternating series of ascending and descending current intensities varied by steps of 50 μA. The current threshold of each rat was defined as the value of stimulus that evoked 50% of the maximal rate of self-stimulation. These intensity values (150–380 μA) were then held constant for the subsequent testing of frequency threshold: the rats were again tested using two alternating series of ascending and descending pulse frequencies. The frequencies increased by 0.1 log unit steps (e.g., 26, 33, 41, 52, 65, 82, 103, 130, 163, 206 Hz). Each frequency was tested within a 180-s trial. During each testing trial, rats received ten priming stimulations at the beginning and nose pokes were recorded only in the last 120 s. A 180-s warm-up trial (65 Hz) followed by a 180-s extinction trial was given before the testing trials. Drug tests began when the rate–frequency curves were stable for at least 3 consecutive days (once daily). A baseline rate–frequency curve was measured (for 66 min) at 3 h before the drug administration. Either scopolamine (1, 10, or 100 μg; n=6) or mecamylamine (5, 10, 25, or 100 μg; n=6) was injected into the PPTg area, and the rate–frequency curve was measured again 15 min after the injection. In each group, vehicle and all doses of drugs were tested in an ascending order for each rat, and a 3-day interval was allowed between injections.
Experiment III: effect of intra-PPTg nicotinic antagonist on nicotine-enhanced LH stimulation reward
Rats were pretreated with nicotine (0.4 mg/kg, s.c.) once daily for 4 days to avoid the depressant effect of acute nicotine administration (Panagis et al. 2000). The procedure of rate–frequency curve testing was the same as in experiment II. Following the baseline measurement, rats were injected intracranially with mecamylamine (5, 10, or 25 μg; n=8) and, 10 min later, subcutaneously with nicotine. The ICSS testing started at 5 min after nicotine injection. Each animal received all doses of mecamylamine as well as the vehicle in an ascending order at 3-day intervals.
Experiment IV: effects of intra-PPTg cholinergic antagonists on extracellular ACh in the VTA
The muscarinic antagonist scopolamine (1, 10, or 100 μg; n=6) or nicotinic antagonist mecamylamine (5, 10, or 25 μg; n=6) was injected into the PPTg following a stable baseline of the ACh level. Microdialysis collections were then continued for 120 min after drug injection. The animals in each drug testing received all doses of the drug as well as the vehicle in an ascending order at 3-day intervals.
Data analysis
At the end of each experiment, rats were perfused intracardially with 10% formalin, and the brains were removed. The accuracy of probe location was verified histologically by cresyl violet staining. Only the animals that showed probe placement in the desired brain region were analyzed. The histological localizations of the stimulating electrodes and of the microdialysis probes are shown in Fig. 1. Self-stimulation thresholds were obtained by fitting the Gompertz growth model to the data (Panagis et al. 2000): \(y = \alpha e - e^{{b{\left( {Xi - X} \right)}}}\) (α, b, and Xi represent the maximum rate, an index of the slope, and threshold, respectively). The shift in mean threshold value and maximum rate of responding produced by vehicles or drugs were subjected to statistical analyses. The data were analyzed by repeated measures ANOVA and Newman–Keuls multiple comparison tests.
Histological localization of tips (arrows) of stimulating electrodes in the LH (left), microdialysis membranes in the VTA (middle) and PPTg (right, filled rectangles), and microinjection probes in the PPTg (right, filled triangles). The numbers by each section indicate the distance (in millimeters) from bregma
Results
LH self-stimulation evoked increased extracellular ACh in VTA as well as PPTg
Self-stimulation of MFB at the level of LH significantly increased the extracellular ACh both in the ipsilateral VTA and PPTg (Fig. 2). ANOVA and post hoc tests indicated that the levels of extracellular ACh in VTA [F (11,77)=4.78, p<0.001, n=8] and PPTg [F (11,77)=5.19, p<0.001, n=8] were significantly higher than the baselines during 1-h self-stimulation (p<0.001) and 20 min after self-stimulation (p<0.05 or p<0.01).
