Abstract
Rationale and Objectives
Conflict procedures are used to study mechanisms underlying the anxiolytic effects of benzodiazepines (BZs). We established a conflict procedure with rhesus monkeys in order to examine the role of GABAA receptors in the anxiolytic-like effects of BZs.
Methods
Four rhesus monkeys responded under a two-component multiple schedule in which responding was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence (suppressed responding) of response-contingent electric shock.
Results
Conventional BZs (alprazolam, flunitrazepam, clonazepam, nitrazepam, lorazepam, bromazepam, diazepam, flurazepam, clorazepate, chlordiazepoxide) engendered increases in the average rates of suppressed responding at low to intermediate doses and decreased the average rates of non-suppressed responding at higher doses. Positive correlations were observed when the therapeutic potencies of BZs in humans were compared with potencies to increase the rates of suppressed responding (R 2=0.83) or decrease the rates of non-suppressed responding (R 2=0.60). The 5-HT1A agonist buspirone increased the rates of suppressed responding, although the effects were modest, whereas the opioid morphine lacked anti-conflict effects. The BZ antagonist flumazenil also modestly increased the rates of suppressed responding. A relatively low dose of flumazenil enhanced, while a high dose blocked, alprazolam’s anti-conflict effects. Compounds selective for α1 subunit-containing GABAA receptors (zolpidem, zaleplon, CL218,872) engendered relatively weak increases in the rates of suppressed responding.
Conclusions
A rhesus monkey conflict procedure was established with predictive validity for therapeutic doses in people and provided evidence that anxiolytic-like effects of BZs can occur with relatively low intrinsic efficacy at GABAA receptors and are reduced by α1GABAA receptor selectivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benzodiazepines (BZs) are highly effective therapeutic agents for the treatment of several disorders, including anxiety disorders (for review, see Nutt 2005). The therapeutic use of BZs is constrained, however, by other effects of these drugs such as impairment of motor coordination, daytime drowsiness, memory deficits, and addiction potential (Griffiths and Weerts 1997; Nutt 2005). Research in the past two decades has revealed the existence of multiple subtypes of the GABAA receptor (Pritchett et al. 1989; for review, see Rudolph et al. 2000) and recent studies have postulated that the diverse behavioral effects of BZs may reflect action at different subtypes of GABAA receptors (McKernan et al. 2000; Rudolph et al. 2000; Rowlett et al. 2005; Lippa et al. 2005). Identification of the subtypes of the GABAA receptor associated with therapeutically beneficial effects and unwanted side effects might lead to the development of more effective anxiolytic medications.
BZs act by allosterically binding to GABAA receptors and enhancing the ability of GABA to increase chloride conductance. GABAA receptors are pentamers constituted from structurally distinct proteins, with each protein family consisting of different subunits (for review, see Rudolph et al. 2000). The majority of GABAA receptors consist of α, β, and γ subunit families. Conventional BZs (e.g., diazepam) bind predominantly to a site on the GABAA receptor that occurs at the interface of the γ2 subunit with any of the α1, α2, α3, or α5 subunits but not on α4- and α6-subunit containing receptors. GABAA receptors containing the α1 subunit (α1GABAA receptors) have been implicated in mediating the sedative and motor effects of BZs, whereas GABAA receptors containing α2 and α3 subunits (α2GABAA and α3GABAA receptors) have been implicated in the anxiolytic effects of BZs (Rudolph et al. 2000; McKernan et al. 2000; Rowlett et al. 2005; but see Lippa et al. 2005). Receptors containing α5 subunits (α5GABAA receptors) comprise a relatively minor population that may play a role in memory processes, but not anxiolysis (Collinson et al. 2002; Crestani et al. 2002).
A common operant conditioning approach to evaluating anxiolytic-like effects is the conflict procedure in which positively reinforced behavior is suppressed by response-contingent administration of a noxious stimulus (e.g., mild electric shock; for review, see Millan 2003). Compounds with anxiolytic effects in people characteristically increase the rates of responding that are suppressed by response-contingent delivery of shock (e.g., Geller and Seifter 1960; Cook and Davidson 1973; Spealman 1979; Kleven and Koek 1999a), and a particular strength of conflict procedures is their predictive validity with respect to therapeutic effects in people. In this regard, positive correlations between the potency of BZs to engender anti-conflict effects and to be clinically effective in humans have been demonstrated in rats and pigeons (Cook and Davidson 1973; Kleven and Koek 1999a).
Another approach to enhancing generalization of preclinical findings to clinical observations is to use a species with close genetic similarity to humans. We report here findings from a conflict procedure developed in rhesus monkeys. The use of rhesus monkeys in conflict procedures is relatively rare (e.g., Glowa et al. 1986) although rhesus monkeys are often used in research on anxiety that employ other approaches, such as observed species-typical behavior in response to presentation of stressors (e.g., Ninan et al. 1982; Kalin and Shelton 1989; for review, see Kalin 2004). In addition, rhesus monkeys are often used in studies on abuse potential using intravenous self-administration techniques (cf. Rowlett et al. 2005). Thus, the overall goal of the present study was to develop a conflict procedure in rhesus monkeys designed to evaluate the anxiolytic potential of drugs administered intravenously which in turn will facilitate comparisons with results from future self-administration studies.
