Abstract
In rodents, stimulation of the nucleus accumbens shell (AcbSh) directly or via its projection to the lateral hypothalamus (LH) attenuates food intake. The ventral pallidum (VP) receives dense projections from the AcbSh and is sensitive to the hedonic aspect of food and motivation for reward. However, the role of accumbal projections to the VP in the regulation of food intake was not well investigated. In the present study conducted on female rats, we examined the effects of stimulation of the AcbSh using optogenetics, or pharmacological inhibition of the rostral VP, or stimulation of projections from the AcbSh to the rostral VP using optogenetics on the consumption of 10% sucrose, lick microstructure and the expression of c-fos mRNA. Stimulation of the AcbSh, inhibition of the rostral VP with muscimol, or stimulation of axonal terminals from the AcbSh to the rostral VP resulted in a decrease in sucrose intake, meal duration, and total number of licks. The licking microstructure analysis showed that optogenetic stimulation of AcbSh or axonal terminals from the AcbSh to the rostral VP decreased the hedonic value of the sucrose. However, inhibition of the rostral VP decreased the motivation, whereas stimulation of the accumbal projections in the rostral VP increased the motivation to drink. This difference could be due to differential involvement of GABAergic and glutamatergic VP neurons. Stimulation of the AcbSh resulted in a decrease of c-fos mRNA expression in the LH and rostral VP, and stimulation of axonal terminals from the AcbSh to the rostral VP decreased c-fos mRNA expression only in the rostral VP. This study demonstrates that in adult female rats, in addition to the already known role of the AcbSh projections to the LH, AcbSh projections to the VP play a major role in the regulation of sucrose intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In modern society, palatable food is ubiquitous, easily accessible, very appealing, and it can be consumed without any metabolic need. This phenomenon can lead to eating disorders such as overconsumption and binge-eating, which are more prevalent in women than men (Cossrow et al. 2016; Asarian and Geary 2013; Hudson et al. 2007) and is strongly associated with a development of severe obesity (Hudson et al. 2007).
In addition to the metabolic system, food intake behavior is also regulated by the brain reward system (Ferrario et al. 2016). The nucleus accumbens shell (AcbSh) is one of the components of this circuit. The majority of the AcbSh neurons are projecting gamma-aminobutyric acidergic (GABAergic) medium spiny neurons (MSNs); the remaining neurons are GABAergic or cholinergic local circuits interneurons (Meredith 1999). AcbSh neurons project to several structures such as the ventral tegmental area or the lateral preoptic area, but the major targets are the lateral hypothalamus (LH) and the ventral pallidum (VP) (Usuda et al. 1998; Heimer et al. 1991).
A large proportion of AcbSh neurons reduced firing rates during sucrose consumption and electrical stimulation of the same neuronal groups abruptly interrupted licking (Krause et al. 2010). A reduction of the AcbSh neurons excitability with glutamate antagonists increases food intake; this increase is abolished by injection of the GABAA agonist muscimol into the LH (Maldonado-Irizarry et al. 1995). Inhibition of the AcbSh neurons with GABA agonists induces an increase in c-Fos expression in LH neurons (Stratford 2005), and associated increase in food intake (Stratford and Kelley 1997). This effect is mediated by dopamine D1-receptor expressing AcbSh MSNs that sent GABAergic projections to the LH, and the activation of their terminals in LH stops feeding (O'Connor et al. 2015). Thus, the role of direct projections from Acbsh neurons to the LH is well characterized in the literature.
The function of AcbSh projections to the VP is less known. The VP contains predominantly GABAergic neurons, but also a proportion of cholinergic and glutamatergic cells (Root et al. 2015; Tooley et al. 2018; Faget et al. 2018). They receive projections from the D1- and D2-receptor expressing AcbSh MSNs (Creed et al. 2016; Robertson and Jian 1995; Lu et al. 1998; Kupchik and Kalivas 2017). Pharmacological studies demonstrated that the blockade of GABAA receptors in the VP increases food consumption (Stratford et al. 1999), specifically the saccharin intake without variation in water intake (Shimura et al. 2006). On the contrary, inhibition of the VP with GABAA agonists decreases both saccharin and water intake, and it increases the aversive responses to the intraoral infusion of saccharin (Shimura et al. 2006). The unilateral lesion of the VP or the LH attenuates the increase of food intake observed after muscimol-induced ipsilateral inhibition of AcbSh (Stratford and Wirtshafter 2012). However, bilateral lesions of the VP produce aphagia and enhanced aversive reactions to sucrose, whereas lesions of the LH only produce aphagia (Cromwell and Berridge 1993).
The AcbSh and the VP are both implicated in regulation of food intake behavior, but the function of the direct projections from the AcbSh to the VP is not clear. We hypothesized that inhibitory control exerted by the AcbSh over the VP plays a major role in a control of sucrose consumption. The goal of our study was to examine whether stimulation of the AcbSh induces a decrease in sucrose intake in adult female rats in our experimental conditions, and to analyze the role of direct projections of the AcbSh to the VP on this behavior.
Materials and methods
All experiments were carried out according to the guidelines of the Canadian Council on Animal Care in Science and Use of Laboratory Animals, and the experimental protocols were approved by the institutional Université Laval animal care committee.
Animals
Female Sprague–Dawley adult rats (250–275 g, n = 51) were purchased from the Canadian Breeding Laboratories (St-Constant, QC, Canada). The rats were housed in individual plastic cages lined with wood shavings and maintained on a 12:12 h light–dark cycle, with an ambient temperature of 23 ± 1 °C. Rats were left undisturbed for at least 5 days to adapt to the new environment. Rats had free access to standard laboratory rat chow (2018 Teklab Global 18% Protein Rodent Diet; 3.1 kcal/g, Harlan Taklab, Montréal, QC, Canada) and tap water.
Stereotaxic surgeries
Each rat was initially deeply anesthetized using 4% isoflurane and placed in a stereotaxic instrument (Kopf Instruments). Surgery procedures were performed under 2.5% isoflurane anesthesia along with systemic (buprenorphine, 0.02 mg/kg, subcutaneous injection) and local [mixture of lidocaine (7 mg/kg) and bupivacaine (3.5 mg/kg), subcutaneous injection] analgesics.
For electrophysiology and behavioral optogenetic experiments we used the adeno-associated virus AAV2-hSyn-ChR2-eYFP (3.9 × 1012GC/ml; UNC Vector Core, Dr. Karl Deisseroth repository). The AAV2-hSyn-eYFP (3.4 × 1012GC/ml; UNC Vector Core, Dr. Karl Deisseroth repository) was used for control. We injected bilaterally 500 nl of AAV solution into the nucleus accumbens shell (1.9 mm rostral to the bregma, 1.3 mm lateral to the midline, 8.3 mm ventral from the skull surface) (Paxinos and Watson 2007) via 30-µm diameter glass micropipette. The injection micropipette was slowly withdrawn 8 min after the end of the infusion. For behavioral optogenetics experiments, rats were bilaterally implanted with an optical fiber (200 µm core, Doric lenses, QC, Canada) dorsal to the nucleus accumbens shell (same coordinates as for virus injection) or to the ventral pallidum (0.3 mm rostral to the bregma, 1.5 mm lateral to the midline, 7.8 mm ventral from the skull surface) (Paxinos and Watson 2007). The cannulas were fixed to the skull using three stainless steel screws and dental cement.
For pharmacological stimulation of GABA receptor in VP, bilateral 26-gauge stainless steel cannulas (Plastics One, Roanoke, VA, USA) were implanted using the stereotaxic technique. The guide cannulas were aimed at the coordinates 0.3 mm rostral to the bregma, 1.5 mm lateral to the midline and 7 mm ventral from the skull surface (Paxinos and Watson 2007). The guide cannulas were fixed to the skull using three stainless steel screws and dental cement. Fitting stainless steel obturators were inserted inside each cannula to prevent blockage and infection.
Each rat received subcutaneous injection of anti-inflammatory meloxicam (1 mg/kg) for 2 days after surgery. Behavioral experiments were performed 3 weeks (for optogenetics) and 1 week (for GABA agonist experiments) after stereotaxic manipulations.
Electrophysiology
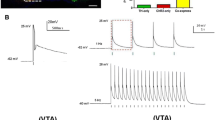
For in vitro experiments, 4 weeks after ChR injection in the AcbSh, rats were deeply anesthetized (60 mg/kg ketamine plus 7.5 mg/kg xylazine). The brains were quickly dissected and maintained in ice-cold artificial cerebrospinal fluid (aCSF) containing: 124 mM NaCl, 2.8 nM KCl, 1.2 mM CaCl2, 2 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3 and 10 mM D-glucose (Sigma-Aldrich, Canada) (pH 7.4), aerated with 95% O2 and 5% CO2. Osmolarity was 300 ± 5 mOsm. Sagittal slices (250 µm) were prepared with a vibratome (VT1200 S, Leica Microsystems Inc., Canada) to obtain sections containing the AcbSh. Slices were transferred to a holding chamber and kept at room temperature for at least 1 h in the same aCSF aerated with 95% O2 and 5% CO2. Then the brain slices were transferred into a submerged recording chamber maintained at 34 °C, containing the perfusion aCSF at a rate of 3 ml/min. ChR-expressing cells in the AcbSh were identified using a CCD camera (PentaMax, Princeton Instrument, NJ, USA) on a fluorescent upright microscope with optic filters for emission wavelengths 488 nm. Using Axon pClamp 9 (Molecular Devices, CA, USA) we obtained somatic whole-cell current-clamp recordings (10–15 MΩ access resistances) with patch pipettes (resistance between 3–5 MOhm) containing the following: 130 mM potassium D-gluconate, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 mM KCl, 2 mM MgCl2, 2 mM ATP, 0.2 mM GTP (Sigma-Aldrich, Canada) and 0.2% Neurobiotin (Vector Laboratories Canada Inc.) at pH 7.2 and 280 mOsm. Neurons were recorded at resting potential (– 65 mV) or a steady depolarizing current was injected to maintain the membrane potential near firing threshold (~ – 50 mV). Optic fiber (50 µm) was placed in the vicinity of the recorded neuron. Blue light pulses (LED, 470 nm, 1–2 mW, 10–500 ms duration) were used to stimulate ChR2-expressing nucleus accumbens neurons. All recorded neurons were intracellularly stimulated with hyper- and depolarizing current pulses in a range between – 100 pA and 300 pA (pulse duration 500 ms) for cell identification. Neurons that displayed regular-spiking firing pattern to depolarizing step currents (Fig. 1a), a firing characteristic of medium spiny GABAergic neurons of the AcbSh (Belleau and Warren 2000), were investigated further for their response to optical stimulation (Fig. 1c, d). Recorded neurons were filled with Neurobiotin, and the images of the recorded neurons were acquired using a confocal microscope (LSM 700, Carl Zeiss Microscopy Gmbh, Germany).
