Abstract
Rationale
Previous studies of mice (Mus domesticus) selectively bred for high voluntary wheel running have suggested that the hyperactivity is associated with dysfunction in the dopaminergic neuromodulatory system and that high-running mice may represent a useful genetic model for attention deficit hyperactivity disorder (ADHD).
Objectives
We tested the hypothesis that mice from the four replicate hyperactive lines would respond differently to methylphenidate (Ritalin), apomorphine (non-selective dopamine agonist), SCH 23390 (selective D1-like dopamine antagonist), and raclopride (selective D2-like dopamine antagonist) than individuals from the four replicate, randomly bred, control lines.
Methods
After animals were habituated (3 weeks) to their cages with attached wheels, drugs were administered via intraperitoneal injections, at night, during peak wheel-running activity. Revolutions on wheels 10–70 min post-injection were used to quantify drug responses.
Results
Ritalin (15 mg/kg and 30 mg/kg) increased wheel running in control lines but decreased running in selected lines. A low-dose (0.125 mg/kg) of apomorphine reduced wheel running by a similar amount in control and selected lines; however, higher doses of apomorphine (0.25 mg/kg and 0.5 mg/kg) produced greater reductions in wheel running in the control lines. SCH 23390 (0.025, 0.05, and 0.1 mg/kg) caused greater reductions in wheel running in control than in selected lines. Raclopride (0.5, 1, and 2 mg/kg) reduced wheel running by a similar amount in control and selected lines.
Conclusions
These results support the interpretation that genetically determined hyperactive wheel-running behavior is associated with altered dopaminergic function in this mouse model. More specifically, results suggest that D1-like (D1 or D5), but not D2-like (D2, D3, or D4), dopamine receptors have reduced function in the high-running mice. The fact that Ritalin decreased wheel running in selected lines further supports their use as an animal model of ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine plays a prominent role in the voluntary control of locomotor behavior (Messier et al. 1992; Baik et al. 1995), and is implicated in drug addiction (Robinson and Berridge 1993) as well as such mental disorders as attention deficit hyperactivity disorder (ADHD) (Solanto et al. 2001). Because Ritalin increases the function of dopamine and norepinephrine (Solanto 1998) and ameliorates symptoms of ADHD, many have proposed that ADHD is caused by reduced function of dopamine (Russell et al. 1995; Sagvolden and Sergeant 1998; Papa et al. 2000) or norepinephrine (Arnsten 2001). Moreover, the dopamine hypothesis of ADHD is supported by neuroimaging (Castellanos 2001; Levy and Swanson 2001) and genetic (Cook et al. 1995; Swanson et al. 2000) studies in humans. A variety of animal models [e.g., spontaneously hypertensive rats (Sagvolden et al. 1992), dopamine transporter (DAT) knockout mice (Gainetdinov et al. 1999), dopamine-depleted rats (Shaywitz et al. 1978)] have been used in attempts to elucidate the molecular basis of altered dopamine function in ADHD.
A good animal model of ADHD (the combined or predominantly hyperactive subtype, DSM-IV, Solanto et al. 2001) should represent the key features of the disorder (Sagvolden 2000; Solanto 2000; Ferguson 2001), such as 24-h hyperactivity in the habituated environment (Porrino et al. 1983) and motor impulsiveness (Sagvolden and Sergeant 1998). Another consideration is that ADHD may not be a discrete behavioral/physiological state, but rather represents one end of a continuum of behavior. Moreover, ADHD is likely affected by multiple environmental factors and multiple genetic loci that may interact with each other in complex ways (Todd 2000).
We have developed a unique animal model to study the neurobiology of genetically determined hyperactive wheel-running behavior and have argued that it has considerable potential as a model for ADHD (Rhodes et al. 2001). Beginning from an outbred base population, selective breeding was used to increase levels of voluntary wheel running in four replicate, genetically variable lines of house mice (Swallow et al. 1998). Four additional lines of mice were randomly bred to serve as controls. The response to selective breeding for a complex phenotype (such as voluntary activity) typically involves changes in allele frequencies at multiple loci, and these alleles may interact in complex ways to produce the phenotype (Falconer and Mackay 1996; Marley et al. 1998; Britton and Koch 2001). The (presumed) polygenic nature of high activity in our selected lines may make them a better model for ADHD than animal models that involve manipulation of single genes. Moreover, the existence of the replicate selected lines allows the possibility of multiple genetic bases for high voluntary wheel-running behavior (Garland 2003; Garland et al. 2002). Finally, because the lines are replicated, differences in drug sensitivity between the selected and control lines can truly be attributed to genetic differences that are related to the selected trait, i.e., hyperactivity (Henderson 1989, 1997). In other models that compare only one selected and one control line (e.g., the spontaneously hypertensive rat–Wistar-Kyoto comparison, Sagvolden et al. 1992), phenotypic differences may result from genes that are entirely unrelated to hyperactivity (or hypertension). This is because, when populations are initially established and then separately propagated for several generations, genetic differences arise through random processes such as genetic drift (Henderson 1989, 1997), in addition to whatever selection is imposed by the investigator.