The rhesus monkeys were trained under a multiple schedule of reinforcement in which food was available under a fixed-ratio (FR) schedule in two components with an interposed FR schedule of mild shock delivery in the second component. The ability of acute injections of a series of BZs to engender increases in the rates of suppressed responding (i.e., anti-conflict effect) was compared to the administration of the conventional BZ anxiolytic, diazepam, and regression analysis was used to assess the degree in which the potencies to induce anti-conflict effects in monkeys predicted therapeutic effects in humans (cf. Cook and Davidson 1973; Kleven and Koek 1999a). Experiments with the BZ antagonist flumazenil and receptor subtype-selective compounds were conducted to explore the role of GABAA receptors in the anti-conflict effects of BZs. The extent to which the anti-conflict effects in this procedure reflect GABAergic-mediated anxiolysis exclusively was evaluated by testing acute administration of the serotonergic anxiolytic, buspirone. The degree in which a general antinociceptive effect contributed to increases in the rates of suppressed responding was evaluated by testing the opioid analgesic, morphine.
Materials and methods
Subjects and surgical procedure
The subjects were four adult rhesus monkeys (Macaca mulatta), two male and two female, with no experimental histories prior to training in the conflict procedure. Monkeys were maintained at 85–95% of their free-feeding weights, individually housed with water available continually, and maintained on a 12 hr lights-on/12 hr lights-off cycle (lights on at 0600 hr). Monkeys received Teklad monkey diet and supplemental feeding (fruits, vegetables, and commercially available primate treats) daily and were given toys and video stimulation when not in an experimental session. The weights of the monkeys ranged from 6.8 to 8.9 kg at the beginning of the experiments, and remained relatively constant throughout the experiments. The animals in this study were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the “Guide for Care and Use of Laboratory Animals” (National Research Council, Department of Health, Education and Welfare Publication No. (NIH) 85-23, revised 1996).
Each monkey was prepared with a chronic indwelling venous catheter according to the procedures described by Carey and Spealman (1998). A polyvinyl chloride catheter (inner diameter: 0.65 mm; outer diameter: 1.35 mm) was implanted in a jugular (internal or external), femoral, or brachial vein under isoflurane anesthesia and aseptic conditions. The proximal end of the catheter terminated above the right atrium, and the distal end was passed subcutaneously to exit in the midscapular region. Monkeys were treated postoperatively with antibiotics and analgesics, and experimental sessions began 5–7 days after surgery.
Apparatus and procedure
Monkeys were seated in primate chairs (Crist Instrument Co., Hagerstown, MD) that were placed inside ventilated and sound-attenuating chambers. A single response lever (model ENV-610M; Med Associates, Georgia, VT) was mounted on the wall of the chamber in front of the monkey. Each press of a lever produced an audible click and was recorded as a response. Food pellets (Formula 0094, 1 g, Bioserve, Frenchtown, NJ) could be delivered to a tray located next to the lever. Mild electric shock (described in detail below) could be delivered to the bottom of the feet via brass electrodes that were fitted to shoes. Red and green lights mounted above the levers could be illuminated to serve as visual stimuli.
Monkeys were trained under a multiple schedule of food reinforcement adapted from Spealman (1979). A daily session consisted of four cycles, each preceded by a 10-min time-out period in which all lights in the chamber were off and responding had no programmed consequences. Each cycle consisted of two components:
-
Component 1 was signaled by red stimulus lights and consisted of an 18-response schedule of food pellet delivery.
-
Component 2, signaled by green stimulus lights, followed immediately and consisted of the FR 18 schedule of food delivery combined with a 20-response, FR schedule of foot shock delivery (1.5–3.0 mA, adjusted for each monkey based on individual performance, 0.25 s duration).
Delivery of a food pellet (FR 18 schedule) was followed by a 10-s time-out in which responding had no programmed consequences. Both components were 5 min in duration, or ended after the monkey obtained five food pellets or received three foot shocks, whichever occurred first.
Sessions were conducted 5 days per week at approximately the same time each day. On training sessions, monkeys received i.v. injections of saline in the fifth minute of each 10-min time-out. The performance of the individual monkeys was considered stable if the average rates of responding (responses/s) for component 1 and component 2 did not vary by ±20% for five consecutive sessions, with no upward or downward trends. Test sessions were initiated once performance was stable, and continued as long as the stability criteria were met on interceding training sessions.
On test sessions (conducted twice a week), i.v. injections of the vehicle or drug were administered in the fifth minute of each time-out. In successive cycles, increasing doses of the test compound were administered using a cumulative dosing procedure, in which the dose of the compound was increased in 1/4 or 1/2 log(10) units. Doses of each test compound were determined at least twice, and dose-response functions consisting of more than three doses were determined by evaluating overlapping cumulative doses in different test sessions.
The experiments were conducted for approximately 4 years, and in order to evaluate possible changes in drug sensitivity over time, tests with the BZ alprazolam were included periodically. Dose-response functions for the individual drugs were determined in different orders for the four monkeys, although testing was usually completed for a compound prior to moving on to the next compound. In drug interaction studies, flumazenil or its vehicle was administered in the fifth minute of the first 10-min time-out period, followed by the determination of three doses of alprazolam. During the course of the study, if a monkey’s performance did not meet the stability criteria or a catheter became dysfunctional and was repaired, training sessions were conducted until the stability criteria were met again and a determination of alprazolam was conducted prior to resuming the tests.
Data analysis
Data were expressed as the mean responses/s (±SEM) for each dose of test compound. Separate Bonferroni t tests were used to compare the average rates of responding for each dose of compound to the average rates of responding that occurred after administration of vehicle or another dose of compound. This was done to evaluate the degree in which individual doses of drug increased the rates of food-maintained responding suppressed by shock presentation (“suppressed responding”) or decreased the rates of food-maintained responding alone (“non-suppressed responding”). For all tests, the family-wise error rate was constrained to p≤0.05.