Electrophysiological validation of optogenetic stimulation experiments. a Typical response of medial spiny neuron from the AcbSh recorded in current-clamp mode to 60 pA 500 ms square depolarizing pulse. b Image showing an example of patched cells. c, d Sample whole-cell patch-clamp recording of neuronal depolarization in the same cell as in the recording in the panel (a), response to 1 mW 500 ms (c) and 1 mW 10 ms (d) blue-light stimulation (465 nm)
Experimental design
All experiments were performed with ad libitum-fed rats during the light period. Body weight (BW) was measured before each session to assess the sucrose intake in milligram per gram BW. During each 30-min experiment, rats had a choice of drinking 10% sucrose solution or water.
Optogenetic behavioral experiments
Rats were habituated for 15 min over 2 days to an operant chamber (MedAssociates, Saint Alban, VT, USA) equipped with two photobeam lickometers to collect time stamps of licking events, with fan and house light on, and water presented on one of the two lickometers attached to the wall. Next, rats were habituated for 15 min over 2 days to the optical cable connected to their head without optical stimulation in the same operant chamber. Thereafter, rats were given access to 10% sucrose solution and water for 30 min over 3–4 days in the operant chamber to counter the neophobia of sucrose solution and habituate them to drink sucrose in the behavioral cage with attached optical cable. Each rat (ChR-injected and control eYFP-injected groups) was then experimented in the operant chamber for 30 min with access to water and 10% sucrose solution, without and with (20 Hz, 10 ms stimulation every 50 ms) blue-light stimulation (465 nm LED delivering 12 mW at the tip of the fiber patch cable; Plexon Inc., Texas, USA). Sessions without and with blue-light stimulation were given in a counterbalanced order to avoid the effect of days on sucrose intake and were separated by at least 24 h. Between session, rats were left undisturbed in their home cages. Consumption of water and 10% sucrose solution was calculated by weighing bottles before and after 30-min access sessions.
Pharmacological experiment
Rats were habituated for 15 min over 2 days to the same operant chamber (MedAssociates, Saint Alban, VT, USA) used for optogenetics experiments and to the manipulation for intra-VP injections by mock injection sessions with removal and insertion of the steel obturators. Then rats were given access to 10% sucrose solution and water for 30 min over 3–4 days in the operant chamber. Thereafter, rats were randomly divided into two groups, and GABA receptors agonists, either muscimol or baclofen, were administered bilaterally into the VP using a within-subject design. Muscimol and baclofen (Tocris Bioscience, Bristol, UK) were dissolved in sterile artificial cerebrospinal fluid (aCSF, Harvard Apparatus, Holliston, MA). The same aCSF was injected as vehicle (0 pmol). Rats received simultaneous bilateral injection of 0.5 μl of either aCSF, muscimol (1800 pmol/µl) or baclofen (1800 pmol/µl), at a flow rate of 0.25 µl/min, via stainless steel bilateral injectors (33-gauge) that projected 1 mm below the tip of the guide cannula. After injection, the injector was left in place for 5 min to ensure drug diffusion and prevent drug leakage at the dorsal sites. The rats were then placed in the operant chamber for 30 min with access to water and 10% sucrose solution. Injections were performed in a counterbalanced order and were separated by at least 48 h. Between sessions, rats were left undisturbed in their home cages.
Estrous cycle
Vaginal smears were collected every day in the light phase and stained with methylene blue (Sigma, Oakville, ON, Canada). The phases of the estrous cycle were determined morphologically after examination of the smear under bright field microscope. Diestrus was characterized by the presence of leukocytes and a low number of nucleated epithelial cells. For the following phase, the proestrus, the majority of cells were nucleated epithelial cells. During estrus, the smear contained predominantly enucleated epithelial cells. Finally, metestrus was characterized by the presence of leukocytes and enucleated epithelial cells. The estrous phases have no impact on sucrose intake (Calvez and Timofeeva 2016), but a decrease in chow intake is present on estrus. All experiments and sacrifices were thus performed during diestrus/metestrus phases, in which there is less impact on chow intake.
Licking microstructure
Licking time stamps were acquired using a multi-channel acquisition system (Tucker-Davis Technologies, Alachua, FL, USA). The number of infrared beam interruptions by tongue protrusion to bottle spout was assessed as the total number of licks performed by rats during 30-min experiment. The lick efficiency was calculated as total number of licks divided by sucrose intake in ml. Then, sucrose licking clusters were determined as a group of high-frequency (3–9 Hz) licks occurring in a run of three or more licks with pauses between clusters as inter-cluster intervals (ICI) of 500 ms or longer. Sucrose licking bursts were determined as a group of high-frequency licks occurring in a run of three or more licks with pauses between bursts as inter-burst intervals (IBI) of 3 s or longer. A custom-written Matlab script (R2010a, The MathWorks™) was used to automatically sort the lick data into lick clusters based on ICI ≥ 500 ms or into lick bursts based on IBI ≥ 3 s. For each experiment, we calculated the meal duration (in second) by adding the duration of all the clusters/bursts recorded in 30 min of sucrose access, the number of clusters/bursts, the cluster/burst size (in licks/cluster or burst), the cluster/burst duration (in second), the mean lick frequency within cluster/burst (licks/second), and the mean inter-lick interval (ILI) within cluster/burst (in ms).
Time spent in the sucrose zone and general locomotor activity
Analysis of the time spent in the sucrose zone
The region in the operant chamber named the sucrose zone, with the lickometer located at the sucrose bottle, was defined as half of the width and quarter of the length (see Fig. 2d). Position and movement of rats in the behavioral cage were recorded on video camera and analyzed with JWatcher software (https://www.jwatcher.ucla.edu/). Time spent by each rat in the sucrose zone, with its head facing or inside the lickometer, and in the other area of the cage were calculated by the software. Percentage time spent in the sucrose zone was calculated by dividing time spent in sucrose lickometer area by total time spent in the cage (30 min). Finally, percentage time spent in the sucrose zone without drinking was calculated by subtracting time spent in sucrose lickometer area by meal duration, and this result was divided by total time spent in the cage (30 min).
Optogenetic stimulation of AcbSh neurons decreases sucrose intake. a Schematic of AAV-hSyn-ChR2-eYFP injection into the AcbSh and implantation of the optical fiber above the injection site of female rats. Fluorescence images showing an example of injection site and optical fiber position in the AcbSh (scale bar 250 µm except for a’’ 60 µm; aca anterior commissure, anterior part, AcbC accumbens nucleus, core, AcbSh accumbens nucleus, shell). On the right, brain atlas drawings (Paxinos and Watson 2007) showing the extent of all ChR injections in the Acb (black: accurate injections, empty circle: misplaced injections). The location of the fiber tips is not shown on this figure but was above the injection sites. b Bar graph showing 10% sucrose intake in mg/g body weight (BW) for 30-min experiment in control (eYFP) and channelrhodopsin (ChR) groups without (black) and with (gray) blue light stimulation (**p < 0.01: significant difference compared to ChR off; ###p < 0.001: significant difference compared to eYFP on). c Bar graph showing 10% sucrose intake in mg/g body weight (BW) for 30-min experiment in ChR rats with misplaced injections without (black) and with (gray) blue light stimulation. d Schematic (top) of the behavioral cage with sucrose (S) and water (W) entries and the delimitation of the sucrose zone (red), and schematic (bottom) of the divisions (red) used in the behavioral cage without sucrose or water to determine the locomotor activity. e Bar graph showing the percentage time spent in the sucrose zone (left) and the general locomotor activity expressed as percentage of the eYFP group (right) for 30-min experiment in control (eYFP) and channelrhodopsin (ChR) groups without (black) and with (gray) blue light stimulation (***p < 0.001: significant difference compared to ChR off; #p < 0.05: significant difference compared to eYFP on). f Bar graph showing plasmatic corticosterone levels in ng/ml 30 min after 30-min experiment with blue light stimulation without sucrose in control (eYFP) and channelrhodopsin (ChR) groups
Analysis of general locomotor activity
The operant chamber was divided into four equal zones (see Fig. 2d). Position and movement of rats in the behavioral cage were recorded on video camera and analyzed with JWatcher software. The general locomotor activity was determined for each rat by counting the number of crossing the borders of zones for 30 min without sucrose bottle. Then, the relative locomotor activity for each rat compared to the control group was calculated as follows: value of a rat/average of the control group × 100.
