Abstract
Aluminum is well recognized as a nephrotoxic agent. Its hazardous effects arise from the high risk of daily exposure. The consumption of fructose also represents a critical health issue that might negatively impact different organs, including the kidneys. To pursue our previous work, this study aimed to investigate the potential renoprotective effects of glycyrrhizic acid (GLYA) on aluminum-induced nephrotoxicity in insulin-resistant rats. Insulin resistance (IR) was induced by adding fructose (10%) in drinking water for 18 weeks. Male Wistar rats were divided into five groups: control (CTRL), aluminum chloride (ALM, 34 mg/kg/day), fructose (FRCT), aluminum plus fructose (AL/FR), and GLYA (rats received AL/FR and treated with 40 mg/kg GLYA daily). AL/FR resulted in abnormal renal function tests and renal tissue injury. This was associated with increased oxidative stress and inflammation in the renal tissue. Moreover, the expressions of the toll-like receptor 4 (TLR4) and its adaptor proteins were increased in AL/FR group. The administration of GLYA mollified AL/FR-induced renal injury, oxidative stress, activation of the TLR4 signaling pathway, and inflammation. In conclusion, we provide evidence for the promising renoprotective effect of GLYA against AL/FR-induced kidney damage in rats. The renoprotection is attributed to the suppression of oxidative stress and inhibition of the TLR4/NF-κB signaling pathway in the kidneys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fructose is a health hazard that is widely used as the main component of the modern diet despite its implication in different organ disorders, including the kidneys. Different studies showed that the consumption of high fructose is implicated in kidney damage (Kizhner and Werman 2002; Nakayama et al. 2010; Aoyama et al. 2012; Oudot et al. 2013). Furthermore, human exposure to aluminum is being increased, particularly in developing countries through the use of utensils made from aluminum. Aluminum leakage is higher when such utensils are used for cooking salty, alkaline, or acidic foods (Lin et al. 1997). Another important source of aluminum exposure is the use of aluminum-containing phosphate binders for the control of secondary hyperparathyroidism (Mudge et al. 2011). Aluminum is implicated in renal osteodystrophy (Flaten, 2001). Different studies also reported the nephrotoxic effects of aluminum in experimental animals (Abdel Moneim et al. 2013; Belaid-Nouira et al. 2013, Al Kahtani et al. 2014).
The various sources of aluminum exposure associated with the increased use of fructose raise the likelihood of contemporaneous exposure to both agents, which exposes humans to many health risks. However, the effect of combined exposure to both fructose and aluminum on different organs is not well studied. In our previous studies, we described the neurotoxic and hepatotoxic effects of a combination of aluminum and fructose in rats (Ali et al. 2019; Zakaria et al. 2020). Further, we reported the protective effects of glycyrrhizic acid (GLYA) in both models. GLYA is a widely used natural sweetener isolated from the roots of Glycyrrhiza species and is being used in the preparation of different pharmaceutical products (Hayashi and Sudo 2009). In addition, GLYA is a promising agent that possesses a wide range of therapeutic effects including anti-inflammatory (Fujisawa et al. 2000), anti-hepatotoxic (Zheng and Lou 2003, Gumpricht et al. 2005), anti-tumor (Hibasami et al. 2006), and antiviral activities (Lin 2003; Hoever et al. 2005).
We hypothesize that exposure to both aluminum and fructose could also impact the kidney negatively. Besides, we also propose that GLY might have a protective effect on kidneys in this model. Therefore, in this study, we investigated the protective effects of glycyrrhizic acid against aluminum-induced nephrotoxicity in insulin-resistant rats and tried to identify the possible molecular mechanisms underlying this effect.
Materials and methods
Drugs and chemicals
Aluminum chloride hexahydrate was purchased from El-Gomhouria Company for Trading Chemicals and Medical Appliances (Cairo, Egypt). Fructose was purchased from El-Nasr Company for Intermediate Chemicals (Giza, Egypt), whereas glycyrrhizic acid (as ammonium salt) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Aluminum chloride and glycyrrhizic acid were dissolved in normal saline just before administration.