The potency of compounds to alter suppressed and non-suppressed responding was estimated by calculating the dose that engendered 50% of the maximum effect (ED50). The ED50 was obtained by converting the maximum increase in the rates of suppressed responding or the maximum suppression of the rates of non-suppressed responding to 100% for individual monkeys, and using these values to calculate the dose corresponding to 50% by log-linear regression analysis. ED50 values were obtained for individual monkeys and averaged with 95% confidence intervals (CIs) calculated to compare potencies between suppressed and non-suppressed responding, and after flumazenil treatment.
In order to compare potencies to alter the rates of suppressed and non-suppressed responding to potencies in humans associated with therapeutic effects, a modification of the procedure of Kleven and Koek (1999a) was used. Potencies for engendering therapeutic effects were obtained from articles by Tallman et al. (1980), Ashton (1994), and Ito et al. (1997). Each article contained a wide range of BZs, with diazepam common to the three reports and therefore served as a comparative standard. The potencies of the BZs reported in an article were divided by the potency of diazepam from that article to obtain a “relative clinical potency”. If a compound was common to two or three of the articles, the average relative clinical potency was used in the analysis. A primary reason for calculating relative clinical potencies in this manner was because the three studies used slightly different methods for calculating absolute potencies. In this respect, Tallman et al. (1980) reported potencies as the lowest dose (mg/70 kg, p.o.) causing an effect in the majority of the subjects. Ashton (1994) calculated potencies as the oral dose (mg/70 kg) corresponding to the plasma half-life of the compound after oral administration. And Ito et al. (1997) reported ranges of oral doses used for treatment of psychiatric disease in the Japanese population (the relative clinical potencies in this case were obtained as the median of the range of therapeutic doses). The specific articles that were used to calculate relative clinical potency for each drug are shown in Table 1.
The relative clinical potencies were compared with the relative potencies in the conflict procedure for both suppressed and non-suppressed responding using linear regression analysis. The relative potencies from the conflict studies were based on the averages calculated for the four monkeys. All relative potencies were converted to log10 values, and linear regression analyses were performed. The fit of the linear regression model was initially evaluated by analysis of variance, corrected for the mean of the observations. Regression coefficients were compared to zero (i.e., no reliable relationship between independent and dependent variables) by t tests, and the correlation coefficient (R 2) was adjusted according to the sample size (i.e., n=9 or 10 compounds).
Drugs
All BZs except chlordiazepoxide were available as the base form. All base forms of BZs (Research Biochemicals Inc., Natick, MA or Sigma-Aldrich, St. Louis, MO) and flumazenil (Hoffman LaRoche, Nutley, NJ) were prepared in a vehicle of 50–80% propylene glycol, 10–40% saline, and 10% ethanol. This vehicle also was used for zolpidem (provided by Dr. Gerard R. Dawson; Merck, Sharp and Dohme, Ltd., Harlow, UK), zaleplon (Wyeth Pharmaceuticals, Princeton, NJ), and CL218,872 (Sigma-Aldrich). Chlordiazepoxide HCl, buspirone HCl, and morphine SO4 (Research Biochemicals, Inc.) were dissolved in 0.9% saline. The drugs were injected in a volume of 0.1–1.0 ml/kg, depending on the dose and solubility.
Results
Alprazolam, buspirone, and morphine
The four monkeys acquired performance at criteria levels after 67 to 173 sessions, depending on the individual animal. Stable performances under training conditions were established as averages that ranged from 0.00 to 0.20 responses/s. The rates of non-suppressed responding of monkeys ranged from 2.1 to 3.9 responses/s at various times during the experiments, which remained stable for several months at a time.
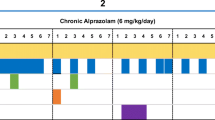
Intravenous administration of the BZ anxiolytic alprazolam engendered a characteristic increase in the rates of suppressed responding at low to intermediate doses and attenuated the rates of non-suppressed responding at higher doses (Fig. 1, left panel). The effects of alprazolam were dose-dependent, with cumulative doses of 0.01, 0.03, and 0.1 mg/kg engendering reliable increases in the mean rates of suppressed responding compared to the response rates observed after the administration of the vehicle (Bonferroni t tests, p<0.05; see Fig. 1). As shown in Fig. 1, the effects of alprazolam on the rates of suppressed and non-suppressed responding did not change over the ∼4-year course of the experiment.
Effects of alprazolam (benzodiazepine), buspirone (5-HT1A agonist), and morphine (mu opioid agonist) in rhesus monkeys responding under a two-component multiple schedule in which responding was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence of response-contingent electric shock (suppressed responding). Alprazolam was determined in the beginning (open square symbol, “Non-Suppressed Responding–First”; closed square, “Suppressed Responding–First”) and at the end (open circle, “Non-Suppressed Responding–Last”; closed diamond, “Suppressed Responding–Last”) of the study. Data are mean±SEM for N=4 rhesus monkeys. Note that *p<0.05 vs. vehicle (“V”) for suppressed responding (the horizontal bracket indicates that each individual point in the 0.01–0.1 mg/kg range of doses was reliably different from vehicle), and †p<0.05 vs. vehicle for non-suppressed responding, Bonferroni t tests
Comparisons of representative anxiolytics with differing mechanisms of action (alprazolam and the 5-HT1A partial agonist buspirone) and an analgesic (the mu opioid agonist morphine) revealed varying degrees of increases in the rates of suppressed responding, although all compounds attenuated the rates of non-suppressed responding (Fig. 1). In this regard, the average maximum increase in the rates of suppressed responding engendered by alprazolam was similar to the mean rates of non-suppressed responding after vehicle administration (Fig. 1, left panel). In contrast, buspirone engendered an increase in the rates of suppressed responding at a single dose (0.1 mg/kg) that was approximately 20% of the rates of non-suppressed responding after vehicle administration (Fig. 1, middle panel). The increase of the buspirone dose to 0.3 mg/kg resulted in an almost complete elimination of the responding. The analgesic opioid agonist morphine did not engender a reliable increase in the rates of suppressed responding until a dose of 3.0 mg/kg completely eliminated responding (Fig. 1, right panel).