Similarities between some of the biological properties of our selected lines and human ADHD are striking. First, each of the selected lines run further on wheels than controls by running shorter, faster bouts (Girard et al. 2001), consistent with motor impulsiveness (Sagvolden and Sergeant 1998). Second, hyperactivity is not specific to running wheels because the selected lines are also hyperactive after acclimating for 24 h to cages without wheels (Rhodes et al. 2001). Third, the dopamine re-uptake blockers cocaine and GBR 12909 reduce wheel running in selected lines (Rhodes et al. 2001). In the present study, we investigated the effects of Ritalin, which shares a similar mode of action as cocaine and GBR 12909, in order to explore further the potential of the selected lines as a model of ADHD.
The aims of this study were, first, to determine whether the high wheel running in the selected mice was reduced by behaviorally appropriate doses of Ritalin, the most common drug used to treat ADHD (Solanto et al. 2001), and, second, to determine whether the D1 or D2 classes of dopamine receptors are altered in hyperactive mice by determining whether the selected-line mice respond differently to drugs that interact specifically with these receptor families.
Methods
Animals
We studied mice from generations 27 and 29 of an artificial selection experiment for high voluntary wheel-running behavior (Swallow et al. 1998). The original progenitors were outbred, genetically variable laboratory house mice (Mus domesticus) of the Hsd:ICR strain. After two generations of random mating, mice were randomly paired and assigned to eight closed lines (ten pairs in each). In each subsequent generation, when the offspring of these pairs were 6–8 weeks old, they were housed individually with access to a running wheel for 6 days. Daily wheel-running activity was monitored using an automated system.
In the four "selected" lines, the highest-running male and female from each family were selected as breeders to propagate the lines to the next generation. Wheel running was quantified as the total number of revolutions run on day 5 and day 6 of the 6-day test. In the four "control" lines, a male and a female were randomly chosen from each family. Within all lines, the chosen breeders were randomly paired except that sibling matings were not allowed.
To supply animals for the experiments presented here, generation-26 and -28 parents were allowed to produce a second litter (i.e., generations 27 and 29). Different groups of mice (n=48, 6 animals per line, 24 control and 24 selected mice) were assigned to each drug. The Ritalin and apomorphine trials were composed of generation-27 mice, whereas generation 29 was the source for the SCH 23390 and raclopride trials. Given that the selected lines appear to have been at a selection limit since approximately generation 16 (Garland 2003), cross-generation comparisons should be valid. To maintain comparability with our previous pharmacological (Rhodes et al. 2001) and locomotor (Girard et al. 2001) studies, only females were studied.
The Principles of Laboratory Animal Care (NIH publication no. 85–23, revised 1985) were followed, and all experiments were approved by the University of Wisconsin Animal Care Committee. Throughout the selection experiment and during this study, water and food [Harlan Teklad Laboratory Rodent Diet (8604); after generation 23, breeding females were given Harlan Teklad Mouse Breeder Diet (7004)] were available ad libitum. Rooms were controlled for temperature (~22°C) and photoperiod 12-h/12-h light/dark (lights on at 0400 hours, Central Standard Time).
Drug protocols and wheel running
Drug trials were conducted according to Rhodes et al. (2001). Drug responses were measured as changes in voluntary wheel running at night during peak activity. Animals were acclimated to cages with running wheels attached (Swallow et al. 1998; Rhodes et al. 2001) for approximately 3 weeks prior to drug injections (age at time of injections was approximately 3 months).
Seven drug trials were conducted. A trial consisted of a series of three intraperitoneal injections, including a vehicle control (0.9% saline) and two different drug doses. The three injections were administered every other day for a total of three injection days and were given in a volume adjusted to the body mass of the animal (5 ml/kg). 5 ml/kg was used instead of the more typical 10 ml/kg because anecdotal evidence suggested that the reduced volume is more comfortable for the mice. Each individual received all three types of injections (vehicle, dose 1, dose 2) over the course of the three injection days (6 days total, including days off). Each mouse per line received the three injections in a different order (one of the six permutations of the three doses), such that possible effects of injection order did not need to be considered in statistical analyses (cross-over design, Milliken and Johnson 1992).