Blood sample and brain preparation
At the end of the behavioral procedure, animals used for optogenetic experiments were placed in the operant chamber for 30 min without 10% sucrose bottle but with blue-light stimulation, and transferred to their home cages for 30 min without access to chow and water. Animals used for pharmacological experiments received a final injection of muscimol or aCSF, and immediately after injection were transferred to their home cages for 30 min without access to chow and water. Then, all rats were deeply anesthetized (60 mg/kg ketamine plus 7.5 mg/kg xylazine), intracardial blood was collected, and they were perfused intracardially with saline followed by a 4% paraformaldehyde solution. Brains were removed and kept in paraformaldehyde for an additional period of 7 days. Brains were then transferred overnight to a solution containing 4% paraformaldehyde and 20% sucrose, before being cut using a sliding microtome (Histoslide 2000, Heidelberg, Germany). Thirty-micron-thick coronal sections were collected and stored at − 20 °C in a cold sterile cryoprotection solution containing 50 mM sodium phosphate buffer, 30% ethylene glycol and 20% glycerol.
Determination of plasma concentrations of corticosterone
Blood samples were centrifuged (3400 RPM for 15 min at 4 °C) and plasma was stored at − 80 °C until the determination of corticosterone plasma levels in duplicate using commercial ELISA kit (Cayman Chemical, Ann Arbor, MI, USA; sensitivity 5.0 pg/ml).
Injection sites and cannula placements
To verify the location of injection sites and probes, brain sections were mounted on poly-L-lysine-coated slides and coverslipped with mounting medium. The expression of yellow fluorescent reporter protein (eYFP) was morphologically confirmed in the AcbSh for injection sites, as well as cannula and optical fiber placements into the AcbSh or into the VP. Only rats with correct injection sites or cannula/optical fiber placements were included in the data analysis.
In situ hybridization for c-fos mRNA
Levels of c-fos mRNA expression in the AcbSh, the VP and the LH were detected using in situ hybridization. Briefly, the sections were mounted on poly-L-lysine coated slides and fixed for 20 min in 4% paraformaldehyde, digested for 25 min at 37 °C with proteinase K (10 µg/ml in 100 mM Tris–HCl containing 50 mM EDTA, pH 8.0), acetylated with acetic anhydride (0.25% in 0.1 M triethanolamine, pH 8.0), and dehydrated through graded concentrations of ethanol. Sections were incubated overnight with antisense [35S]-labeled cRNA probes (107 cpm/ml) for c-fos (generated from the 2116-bp fragment of rat c-fos cDNA, GenBank accession number X06769.1; Dr I Verma, Salk Institute, La Jolla, CA) at 60 °C. Slices were rinsed with sodium chloride-sodium citrate solution, digested with ribonuclease-A (20 µg/ml) at 37 °C, washed in descending concentrations of sodium chloride-sodium citrate solutions and dehydrated through an ethanol gradient. Slides were defatted in toluene, dipped in nuclear track beta-2 nuclear emulsion (Eastman Kodak, Rochester, NY, USA), and exposed for 10 days before developing. Tissues were counterstained with thionin, dehydrated through graded concentrations of ethanol, cleared in toluene and coverslipped with mounting medium.
Image analyses
Slides were examined with dark-field microscopy using an Olympus BX61 microscope (Olympus Canada, Richmond Hill, ON, Canada). Images were acquired with a DVC-2000C digital camera (DVC Company Inc., Austin, TX, USA) and analyzed with Stereo Investigator software (MBF Bioscience, Williston, VT, USA). The system was calibrated for each set of analyses to prevent saturation of the integrated signal. Mean optical density (OD) was obtained by measuring the OD of pixels of the positive hybridization signal in sections of the nucleus accumbens shell (from 2.20 to 1.00 mm rostral to the bregma), the rostral ventral pallidum (from 0.40 rostral to 0.12 mm caudal to the bregma), the caudal ventral pallidum (from 0.12 to 0.50 mm caudal to the bregma) and the lateral hypothalamus (from 2.00 to 3.50 mm caudal to the bregma), and subtracting the background readings taken from the areas immediately surrounding the analyzed region, using homemade script on Matlab (R2010a, The MathWorks™). A threshold depicting positive hybridization signal was set for pixels with OD > 5 times the background for all micrographs captured with the same luminosity. The OD measurements were performed in the regions of interest outlined bilaterally on five to six (AcbSh), three to four (rostral or caudal VP) and seven to eight (LH) consecutive brain sections. The individual score of each rat was normalized to the mean value of the control group (mean value of each rat / mean value of the control group × 100) to obtain the relative expression of c-fos mRNA.
Statistical analysis
Statistical analyses were performed using GraphPad Prism statistical software, V6.04 (GraphPad Software Inc, La Jolla, CA, USA). The sample size was calculated using the resource equation approach for comparison of two groups with two repeated measurements (Arifin and Zahiruddin 2017). Results are presented as mean ± standard error of mean (SEM). For sucrose intake, lick microstructure parameters and percentage time in sucrose zone for optogenetic experiments, two-way repeated-measures ANOVA was used to detect the main and interactive effects of treatment (ChR or eYFP) and light (off or on). Post-hoc comparisons between the groups were performed using Bonferroni’s multiple comparison when the main and/or interactive ANOVA effects were significant. For GABA agonist experiments, sucrose intake for missed injections, locomotion, plasma corticosterone and c-fos mRNA analyses, Student’s t-test was used. Results were considered significant with p-value < 0.05.
Results
Electrophysiological validation of optogenetic stimulation experiments
We injected AAV2-hSyn-ChR2-eYFP in the AcbSh of female rats (n = 6), and 4 weeks later we made acute slice preparations through the AcbSh for whole-cell patch clamp recordings. The mean resting potential of recorded neurons was − 65.24 ± 3.28 mV. A depolarizing current pulse for 500 ms induced depolarization of the neurons accompanied with spikes (Fig. 1a). The patched cells had morphological feature of medial spiny neurons (Fig. 1b). Then, patched neurons were optically stimulated by 465 nm blue light emitted by an optical fiber placed near to the cell bodies. We found that the firing response to a 1 mW light stimulation for 500 ms was similar to the stimulation with intracellularly applied current pulse (Fig. 1a, c). Short-lasting blue light pulses (10 ms) were also able to trigger action potentials (Fig. 1d). Application of trains of 10 pulses lasting 10 ms each with a 10-Hz frequency at the resting membrane potential induced action potential response to the first pulse only. However, if the cell was depolarized by 3–5 mV from the resting potential, that imitates in vivo situation, most of the pulses were able to trigger spikes (data not shown). Very similar results with 20 Hz stimulation were obtained in another study (Larson et al. 2015). Therefore, optogenetic stimulation with ChR2 efficiently drives action potentials in AcbSh.
Optogenetic stimulation of AcbSh neurons decreases sucrose intake in female rats
Only animals with morphologically identified position of injection site and optical fibers in AcbSh (Fig. 2a) in control and ChR group were analyzed. As additional control, we separately analyzed rats in which the injection site was outside AcbSh. Two-way ANOVA revealed significant effects of optogenetic stimulation of AcbSh (F1,8 = 7.294, p = 0.0270), treatment (F1,8 = 14.06, p = 0.0056) and interaction (F1.8 = 11.74, p = 0.0090) on 10% sucrose intake in control (eYFP, n = 5) and experimented (ChR, n = 5) groups (Fig. 2b). ChR rats had a significantly lower sucrose intake with the light on compared to light off (p = 0.0050), and also compared to the eYFP rats (p = 0.0002). The 10% sucrose intake of ChR rats with injections outside AcbSh (n = 3) was not significantly different (Paired t-test, p = 0.8868; Fig. 2c).
Next, we analyzed the percentage time spent in the sucrose zone during the 30 min-experiment (Fig. 2d). Two-way ANOVA revealed significant effects of optogenetic stimulation of AcbSh (F1,8 = 12.36, p = 0.0079) and interaction (F1.8 = 23.07, p = 0.0014) but no effect of treatment (F1,8 = 1.783, p = 0.2185) on percentage time spent in the sucrose zone (Fig. 2e). Post-hoc analysis revealed that ChR rats spent significantly less time in the sucrose zone with the light on compared to light off (p = 0.0007), and also compared to the eYFP rats (p = 0.0296). Finally, the general locomotor activity was not modified by the optogenetic stimulation of the AcbSh in the ChR group compared to the control group (Unpaired t test, p = 0.6988; Fig. 2d, e), as well as the corticosterone levels were unchanged (Unpaired t test, p = 0.1637; Fig. 2f).
Therefore, we conclude that optogenetic stimulation of AcbSh decreases sucrose intake in female rats in our experimental conditions. Small heating produced by optogenetic stimulation could potentially affect activities of AcbSh (Owen et al. 2019). However, in our experiments the effects on sucrose intake observed during optogenetic stimulation were present in rats with ChR expression in the AcbSh, but not in control rats.