Anti-conflict effects of conventional BZs
Figure 2 shows the effects of a series of ten BZs (including the redetermination of alprazolam that occurred during this phase of the study) for which therapeutic potencies in humans were available. All BZs engendered reliable increases in the mean rates of suppressed responding compared to the mean rates after vehicle administration (Bonferroni t tests, p<0.05). In addition, all compounds, except nitrazepam, decreased the mean rates of non-suppressed responding compared to the vehicle over the dose ranges tested (Bonferroni t tests, p<0.05). All BZs induced an increase in the rates of suppressed responding at a dose or doses below those inducing a decrease in the rates of non-suppressed responding (Fig. 2, compare open and filled symbols).
Effects of clinically available benzodiazepines in rhesus monkeys responding under a two-component multiple schedule in which responding was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence of response-contingent electric shock (suppressed responding). Data are mean±SEM for N=4 rhesus monkeys. Horizontal dashed and solid lines represent the upper and lower SEMs for vehicle in the non-suppressed and suppressed components, respectively. Note that *p<0.05 vs. vehicle for suppressed responding (closed symbols), and †p<0.05 vs. vehicle for non-suppressed responding (open symbols), Bonferroni t tests
The ten conventional BZs demonstrated a relatively broad range of potencies, with clonazepam as the most potent and chlordiazepoxide as the least potent for both suppressed and non-suppressed responding (Table 1). Overall, the rank order of the potency for the rates of suppressed and non-suppressed responding was similar but with the primary exception being a lack of effect of nitrazepam on the rates of non-suppressed responding. In order to compare the differences in potencies to engender an increase in the rates of suppressed responding vs. a decrease in the rates of non-suppressed responding, ratios were calculated as the mean ED50 for non-suppressed responding divided by the mean ED50 for suppressed responding. Based on the ratio values (excluding nitrazepam), the ED50 values for inducing an increase in the rates of suppressed responding were 3.3- to 50-fold lower than the corresponding ED50 values for decreases in the rates of non-suppressed responding (reliably different via nonoverlapping 95% CIs, Table 1).
The regression analysis of the relationship between relative potency in monkeys with relative potency in humans revealed reliable relationships for both suppressed and non-suppressed responding (Fig. 3). This relationship was stronger for suppressed responding compared to non-suppressed responding (adjusted R 2 for suppressed responding was 0.83 compared to 0.60 for non-suppressed responding). Regression equations were generated for both types of responding (Table 2). For each equation, the overall linear regression model reliably fit the data [suppressed responding, F(1,9)=45.6, p<0.05; non-suppressed responding, F(1,8)=13.3, p<0.05]. Individual coefficients revealed a positive linear relationship; with slopes, but not y-intercepts, reliably different from zero (Table 2). The standard error of the estimate for suppressed responding was lower than the corresponding value for non-suppressed responding, suggesting relatively greater deviation from the predicted model for the latter type of responding.
Regression analysis of relative potencies for clinically available benzodiazepines in human and rhesus monkey. Relative potencies for human were based on doses that engendered therapeutic effects relative to diazepam and were obtained from articles by Tallman et al. (1980), Ashton (1994) and Ito et al. (1997). Relative potencies for rhesus monkeys were based on ED50 values (i.e., dose inducing 50% of the maximum effect) relative to diazepam, obtained in the conflict procedure
Effects of Flumazenil
Flumazenil alone engendered reliable increases in the rates of suppressed responding relative to vehicle levels at doses of 0.3, 3.0, and 5.6 mg/kg (Bonferroni t tests, p<0.05; Fig. 4, right panel). Flumazenil did not alter the rates of non-suppressed responding over the dose range tested (5.6 mg/kg was the maximum dose that could be tested due to this compound’s solubility). The maximum increases in the rates of suppressed responding engendered by flumazenil were lower than the rates of non-suppressed responding after vehicle administration.
Effects of the benzodiazepine antagonist flumazenil in rhesus monkeys responding under a two-component multiple schedule in which responding was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence of response-contingent electric shock (suppressed responding). Other details as in Fig. 1
The administration of flumazenil prior to the determination of the alprazolam dose-response function resulted in effects on the rates of suppressed and non-suppressed responding that markedly differed as a function of the dose (Fig. 5). As can be seen in the top panel of Fig. 5, pretreatment with 0.3 mg/kg of flumazenil prior to 0.003 and 0.01 mg/kg of alprazolam resulted in a reliable increase in the response rates compared to these doses of alprazolam alone (Fig. 5, top panel; Bonferroni t test, p<0.05). An ED50 value could not be computed for this enhancement of alprazolam’s effects because the effects on the rates of responding at the lower alprazolam doses were above, but not below 50% of the maximum effect in the majority of the monkeys. The 0.3 mg/kg dose of flumazenil had no effect on alprazolam-induced decreases in the rates of non-suppressed responding (Fig. 5, bottom panel).