Mice were injected in the same sequence each day so that a mouse always received its injection at approximately the same time of day. It usually took less than 2 min to capture, inject, and return a mouse to its home cage. Injections began 2 h after lights off, during peak activity (unpublished observations; see also Fig. 1 in Girard et al. 2001).
Drugs were dissolved in sterile saline, then filtered through a 0.22-µm syringe-driven filter unit (Millipore, Bedford, MA), and were prepared immediately before injections each day. Ritalin was administered at 15 mg/kg and 30 mg/kg. Apomorphine was administered in two separate trials (separated by a 1-week interval), a low-dose (0.125 mg/kg and 0.25 mg/kg) and a high-dose trial (0.5 mg/kg, and 1 mg/kg). SCH 23390 was also administered in two separate trials (0.025 mg/kg and 0.05 mg/kg then 0.05 mg/kg and 0.1 mg/kg) to extend the dose range. Similarly, raclopride was administered in two trials (0.5 mg/kg and 1.0 mg/kg then 1.0 mg/kg and 2.0 mg/kg). Doses were chosen after consulting the literature [for Ritalin (Gainetdinov et al. 1999), for apomorphine (Cabib and Puglisi-Allegra 1985), for SCH 23390 (Ichihara et al. 1993; Womer et al. 1994), for raclopride (Simon et al. 2000)]. However, in all of these studies, drugs were administered during the day and wheel running was not used to measure drug responses. Therefore, we also conducted preliminary studies to determine behaviorally equivalent doses for all four drugs.
As in our previous study (Rhodes et al. 2001), wheel rotations were monitored via computer in 1-min intervals throughout each trial. Running distance was computed as revolutions multiplied by 1.12 m, the circumference of the wheels (Swallow et al. 1998). An average running speed was calculated as total revolutions divided by number of 1-min intervals with any revolutions (Koteja and Garland 2001).
To compare responses between selected and control lines, proportional, rather than absolute changes in wheel rotations following drug injections were statistically analyzed because baseline running differed substantially between selected and control lines (by a factor of approximately three). Individual proportional responses for each drug dose were calculated by dividing total revolutions in the 10–70 min period after drug administration by total revolutions in the 10–70 min period after the saline injection. The first 10 min were not included because wheel running was significantly suppressed by the vehicle injection during this period (see Figures and Rhodes et al. 2001). Data beyond 70 min were excluded because inspection of the data suggested that the greatest effects of all the drugs occurred prior to 70 min except for Ritalin, where the greatest effect extended to 100 min in control mice. However, for Ritalin, we decided the 10–70 min was a good compromise because, in selected mice, the effect of Ritalin vanished after 50 min, and we did not wish to dilute the effect in selected mice by including 30 extra minutes of noise.
Statistics
SAS (SAS Institute Inc.) PROC MIXED (which employs Restricted Maximum Likelihood) was used to analyze the data. Line was always entered as a random effect, nested within the fixed effect of "linetype" (selected or control). The lines were separately propagated for 27 generations; therefore, individuals in a given generation do not represent independent data points and must be nested within the populations from which they arose (Henderson 1989, 1997). Degrees of freedom for testing the linetype effect were always 1 and 6. Stage of the estrus cycle was not measured and hence was not entered as a cofactor in any analyses.
Baseline wheel running was compared between selected and control lines by considering mean total revolutions during the 2 days preceding injections in a pooled analysis of all the animals. Variance was estimated separately for control and selected lines. Data were analyzed using a two-way analysis of covariance (ANCOVA), with linetype and drug trial as factors and wheel freeness (total number of revolutions produced by the wheel after being accelerated to constant velocity, an inverse measurement of how resistant the wheel is to continued rotation) as a covariate.
Proportional drug responses (total revolutions 10–70 min after drug injection divided by total revolutions 10–70 min after saline injection) were analyzed separately for each drug dose to test for differences between selected and control lines. In the cases where a dose was replicated in two separate drug trials (SCH 23390 0.05 mg/kg, raclopride 1.0 mg/kg), individual responses were averaged over the replicates prior to analysis. To improve normality of residuals, the proportional responses were transformed by raising to an exponent <1 (residuals were highly positively skewed otherwise).