Experimental animals
Adult male Wistar rats weighing 100 ± 20 g were used in the present study. The animals were supplied from the National Research Center, Cairo, Egypt. Rats were allowed 1 week for acclimatization before starting experimental manipulations. Optimal rat housing conditions were maintained throughout the study, including temperature (23 ± 2 °C), humidity (60 ± 10%), and 12-h light/dark cycle. Rats had access to water and chow diet ad libitum. The study protocol and animal handling procedures were approved by the Ethical Committee for Animal Handling at Zagazig University (ECAH ZU), Faculty of Pharmacy, Zagazig University, Egypt, based on the recommendations of the Weatherall report. Reducing the number of used rats and suffering was of utmost consideration.
Study protocol
The study embraced five groups of six rats each. Group I (CTRL) comprised negative control rats that were administered the vehicle only. Group II (ALM) comprised rats that were administered aluminum chloride (34 mg/kg/day). Group III (FRUCT) comprised rats that were administered 10% (w/v) fructose. Group IV (AL/FR) comprised rats that were administered 10% (w/v) fructose plus aluminum chloride (34 mg/kg/day). Group V (GLYA) comprised rats that were administered 10% (w/v) fructose plus aluminum chloride (34 mg/kg/day) plus glycyrrhizic acid (40 mg/kg/day). Fructose was administered in drinking water for 18 weeks, whereas aluminum chloride and glycyrrhizic acid were administered per oral gavage between 9:00 AM and 11:00 AM for 12 consecutive weeks starting from the 7th week. The doses of aluminum chloride and glycyrrhizic acids were chosen based on our previous work and other published studies (Abdel Moneim et al. 2013; El-Kenawy Ael et al. 2014; Ali et al. 2019; Zakaria et al. 2020).
Blood and tissue sampling
Blood samples were collected from the retro-orbital sinus under anesthesia, in clean, dry centrifuge tubes. The tubes were left to clot for 15 min at room temperature. The serum was separated by centrifugation at 1000 g for 30 min. Rats were euthanized by cervical dislocation, and then, a longitudinal cut was made to open the abdominal cavity. Both kidneys were dissected, removed, and rinsed with normal saline. One kidney was fixed in 10% formalin for histopathological investigation and immunohistochemical staining. The other kidney was immersed immediately in liquid nitrogen and then stored at − 80 °C for further biochemical measurements.
Measurement of glycemic markers
Fasting blood glucose level was measured from a drop of blood obtained from the rat tail tip using GlucoSure Star, an automatic blood glucose meter supplied by Apex Biotechnology (Hsinchu, Taiwan). Fasting serum insulin level was measured by enzyme-linked immunosorbent assay (ELISA) technique using a rat kit supplied by DRG Instruments (Marburg, Germany). Insulin resistance (IR) was calculated using the homeostasis model assessment (HOMA) index for IR using the following formula: HOMA-IR = [fasting blood glucose (mg/dL) × fasting insulin level (μU/mL)]/405 (Matthews et al. 1985).
Measurement of renal function biomarkers
Serum cystatin C (Cys-C) level was measured by the ELISA technique using a commercial rat kit (code: CSB-E08385r) provided by Cusabio (Wuhan, China). Serum creatinine (SCr), urea, and uric acid levels were measured colorimetrically using kits supplied by Diamond Diagnostics (Cairo, Egypt).
Measurement of oxidative stress markers and tumor necrosis factor-alpha (TNF-⍺) in renal tissue homogenate
Colorimetry-based methods were used for the measurement of catalase (CAT) activity, superoxide dismutase (SOD) activity, and malondialdehyde (MDA) level using kits supplied by Biodiagnostic (Giza, Egypt). The ratio of total glutathione (GSH)/oxidized glutathione (GSSG) was measured colorimetrically using a kit supplied by Elabscience Biotechnology Inc. (Houston, TX, USA). Quantitative assessment of TNF-⍺ was done using an ELISA-based kit supplied by R&D Systems (Minneapolis, MN, USA).
Quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) for the gene expression of TLR4, TRIF, and MyD88 in renal tissue homogenate
Total RNA was isolated from kidney tissue using the Qiagen RNeasy kit (Hilden, Germany). The synthesis of the first-stand cDNA and RT-PCR were performed as described in detail in our previous study (Ali et al. 2019). Primer sequences (5′-3′) for TLR4, TRIF, MyD88, and β-actin were as follows: forward primers CTACCTCGAGTGGGAGGACA, TGGATATCTCCCCTTCGCCT, TGTCTCCCCTGACATGCCTA, TATCCTGGCCTCACTGTCCA, respectively, and reverse primers TGCTACTTCCTTGTGCCCTG, TGGAGGTGAGACAGACCCTT, TTTTGTGTGTCCGCTGCTTG, AACGCAGCTCAGTAACAGTC, respectively. The relative expressions of the studied genes were calculated using the delta-delta Ct method as described by Livak and Schmittgen (2001), and the values were expressed as fold expression of the control after normalization to β-actin gene expression.