Optogenetic stimulation of AcbSh neurons modifies lick microstructure
For the total number of licks, two-way ANOVA revealed a tendency for interaction (F1,8 = 4.893, p = 0.0579) but no effect of optogenetic stimulation of AcbSh (F1.8 = 3.135, p = 0.1146) and treatment (F1,8 = 3.194, p = 0.1117) (Fig. 3a). Post hoc analysis showed that ChR rats had a significantly lower total number of licks with the light on compared to light off (p = 0.0453) and compared to the control group with light on (p = 0.0241). For the lick efficiency, two-way ANOVA revealed no significant effect of light (F1,8 = 0.0058, p = 0.9410), treatment (F1,8 = 0.4472, p = 0.5225) or interaction (F1.8 = 0.0013, p = 0.9719) (Fig. 3b). Then we analyzed in detail the microstructural organization of the licking behaviors. Rodents lick in stereotypic oromotor patterns characterized by runs of consecutive licks (cluster or burst) separated by pauses (inter-cluster or inter-burst intervals). Studies have shown that a choice of time pause between licks to separate them to clusters affects the overall conclusions (Spector et al. 1998; Johnson 2018). To ensure robustness of our findings, we used two different intervals: 0.5 s, called here inter-cluster intervals (ICI), and 3 s, called here inter-burst intervals (IBI) (see ‘licking microstructure’ in methods part for more details). The total meal duration as well as cluster/burst duration was significantly decreased in ChR group with the light on compared to light off (Fig. 3c, f, Table 1). The burst size was also significantly decreased, and a tendency to decrease is observed for the cluster size (Fig. 3e, Table 1). The number of clusters / bursts, the licking frequency within cluster/burst and the interlick intervals were not significantly different (Fig. 3d, g, h, Table 1). To analyze the time spent in the sucrose zone without drinking, we have subtracted the total time of meal duration (duration of all the clusters/bursts recorded during the 30-min sucrose access) from the total time spent in sucrose zone (Fig. 2e). Rats of both groups, with or without stimulation, spent the same amount of time in the sucrose zone without drinking (Table 1). The meal duration of ChR rats with AcbSh stimulation was lower compared to the other conditions (Fig. 3c and Table 1), so the total percentage time spent in the sucrose zone was lower for this group than the others (Fig. 2e).
Optogenetic stimulation of AcbSh neurons modifies lick microstructure. Bar graphs showing total number of licks (a), licking efficiency (b), meal duration (c), number of bursts (d), burst size (e), burst duration (f), lick frequency (g) and interlick intervals (h) for 30-min experiment in control (eYFP) and channelrhodopsin (ChR) groups without (black) and with (gray) blue light stimulation (*p < 0.05 and ***p < 0.001: significant difference compared to ChR off; #p < 0.05 and ##p < 0.01: significant difference compared to eYFP on; ILI interlick intervals, ms millisecond, s second)
We conclude that the decrease in sucrose intake triggered with AcbSh optogenetic activation was due to a decrease in total number of licks, and as follow in meal duration. The lick microstructure analyses showed that this reduction primarily depended on a decrease in cluster/burst duration and cluster/burst size, but not on variation of lick frequency or number of clusters/bursts.
Optogenetic stimulation of AcbSh neurons decreases c-fos mRNA expression in the rostral VP and LH
At the end of the sucrose intake experiment, the AcbSh was optogenetically stimulated for 30 min and then the rats were killed. C-fos mRNA levels were higher in the AcbSh (Unpaired t-test, p = 0.0138; Fig. 4a) and lower in the LH (Unpaired t-test, p = 0.0085; Fig. 4b) in ChR group compared to control rats. In the VP, the c-fos mRNA expression was lower in the rostral part (Unpaired t-test, p = 0.0082; Fig. 4c) but not significantly different in the caudal part (Unpaired t-test, p = 0.6577; Fig. 4d) in ChR group compared to eYFP rats.
Optogenetic stimulation of AcbSh neurons decreases c-fos mRNA expression in the rostral VP and LH. a Dark-field images showing the positive hybridization signal of c-fos mRNA in the AcbSh of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; aca anterior commissure, anterior part, AcbC accumbens nucleus, core, AcbSh accumbens nucleus, shell). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the AcbSh 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups (*p < 0.05). b Dark-field images showing the positive hybridization signal of c-fos mRNA in the lateral hypothalamus (LH, white shape) of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; 3V third ventricle, f fornix, ic internal capsule, opt optic tract). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the LH 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups (**p < 0.01). c Dark-field images showing the positive hybridization signal of c-fos mRNA in the rostral ventral pallidum (VP, white shape) of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; 3V third ventricle, aca anterior commissure, anterior part). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the rostral part of the VP 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups (**p < 0.01). (d) Dark-field images showing the positive hybridization signal of c-fos mRNA in the caudal ventral pallidum (VP, white shape) of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; 3V third ventricle, ac anterior commissure, LV lateral ventricle). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the caudal part of the VP 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups
These results are in accordance with previous study showing that activation of AcbSh neurons reduces activity in LH neurons (Stratford 2005; O'Connor et al. 2015). They also show that AcbSh stimulation reduces the activity of rostral VP neurons in female rats. Because the AcbSh sends GABAergic projections to the VP, we then tested the effect of the inhibition of the rostral VP in our condition of sucrose intake in female rats.
Pharmacological inhibition of rostral VP neurons decreases sucrose intake in female rats
After bilateral infusions of the GABAA receptor agonist muscimol into the rostral VP (Fig. 5a, n = 5), the 10% sucrose intake was significantly decreased compared to 10% sucrose intake after injection of aCSF (Paired t-test, p = 0.0010; Fig. 5b). The 10% sucrose intake of rats with cannulas placed outside VP (n = 3) was not significantly different (Paired t-test, p = 0.0805; Fig. 5c). The percentage time spent in the sucrose zone during the 30-min experiment was not significantly different after aCSF or muscimol injection (Paired t-test, p = 0.2383; Fig. 5d). We did not perform a behavioral experiment with bilateral infusions of muscimol into the VP without the presence of the sucrose solution; therefore, the general locomotor activity was not evaluated in our study (see “Discussion”). Finally, the corticosterone level was not modified by the muscimol injection into the VP compared to the control group (Unpaired t test, p = 0.4273; Fig. 5e).
Pharmacological inhibition of rostral VP neurons with muscimol decreases sucrose intake. a Schematic of implantation of the cannula above the rostral VP of female rats and insertion of the injector that projected 1 mm below the tip of the guide cannula. Below is an image showing an example of cannula and injector position (scale bar 250 µm; aca anterior commissure, anterior part, CPu caudate putamen, och optic chiasm, VP ventral pallidum). On the right, brain atlas drawings (Paxinos and Watson 2007) showing the injection sites in the VP (black: accurate injections, empty circle: misplaced injections). b Bar graph showing 10% sucrose intake in mg/g body weight (BW) for 30-min experiment after injection of aCSF (control) or muscimol (**p < 0.01). c Bar graph showing 10% sucrose intake in mg/g body weight (BW) for 30-min experiment after injection of aCSF (control) or muscimol in rats with misplaced cannula. d Schematic of the behavioral cage with sucrose (S) and water (W) entries and the delimitation of the sucrose zone (red), and bar graph showing the percentage time spent in the sucrose zone for 30-min experiment after injection of aCSF (control) or muscimol. e Bar graph showing plasmatic corticosterone levels in ng/ml 30 min after injection of aCSF (control) or muscimol. f Bar graph showing total number of licks for 30-min experiment after injection of aCSF (control) or muscimol (**p < 0.01). g Bar graph showing licking efficiency for 30-min experiment after injection of aCSF (control) or muscimol
We also injected the GABAB receptor agonist baclofen into the rostral VP (n = 4), and the 10% sucrose intake was significantly decreased compared to 10% sucrose intake after aCSF (Paired t-test, p = 0.0165; data not shown), but the effect was less pronounced than after muscimol injection (38.35 ± 4.87 mg/g BW for control versus 31.97 ± 3.84 mg/g BW for baclofen group). The 10% sucrose intake of rats with cannulas placed outside VP (n = 3) was not significantly different (Paired t-test, p = 0.1604; data not shown). Finally, the percentage time spent in the sucrose zone during the 30-min experiment was not significantly different after aCSF or baclofen injection (Paired t-test, p = 0.3582; data not shown).
Therefore, we conclude that pharmacological activation of GABAA, and to a minor extent GABAB receptors within the rostral VP, is sufficient to reduce sucrose intake in female rats.
Pharmacological inhibition of rostral VP neurons modifies lick microstructure
The total number of licks was significantly decreased after muscimol injection into the rostral VP compared to aCSF injection (Paired t-test, p = 0.0031; Fig. 5f), but the lick efficiency was not modified (Paired t-test, p = 0.9594; Fig. 5g). Then we analyzed in detail the microstructure of the licks by dividing them in clusters (ICI; 0.5 s) or bursts (IBI; 3 s). We found that the injection of muscimol into the rostral VP significantly decreased the meal duration, the number of clusters/bursts, and the burst size compared to the injection of aCSF (Table 2). The decrease in burst duration was close to the significance, and there were close to significance effects on the licking frequency within cluster and the interlick intervals in clusters (Table 2). No significant difference was observed between the two conditions for the cluster size, the cluster duration, the licking frequency within burst or the interlick intervals in bursts (Table 2). To analyze the time spent in the sucrose zone without drinking, we have subtracted the meal duration (Table 2) from the total time spent in sucrose zone (Fig. 5d). Paired t-test revealed a tendency to an increase of percentage time spent in the sucrose zone without drinking for ICI = 0.5 s, and a significant increase for IBI = 3 s (Table 2). Thus, rats after muscimol injection spent the same amount of total time in the sucrose zone than after aCSF injection (Fig. 5d), but their meal duration was shorter and their time spent in the sucrose zone without drinking was longer than controls (Table 2).