Absolute responses (total revolutions 10–70 min post-injection) were analyzed separately for selected and control lines to determine the effect of the drug doses in each linetype. One-way repeated-measures ANCOVAs were used, with dose as the repeated measures factor (because the doses were applied to the same individual) and wheel freeness as the covariate. The compound symmetric covariance structure was used, which assumes that the correlation between responses within individuals is the same for all pair-wise comparisons between drug doses (e.g. correlation between responses for dose 1 and dose 2 is equal to correlation between responses for dose 1 and dose 3, within individuals).
Results
Baseline wheel running
As expected, mice from selected lines ran farther than controls throughout the study. Considering the 2 days preceding drug injections (in a pooled analysis of all the animals), selected mice ran on average 18.0±0.95 km/day (least square adjusted mean ±standard error) versus 6.4±0.42 km/day in control mice (F 1,6=123.44, P<0.0001). For unknown reasons, animals in the apomorphine trial (both selected and control) ran approximately 2 km less than in other drug trials (F 3,172=5.32, P=0.002). Otherwise, average levels of wheel running 2 days preceding injections were similar among Ritalin, SCH 23390, and raclopride trials. Wheel freeness was positively correlated with running distance (F 1,172=9.24, P=0.003), but this covariate explained only a small fraction (approximately 2%) of the total variation in wheel running.
Ritalin
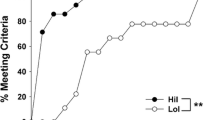
Selected and control mice responded differently to Ritalin (Table 1, Fig. 1). Ritalin increased running distance in control lines (one-way repeated-measures ANCOVA, F 2,46=8.73, P<0.001; Table 2, Fig. 1) but decreased it in selected lines (F 2,44=6.76, P=0.003; Table 2, Fig. 1). The decreased distance in selected mice resulted from decreased average running speed, not duration of running; whereas, in control lines, both speed and duration contributed to the increased running distance (Table 2). In selected lines, Ritalin had the greatest effect 10–40 min post-injection (Fig. 1), when Ritalin decreased average running speed from 2.00±0.17 km/h (after saline) to 1.38±0.15 km/h (after 15 mg/kg) to 1.01±0.12 km/h (after 30 mg/kg). In control lines, Ritalin had the greatest effect during the 50- to 100-min time period (Fig. 1), when it increased total distance (over the 50-min time period) from 0.51±0.08 km (saline) to 0.68±0.05 km (15 mg/kg) to 0.74±0.06 km (30 mg/kg).
Mean wheel running (revolutions) ± SEM is plotted in 10-min increments 1 h before and 2 h after an injection of either saline or Ritalin (15 mg/kg or 30 mg/kg) (n=24 per data point). Data points are plotted at the mid-point of the interval (i.e., 5 on the x-axis represents the 0-min to 10-min interval). Data for control-line mice are plotted on the left, selected on the right. The range of the y-axes differ between left and right graphs to facilitate comparison of proportional responses between control and selected mice. Ritalin increased wheel running in control lines and decreased running in selected lines
Apomorphine
Apomorphine decreased wheel-running distance in both control (one-way repeated-measures ANCOVA, F 4,92=21.22, P<0.0001) and selected lines (F 4,92=19.57, P<0.0001; Table 2). However, selected mice were less sensitive to the activity-reducing effects of medium doses (0.25 mg/kg and 0.5 mg/kg) of apomorphine than controls (Table 1, Fig. 2). The lowest dose (0.125 mg/kg) decreased wheel running by approximately 40% in control lines and 30% in selected lines, and there were no significant differences in the proportional responses between selected and control lines for this low dose (Table 1; Fig. 2A, B). However, the medium doses (0.25 mg/kg and 0.5 mg/kg) caused a proportionally greater response in control than selected lines (Table 1, Fig. 2C, D). In control mice, 0.25, 0.5, and 1 mg/kg all produced severe, comparable reductions in wheel running (approximately 80% reductions). In contrast, in selected mice, 0.5 mg/kg and 0.25 mg/kg produced only a slight reduction in wheel running (approximately 40%) comparable to the response after the 0.125-mg/kg dose. Substantial reductions in wheel running (approximately 80%) in selected mice required the highest dose, 1 mg/kg, and at this high dose there were no significant differences in the proportional response between selected and control lines (Table 1).