Immunohistochemical staining of nuclear factor-kappa B (NF-κB)
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded kidney sections with a thickness of 5 μm. The sections were washed with phosphate-buffered saline (PBS; pH 7.4) and treated with 0.3% hydrogen peroxide solution for 20 min to halt endogenous peroxidase activity. After three washes with PBS, the sections were incubated with a primary anti-NF-κB p65 antibody (Fisher Scientific, Rockford, IL, USA) in a humidified chamber at 37 °C for 3 h. A biotinylated secondary antibody and streptavidin peroxidase were consecutively applied at 37 °C for 15 min each. 3,3′-Diaminobenzidine (DAB, Sigma-Aldrich, St., Louis, MO, USA) was then added to visualize antibody binding by the formation of a brown color due to the oxidation of DAB. Nuclei were counterstained using Mayer’s hematoxylin (Sigma-Aldrich, St., Louis, MO, USA), and the average optical density of positive NF-κB staining was measured in using Leica Qwin 500 (Leica Microsystems GmbH, Wetzlar, Germany).
Histopathological examination
Renal tissue samples were fixed using 10% neutral buffered formalin and then dehydrated in ethyl alcohol of increasing concentration (70%, then 90%, then 100%). The samples were cleared in xylol, followed by impregnation and embedding in paraffin wax. Sections (5-μm thick) were cut using a rotatory microtome. Samples were then stained with hematoxylin and eosin (H&E) stain and inspected under a light microscope.
Statistical analysis
Statistical analysis and graph creation were done using the R language for statistical computing version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad prism v.8 (GraphPad Software, San Diego, CA, USA). Results were expressed as the mean ± standard error of the mean (SEM). Significance of difference between group means was tested using ordinary one-way analysis of variance (ANOVA) followed by Bonferroni’s test for pairwise comparisons or Welch’s ANOVA followed by Tamhane’s T2 post hoc test with p < 0.05 considered significant.
Results
Glycyrrhizic acid alleviates hyperinsulinemia and IR in AL/FR-treated rats
Rats from FRCT or AL/FR group exhibited comparable blood glucose level and hyperinsulinemia that were significantly higher compared with the CTRL group. Similarly, rats from FRCT or AL/FR group had significantly elevated HOMA-IR compared with either the CTRL or ALM group. On the other hand, the administration of glycyrrhizic acid significantly alleviated hyperinsulinemia and IR by 47.6% and 48.2%, respectively, compared with AL/FR group (Table 1).
Glycyrrhizic acid improves kidney function biomarkers in AL/FR-treated rats
As illustrated in Fig. 1, rats from ALM, FRCT, and AL/FR exhibited significant elevations in serum cystatin C level (84.4 ± 3.2, 50.9 ± 3.9, and 143 ± 5.5, respectively, vs. 27.8 ± 2.1 pg/mL), creatinine level (0.51 ± 0.013, 0.45 ± 0.015, and 0.55 ± 0.03, respectively, vs. 0.32 ± 0.014 mg/dL), and urea level (53.5 ± 2.3, 45.2 ± 1.8, and 60.3 ± 4.6, respectively, vs. 31 ± 1.2 mg/dL) compared with CTRL rats. However, the serum level of uric acid was significantly elevated only in AL/FR rats (2.3 ± 0.17 vs. 0.84 ± 0.05 mg/dL), but it was not significantly altered in either ALM or FRCT group compared with CTRL group. The deleterious effects of ALM and FRCT were comparable except serum cystatin C that was significantly higher in the ALM group compared with the FRCT group. The elevations in these parameters induced by the combination of aluminum chloride and fructose (AL/FR) were significantly higher compared to the FRCT group. In contrast, compared with the ALM group, the elevations of serum cystatin C and uric acid were only significant. On the other hand, the administration of GLYA significantly reduced serum cystatin C by 63%, creatinine by 31%, urea by 38%, and uric acid by 55% compared with AL/FR group.