For the group with baclofen injection into rostral VP, no significant difference was found for the different parameters of the lick microstructure: the total number of licks (Paired t-test, p = 0.2961; data not shown), lick efficiency (Paired t-test, p = 0.1578; data not shown), but also the meal duration (Paired t-test, p = 0.1481 for ICI and p = 0.1257 for IBI), number of clusters/bursts (Paired t-test, p = 0.9205 for ICI and p = 0.2746 for IBI), cluster/burst size (Paired t-test, p = 0.4350 for ICI and p = 0.5349 for IBI), cluster/burst duration (Paired t-test, p = 0.8487 for ICI and p = 0.3514 for IBI), licking frequency (Paired t-test, p = 0.1153 for ICI and p = 0.0983 for IBI) and interlick intervals (Paired t-test, p = 0.1015 for ICI and p = 0.0986 for IBI) (data not shown). To analyze the time spent in the sucrose zone without drinking, we have subtracted the meal duration from the total time spent in sucrose zone. Paired t-test revealed no significant difference for the percentage time spent in the sucrose zone without drinking for ICI = 0.5 s (p = 0.4699) and for IBI = 3 s (p = 0.4652). Rats after baclofen injection spent the same total amount of time in the sucrose zone, the same amount of time in this zone without drinking, and they have comparable meal duration, compared to aCSF group.
Therefore, although baclofen injections into the VP reduced to some extent the sucrose intake, the meal duration and lick microstructure were not significantly different compared to control. This can be explained by the fact that cumulative effects of subtle changes in different parameters of lick microstructure were responsible for the overall reduction of sucrose intake.
We conclude that activation of GABAA or GABAB receptors within the rostral VP affects licking microstructure differently. The stimulation of GABAA receptors mainly modified the total number of licks, meal duration and number of clusters/bursts, whereas the stimulation of GABAB receptors affected more the licking frequency and efficiency (close to but did not reach the significance).
The stimulation of AcbSh neurons and the inhibition of rostral VP neurons induce a decrease of 10% sucrose intake in female rats. We then tested whether the stimulation of the rostral VP axonal terminals from the AcbSh neurons would be sufficient to decrease the sucrose intake in female rats.
Optogenetic stimulation of rostral VP axonal terminals from the AcbSh decreases sucrose intake in female rats
In these experiments, the presence and the location of the injection sites in the AcbSh and the location of the optical fibers above the rostral VP were verified for each rat (Fig. 6a). Two-way ANOVA revealed significant effects of optogenetic stimulation of VP axonal terminals (F1,10 = 8.762, p = 0.0143) and interaction (F1.10 = 11.62, p = 0.0067), but no effect of treatment (F1,10 = 1.575, p = 0.2381) on 10% sucrose intake in control (eYFP, n = 6) and experimented (ChR, n = 6) groups (Fig. 6b). Rats that had ChR injection in AcbSh had a significantly lower sucrose intake with the light on compared to light off (p = 0.0023), and a tendency to a decrease was observed between ChR and eYFP rats with light on (p = 0.0696). The 10% sucrose intake for a rat with misplaced optical fiber (n = 1) was similar with and without stimulation (Fig. 6c).
Optogenetic stimulation of rostral VP axonal terminals from the AcbSh decreases sucrose intake. a Schematic of AAV-hSyn-ChR2-eYFP injection into the AcbSh and implantation of the optical fiber above the rostral VP of female rats. Fluorescence images showing an example of injection site (scale bar 250 µm except for a’’ 60 µm). On the right, brain atlas drawings (Paxinos and Watson 2007) showing the area covered by all ChR injections in the AcbSh. Below are images showing an example of optical fiber position and the axonal terminals from the AcbSh in the rostral VP (scale bar 250 µm except for a** 60 µm). On the right, schematics (Paxinos and Watson 2007) showing the location of the fiber tips in the VP (black circle: accurate locations, empty circle: misplaced location) (aca anterior commissure, anterior part, AcbC accumbens nucleus, core, AcbSh accumbens nucleus, shell, CPu caudate putamen, och optical chiasm, VP ventral pallidum). b Bar graph showing 10% sucrose intake in mg/g body weight (BW) for 30-min experiment in control (eYFP) and channelrhodopsin (ChR) groups without (black) and with (gray) blue light stimulation (**p < 0.01: significant difference compared to ChR off). c Bar graph showing 10% sucrose intake in mg/g body weight (BW) for 30-min experiment in the ChR rat with misplaced optical fiber without (black) and with (gray) blue light stimulation. d Schematic of the behavioral cage with sucrose (S) and water (W) entries and the delimitation of the sucrose zone (red), and bar graph showing the percentage time spent in the sucrose zone for 30-min experiment in control (eYFP) and channelrhodopsin (ChR) groups without (black) and with (gray) blue light stimulation. e Schematic of the divisions (red) used in the behavioral cage without sucrose or water to determine the locomotor activity, and bar graph showing the general locomotor activity expressed as percentage of the eYFP group, for 30-min experiment in control (eYFP) and channelrhodopsin (ChR) groups with blue light stimulation. f Bar graph showing plasmatic corticosterone levels in ng/ml 30 min after 30-min experiment blue light stimulation without sucrose in control (eYFP) and channelrhodopsin (ChR) groups
We evaluated the percentage time spent in the sucrose zone during the 30-min experiment. Two-way ANOVA revealed no effect of optogenetic stimulation of rostral VP axonal terminals (F1,10 = 3.878, p = 0.0773), treatment (F1,10 = 0.07541, p = 0.7892) and interaction (F1.10 = 0.7180, p = 0.4166) on percentage time spent in the sucrose zone (Fig. 6d). Finally, the general locomotor activity was not modified by the optogenetic stimulation of VP axonal terminals from the AcbSh in the ChR injected rats compared to the control group (Unpaired t test, p = 0.5390; Fig. 6e), as well as the corticosterone levels (Unpaired t test, p = 0.7461; Fig. 6f).
Therefore, we conclude that optogenetic stimulation of rostral VP axonal terminals from the AcbSh decreases sucrose intake in female rats in our experimental conditions.
Optogenetic stimulation of rostral VP axonal terminals from the AcbSh modifies lick microstructure
For the total number of licks, two-way ANOVA revealed significant effects of optogenetic stimulation of VP axonal terminals (F1,10 = 11.22, p = 0.0074) and interaction (F1.10 = 5.745, p = 0.0375), but no effect of treatment (F1,10 = 0.05753, p = 0.8153) (Fig. 7a). Post hoc analysis showed that rats with AcbSh axons expressing ChR within the VP had a significantly lower total number of licks with the light on compared to light off (p = 0.0045). For the lick efficiency, two-way ANOVA revealed no significant effect of optogenetic stimulation of AcbSh axonal terminals within VP (F1,10 = 2.825, p = 0.1237), treatment (F1,10 = 3.842, p = 0.0784) or interaction (F1.10 = 0.4404, p = 0.5219) (Fig. 7b). Then we analyzed in details the microstructure of the licks by divided them into clusters (ICI; 0.5 s) or bursts (IBI; 3 s). The meal duration was significantly decreased in ChR group with the light on compared to light off (Fig. 7c, Table 3). The burst size and burst duration were also significantly decreased, and the cluster size and cluster duration tended to decrease (Fig. 7e, f, Table 3). The number of bursts was increased (Fig. 7d, Table 3). The licking frequency within cluster was slightly, but significantly increased, whereas the interlick intervals in clusters were decreased (Table 3). The other parameters were not significantly different (Fig. 3g, h, Table 3). To analyze the time spent in the sucrose zone without drinking, we have subtracted the meal duration from the total time spent in sucrose zone (Fig. 6d). Rats of both group, with or without stimulation, spent the same amount of time in the sucrose zone without drinking (Table 3). The meal duration of ChR rats with stimulation of the VP axonal terminals from the AcbSh was decreased compared to the other conditions, but the total percentage time spent in the sucrose zone was comparable. However, a tendency to the decrease was observed for the total percentage time spent in the sucrose zone; the p-value for the effect of optogenetic stimulation of VP axonal terminals was p = 0.0773.
Optogenetic stimulation of rostral VP axonal terminals from the AcbSh modifies lick microstructure. Bar graphs show total number of licks (a), licking efficiency (b), meal duration (c), number of bursts (d), burst size (e), burst duration (f), lick frequency (g) and interlick intervals (h) for 30-min experiment in control (eYFP) and channelrhodopsin (ChR) groups without (black) and with (gray) blue light stimulation (**p < 0.01 and ****p < 0.0001: significant difference compared to ChR off; ILI interlick intervals, ms millisecond, s second)
We conclude that the decrease in sucrose intake observed after inhibition of rostral VP neurons arising from the stimulated AcbSh axons was due to a decrease in total number of licks and meal duration; this is similar to the results obtained with optogenetic stimulation of AcbSh. However, the lick microstructure analyses showed a more complex behavior.
Optogenetic stimulation of rostral VP axonal terminals from the AcbSh decreases c-fos mRNA expression in the rostral VP, but not in the LH
At the end of the sucrose intake experiment, rostral VP axonal terminals from the AcbSh were optogenetically stimulated for 30 min and then the rats were killed. C-fos mRNA levels were higher in the AcbSh of ChR rats (Unpaired t-test, p = 0.0398; Fig. 8a), but were not significantly different between the two groups in the LH (Unpaired t-test, p = 0.1993; Fig. 8b). Finally, c-fos mRNA expression was lower in the rostral part of the VP (Unpaired t-test, p = 0.0103; Fig. 8c) and not significantly different in the caudal part of the VP (Unpaired t-test, p = 0.8789; Fig. 8d) in ChR group compared to control rats.