Mean wheel running (revolutions) ± SEM is plotted in 10-min increments 1 h before and 2 h after an injection of a low dose of apomorphine (A, B), a medium dose of apomorphine (C, D), raclopride (E, F), and SCH 23390 (G, H) (n=24 per data point). Data points are plotted at the mid-point of the interval (i.e., 5 on the x-axis represents the 0-min to 10-min interval). Data for control-line mice are plotted in the left column, selected on the right. The range of the y-axes differ between left and right columns to facilitate comparison of proportional responses between control and selected mice. Control and selected mice responded similarly to a low dose of apomorphine (mixed D1-/D2-like agonist), but were less sensitive than controls to a medium dose of apomorphine. Selected and control mice responded similarly to raclopride (D2-like antagonist), but were less sensitive than controls to SCH 23390 (D1-like antagonist). Taken together, results suggest that D1-like but not D2-like receptors have reduced function in selected, hyperactive wheel running mice
Raclopride
Raclopride decreased wheel running in both control (F 3,66=10.10, P<0.0001) and selected lines (F 3,67=38.03, P<0.0001; Table 2), and there were no significant differences in the proportional responses between selected and control lines (Table 1, Fig. 2E, F).
SCH 23390
SCH 23390 decreased wheel running in both control (F 3,68=43.59, P<0.0001) and selected lines (F 3,65=17.24, P<0.0001; Table 2). However, the proportional responses were significantly greater in control than in selected lines (Table 1, Fig. 2 G, H), indicating that selected mice were less sensitive to the activity-reducing effects of SCH 23390.
Wheel freeness was occasionally a significant positive predictor of wheel running distance after drug injections, but explained very little of the total variation in wheel running. The random effect of line nested within linetype was not significant in any analyses.
Discussion
Results support the hypothesis that genetically determined hyperactive wheel-running behavior is associated with altered dopaminergic function (Rhodes et al. 2001). The selected, hyperactive mice were less sensitive than controls to the mixed D1-/D2-like dopamine receptor agonist, apomorphine (Thal et al. 1978). Selected mice were also less sensitive than controls to the specific D1-like antagonist, SCH 23390 (Andersen 1988), but were normally sensitive to the specific D2-like antagonist, raclopride (Andersen 1988). Taken together, these results suggest that hyperactive wheel running is associated with reduced function of D1-like (D1 or D5) but not D2-like (D2, D3, or D4) dopamine receptors (see Jarvie and Caron 1993 for a review of dopamine receptor families). Reduced function of D1-like receptors in hyperactive mice could explain why drugs that facilitate dopamine function (e.g., Ritalin, cocaine, GBR 12909) ameliorate the hyperactivity (Rhodes et al. 2001). The fact that Ritalin decreased wheel running (P=0.003) in selected lines supports their use as a model of ADHD.
Ritalin
Ritalin might have reduced wheel-running in the selected, hyperactive lines by increasing the function of dopamine and/or norepinephrine (Solanto 1998). Previous studies support a role for dopamine, because dopamine reuptake blockers GBR 12909 and cocaine also reduced wheel running in hyperactive but not control lines (Rhodes et al. 2001). It is possible that reduced function of D1-like receptors underlies hyperactive wheel-running, and that Ritalin corrects the deficit by increasing dopaminergic function in these regions. Reduced dopaminergic function is implicated in other animal models of genetic hyperactivity (Shaywitz et al. 1978; Russell et al. 1998). Furthermore, dopaminergic function may have been altered in selected mice in association with increased motivation (Koob 1996; Salamone 1996) for running, or possibly addiction to wheel running (Berridge and Robinson 1998; Nestler et al. 2001; Werme et al. 2002).
The doses of Ritalin chosen for this study demonstrate what can be viewed as a classic "paradoxical" effect in hyperactive versus control mice: activity was reduced in hyperactive mice but increased in control mice. This paradoxical effect is consistent with the observation in rodents that responses to stimulant drugs (e.g., cocaine, Ritalin, amphetamine) depend on baseline level of response, with low-baseline responders being stimulated and high-baseline responders being depressed (inverse rate-dependent effect) (Sanger and Blackman 1976; Robbins and Sahakian 1979; Solanto 1998; Rhodes et al. 2001). In humans, it is widely believed that there is no paradoxical effect for clinically relevant doses of stimulants (Solanto 1998). However, this is based on one report that amphetamine decreased activity in 14 "normal" boys between the ages of 6 years and 12 years selected by the following criteria: superior school performance, good coordination and good peer relationships (Rapoport et al. 1978). More recently it was found that clinical doses of amphetamine increased activity in 12 "normal" adults (8 males and 4 females, mean age 32 years) chosen using newspaper advertisements and by word of mouth (Greenwald et al. 1998). Taken together, results are consistent with an inverse rate-dependent effect of stimulants in humans over the clinically relevant dose range. One body-mass-scaling-equation (Morris 1999) translates the human dose range for Ritalin [0.3–0.7 mg/kg (Solanto et al. 2001)] into 2–5 mg/kg for mice. Although the doses we administered were higher, 15 mg/kg and 30 mg/kg, they were behaviorally relevant because they produced opposite effects in selected and control mice, did not reduce wheel running in selected lines to levels below controls (Fig. 1), and reduced the speed not the duration of running in selected mice (Table 2).