LH self-stimulation increased extracellular ACh in the ipsilateral VTA and PPTg. a VTA. The mean lever-pressing rate was 65±2 responses per minute (n=8). b PPTg. The lever-pressing rate was 76±5 responses per minute (n=8). Values are mean±SEM; **p<0.01 indicates within group difference from baselines
Intra-PPTg muscarinic antagonist reduced frequency threshold for LH stimulation reward
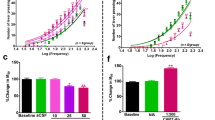
Scopolamine locally infused into the PPTg area reduced the frequency threshold of rewarding LH stimulation (Fig. 3). Repeated measures ANOVA revealed a significant treatment effect [F (3,15)=3.62, p<0.05, n=6], and post hoc tests found that the significant effect was at the highest dose of 100 μg (p<0.05). Scopolamine did not affect the maximum rate of responding at any dose (data are not shown).
Top: intra-PPTg mAChR antagonist scopolamine (100 μg) reduced the frequency threshold with shifts of −13.35±2.15% (mean±SEM) from baseline (n=6, *p<0.05 indicates difference from vehicle group). Bottom: plots represent the examples of rate–frequency curve shifts induced by vehicle (left) and scopolamine of 100 μg (right)
Intra-PPTg nicotinic antagonist had no effect on the normal LH self-stimulation reward but blocked nicotine-enhanced rewarding effect of LH self-stimulation
Intra-PPTg mecamylamine in doses ranging from 5 to 100 μg generally did not alter frequency threshold for LH stimulation reward (Fig. 4a). However, mecamylamine inhibited the nicotine-enhanced rewarding effects of LH stimulation (Fig. 4b). Repeated measures ANOVA results showed a significant treatment effect [F (4,28)=4.76, p<0.01, n=8]. Nicotine (0.5 mg/kg, s.c.) enhanced effectiveness of LH stimulation as evidenced by significant lowering of the frequency thresholds (Ringer + Nic vs Ringer + Sal, P<0.01); mecamylamine suppressed the nicotine potentiation of LH stimulation reward, at the higher doses of 10 (Ringer + Nic vs Mec10 + Nic, p<0.05) or 25 μg (Ringer + Nic vs Mec25 + Nic, p<0.01). No significant shift in the maximum rate of responding by each treatment was found (data are not shown).
a Intra-PPTg nAChR antagonist mecamylamine did not significantly alter the threshold (n=6). b Nicotine (0.5 mg/kg, s.c.) raised the LH stimulation reward as evidenced by significant lowering of frequency threshold (−13.09±2.057% shift from predrug treatment). Mecamylamine (10 or 25 μg/μl) effectively blocked this potentiation. Values are mean±SEM; n=8; *p<0.05, **p<0.01
Intra-PPTg muscarinic but not nicotinic antagonist affected extracellular ACh concentration in VTA
Intra-PPTg injections of scopolamine enhanced the levels of extracellular ACh in the ipsilateral VTA (Fig. 5). There were significant treatment [F (3,160)=12.10, p<0.001], time [F (8,160)=12.89, p<0.001] and treatment × time interaction effects [F (24,160)=7.51, p<0.001]. Figure 5b showed that the major increase in extracellular ACh induced by injection of 100 μg scopolamine continued for 120 min (p<0.05 compared with vehicle group; p<0.001 with the baselines before injection). The effect of 10 μg scopolamine was far less and shorter-lasting than that of 100 μg, inducing an increase marked only within 20–40 min after injection (compared with baselines, p<0.01). No significant effects of 1 μg were found in this experiment. Mecamylamine at the doses of 5, 10, and 25 μg did not significantly affect the levels of extracellular ACh (Fig. 5c).
Intra-PPTg mAChR antagonist scopolamine increased extracellular ACh in VTA, whereas nAChR antagonist mecamylamine did not result in significant alteration in ACh level. a Schematic representation of a microdialysis probe placed into VTA and a drug-injector into the ipsilateral PPTg. b Intra-PPTg 100 μg scopolamine caused a marked and long-lasting increase in extracellular ACh in VTA. The lower dose of 10 μg was less effective than that of 100 μg. c No significant effects of mecamylamine at any dose (5, 10, 25 μg) were found (p>0.05). Values are mean±SEM. ***p<0.001 indicates within-group differences from baselines, and †p<0.05 indicates between-group differences from the vehicle group. N=6 for each drug testing
Discussion
The present findings support the hypothesis that LH-MFB self-stimulation may exert its rewarding effects by acting through the ascending cholinergic pathway from PPTg to VTA. We observed that LH self-stimulation induced concurrent ACh release in the PPTg and VTA. Antimuscarinic scopolamine injected into PPTg significantly promoted the LH stimulation reward, suggesting that the mAChRs expressed on the cholinergic neurons within PPTg play an inhibitory role on the BSR. The present work represents the first demonstration of functional roles of PPTg nAChR in the BSR, i.e., the nAChR exerts a beneficial effect on the nicotine potentiation of BSR yet has no effect on the basal BSR. In vivo microdialysis studies detected consistent ACh transmission in VTA that may subserve the behavioral phenomena after administration of scopolamine or mecamylamine into the PPTg.