Changes in renal function biomarkers in rats administered aluminum chloride, fructose, aluminum chloride plus fructose, or aluminum chloride plus fructose and treated with glycyrrhizic acid. CTRL, control rats; ALM, rats received aluminum chloride (34 mg/kg/day); FRCT, rats received fructose (10%) in drinking water; AL/FR, rats received a combination of ALM and FRCT; GLYA, rats received AL/FR plus glycyrrhizic acid (40 mg/kg/day). Values are expressed as mean ± SEM (n = 3–4); statistical analysis was performed using ordinary one-way ANOVA, followed by Bonferroni’s post-test: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001; ns, non-significant
Glycyrrhizic acid alleviates the histopathological changes in renal tissue in AL/FR-treated rats
Figure 2 depicts representative photomicrographs of the renal tissue from different treatment groups. CTRL group showed a typical histological picture of the renal tissue (Fig. 2a). ALM group had many degenerated and cast-filled tubules. Mononuclear cellular infiltration and some areas of hemorrhage were observed (Fig. 2b). Similarly, the FRCT group showed degenerated tubules, areas of hemorrhage, and marked mononuclear cellular infiltration (Fig. 2c). AL/FR group showed vacuolated and cast-filled tubules, marked mononuclear cellular, multiple areas of hemorrhage, and shrunken renal corpuscles (Fig. 2d–e). On the other hand, the administration of GLYA reversed the histopathologic alterations induced by AL/FR and resulted in a histological picture very close to the CTRL group (Fig. 2f).
Histopathological examination of the renal tissue. Representative photomicrographs (H&E × 200) are depicted from a CTRL rats showing normal histological structure with renal corpuscles (RC) surrounded by Bowman’s capsule (black arrow), subcapsular space (blue arrow), proximal convoluted tubule (P), and distal convoluted tubule (D); b ALM-treated rats showing degenerated tubules (blue arrow), cast-filled tubules (black arrow), mononuclear cellular infiltration (double arrows), hemorrhage (H), and shrunken renal corpuscle (RC); c FRCT-treated rats showing degenerated tubules (single arrows), hemorrhage (H), marked mononuclear cellular infiltration (double arrows); d AL/FR-treated rats showing cast-filled tubules (single arrows), vacuolated tubules (V), mononuclear cellular infiltration (double arrows), and hemorrhage (H); e AL/FR-treated rats showing degenerated tubules (arrow), hemorrhage (H), shrunken renal corpuscle (RC), and marked mononuclear cellular infiltration (double arrows); f GLYA-treated rats showing marked improvement of the renal tissue with a histological picture very close to that of the CTRL group. The renal corpuscle (RC) is surrounded by Bowman’s capsule (black arrow) and subcapsular space (blue arrow), with little mononuclear cellular infiltration (double arrows) and few vacuolated tubules (V)
Glycyrrhizic acid suppresses renal oxidative stress and enhances the activity of antioxidative enzymes in AL/FR-treated rats
The administration of ALM, FRCT, and their combination AL/FR significantly reduced SOD activity (0.22 ± 0.02, 0.68 ± 0.11, and 0.19 ± 0.07, respectively, vs. 2.37 ± 0.26 U/g tissue), CAT activity (46.5 ± 13.1, 52.7 ± 3.3, and 43.4 ± 1.6, respectively, vs. 121 ± 3.6 U/g tissue), and GSH/GSSG ratio (0.4 ± 0.03, 0.88 ± 0.1, and 0.35 ± 0.05, respectively, vs. 4.63 ± 0.85) compared with CTRL group. However, the levels of renal MDA were significantly increased in ALM, FRCT, and AL/FR groups (133 ± 9.1, 117 ± 4.7, and 130 ± 3.4 nmol/g tissue, respectively) compared with CTRL group (10.5 ± 0.15 nmol/g tissue). These alterations were not statistically significant among ALM, FRCT, and AL/FR groups. GLYA-treated rats had significantly increased SOD activity by 747%, CAT activity by 112%, and GSH/GSSG ratio by 586% and significantly decreased MDA level by 58% compared with AL/FR group (Fig. 3).