Optogenetics stimulation of rostral VP axonal terminals from the AcbSh decreases c-fos mRNA expression in the rostral VP but not in the LH. a Dark-field images showing the positive hybridization signal of c-fos mRNA in the AcbSh of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; aca anterior commissure, anterior part, AcbC accumbens nucleus, core, AcbSh accumbens nucleus, shell). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the AcbSh 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups (*p < 0.05). b Dark-field images showing the positive hybridization signal of c-fos mRNA in the lateral hypothalamus (LH, white shape) of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; 3V third ventricle, f fornix, ic internal capsule, opt optic tract). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the LH 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups. c Dark-field images showing the positive hybridization signal of c-fos mRNA in the rostral ventral pallidum (VP, white shape) of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; 3V third ventricle, aca anterior commissure, anterior part). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the rostral part of the VP 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups (*p < 0.05). d Dark-field images showing the positive hybridization signal of c-fos mRNA in the caudal ventral pallidum (VP, white shape) of control (eYFP) and channelrhodopsin (ChR) groups (scale bar 250 µm; 3V third ventricle, ac anterior commissure). Bar graph showing relative levels of c-fos mRNA expression expressed as percentage of the eYFP group in the caudal part of the VP 30 min after 30-min blue light stimulation in control (eYFP) and channelrhodopsin (ChR) groups
We conclude that the stimulation of the AcbSh axonal terminals within VP induced an inhibition of the rostral VP. The effects of this stimulation were specific to the rostral VP because the stimulation of the VP axonal terminals from the AcbSh did not affect the LH activity.
Discussion
In this study, we observed that stimulation of the AcbSh, inhibition of the rostral VP and stimulation of projections from the AcbSh to the rostral VP induced a decrease in sucrose intake. AcbSh-regulated feeding via opioid system is independent of the caudal VP for the motivation or ‘wanting’ component of reward, but not for the hedonic aspect of reward or ‘liking’ (Taha et al. 2009; Smith and Berridge 2007). Here, we demonstrate that the projections from the medial part of the AcbSh to the rostral VP were sufficient to control sucrose consumption, with different effects on ‘wanting’ and ‘liking’.
The role of the AcbSh and VP in feeding behavior was previously investigated (Basso and Kelley 1999; Krause et al. 2010; O'Connor et al. 2015; Prado et al. 2016; Stratford 2005; Smith et al. 2009). However, the role of accumbal projections to the rostral VP in the regulation of food intake is still unclear. We investigated different parameters of feeding behavior after various experiments involving these two regions (Table 4). The sucrose intake was reduced in all our experimental settings; inhibition of rostral VP by the AcbSh was sufficient to reduce sucrose consumption in female rats. The corticosterone levels were not different, indicating that the decrease was not the consequence of different stress levels between groups (Hermans et al. 2014). We found that, in our experimental design, the total time spent by animals in sucrose zone was significantly decreased with stimulation of AcbSh; a tendency to decrease was observed with stimulation of some subset of axons from the AcbSh to the rostral VP. The inhibition of the rostral VP with muscimol did not modify the total time spent in the sucrose zone, but the meal duration was shorter and the time spent in the sucrose zone without drinking was longer than in controls. The reduction in sucrose intake in all experiments was primarily the result of decreases in total licks number and meal duration, except for activation of GABAB receptors in the VP for which the reduction in sucrose intake could be due to modifications in licking frequency and efficiency that were close to, but did not reach the level of significance.
We analyzed the lick microstructure organization in order to better understand the decrease in sucrose intake that we observed in each experiment. Studies show that a variation in cluster size/duration is positively correlated with the hedonic value of the stimulus (palatability), whereas a variation in the number of clusters is positively correlated with physiological need (Davis and Smith 1992; Spector and St John 1998; Spector et al. 1998). In our study, the animals were ad libitum fed so there was no increase in physiological need for food consumption; we thus associated a variation in number of clusters as a modification in motivation to consume palatable food. Stimulation of the AcbSh induced a decrease in cluster/burst duration and burst size, reflecting a decrease in palatability, but did not modify the motivation to drink (no change in number of clusters/bursts) and licking frequency. The rostromedial AcbSh contains a hotspot involved in hedonic ‘liking’ reactions to a sucrose taste (Pecina and Berridge 2005). In this region, manipulations of the opioid signaling through agonists or antagonists affect palatable food intake with preferential effects on palatability (Scheggi et al. 2013; Shin et al. 2010; Castro and Berridge 2014a, b; Hanlon et al. 2004; Taha et al. 2009), but increase alcohol consumption by enhancing the motivation (Richard and Fields 2016). The µ-opioid receptors are Gi/Go-protein coupled receptors (Connor and Christie 1999). The infusion of the µ-opioid receptor agonist DAMGO into the nucleus accumbens increases c-Fos expression in the VP and the LH, whereas the infusion of the µ-opioid receptor antagonist naloxone into the nucleus accumbens decreases c-Fos expression in the VP and the LH (Smith and Berridge 2007). Thus, the modification in food consumption observed after manipulations of opioid receptors in the AcbSh could be due to the involvement of these two regions.
Unlike stimulation of the AcbSh, inhibition of the rostral VP with muscimol induced a decrease in number of clusters/bursts reflecting a decrease in the motivation to drink and a tendency to decrease the licking frequency within cluster. Inhibition of the rostral VP had less effect on the palatability (no significant change in cluster size and cluster duration, tendency to decrease the burst duration and significant decrease in burst size). In previous studies, the injection of GABAA receptor antagonist bicuculline in all parts of the VP increased food intake, with no effect on hedonic reactions to sucrose but enhancing the motivation to consumption (Shimura et al. 2006; Smith et al. 2009; Smith and Berridge 2005; Reichard et al. 2019a, b). We observed a decrease in sucrose intake with preferential effects on motivation after muscimol injection in the rostral VP. Muscimol injection in the caudal VP containing an hedonic hotspot decreased the intake of fluids, but increased aversive reactions to sweetness (Shimura et al. 2006; Ho and Berridge 2014; Khan et al. 2020) and decreased hedonic value of the tasting (Taha et al. 2009); opioid receptor activation in the caudal VP increases sucrose palatability (Wassum et al. 2009). A functional heterogeneity of the rostral (mainly motivation) versus caudal (motivation and hedonic value) VP after muscimol infusions is supported by reports in other studies (Mahler et al. 2014; Smith et al. 2009; Ho and Berridge 2014). The baclofen injection in the rostral VP induced a decrease in sucrose intake mainly by affecting the licking frequency and efficiency (these parameters were close to, but did not reach the significance), suggesting that the two GABAergic receptors in the VP play a slightly different role in sucrose intake regulation. Different modulations of a function by muscimol and baclofen were previously demonstrated (Manfridi et al. 2001; Kenigsberg et al. 1998; Waraczynski 2008). Moreover, the weaker effect on sucrose intake of baclofen infusion compared to muscimol infusion could be due to the low level of GABAB receptors expression in the VP (Margeta-Mitrovic et al. 1999).
It has been shown that bilateral infusions of muscimol into the VP decrease locomotion and rearing activity (Subramanian et al. 2018; van den Bos and Cools 2003). The general locomotor activity of the rats infused with muscimol was not analyzed in our study. However, during the experiment in the presence of the sucrose solution, the bilateral infusions of muscimol into the rostral VP did not modify the total time spent by the rats in the sucrose zone, but the rats’ behavior in this zone was different from the control animals. The meal duration was decreased whereas the percentage time spent without drinking was increased compared to the control group. This result and the lick microstructure analysis of the sucrose intake after VP muscimol infusion lead us to the conclusion that the inhibition of the rostral VP seems to induce a decrease in the motivation to drink. The rostral VP has been involved in reward-seeking behaviors (Smith et al. 2009; Mahler et al. 2014), and the decrease in locomotion observed after bilateral infusions of muscimol into the VP could be associated with a decrease in motivation (Taha et al. 2009). The bilateral infusions of bicuculline into the VP produce pivoting and gnawing movements and in a presence of food produce compulsive ingestion without increasing locomotion (Zahm et al. 2014; Reichard et al. 2019a, b). Finally, injection of the GABAB receptor antagonist phaclofen into the VP has no effect on motor activity (Austin and Kalivas 1990).
Like overall stimulation of the AcbSh, stimulation of AcbSh axonal terminals within rostral VP significantly decreased the burst size and duration (a tendency to decrease was observed for the cluster size and cluster duration), reflecting a decrease in palatability (Table 4). It also increased the number of bursts and the lick frequency within cluster, reflecting an increase in motivation; this result is the opposite of the behavior observed after overall inhibition of the rostral VP by muscimol and baclofen, inducing a decrease in the number of clusters/bursts or in licking frequency. The VP receives projections from both accumbal D1- and D2-receptor expressing MSNs (Creed et al. 2016; Kupchik and Kalivas 2017; Robertson and Jian 1995; Lu et al. 1998), but the role of these two sub-populations is not clear. Activation of D1- and D2-receptor expressing MSNs in the Acb increases motivation during progressive ratio task and reward-predictive cues in mice (Soares-Cunha et al. 2016). The D2-receptor expressing MSNs activation induces a modulation of the ventral tegmental area (VTA) dopaminergic neurons via a reduction in the firing rate of the VP neurons (Soares-Cunha et al. 2018). However, another study has shown that a decrease in inhibitory transmission from D2-receptor MSNs neurons to the VP is sufficient to enhance motivation (Gallo et al. 2018). Authors proposed that differences in the design of the experiment or in the time window of the pathway manipulation could explain these different results. Moreover, the increase in ingestion observed after bicuculline infusions into the VP was attenuated by D2, but not D1 receptor antagonist; the preference for sweet over chow pellets was maintained in the presence of D1 and D2 antagonists (Reichard et al. 2019b). In the cited study, the D1 and D2 antagonists were given systemically; the effects were not specific to the AcbSh and could be due to D1 and D2 signaling from other brain regions. Activation of accumbal D1-, but not D2-receptor expressing MSNs’ projections to the LH stops food consumption (O'Connor et al. 2015). Another study shows that D1-receptor expressing neurons in the ventral striatum mediates the gustatory actions of sugars, that their activation increased licking for sucralose, and that optogenetic activation of projections from the ventral striatum to the VP produced an increase in sucralose intake that was blocked by VP activation (Tellez et al. 2016). However, important divergences in the design of their experiments could explain the differences with our results, as well as the fact that they stimulated the ventral striatum and not specifically the shell part of the nucleus accumbens. Our optogenetics experiment induced an activation of axonal terminals from AcbSh neurons projecting to the VP, regardless of the expression of D1- or D2-receptor expressing neurons. We thus suggest that the motivation to eat and the palatability were differently impacted by the stimulation of axonal terminals from both populations, inducing an overall decrease in sucrose intake (Taha and Fields 2005). Considering these various results, we can assume that different context (palatable vs regular food; starved vs satiated) or different phases of feeding behavior (foraging, approach or consumption) induce differential activation of D1- and/or D2-receptor expressing MSNs, producing distinct responses. Moreover, the activation of accumbal neurons, regardless of the expression of D1- or D2-receptor expressing MSNs in our study can explain why we observed a decrease in sucrose intake, whereas other studies showed a stop of consumption (O'Connor et al. 2015; Krause et al. 2010). Future experiments on the specific activation of D1- or D2-receptor expressing MSNs’ projections to the VP should be performed to understand the specific role of these accumbal outputs in sucrose intake regulation.