Effects of alprazolam, alone or after i.v. pretreatments with flumazenil, in rhesus monkeys responding under a two-component multiple schedule in which responding was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence of response-contingent electric shock (suppressed responding). Note that *p<0.05, flumazenil pretreatment vs. alprazolam alone, Bonferroni t tests. Data are means±SEM for N=4 monkeys
When the dose of flumazenil was increased to 3.0 mg/kg, the alprazolam-induced increases in the rates of suppressed responding were reliably enhanced at 0.003 mg/kg of alprazolam, but reliably attenuated at 0.03 mg/kg of alprazolam (Fig. 5, upper panel). Higher doses of alprazolam were not altered by 3.0 mg/kg of flumazenil, and the alprazolam-induced decreases in the rates of non-suppressed responding were unaltered by flumazenil (Fig. 5, bottom panel). The evaluation of potency values indicated that the ED50 for the alprazolam-induced increase in the rates of suppressed responding after 3.0 mg/kg of flumazenil was reliably increased by approximately 6-fold [ED50 for alprazolam: 0.011 mg/kg (95% CI=0.009–0.014); ED50 for alprazolam plus flumazenil: 0.065 mg/kg (95% CI=0.056–0.077).
Compounds with α1GABAA receptor selectivity
As shown in the left panel of Fig. 6, the α1GABAA-preferring full agonist zolpidem engendered a relatively modest increase in the rates of suppressed responding relative to the vehicle levels, with a reliable effect evident at only a single dose (0.1 mg/kg, Bonferroni t test, p<0.05). Zolpidem also reliably decreased the rates of non-suppressed responding at 0.3 and 1.0 mg/kg, with the latter dose nearly eliminating responding (Bonferroni t tests, p<0.05). A very similar pattern of results was observed with the α1GABAA-preferring full agonist zaleplon and the α1GABAA-preferring partial agonist CL218,872 (Fig. 6, middle and right panels, respectively). Both compounds engendered modest increases in the rates of suppressed responding at single doses (zaleplon, 0.1 mg/kg; CL218,872, 1.0 mg/kg), and both compounds markedly decreased the rates of non-suppressed responding compared to the vehicle, with zaleplon eliminating responding completely at 1.0 mg/kg and CL218,872 nearly eliminating responding at 3.0 mg/kg (Bonferroni t tests, p<0.05).
Effects of zolpidem, zaleplon, and CL218,872 (agonists selective for α1 subunit-containing GABAA receptors) in rhesus monkeys responding under a two-component multiple schedule in which responding was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence of response-contingent electric shock (suppressed responding). Other details as in Fig. 1
Discussion
The procedures that evaluate the anxiolytic effects of drugs based on the concept of experimentally induced conflict most frequently use rodents as subjects (e.g., Geller-Seifter and Vogel conflict procedures; for review, see Millan 2003). Similar conflict procedures in nonhuman primate species have been developed, but are less common (e.g., Sepinwall et al. 1978; Spealman 1979; Paronis and Bergman 1999). In particular, the use of rhesus monkeys is relatively rare (e.g., Glowa et al. 1986). In this study, the rhesus monkeys showed characteristic anti-conflict effects when administered with BZs. These findings are consistent with the findings of Glowa et al. (1986) where a different conflict procedure was used (i.e., fixed interval schedules of food and shock presentation vs. the FR schedules in the present study). Thus, BZs induced an increase in the rates of suppressed responding at doses lower than those that decreased the rates of non-suppressed responding. Overall, our findings with rhesus monkeys were notably concordant with the extant literature on the anti-conflict effects of BZ-type compounds (cf. Kleven and Koek 1999a) although some exceptions were evident (e.g., effects of α1GABAA agonists in rhesus vs. squirrel monkeys), as described below.
The 5-HT1A partial agonist buspirone, a compound devoid of BZ-site activity but used clinically as an anxiolytic, also engendered an increase in the rates of suppressed responding, albeit a modest one. Nonetheless, these preliminary findings with buspirone raise the possibility that the rhesus monkey conflict procedure might be sensitive to anxiolytics with serotonergic mechanisms of action. This potential use of the rhesus monkey conflict procedure requires an evaluation of other serotonergic anxiolytics, in particular serotonin reuptake inhibitors that are currently the drugs of choice for treating anxiety disorders (cf. Nutt 2005). In contrast with the findings of BZs and buspirone, the opioid agonist morphine lacked anti-conflict effects, suggesting that the increase in the rates of suppressed responding by BZs was not likely due to an analgesic effect.
In the present study, the anti-conflict effects of a series of conventional BZs were evaluated. Although these compounds are similar with respect to pharmacological action, conventional BZs often have quite different clinical indications (e.g., flurazepam and nitrazepam are used clinically as hypnotics rather than anxiolytics; Ashton 1994; Dündar et al. 2004). Based on anti-conflict effects, however, there were no clear patterns that differentiated anxiolytics from hypnotics. For example, although nitrazepam did not attenuate non-suppressed responding, the hypnotic flurazepam engendered a clear decrease in the rates of non-suppressed responding. The lack of relationship of the clinical indications with effects in the conflict procedure is consistent with the idea that the use of a BZ as an anxiolytic vs. hypnotic reflects factors other than mechanism of action at GABAA receptors, such as pharmacokinetics (Ashton 1994; Kleven and Koek 1999a).
Conflict procedures in rodents and pigeons have documented predictive validity with respect to clinical effectiveness in humans (Cook and Davidson 1973; Kleven and Koek 1999a). In the present study, our analysis of the potencies to induce anti-conflict effects in rhesus monkeys with therapeutic doses in humans revealed a strong positive correlation (R 2=0.83), suggesting that this procedure also has predictive validity. A reliable relationship between human therapeutic dose and the dose to attenuate non-suppressed responding was also found, although the relationship was weaker (R 2=0.60). Because of the relatively strong positive correlation between anti-conflict and therapeutic potency, this approach may prove useful in predicting the potency of a newer anxiolytic drug in humans, relative to diazepam as a comparison. For example, the functionally selective α2,3GABAA ligand, SL651498, engendered an increase in the rates of suppressed responding in rhesus monkeys with an ED50 value of 0.70 mg/kg, i.v. (Licata et al. 2005), i.e., 6.36-fold less potent than diazepam in the present study. Thus, based on the regression coefficients for the rates of suppressed responding shown in Table 2, SL651498 is expected to be 4.1-fold less potent than diazepam as an oral therapeutic in humans. As a cautionary note, because the therapeutic potencies were derived by slightly different methods, estimations of relative therapeutic potencies based on these conflict data should be considered an initial estimate, rather than a precise calculation of a therapeutic dose.