Changes in renal oxidative stress markers in rats administered aluminum chloride, fructose, aluminum chloride plus fructose, or aluminum chloride plus fructose and treated with glycyrrhizic acid. a superoxide dismutase (SOD) activity, b catalase (CAT) activity, c malondialdehyde (MDA), and d ratio of reduced (GSH)/oxidized (GSSG) glutathione. CTRL, control rats; ALM, rats received aluminum chloride (34 mg/kg/day); FRCT, rats received fructose (10%) in drinking water; AL/FR, rats received a combination of ALM and FRCT; GLYA, rats received AL/FR plus glycyrrhizic acid (40 mg/kg/day). Values are expressed as mean ± SEM (n = 3), statistical analysis was performed using ordinary one-way ANOVA, followed by Bonferroni’s post-test: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001; ns, non-significant
Glycyrrhizic acid exerts an anti-inflammatory effect in renal tissue of AL/FR-treated rats
As shown in Fig. 4, the administration of ALM, FRCT, and AL/FR resulted in significant increases in NF-κB expression by 8.5-fold, 8.2-fold, and 10.2-fold, respectively, compared with the CTRL group. The difference in NF-κB expression between ALM and FRCT or ALM and AL/FR was not significant; however, there was a significant increase in NF-κB expression in AL/FR group compared with the FRCT group (Bonferroni’s adjusted P = 0.023). The administration of GLYA significantly diminished NF-κB expression by 4.7-fold compared with AL/FR group.
Changes in renal NF-κB expression in rats administered aluminum chloride, fructose, aluminum chloride plus fructose, or aluminum chloride plus fructose and treated with glycyrrhizic acid. Representative immunohistochemical photos (× 400) from a control group showing very weak immunoreaction (arrow); b, c, d ALM, FRCT, and AL/FR groups, respectively, showing strong positive immunoreaction (arrow); and e GLYA group showing weak immunoreaction (arrow). f shows the quantification of NF-κB expression. CTRL, control rats; ALM, rats received aluminum chloride (34 mg/kg/day); FRCT, rats received fructose (10%) in drinking water; AL/FR, rats received a combination of ALM and FRCT; GLYA, rats received AL/FR plus glycyrrhizic acid (40 mg/kg/day). Values are expressed as mean ± SEM (n = 6); statistical analysis was performed using ordinary one-way ANOVA, followed by Bonferroni’s post-test: *p ≤ 0.05, ****p ≤ 0.0001; ns, non-significant
Furthermore, renal TNF-⍺ level was significantly increased in both ALM (162 ± 18.4 pg/g tissue) and AL/FR (167 ± 22.6 pg/g tissue) groups compared with either CTRL (30.9 ± 2.9 pg/g tissue) or FRCT (87.1 ± 11.1 pg/g tissue) group. The difference observed between CRTL and FRCT was not statistically significant (adjusted P = 0.149). The administration of GLYA significantly lowered the TNF-⍺ level by 58% compared with AL/FR group (Fig. 5a).
Changes in renal TNF-ɑ level and TLR4 pathway expression in rats administered aluminum chloride, fructose, aluminum chloride plus fructose, or aluminum chloride plus fructose and treated with glycyrrhizic acid. a Tumor-necrosis factor-alpha (TNF-⍺), b toll-like receptor 4 (TLR4), c myeloid differentiation primary response 88 (MyD88), and d TIR-domain-containing adapter-inducing interferon-β (TRIF). CTRL, control rats; ALM, rats received aluminum chloride (34 mg/kg/day); FRCT, rats received fructose (10%) in drinking water; AL/FR, rats received a combination of ALM and FRCT; GLYA, rats received AL/FR plus glycyrrhizic acid (40 mg/kg/day). Values are expressed as mean ± SEM (n = 3); statistical analysis was performed using ordinary one-way ANOVA, followed by Bonferroni’s post-test: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001; ns, non-significant
Glycyrrhizic acid inhibits TLR4 pathway in renal tissue of AL/FR-treated rats
As illustrated in Fig. 5b–d, the administration of ALM, FRCT, and AL/FR resulted in significant increases in the gene expression of TLR4 (6.8-fold, 5.5-fold, and 7.9-fold, respectively), MyD88 (8.4-fold, 5.8-fold, and 9.8-fold, respectively), and TRIF (12.2-fold, 6.8-fold, and 12.2-fold, respectively) compared with CTRL group. The effects of ALM and AL/FR were almost comparable. The expressions of MyD88 and TRIF were significantly higher in the AL/FR group compared with the FRCT group. Although the expression of TLR4 was increased in AL/FR group by 43% compared to the FRCT group; however, such a difference did not reach the statistical significance (adjusted P = 0.092). GLYA significantly inhibited the expression of TLR4, MyD88, and TRIF by 62%, 64%, and 63%, respectively, compared with AL/FR group.