Activation of the ascending cholinergic pathway from PPTg to VTA contributes to the rewarding effect of MFB self-stimulation
The LH self-stimulation reward appears to depend on the dopaminergic and glutamatergic neurotransmission in the VTA, and the activation of descending myelinated fibers afferent to the DA neurons of VTA is required (Yeomans 1989; You et al. 2001). Given the cholinergic projections directly onto the dopaminergic and GABAergic neurons in VTA (Oakman et al. 1995; Garzon et al. 1999), PPTg and the adjacent laterodorsal tegmental nucleus (LDTg) are thought to monosynaptically activate the VTA DA neurons during MFB self-stimulation (Yeomans et al. 1993). In the present study, LH self-stimulations caused concurrent release of ACh in the PPTg and VTA (Fig. 2). These results extend the previous finding that LH self-stimulation induced an ACh release in VTA (Rada et al. 2000; Nakahara et al. 2001) and suggest that rewarding stimulation of LH-MFB could activate the cholinergic pathway from PPTg to VTA. In the VTA, endogenous ACh release and consequent activation of AChR may help amplify the excitatory effects of glutamate and DA and probably also attenuates inhibitory GABAergic transmission, implicated broadly in drug- or non-drug-related reward function (Grillner et al. 2000; Kelley 2002; Ikemoto and Wise 2004). Here we would generalize this notion to the significance of cholinergic excitation by LH-MFB self-stimulation: LH-stimulation-induced endogenous ACh release and subsequent AChR activation trigger or amplify neuronal excitation of dopaminergic cells and glutamatergic axon terminals in the VTA. Consistent with this point of view, ACh directly injected into the VTA has been shown to promote the rewarding effects of hypothalamic stimulation (Redgrave and Horrell 1976); activation or inactivation of AChRs in VTA, in particular the mAChR, could up- or down-regulate the LH self-stimulation reward, respectively (for review, see Yeomans et al. 2001). The LH self-stimulations may act in two possible ways to induce the ACh release in VTA. One is to stimulate, directly or indirectly, the cholinergic inputs in VTA to lead to presynaptic release of ACh. The other is to activate the VTA-projecting cholinergic neurons in PPTg and LDTg that in turn elicit the release of ACh in VTA. Our data of the coincident ACh releases in VTA and PPTg by self-stimulation provide direct evidence in favor of the latter route. Further anatomic or electrophysiological evidence is needed to identify the former one.
It should be noted that the VTA also receives significant cholinergic innervation from the LDTg and PPTg projects dense cholinergic inputs also into the SNc (Oakman et al. 1995). So far, it has been suggested that projections of the LDTg to VTA appear more important than that of PPTg-VTA in modulation of the activity of dopaminergic VTA neurons; on the other hand, the PPTg is considered to be important for regulation of the activity of SNc DA-containing neurons. Lesions of the LDTg, but not the PPTg, attenuate DA efflux in NAc induced by intra-VTA neostigmine (Blaha et al. 1996). In vivo electrochemical studies showed that intra-PPTg scopolamine and M2/4 muscarinic antagonist enhance the striatal DA efflux in rats, suggesting that via mAChRs, PPTg may regulate the SNc DA cell activity in basal status (Chapman et al. 1997; Miller and Blaha 2004). Briefly, it seems that the LDTg and PPTg regulate the DA neuronal activity in VTA and SNc, respectively (Forster and Blaha 2000, 2003). In fact, however, there is considerable overlap in the midbrain and diencephalic projections of the LDTg and PPTg (Satoh and Fibiger 1986). There are cholinergic interconnections between the LDTg and PPTg, and the cholinergic input to PPTg appears to originate from the contralateral PPTg and ipsilateral LDTg (Semba and Fibiger 1992). Therefore, it would not be easy to define the functionally distinct pathways of LDTg-VTA and PPTg-SNc in reward-related behaviors. The previous finding that unilateral PPTg or LDTg lesions do not disrupt the LH and VTA self-stimulation reward suggests that there exists no exclusive neural circuit underlying the MFB self-stimulation reward (Waraczynski and Perkins 1998). In this regard, the present study emphasizes the importance of a cholinergic PPTg-VTA pathway for LH self-stimulation reward and thus contributes to a more comprehensive understanding of the function of cholinergic projections from mesopontine tegmentum to midbrain DA system.