General inhibition of the rostral VP mainly induced a decrease in motivation, whereas the inhibition of the rostral VP via stimulation of axonal terminals from accumbal neurons induced an increase in motivation and a decrease in palatability. The VP not only contains predominantly GABAergic neurons, but also glutamatergic cells (Faget et al. 2018; Tooley et al. 2018; Root et al. 2015). These two populations project to similar targets but they drive the opposite behavior (Faget et al. 2018). The GABAergic neurons elicit positive reinforcement via projections to the VTA while activation of glutamatergic neurons induces behavioral avoidance via projections to the lateral habenula (Faget et al. 2018). Furthermore, the ablation of glutamatergic VP neurons increased reward responses and abolished taste aversion to sucrose (Tooley et al. 2018). Nonselective stimulation of the VP induces a robust place preference (Tooley et al. 2018); our pharmacological experiment induced a non-specific inhibition of VP neurons leading to a decrease in motivation. On the contrary, stimulation of the axonal projections from the AcbSh could target specific neurons in the VP. The AcbSh sends inputs to both VP populations (Tooley et al. 2018; Root et al. 2015), but specific projections of D1- and D2-receptor expressing MSNs on GABAergic and glutamatergic VP neurons are not known. Future experiments on the specific projections of MSN populations to different populations in the VP should be performed to better understand the neuroanatomy of reward network.
In our study we used rhythmic optogenetic stimulation of AcbSh or AcbSh terminals within VP. We cannot confirm that every stimulus applied to the AcbSh elicited action potentials. Therefore, the effect of AcbSh stimulation was validated by the increase in c-fos mRNA expression. We found a decrease in c-fos mRNA expression in the LH and the rostral part of the VP; these two regions receive intense GABAergic projections from the AcbSh (Heimer et al. 1991; Usuda et al. 1998). The role of projections from the AcbSh to the LH in the regulation of palatable food intake is well known in the literature (Maldonado-Irizarry et al. 1995; O'Connor et al. 2015; Stratford 2005; Urstadt et al. 2013). Indeed, it has been shown that GABAergic neurons in the LH receive projections from D1-receptor expressing accumbal neurons, and the manipulation of this pathway modulates consummatory actions irrespective of palatability (O'Connor et al. 2015). Because the largest input to the VP is from the Acb (Zahm et al. 1985; Tooley et al. 2018), we focused on the VP (Stratford et al. 1999; Tindell et al. 2006; Root et al. 2015) and demonstrated that stimulation of axonal projections from the AcbSh to the VP induced a decrease in sucrose intake mainly via the decrease in the hedonic value of the solution. This stimulation also induced a decrease in c-fos mRNA expression in the rostral VP and no difference in the LH. Preliminary data in our laboratory showed that individual AcbSh neurons rarely project simultaneously to both VP and LH (data not shown), the two main target regions of the AcbSh (Usuda et al. 1998), but these results need to be confirmed.
We observed an increase in c-fos mRNA expression in the AcbSh after stimulation of axonal projections in the VP, suggesting that optogenetics stimulation of axonal projections in the VP could antidromically activates AcbSh neurons. This effect could be resulting in activation of inputs to the VTA. However, the VTA receives projections from both AcbSh and VP; it is difficult to differentiate if the potential effect on the VTA could be the consequence of the inhibition of the VP neurons by accumbal projections, or of the antidromic activation of accumbal neurons. The licking microstructure results were different between direct stimulation of the AcbSh and stimulation of the axonal terminals in the VP. Thus, the behavioral results observed after stimulation of axonal terminals to the VP were likely due to projections from the AcbSh to the rostral VP. The increase in c-fos mRNA expression observed in the AcbSh can be the consequence of disinhibition of regions that project to the AcbSh following inhibition of GABAergic VP neurons. In our experiment, we stimulated one specific pathway but in the normal condition, different regions interact to induce a specific behavior.
In conclusion, this study shows that stimulation of projections from the AcbSh to the rostral VP results in a reduction in sucrose consumption in female rats. This reduction is mainly the consequence of a decrease in hedonic value of the solution. However, a more complicated pathway between D1- and D2-receptor expressing MSNs neurons in the accumbens and GABAergic and glutamatergic neurons in the VP needs to be assessed. It would also be important to investigate whether activation of projections from the AcbSh to the rostral VP could counterbalance the overconsumption of sucrose observed in a binge-like eating disorders rat model (Calvez and Timofeeva 2016).
References
Arifin WN, Zahiruddin WM (2017) Sample size calculation in animal studies using resource equation approach. Malays J Med Sci MJMS 24(5):101–105. https://doi.org/10.21315/mjms2017.24.5.11
Asarian L, Geary N (2013) Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305(11):R1215-1267. https://doi.org/10.1152/ajpregu.00446.2012
Austin MC, Kalivas PW (1990) Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharmacol Exp Therap 252(3):1370–1377
Basso AM, Kelley AE (1999) Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci 113(2):324–336
Belleau ML, Warren RA (2000) Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol 84(5):2204–2216. https://doi.org/10.1152/jn.2000.84.5.2204
Calvez J, Timofeeva E (2016) Behavioral and hormonal responses to stress in binge-like eating prone female rats. Physiol Behav 157:28–38. https://doi.org/10.1016/j.physbeh.2016.01.029
Castro DC, Berridge KC (2014a) Advances in the neurobiological bases for food “liking” versus “wanting.” Physiol Behav 136:22–30. https://doi.org/10.1016/j.physbeh.2014.05.022
Castro DC, Berridge KC (2014b) Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting.” J Neurosci 34(12):4239–4250. https://doi.org/10.1523/jneurosci.4458-13.2014
Connor M, Christie MD (1999) Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol 26(7):493–499. https://doi.org/10.1046/j.1440-1681.1999.03049.x
Cossrow N, Pawaskar M, Witt EA, Ming EE, Victor TW, Herman BK, Wadden TA, Erder MH (2016) Estimating the prevalence of binge eating disorder in a community sample from the United States: comparing DSM-IV-TR and DSM-5 criteria. J Clin Psychiatry 77(8):e968-974. https://doi.org/10.4088/JCP.15m10059
Creed M, Ntamati NR, Chandra R, Lobo MK, Luscher C (2016) Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron 92(1):214–226. https://doi.org/10.1016/j.neuron.2016.09.001
Cromwell HC, Berridge KC (1993) Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res 624(1–2):1–10
Davis JD, Smith GP (1992) Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106(1):217–228
Faget L, Zell V, Souter E, McPherson A, Ressler R, Gutierrez-Reed N, Yoo JH, Dulcis D, Hnasko TS (2018) Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat Commun 9(1):849. https://doi.org/10.1038/s41467-018-03125-y
Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC (2016) Homeostasis meets motivation in the battle to control food intake. J Neurosci 36(45):11469–11481. https://doi.org/10.1523/jneurosci.2338-16.2016
Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS, Moore H, Javitch JA, Kellendonk C (2018) Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat Commun 9(1):1086. https://doi.org/10.1038/s41467-018-03272-2
Hanlon EC, Baldo BA, Sadeghian K, Kelley AE (2004) Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology 172(3):241–247. https://doi.org/10.1007/s00213-003-1654-0
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C (1991) Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41(1):89–125
Hermans EJ, Henckens MJ, Joels M, Fernandez G (2014) Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37(6):304–314. https://doi.org/10.1016/j.tins.2014.03.006
Ho CY, Berridge KC (2014) Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. Eur J Neurosci 40(10):3556–3572. https://doi.org/10.1111/ejn.12720
Hudson JI, Hiripi E, Pope HG Jr, Kessler RC (2007) The prevalence and correlates of eating disorders in the National Comorbidity Survey replication. Biol Psychiat 61(3):348–358. https://doi.org/10.1016/j.biopsych.2006.03.040
Johnson AW (2018) Characterizing ingestive behavior through licking microstructure: underlying neurobiology and its use in the study of obesity in animal models. Int J Dev Neurosci 64:38–47. https://doi.org/10.1016/j.ijdevneu.2017.06.012
Kenigsberg RL, Hong Y, Théorêt Y (1998) Cholinergic cell expression in the developing rat medial septal nucleus in vitro is differentially controlled by GABAA and GABAB receptors. Brain Res 805(1–2):123–130. https://doi.org/10.1016/s0006-8993(98)00690-8
Khan HA, Urstadt KR, Mostovoi NA, Berridge KC (2020) Mapping excessive “disgust” in the brain: ventral pallidum inactivation recruits distributed circuitry to make sweetness “disgusting.” Cognit Affect Behav Neurosci 20(1):141–159. https://doi.org/10.3758/s13415-019-00758-4