An unexpected finding from the present study was a relatively modest but reliable increase in the rates of suppressed responding engendered by flumazenil. This finding is consistent with an earlier study with rats as subjects (Feldon et al. 1983); however, many studies have shown flumazenil to be devoid of anti-conflict effects in other species (e.g., Barrett et al. 1985; Martin et al. 1993; Paronis and Bergman 1999). In contrast, reports using other models of anxiolysis have demonstrated effects of flumazenil suggestive of weak anxiolytic-like activity. For example, recent studies have shown flumazenil to have anxiolytic-like activity in procedures based on “innate” anxiety (i.e., elevated-plus maze, Belzung et al. 2000; social conflict test, Uhlirova et al. 2004).
In addition to anxiolytic-like effects, flumazenil has been shown to have moderate anti-convulsant and muscle relaxant effects (Nutt et al. 1982; Kawasaki et al. 1984; Marescaux et al. 1984) and has been trained as a discriminative stimulus (e.g., Acri et al. 1995; Gerak and France 1999; Smith and Bickel 1999). In an early study by Barrett et al. (1985), flumazenil enhanced the effects of chlordiazepoxide on responding maintained by shock presentation in squirrel monkeys. Although flumazenil might cause a degree of positive allosteric modulation, other reports have provided evidence that flumazenil may have inverse agonist effects. For example, some studies have shown that flumazenil may induce anxiogenic effects in humans (e.g., Duka et al. 1986; Lavie 1987), and can increase the frequency of panic attacks in patients with panic disorder (Nutt et al. 1990). Seemingly opposing effects of flumazenil have been found in animal studies as well, a striking example being the demonstration of an agonist-like enhancement of feeding behavior and an inverse agonist-like attenuation of social behaviors observed at the same dose of flumazenil in squirrel monkeys (Weerts et al. 1993).
The paradoxical effects of flumazenil may reflect differential action at GABAA receptor subtypes. In this respect, flumazenil has been shown to induce a moderate degree of GABA potentiation of chloride currents in GABAA receptors containing α2 and α3 subunits (Harvey et al. 2002), both of which have been proposed to play key roles in anxiolysis (Rudolph et al. 2000; Atack et al. 2005). However, Harvey et al. 2002 also reported a modest inverse agonist activity for flumazenil at α1 and α5 subunit-containing GABAA receptors. Altogether, these observations raise the possibility that flumazenil may have mixed partial agonist/partial inverse agonist effects that may reflect intrinsic efficacy differences at GABAA receptor subtypes; and that under certain conditions flumazenil may have behavioral effects similar to BZs and BZ-site inverse agonists. Alternatively, flumazenil might exert behavioral effects through its binding to BZ-insensitive sites (i.e., α4 and α6GABAA receptors; Acri et al. 1995), although no evidence of a role for these sites in anxiolysis exists to date.
Partial agonists can act as antagonists on the effects of higher efficacy compounds under appropriate conditions (e.g., when the partial agonist has no effect; cf. Lelas et al. 2001). Thus, a partial agonist can, in theory, both enhance and antagonize the effects of a full agonist, depending on the dose and the level of effect exerted by the partial agonist (for review, see Kenakin 1997). Such a phenomenon was observed in the present study for flumazenil administered prior to the determination of alprazolam dose-response functions, providing further support for the idea that flumazenil has partial agonist activity in the rhesus monkey conflict procedure. In addition, flumazenil was ineffective in blocking the alprazolam-induced decreases in the rates of non-suppressed responding. This observation is consistent with the findings of other procedures (Rowlett and Woolverton 1996; Paronis and Bergman 1999), although the factors responsible for this difference in sensitivity between suppressed and non-suppressed responding are unknown.
Recent research using transgenic mice and subtype-selective compounds has implicated the α2GABAA and/or α3GABAA receptor, but not the α1GABAA or α5GABAA receptor, as important for BZ-induced anxiolysis (McKernan et al. 2000; Atack et al. 2005; for review, see Rudolph et al. 2000). This is consistent with the findings of the present study that showed that full agonists with selectivity for the α1GABAA receptor (zolpidem, zaleplon) show little or no anti-conflict effects (cf. Rowlett et al. 2005). The α1GABAA receptor-preferring partial agonist CL218,872 showed effects that were similar to those of zolpidem and zaleplon, suggesting that intrinsic efficacy at the α1GABAA receptor is not a key mediator of the anti-conflict effects of these ligands. Relatively weak anxiolytic-like effects also have been found in zolpidem and related compounds in both conflict and innate fear-based procedures in both rodents and pigeons (Griebel et al. 1996; Kleven and Koek 1999b). A notable difference between the present study and a previous report by Paronis et al. (2001) was the degree of increase in the rates of suppressed responding by the α1GABAA receptor-preferring agonists zolpidem and zaleplon. In the previous report, the anti-conflict effects of zolpidem and zaleplon were similar to a conventional BZ, midazolam, in squirrel monkeys; whereas the effects of zolpidem and zaleplon in the present study were less robust than conventional BZs. Because both studies used a similar multiple schedule of suppressed and non-suppressed responding maintained by food and shock, it is feasible that this observation reflects species differences between rhesus and squirrel monkeys, although other factors such as procedural variables (e.g., different response requirements, different routes of administration) cannot be discounted.