Roles of the muscarinic receptors of PPTg in hypothalamic stimulation reward
Intra-PPTg muscarinic antagonist scopolamine (100 μg) significantly enhanced the LH stimulation reward as evidenced by the decrease in frequency threshold (Fig. 3). The result confirmed and extended what has been found by Yeomans et al. (1993) by demonstrating the dose–response effects of scopolamine. It has been demonstrated that muscarinic antagonists, e.g., scopolamine injected into VTA, reduce the rewarding effects of MFB stimulation by inactivating the excitatory mAChRs on DA cells and glutamate terminals, whereas the agonists have opposite effects (Yeomans et al. 2001). Data from in vivo electrochemical study that intra-VTA scopolamine can effectively attenuate basal DA efflux in the nucleus accumbens (NAc) and striatum provided further support for this notion (Miller and Blaha 2005). Results above suggest that mAChRs in the VTA plays an excitatory role in MFB stimulation reward. However, within PPTg, mAChRs act as the autoreceptors in inhibitory feedback regulation of the cholinergic neuronal activity (Vilaro et al. 1994; Roth et al. 1996). Thus, it would not be surprising that at the level of PPTg, antimuscarinic scopolamine disinhibits cholinergic neurons and increases the cholinergic activation of dopaminergic and glutamatergic transmission in VTA, which serve to promote the rewarding effects of LH stimulation. This speculation was supported by the subsequent experiment (IV) in which we found that intra-PPTg scopolamine at the dose of 100 μg caused a remarkable, i.e., much higher and longer-lasting, elevation of ACh output in the VTA. Scopolamine at the lower dose of 10 μg, which was not optimal to significantly shift the threshold for LH self-stimulation, induced only a slight increase in ACh (Fig. 5). These results add to evidence and give a neurochemical interpretation for the inhibitory effects of muscarinic receptors in the PPTg on the BSR.
Roles of the nicotinic receptors of PPTg in hypothalamic stimulation reward
It has been demonstrated that systemic nicotine results in an enhancement of dopamine release in NAc (Nisell et al. 1994; Schilstrom et al. 1998) and a potentiation in BSR via the VTA nicotinic receptors (Panagis et al. 2000). As one of the major sources of cholinergic input to VTA, PPTg has been demonstrated to play an important role in nicotine self-administration (for review, see Picciotto and Corrigall 2002). In the present study, we for the first time tested the nicotinic receptors within PPTg for its roles in the MFB self-stimulation reward in nicotine-treated and nontreated rats, respectively. Nicotinic antagonist mecamylamine injected into PPTg did not substantially affect the LH self-stimulation reward in non-drug-treated rats (Fig. 4a). A similar outcome was previously found at the level of VTA that mecamylamine (10, 30, 100, 300 μg) produced no statistically significant change in the rewarding threshold for LH stimulation (Yeomans and Baptista 1997). The lack of effect of intra-PPTg mecamylamine could be due to the fact that intra-PPTg mecamylamine did not alter ACh output in VTA (Fig. 5). These results indicated that the nicotinic receptors in PPTg and VTA do not play important roles in basal BSR function. However, intra-PPTg mecamylamine (10, 25 μg) dramatically diminished the nicotine-enhanced rewarding effectiveness of LH stimulation (Fig. 4b). The data acquired from intra-PPTg mecamylamine would be somewhat analogous to the previous findings: systemic or intra-VTA antinicotinics, which are able to block the nicotine potentiation of BSR, fail to affect the normal BSR function (Ivanova and Greenshaw 1997; Wise et al. 1998; Panagis et al. 2000; Harrison et al. 2002). Intra-VTA mecamylamine reduces the extracellular DA detected by in vivo microdialysis in the NAc of chronically nicotine-treated rats, but not in non-drug-treated rats (Hildebrand et al. 1999). These data together suggest that nicotinic antagonism acts on certain substrates directly affected by nicotine.