Krause M, German PW, Taha SA, Fields HL (2010) A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci 30(13):4746–4756. https://doi.org/10.1523/jneurosci.0197-10.2010
Kupchik YM, Kalivas PW (2017) The direct and indirect pathways of the nucleus accumbens are not what you think. Neuropsychopharmacology 42(1):369–370. https://doi.org/10.1038/npp.2016.160
Larson EB, Wissman AM, Loriaux AL, Kourrich S, Self DW (2015) Optogenetic stimulation of accumbens shell or shell projections to lateral hypothalamus produce differential effects on the motivation for cocaine. J Neurosci 35(8):3537–3543. https://doi.org/10.1523/jneurosci.1524-14.2015
Lu XY, Ghasemzadeh MB, Kalivas PW (1998) Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience 82(3):767–780. https://doi.org/10.1016/s0306-4522(97)00327-8
Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17(4):577–585. https://doi.org/10.1038/nn.3664
Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15(10):6779–6788
Manfridi A, Brambilla D, Mancia M (2001) Sleep is differently modulated by basal forebrain GABA(A) and GABA(B) receptors. Am J Physiol Regul Integr Comp Physiol 281(1):R170-175. https://doi.org/10.1152/ajpregu.2001.281.1.R170
Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI (1999) Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol 405(3):299–321
Meredith GE (1999) The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci 877:140–156
O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, Luscher C (2015) Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88(3):553–564. https://doi.org/10.1016/j.neuron.2015.09.038
Owen SF, Liu MH, Kreitzer AC (2019) Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci 22(7):1061–1065. https://doi.org/10.1038/s41593-019-0422-3
Paxinos G, Watson C (eds) (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier Inc., Burlington
Pecina S, Berridge KC (2005) Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25(50):11777–11786. https://doi.org/10.1523/jneurosci.2329-05.2005
Prado L, Luis-Islas J, Sandoval OI, Puron L, Gil MM, Luna A, Arias-Garcia MA, Galarraga E, Simon SA, Gutierrez R (2016) Activation of glutamatergic fibers in the anterior NAc shell modulates reward activity in the aNAcSh, the lateral hypothalamus, and medial prefrontal cortex and transiently stops feeding. J Neurosci 36(50):12511–12529. https://doi.org/10.1523/jneurosci.1605-16.2016
Reichard RA, Parsley KP, Subramanian S, Stevenson HS, Schwartz ZM, Sura T, Zahm DS (2019) The lateral preoptic area and ventral pallidum embolden behavior. Brain Struct Funct 224(3):1245–1265. https://doi.org/10.1007/s00429-018-01826-0
Reichard RA, Parsley KP, Subramanian S, Zahm DS (2019) Dissociable effects of dopamine D1 and D2 receptors on compulsive ingestion and pivoting movements elicited by disinhibiting the ventral pallidum. Brain Struct Funct 224(5):1925–1932. https://doi.org/10.1007/s00429-019-01879-9
Richard JM, Fields HL (2016) Mu-opioid receptor activation in the medial shell of nucleus accumbens promotes alcohol consumption, self-administration and cue-induced reinstatement. Neuropharmacology 108:14–23. https://doi.org/10.1016/j.neuropharm.2016.04.010
Robertson GS, Jian M (1995) D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience 64(4):1019–1034. https://doi.org/10.1016/0306-4522(94)00426-6
Root DH, Melendez RI, Zaborszky L, Napier TC (2015) The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130:29–70. https://doi.org/10.1016/j.pneurobio.2015.03.005
Scheggi S, Secci ME, Marchese G, De Montis MG, Gambarana C (2013) Influence of palatability on motivation to operate for caloric and non-caloric food in non food-deprived and food-deprived rats. Neuroscience 236:320–331. https://doi.org/10.1016/j.neuroscience.2013.01.027
Shimura T, Imaoka H, Yamamoto T (2006) Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci 23(6):1596–1604. https://doi.org/10.1111/j.1460-9568.2006.04689.x
Shin AC, Pistell PJ, Phifer CB, Berthoud HR (2010) Reversible suppression of food reward behavior by chronic mu-opioid receptor antagonism in the nucleus accumbens. Neuroscience 170(2):580–588. https://doi.org/10.1016/j.neuroscience.2010.07.017
Smith KS, Berridge KC (2005) The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci 25(38):8637–8649. https://doi.org/10.1523/jneurosci.1902-05.2005
Smith KS, Berridge KC (2007) Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27(7):1594–1605. https://doi.org/10.1523/jneurosci.4205-06.2007
Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009) Ventral pallidum roles in reward and motivation. Behav Brain Res 196(2):155–167. https://doi.org/10.1016/j.bbr.2008.09.038
Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, Sousa N, Rodrigues AJ (2016) Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun 7:11829. https://doi.org/10.1038/ncomms11829
Soares-Cunha C, Coimbra B, Domingues AV, Vasconcelos N, Sousa N, Rodrigues AJ (2018) Nucleus accumbens microcircuit underlying D2-MSN-driven increase in motivation. eNeuro. https://doi.org/10.1523/eneuro.0386-18.2018
Spector AC, Klumpp PA, Kaplan JM (1998) Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112(3):678–694
Spector AC, St John SJ (1998) Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol 274(6):R1687-1703. https://doi.org/10.1152/ajpregu.1998.274.6.R1687
Stratford TR (2005) Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res 1048(1–2):241–250. https://doi.org/10.1016/j.brainres.2005.05.002
Stratford TR, Kelley AE (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17(11):4434–4440
Stratford TR, Kelley AE, Simansky KJ (1999) Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res 825(1–2):199–203
Stratford TR, Wirtshafter D (2012) Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit. Behav Brain Res 226(2):548–554. https://doi.org/10.1016/j.bbr.2011.10.014
Subramanian S, Reichard RA, Stevenson HS, Schwartz ZM, Parsley KP, Zahm DS (2018) Lateral preoptic and ventral pallidal roles in locomotion and other movements. Brain Struct Funct 223(6):2907–2924. https://doi.org/10.1007/s00429-018-1669-2
Taha SA, Fields HL (2005) Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci 25(5):1193–1202. https://doi.org/10.1523/jneurosci.3975-04.2005
Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL (2009) Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience 161(3):718–733. https://doi.org/10.1016/j.neuroscience.2009.03.057
Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE (2016) Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci 19(3):465–470. https://doi.org/10.1038/nn.4224
Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW (2006) Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol 96(5):2399–2409. https://doi.org/10.1152/jn.00576.2006
Tooley J, Marconi L, Alipio JB, Matikainen-Ankney B, Georgiou P, Kravitz AV, Creed MC (2018) Glutamatergic ventral pallidal neurons modulate activity of the habenula-tegmental circuitry and constrain reward seeking. Biol Psychiat 83(12):1012–1023. https://doi.org/10.1016/j.biopsych.2018.01.003
Urstadt KR, Kally P, Zaidi SF, Stanley BG (2013) Ipsilateral feeding-specific circuits between the nucleus accumbens shell and the lateral hypothalamus: regulation by glutamate and GABA receptor subtypes. Neuropharmacology 67:176–182. https://doi.org/10.1016/j.neuropharm.2012.10.027
Usuda I, Tanaka K, Chiba T (1998) Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res 797(1):73–93
van den Bos R, Cools AR (2003) Switching to cue-directed behavior: specific for ventral striatal dopamine but not ventral pallidum/substantia innominata gaba as revealed by a swimming-test procedure in rats. Neuroscience 118(4):1141–1149. https://doi.org/10.1016/s0306-4522(03)00058-7
Waraczynski M (2008) GABA receptor agonism in the sublenticular central extended amygdala impairs medial forebrain bundle self-stimulation but GABA blockade does not enhance it. Behav Brain Res 187(2):396–404. https://doi.org/10.1016/j.bbr.2007.10.001
Wassum KM, Ostlund SB, Maidment NT, Balleine BW (2009) Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci USA 106(30):12512–12517. https://doi.org/10.1073/pnas.0905874106
Zahm DS, Schwartz ZM, Lavezzi HN, Yetnikoff L, Parsley KP (2014) Comparison of the locomotor-activating effects of bicuculline infusions into the preoptic area and ventral pallidum. Brain Struct Funct 219(2):511–526. https://doi.org/10.1007/s00429-013-0514-x
Zahm DS, Zaborszky L, Alones VE, Heimer L (1985) Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res 325(1–2):317–321
Acknowledgements
This work was financially supported by the Natural Sciences and Engineering Research Council of Canada and Canadian Institutes of Health Research. We thank Dr. Christophe Lenglos for the help with Matlab programming, and Dr. Arojit Mitra for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased on September 28, 2017: Timofeeva Elena.
Rights and permissions
About this article
Cite this article
Chometton, S., Guèvremont, G., Seigneur, J. et al. Projections from the nucleus accumbens shell to the ventral pallidum are involved in the control of sucrose intake in adult female rats. Brain Struct Funct 225, 2815–2839 (2020). https://doi.org/10.1007/s00429-020-02161-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-020-02161-z