We have recently explored the role of GABAA receptor subtypes in the anxiolytic-like effects of BZs by evaluating the anti-conflict effects of the “functionally” selective agonists, L-838,417 and SL651498 (Licata et al. 2005; Rowlett et al. 2005). These agonists are considered functionally selective because they have either zero or relatively low intrinsic efficacy at α1GABAA receptors, but partial to high efficacy at α2,3GABAA receptors. Both compounds engendered anti-conflict effects that were similar to the effects observed with conventional BZs, and neither compound markedly attenuated non-suppressed responding. Collectively, the findings of the present study with compounds having selective affinity for α1GABAA receptors along with the previous results with functionally selective ligands, clearly support the idea that α1GABAA receptors do not play a primary role in the anxiolytic effects of BZ-type compounds (but see, Silveri et al. 2005).
In conclusion, the research reported here shows that a conflict procedure can be reliably established in rhesus monkeys with anti-conflict effects that are stable over time and robust when evaluating BZ-type drugs administered via the i.v. route. Our results with flumazenil raise the possibility that the anti-conflict effects of BZs might occur even with a very low level of intrinsic efficacy. On the other hand, selectivity for α1GABAA receptors results in compounds having relatively weak anxiolytic-like behavior. Overall, these results demonstrate the feasibility of developing a conflict procedure in rhesus monkeys, which should provide a useful approach for evaluating mechanisms of action underlying the anxiolytic-like effects of BZ-type compounds in a primate species.
References
Acri JB, Wong G, Witkin JM (1995) Stereospecific transduction of behavioral effects via diazepam-insensitive GABAA receptors. Eur J Pharmacol 278:213–223
Ashton H (1994) Guidelines for the rational use of benzodiazepines, when and what to use. Drugs 48:25–40
Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, Cook SM, Collins I, Wafford K, McKernan RM, Dawson GR (2005) Anxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptors. Brit J Pharmacol 144:357–366
Barrett JE, Brady LS, Witkin JM (1985) Behavioral studies with anxiolytic drugs. I. Interactions of the benzodiazepine antagonist Ro 15-1788 with chlordiazepoxide, pentobarbital and ethanol. J Pharmacol Exp Ther 233:554–559
Belzung C, Le Guisquet, Crestani F (2000) Flumazenil induces benzodiazepine partial agonist-like effects in BALB/c but not C57BL/6 mice. Psychopharmacology 148:24–32
Carey G, Spealman RD (1998) Models of neurological disease (substance abuse): self-administration in monkeys, In: Enna S, Williams M, Ferkany J, Kenakin T, Portsolt R, Sullivan J (eds) Current protocols in pharmacology, Wiley, New York, pp10.5.1–10.5.15
Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Out FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW (2002) Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci 22:5572–5580
Cook L, Davidson AB (1973) Effects of behaviorally active drugs in a conflict-punishment procedure in rats. In: Garattini S, Mussini E, Randall LO (eds) The benzodiazepines. Raven, New York, pp327–345
Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U (2002) Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA 99:8980–8985
Dündar Y, Dodd S, Strobl J, Boland A, Dickson R, Walley T (2004) Comparative efficacy of newer hypnotic drugs for the short-term management of insomnia: a systematic review and meta-analysis. Hum Psychopharmacol Clin Exp 19:305–322
Duka T, Ackenheil M, Noderer J, Doenicke A, Dorow R (1986) Changes in noradrenaline plasma levels and behavioural responses induced by benzodiazepine agonists with the benzodiazepine antagonist Ro 15-1788. Psychopharmacology 90:351–357
Feldon J, Lerner T, Levin D, Myslobodsky M (1983) A behavioral examination of convulsant benzodiazepine and GABA antagonist, Ro 5-3663, and benzodiazepine-receptor antagonist Ro 15-1788. Pharmacol Biochem Behav 19:39–41
Geller I, Seifter J (1960) The effects of meprobamate, barbiturate, d-amphetamine and promazine on experimentally-induced conflict in the rat. Psychopharmacologia 1:482–492
Gerak LR, France CP (1999) Discriminative stimulus effects of flumazenil in untreated and in diazepam-treated rhesus monkeys. Psychopharmacology 146:252–261
Glowa JR, Skolnick P, Paul SM (1986) Effects of beta-carboline-3-carboxylic acid ethyl ester on suppressed and non-suppressed responding in the rhesus monkey. Eur J Pharmacol 129:39–47
Griebel G, Sanger DJ, Perrault G (1996) Further evidence for differences between non-selective and BZ-1 (ω1) selective, benzodiazepine receptor ligands in murine models of “state” and “trait” anxiety. Neuropharmacology 35:1081–1091
Griffiths RR, Weerts EM (1997) Benzodiazepine self-administration in humans and laboratory animals—implications of problems of long-term use and abuse. Psychopharmacology 134:1–37
Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL (2002) The GABAA receptor α1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci 22:3765–3775
Ito K, Asakura A, Yamada Y, Nakamura K, Sawada U, Iga T (1997) Prediction of the therapeutic dose for benzodiazepine anxiolytics based on receptor occupancy theory. Biopharm Drug Dispos 18:293–303
Kalin NH (2004) Studying non-human primates: a gateway to understanding anxiety disorders. Psychopharmacol Bull 38:8–13
Kalin NH, Shelton SE (1989) Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science 243:1718–1721