The mechanisms by which nAChRs in PPTg and VTA might be acting to produce differential effects on BSR when in the presence or absence of nicotine are not readily understood and discussed at this time. Acute or repeated administration of nicotine has been known to result in an nAChR-mediated increase in cortical, hippocampal, and striatal ACh release (Summers et al. 1994; Summers and Giacobini 1995; Tani et al. 1998; Reid et al. 1999; Arnold et al. 2003). It is thus tempting to speculate about a potential cholinergic mechanism in PPTg and VTA mediating the effects of mecamylamine on nicotine-enhanced LH self-stimulation reward. This could be tested in the further study to see whether nicotine induces ACh release in the PPTg and VTA, and if so, whether the nicotine-induced ACh release is blocked by intra-PPTg mecamylamine. One could also speculate about several potential mechanisms other than the cholinergic. Lanca et al. (2000) revealed that systemically administered nicotine induces strong c-fos expression in the noncholinergic neurons in PPTg. Our previous work found that LH-stimulation-induced c-fos expression is restricted mostly to the GABAergic neurons in PPTg (Nakahara et al. 2001). It has been found that there are moderate amounts of α4, β2, and α7 subunits in the PPTg; however, the precise localization of nAChRs is still unclear (Ryan and Loiacono 2000). In this respect, identification of the nicotinic synaptic circuits in the PPTg could lead to a clearer explanation for the above intrinsic phenomena.
References
Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P (2004) An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience 125:349–358
Arnold HM, Nelson CL, Sarter M, Bruno JP (2003) Sensitization of cortical acetylcholine release by repeated administration of nicotine in rats. Psychopharmacology (Berl) 165:346–358
Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P (1996) Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci 16:714–722
Chapman CA, Yeomans JS, Blaha CD, Blackburn JR (1997) Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience 76:177–186
Corrigall WA, Coen KM, Adamson KL, Chow BLC (1999) Manipulations of mu-opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self-administration in rats. Psychopharmacology (Berl) 145:412–417
Corrigall WA, Coen KM, Zhang J, Adamson KL (2001) GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl) 158:190–197
Corrigall WA, Coen KM, Zhang J, Adamson KL (2002) Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology (Berl) 160:198–205
Diederich K, Koch M (2005) Role of the pedunculopontine tegmental nucleus in sensorimotor gating and reward-related behavior in rats. Psychopharmacology (Berl) 179:402–408
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973
Forster GL, Blaha CD (2000) Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci 12:3596–3604
Forster GL, Blaha CD (2003) Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci 17:751–762
Garzon M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM (1999) Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. J Comp Neurol 410:197–210
Grillner P, Berretta N, Bernardi G, Svensson TH, Mercuri NB (2000) Muscarinic receptors depress GABAergic synaptic transmission in rat midbrain dopamine neurons. Neuroscience 96:299–307
Harrison AA, Gasparini F, Markou A (2002) Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 160:56–66
Hildebrand BE, Panagis G, Svensson TH, Nomikos GG (1999) Behavioral and biochemical manifestations of mecamylamine-precipitated nicotine withdrawal in the rat: role of nicotinic receptors in the ventral tegmental area. Neuropsychopharmacology 21:561–574
Ikemoto S, Wise RA (2004) Mapping of chemical trigger zones for reward. Neuropharmacology 47:190–201
Ivanova S, Greenshaw AJ (1997) Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology (Berl) 134:187–192
Kelley AE (2002) Nicotinic receptors: addiction’s smoking gun? Nat Neurosci 8:447–449
Kippin TE, van der Kooy D (2003) Excitotoxic lesions of the tegmental pedunculopontine nucleus impair copulation in naive male rats and block the rewarding effects of copulation in experienced male rats. Eur J Neurosci 18:2581–2591
Lanca AJ, Adamson KL, Coen KM, Chow BLC, Corrigall WA (1999) The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience 96:735–742
Lanca AJ, Sanelli TR, Corrigall WA (2000) Nicotine-induced Fos expression in the pedunculopontine messencephalic tegmentum in the rat. Neuropharmacology 39:2808–2817
Mena-Segovia J, Bolam JP, Magill PJ (2004) Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci 27:585–588
Miller AD, Blaha CD (2004) Nigrostriatal dopamine release modulated by mesopontine muscarinic receptors. Neuroreport 15:1805–1808