Reduced function of D1-like receptors in hyperactive mice
It has been proposed that wheel running is naturally rewarding and addictive (Sherwin 1998; Nestler et al. 2001; Werme et al. 2002), and recent evidence suggests that D1-like receptors play an important role in mediating neuronal plasticity associated with addiction (Beurrier and Malenka 2002). Therefore, it is not surprising that D1-like receptors are implicated in the high wheel-running behavior of the selected mice. Several mechanisms could contribute to the reduced sensitivity of selected mice to SCH 23390, including reduced expression of the D1-like receptors, altered coding sequence or post-transcriptional or post-translational modification of D1-like receptors, differences in the numerous second messengers which are down-stream from the D1- like receptors (Vallone et al. 2000), structural differences in the anatomy of dopaminergic projections in the brain, or changes in other receptor systems that interact with D1-receptor signaling (Kelley and Cador 1988; Smith-Roe and Kelley 2000).
Agonists and antagonists
Consistent with effects of apomorphine on locomotor activity (as measured by photobeams in cages without wheels) in DBA and BALB inbred mouse strains (Cabib and Puglisi-Allegra 1985), the non-selective dopamine agonist apomorphine dose dependently reduced wheel running in both control and selected mice over a range of doses (Table 2). However, in C57BL/6, the same doses had a biphasic effect on photobeam activity, with the lowest dose decreasing activity and the highest doses increasing it (Cabib and Puglisi-Allegra 1985). Therefore, comparisons across studies using different strains of mice should be made with caution. Moreover, apomorphine can have opposite effects on different measures of locomotion in the same strain (Cabib and Puglisi-Allegra 1985). Wheel running and cage activity are different behaviors that could be mediated by different dopaminergic mechanisms (as suggested previously by Rhodes et al. 2001). We measured drug responses at night, during peak activity, after animals had acclimated to their cages with attached wheels for 3 weeks. Typically, studies employing photobeam cages measure locomotion during the day, when nocturnal rodents are normally sleeping. Moreover, animals are typically moved from their home cage to the experimental cage for the testing period. We have argued previously (Rhodes et al. 2001) that photobeam activity measured in this manner represents reactivity to a novel environment, which is different from activity in an acclimated environment.
The dopamine receptor agonist apomorphine and antagonists SCH 23390 or raclopride, seemingly opposing treatments, both reduced wheel running in selected and control mice. Agonists and antagonists of dopamine receptors have produced behaviorally similar responses in other model systems, such as cocaine-seeking behavior in rats (Weissenborn et al. 1996; Alleweireldt et al. 2002). Alleweireldt et al. (2002) attributed similar effects of dopamine agonists and antagonists, which reduce cocaine-seeking behavior, to complex interactions of receptor subtypes. Dopamine receptors can modulate excitatory or inhibitory neurons (Pennartz et al. 1992) via inhibitory or excitatory signaling pathways (Smialowski and Bijak 1987), and concurrent activation of combinations of dopamine receptors determine behavioral phenotypes (Capper-Loup et al. 2002). Therefore, it is difficult to predict the behavioral effect of an agonist (such as apomorphine) based on knowledge of an antagonist effect.
Selected, hyperactive mice, displayed significantly reduced sensitivity to apomorphine only for the medium (0.25, 0.5 mg/kg) doses, not for the low (0.125 mg/kg) or high (1 mg/kg) dose. The low dose may have been too low to affect wheel running substantially enough in control lines to detect differences between control and selected mice, and the high dose may have produced near maximum (i.e., ceiling) effects in both control and selected mice (Table 1), again obscuring differences. In addition, it is possible that the behavioral response to the low dose was primarily mediated by activation of high-affinity D2-like dopamine receptors (Vallone et al. 2000), which may not function differently in selected mice.
Summary
Results support the hypothesis that genetic hyperactivity on running wheels is associated with reduced dopamine function. Mice from lines that had been selectively bred for high wheel running were less sensitive than controls to drugs that interact with D1-like but not D2-like receptors, which suggests that hyperactive mice have reduced function of D1-like receptors in the brain. The differential response to Ritalin in control versus hyperactive mice supports the use of the selected lines as a model of ADHD. The replicate, selectively bred, hyperactive lines represent a powerful animal model to investigate the underlying neural basis of genetic hyperactivity. Future investigations are needed to investigate the possible role of other neurotransmitter systems, such as norepinephrine (Arnsten 2001). In addition, future investigations are needed to determine which genes have changed in the selectively bred lines. Genes that affect dopamine function, in particular D1-like receptors (D1 or D5), are likely candidates.