Kawasaki K, Kodama M, Matsushita A (1984) An imidazodiazepine derivative, Ro 15-1788, behaves as a weak partial agonist in the crossed extensor reflex. Eur J Pharmacol 102:147–150
Kenakin T (1997) Pharmacological analysis of drug-receptor interaction, 3rd edn. Raven, New York
Kleven MS, Koek W (1999a) Effects of benzodiazepine agonists on punished responding in pigeons and their relationship with clinical doses in humans. Psychopharmacology 141:206–212
Kleven MS, Koek W (1999b) Effects of different classes of partial benzodiazepine agonists on punished and unpunished responding in pigeons. Psychopharmacology 144:405–410
Lavie P (1987) Ro 15-1788 decreases hypnotic effects of sleep deprivation. Life Sci 41:227–233
Lelas S, Rowlett JK, Spealman RD (2001) Isobolographic analysis of chlordiazepoxide and triazolam combinations in squirrel monkeys discriminating triazolam. Psychopharmacology 158:181–189
Licata SC, Platt DM, Cook JM, Sarma PVVS, Griebel G, Rowlett JK (2005) Contribution of GABAA receptor subtypes to the anxiolytic-like, motor, and discriminative stimulus effects of benzodiazepines: studies with the functionally selective ligand SL 651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one]. J Pharmacol Exp Ther 313:1118–1125
Lippa A, Czobor P, Stark J, Beer B, Kostakis E, Gravielle M, Bandyopadhyay S, Russek SJ, Gibbs TT, Farb DH, Skolnick P (2005) Selective anxiolysis produced by ocinaplon, a GABAA receptor modulator. Proc Natl Acad Sci USA 102:7380–7385
Marescaux C, Micheletti G, Vergnes M, Depaulis A, Rumbach L, Warter J-M (1984) Biphasic effects of Ro 15-1788 on spontaneous petit mal-like seizures in rats. Eur J Pharmacol 102:355–359
Martin JR, Schoch P, Jenck F, Moreau JL, Haefely WE (1993) Pharmacological characterization of benzodiazepine ligands with intrinsic efficacies ranging from high to zero. Psychopharmacology 111:415–422
McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA α1 subtype. Nat Neurosci 3:587–592
Millan MJ (2003) The neurobiology and control of anxious states. Prog Neurobiol 70:83–244
Ninan PT, Insel TM, Cohen RM, Cook JM, Skolnick P, Paul SM (1982) Benzodiazepine receptor-mediated experimental “anxiety” in primates. Science 218:1332–1334
Nutt DJ (2005) Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr 10:49–56
Nutt DJ, Cowen PJ, Little HJ (1982) Unusual interactions of benzodiazepine receptor antagonists. Nature 295:436–438
Nutt DJ, Glue P, Lawson C, Wilson S (1990) Flumazenil provocation of panic attacks. Evidence for altered benzodiazepine receptor sensitivity in panic disorder. Arch Gen Psychiatry 47:917–925
Paronis CA, Bergman J (1999) Apparent pA2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther 290:1222–1229
Paronis CA, Cox ED, Cook JM, Bergman J (2001) Different types of GABAA receptors may mediate the anticonflict and response rate-decreasing effects of zaleplon, zolpidem, and midazolam in squirrel monkeys. Psychopharmacology 156:461–468
Pritchett DB, Lüddens H, Seeburg PH (1989) Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science 245:1389–1392
Rowlett JK, Woolverton WL (1996) Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA2 and pKB analysis from behavioral studies. Psychopharmacology 128:1–16
Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR (2005) Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA 102:915–920
Rudolph U, Crestani F, Möhler H (2000) GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci 22:188–194
Sepinwall J, Grodsky FS, Cook L (1978) Conflict behavior in the squirrel monkey: effects of chlordiazepoxide, diazepam and n-desmethyldiazepam. J Pharmacol Exp Ther 204:88–102
Silveri S, Bruni F, Costagli C, Costanzo A, Guerrini G, Ciciani G, Gratteri P, Besnard F, Costa B, Montali M, Martini C, Fohlin J, De Siena G, Aiello PM (2005) A novel selective GABAAα1 receptor agonist displaying sedative and anxiolytic-like properties in rodents. J Med Chem 48:6756–6760
Smith BJ, Bickel WK (1999) Flumazenil discrimination by humans under a two-response and a novel-response procedure. J Pharmacol Exp Ther 291:1257–1268
Spealman RD (1979) Comparison of drug effects on responding punished by pressurized air or electric shock delivery in squirrel monkeys: pentobarbital, chlordiazepoxide, d-amphetamine and cocaine. J Pharmacol Exp Ther 209:309–315
Tallman JF, Paul SM, Skolnick P, Gallager DW (1980) Receptors for the age of anxiety: pharmacology of the benzodiazepines. Science 207:274–281
Uhlirova L, Sustkova-Fiserova M, Krsiak M (2004) Behavioral effects of flumazenil in the social conflict test in mice. Psychopharmacology 171:259–269
Weerts EM, Tornatzky W, Miczek KA (1993) Prevention of the pro-aggressive effects of alcohol in rats and squirrel monkeys by benzodiazepine receptor antagonists. Psychopharmacology 111:144–152
Acknowledgements
The authors thank Dr. Annemarie Duggan, Dr. Stefan Tiefenbacher, and Bethann Johnson for technical assistance. We thank Dr. Donna Platt and Ms. Angela Duke for comments on an earlier draft of this manuscript, and we are especially grateful to Dr. Roger Spealman for assistance in the development of this conflict procedure.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by U.S.P.H.S. grants DA11792 and RR00168
Rights and permissions
About this article
Cite this article
Rowlett, J.K., Lelas, S., Tornatzky, W. et al. Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology 184, 201–211 (2006). https://doi.org/10.1007/s00213-005-0228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0228-8