Miller AD, Blaha CD (2005) Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci 21:1837–1846
Nakahara D, Ishida Y, Nakamura M, Furuno N, Nishimori T (2001) Intracranial self-stimulation induces Fos expression in GABAergic neurons in the rat mesopontine tegmentum. Neuroscience 106:633–641
Nisell M, Nomikos GG, Svensson TH (1994) Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 16:36–44
Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK (1995) Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci 15:5859–5869
Olmstead MC, Munn EM, Franklin KBJ, Wise RA (1998) Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci 18:5035–5044
Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson’s disease. Brain 123:1767–1783
Panagis G, Kastellakis A, Spyraki C, Nomikos G (2000) Effects of methyllycaconitine (MLA), an α7 nicotinic receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology (Berl) 149:388–396
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, San Diego
Picciotto MR, Corrigall WA (2002) Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci 22:3336–3341
Rada PV, Mark GP, Yeomans JJ, Hoebel BG (2000) Acetylcholine release in ventral tegmental area by hypothalamic self-stimulation, eating and drinking. Pharmacol Biochem Behav 65:375–379
Redgrave P, Horrell RI (1976) Potentiation of central reward by localized perfusion of acetylcholine and 6-hydroxytryptamine. Nature 262:305–307
Reid RT, Lloyd GK, Rao TS (1999) Pharmacological characterization of nicotine-induced acetylcholine release in the rat hippocampus in vivo: evidence for a permissive dopamine synapse. Br J Pharmacol 127:1486–1494
Roth MT, Fleegal MA, Lydic R, Baghdoyan HA (1996) Pontine acetylcholine release is regulated by muscarinic autoreceptors. Neuroreport 7:3069–3072
Ryan RE, Loiacono RE (2000) Nicotinic receptor subunit mRNA in the thalamus of the rat: relevance to schizophrenia? Neuroreport 11:3693–3698
Satoh K, Fibiger HC (1986) Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol 253:277–302
Schilstrom B, Svensson HM, Svensson TH, Nomikos GG (1998) Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience 85:1005–1009
Semba K, Fibiger HC (1992) Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol 323:387–410
Steininger TL, Rye DB, Wainer BH (1992) Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol 321:515–543
Summers KL, Giacobini E (1995) Effects of local and repeated systemic administration of (−) nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res 20:753–759
Summers KL, Cuadra G, Naritoku D, Giacobini E (1994) Effects of nicotine on levels of acetylcholine and biogenic amines in rat cortex. Drug Dev Res 31:108–119
Tani Y, Saito K, Imoto M, Ohno T (1998) Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain—an in vivo microdialysis study. Eur J Pharmacol 351:181–188
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Vilaro MT, Palacios JM, Mengod G (1994) Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Brain Res Mol Brain Res 21:30–46
Waraczynski M, Perkins M (1998) Lesions of pontomesencephalic cholinergic nuclei do not substantially disrupt the reward value of medial forebrain bundle stimulation. Brain Res 800:154–169
Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240
Wise RA, Marcangione C, Bauco P (1998) Blockade of the reward-potentiating effects of nicotine on lateral hypothalamic brain stimulation by chlorisondamine. Synapse 29:72–79
Yeomans JS (1989) Two substrates for medial forebrain bundle self-stimulation: myelinated axons and dopamine axons. Neurosci Biobehav Rev 13:91–98
Yeomans JS, Baptista M (1997) Both nicotine and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacol Biochem Behav 57:915–921
Yeomans JS, Mathur A, Tampakeras M (1993) Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav Neurosci 107:1077–1087
Yeomans J, Forster G, Blaha C (2001) M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life Sci 68:2449–2456
You ZB, Chen YQ, Wise RA (2001) Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience 107:629–639
Acknowledgements
We thank Dr. Gregory V.G. O’Dowd for editing the manuscript. This research was supported in part by a Grant-in-Aid for Scientific Research, the Ministry of Education, Science, Sports, and Culture of Japan and by the Smoking Research Foundation Grant for Biomedical Research (Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Nakamura, M., Kawamura, T. et al. Roles of pedunculopontine tegmental cholinergic receptors in brain stimulation reward in the rat. Psychopharmacology 184, 514–522 (2006). https://doi.org/10.1007/s00213-005-0252-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0252-8