References
Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL (2002) Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 159:284–293
Andersen PH (1988) Comparison of the pharmacological characteristics of [3H]raclopride and [3H]SCH 23390 binding to dopamine receptors in vivo in mouse brain. Eur J Pharmacol 146:113–120
Arnsten AFT (2001) Dopaminergic and noradrenergic influences on cognitive function mediated by prefrontal cortex. In: Solanto MV, Arnsten AFT, Castellanos FX (eds) Stimulant drugs and ADHD. Oxford University Press, Oxford, pp 185–208
Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377:424–428
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Beurrier C, Malenka R (2002) Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J Neurosci 22:5817–5822
Britton SL, Koch LG (2001) Animal genetic models for complex traits of physical capacity. Exerc Sport Sci Rev 29:7–14
Cabib S, Puglisi-Allegra S (1985) Different effects of apomorphine on climbing behavior and locomotor activity in three strains of mice. Pharmacol Biochem Behav 23:555–557
Capper-Loup C, Canales JJ, Kadaba N, Graybiel AM (2002) Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. J Neurosci 22:6218–6227
Castellanos FX (2001) Neuroimaging studies of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX (eds) Stimulant drugs and ADHD. Oxford University Press, Oxford, pp 243–258
Cook EH Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL (1995) Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet 56:993–998
Falconer D, Mackay T (1996) Introduction to quantitative genetics, 4th edn. Longman, Essex
Ferguson S (2001) A review of rodent models of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX (eds) Stimulant drugs and ADHD. Oxford University Press, Oxford, pp 209–220
Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283:397–401
Garland T (2003) Selection experiments: an underutilized tool in biomechanics and organismal biology. In: Gasc JP, Casinos A, Bels VL (eds) Vertebrate biomechanics and evolution, vol I. Bios Scientific Publishers, Oxford, pp 23–56
Garland T Jr, Morgan M, Swallow J, Rhodes J, Girard I, Belter J, Carter P (2002) Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evolution 56:1267–1275
Girard I, McAleer MW, Rhodes JS, Garland T Jr (2001) Selection for high voluntary wheel-running increases speed and intermittency in house mice (Mus domesticus). J Exp Biol 204:4311–4320
Greenwald MK, Schuster CR, Johanson CE, Jewell J (1998) Automated measurement of motor activity in human subjects: effects of repeated testing and d-amphetamine. Pharmacol Biochem Behav 59:59–65
Henderson N (1989) Interpreting studies that compare high- and low-selected lines on new characters. Behav Genet 19:473–502
Henderson N (1997) Spurious association in unreplicated selected lines. Behav Genet 27:145–154
Ichihara K, Nabeshima T, Kameyama T (1993) Mediation of dopamine D1 and D2 receptors in the effects of GBR 12909 on latent learning and locomotor activity in mice. Eur J Pharmacol 234:155–163
Jarvie KR, Caron MG (1993) Heterogeneity of dopamine receptors. Adv Neurol 60:325–333
Kelley AE, Cador M (1988) Behavioral evidence for differential neuropeptide modulation of the mesolimbic dopamine system. Ann NY Acad Sci 537:415–434
Koob GF (1996) Hedonic valence, dopamine and motivation. Mol Psychiatry 1:186–189
Koteja P, Garland T Jr (2001) Forum. Response to R. Eikelboom. Anim Behav 61:F25–F26
Levy F, Swanson JM (2001) Timing, space and ADHD: the dopamine theory revisited. Aust N Z J Psychiatry 35:504–511
Marley RJ, Arros DM, Henricks KK, Marley ME, Miner LL (1998) Sensitivity to cocaine and amphetamine among mice selectively bred for differential cocaine sensitivity. Psychopharmacology 140:42–51
Messier C, Mrabet O, Destrade C (1992) Locomotor bias produced by intra-accumbens injection of dopamine agonists and antagonists. Pharmacol Biochem Behav 41:177–182
Milliken GA, Johnson DE (1992) Analysis of messy data. Chapman and Hall, New York
Morris TH (1999) Dose estimation among species. In: Hawk CT, Leary SL (eds) Formulary for laboratory animals. Iowa State University Press, Iowa
Nestler EJ, Barrot M, Self DW (2001) Delta fosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA 98:11042–11046
Papa M, Sellitti S, Sadile AG (2000) Remodeling of neural networks in the anterior forebrain of an animal model of hyperactivity and attention deficits as monitored by molecular imaging probes. Neurosci Biobehav Rev 24:149–156
Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH (1992) Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol 67:1325–1334
Porrino LJ, Rapoport JL, Behar D, Sceery W, Ismond DR, Bunney WE Jr (1983) A naturalistic assessment of the motor activity of hyperactive boys. I. Comparison with normal controls. Arch Gen Psychiatry 40:681–687
Rapoport JL, Buchsbaum MS, Zahn TP, Weingartner H, Ludlow C, Mikkelsen EJ (1978) Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science 199:560–563
Rhodes JS, Hosack GR, Girard I, Kelley AE, Mitchell GS, Garland T Jr (2001) Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology 158:120–131
Robbins TW, Sahakian BJ (1979) "Paradoxical" effects of psychomotor stimulant drugs in hyperactive children from the standpoint of behavioural pharmacology. Neuropharmacology 18:931–950
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J (1995) Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder—the spontaneously hypertensive rat. Brain Res 676:343–351
Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J (1998) Differences between electrically-, ritalin- and d-amphetamine-stimulated release of [3H]dopamine from brain slices suggest impaired vesicular storage of dopamine in an animal model of attention-deficit hyperactivity disorder. Behav Brain Res 94:163–171
Sagvolden T (2000) Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 24:31–39
Sagvolden T, Sergeant JA (1998) Attention deficit/hyperactivity disorder—from brain dysfunctions to behaviour. Behav Brain Res 94:1–10
Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G (1992) The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol 58:103–112
Salamone JD (1996) The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods 64:137–149
Sanger DJ, Blackman DE (1976) Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav 4:73–83
Shaywitz BA, Klopper JH, Gordon JW (1978) Methylphenidate in 6-hydroxydopamine-treated developing rat pups. Effects on activity and maze performance. Arch Neurol 35:463–469
Sherwin CM (1998) Voluntary wheel running: a review and novel interpretation. Anim Behav 56:11–27
Simon VM, Parra A, Minarro J, Arenas MC, Vinader-Caerols C, Aguilar MA (2000) Predicting how equipotent doses of chlorpromazine, haloperidol, sulpiride, raclopride and clozapine reduce locomotor activity in mice. Eur Neuropsychopharmacol 10:159–164
Smialowski A, Bijak M (1987) Excitatory and inhibitory action of dopamine on hippocampal neurons in vitro. Involvement of D2 and D1 receptors. Neuroscience 23:95–101
Smith-Roe SL, Kelley AE (2000) Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 20:7737–7742
Solanto MV (1998) Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 94:127–152
Solanto MV (2000) Clinical psychopharmacology of AD/HD: implications for animal models. Neurosci Biobehav Rev 24:27–30
Solanto MV, Arnsten AFT, Castellanos FX (2001) Stimulant drugs and ADHD. Oxford University Press, Oxford
Swallow JG, Carter PA, Garland T Jr (1998) Artificial selection for increased wheel-running behavior in house mice. Behav Genet 28:227–237
Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, Murias M, Moriarity J, Barr C, Smith M, Posner M (2000) Dopamine genes and ADHD. Neurosci Biobehav Rev 24:21–25
Thal L, Creese I, Snyder SH (1978) 3H-Apomorphine interactions with dopamine receptors in calf brain. Eur J Pharmacol 49:295–299
Todd RD (2000) Genetics of attention deficit/hyperactivity disorder: are we ready for molecular genetic studies? Am J Med Genet 96:241–243
Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24:125–132
Weissenborn R, Deroche V, Koob GF, Weiss F (1996) Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology 126:311–322
Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S (2002) Delta fosB regulates wheel running. J Neurosci 22:8133–8138
Womer DE, Jones BC, Erwin VG (1994) Characterization of dopamine transporter and locomotor effects of cocaine, GBR 12909, epidepride, and SCH 23390 in C57BL and DBA mice. Pharmacol Biochem Behav 48:327–335
Acknowledgements
Supported by NSF grants IBN-9728434 and IBN-0212567 to T.G., and NIH grant P30 HD03352 (P.I.: Terrence R. Dolan). J.S.R. was supported by NINDS fellowship NS42872–02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rhodes, J.S., Garland, T. Differential sensitivity to acute administration of Ritalin, apormorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology 167, 242–250 (2003). https://doi.org/10.1007/s00213-003-1399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1399-9