Discussion
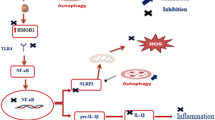
The present study investigated the possible protective effects of glycyrrhizic acid against aluminum-induced nephrotoxicity in insulin-resistant rats and identified the possible molecular mechanism of action underlying this effect. The results showed that glycyrrhizic acid mollifies the aluminum-induced nephrotoxicity in insulin-resistant rats through a TLR4-dependent mechanism. The nephroprotective effect of glycyrrhizic acid involves the suppression of oxidative stress and inhibition of TLR4/TNF-⍺/NF-KB signaling pathway.
Aluminum might cause nephrotoxicity due to its tendency to accumulate in kidneys generating reactive oxygen species (ROS), causing inflammation (Kutlubay et al. 2007). In addition, excessive fructose intake can lead to glomerular hypertension, renal inflammation, and tubulointerstitial injury in animals (Nakayama et al. 2010). Fructose can also expedite the progression of chronic kidney disease (Gersch et al. 2007).
We postulated that fructose might exacerbate aluminum-induced nephrotoxicity. Hence, we treated rats with ALM after the induction of IR by the administration of fructose (10%) in drinking water. FRCT-induced IR was associated with hyperinsulinemia and increased HOMA-IR index. The combination of AL/FR caused renal impairment that was manifested as marked deterioration in kidney function tests. Serum cystatin C, creatinine, and urea were significantly elevated by AL/FR administration compared with CTRL rats. The differences were significant between AL/FR and ALM only regarding cystatin C, which might be attributed to the superior sensitivity of cystatin C as a marker of renal function over creatinine (Dharnidharka et al. 2002). Likewise, the combined administration of AL/FR caused a significant elevation of serum uric acid level compared with either ALM or FRCT alone, while the effects of ALM and FRCT were comparable. These results point to the additive toxic effects of both agents when combined.
The observed aberrations of the biochemical parameters were also evident on the histological level. The administration of ALM or FRCT alone caused renal tubular degeneration, mononuclear cellular infiltration, and the appearance of small areas of hemorrhage. These results are in harmony with previous studies (Qiao et al. 2018). The administration of AL/FR caused additive damage in the form of markedly dilated and congested blood vessels, mononuclear cellular infiltration, shrunken renal corpuscle cells, and many areas of hemorrhage. This further supports the postulation that ALM-induced nephrotoxicity is aggravated, at least partly, by FRCT-induced IR.
GLYA is the main bioactive constituent of licorice root and has been widely used in folk medicine due to its antioxidant and anti-inflammatory properties (Feng et al. 2007, Fiore et al. 2008). In our previous work, we reported the protective effects of GLYA on AL/FR-induced neurotoxicity (Ali et al. 2019) and hepatotoxicity (Zakaria et al. 2020) making it interesting to explore the renoprotective effects of GLYA in this model. In the present study, the administration of GLYA remarkably improved FRAL-induced IR, which is supported by other studies (Takii et al. 2001; Sen et al. 2011; Sil et al. 2013; Guo et al. 2016). In addition, the administration of GLYA markedly improved AL/FR-induced renal impairment and histological changes that were evident as restoration of normal histological structure of renal cells and the marked reduction of congestion in the blood vessels.
The generation of reactive oxygen species (ROS) has been reported as a key mechanism implicated in the pathogenesis of ALM-induced tubular degeneration through oxidative damage to cellular lipids, proteins, and DNA (Kutlubay et al. 2007). This was also reproducible in our study. The administration of ALM or AL/FR significantly elevated the renal MDA level, which is a marker of ROS-induced peroxidation of polyunsaturated fatty acids. This was associated with a significant reduction in GSH/GSSG ratio, presumably due to the consumption of reduced GSH in an attempt to detoxify the excessively generated ROS. Moreover, ALM and AL/FR reduced the activity of the antioxidant enzymes, SOD, and CAT. These results are supported by other studies showing similar effects of aluminum on renal tissue (Mahieu et al. 2003, Sargazi et al. 2006). However, no significant differences were observed between ALM and AL/FR regarding these markers of oxidative stress.
Furthermore, the results of the current study showed that ALM caused a significant increase in renal TNF-α level, which is consistent with other studies reporting renal inflammation following the exposure to aluminum (Al Dera 2016). The administration of AL/FR increased TNF-α to a level comparable to ALM alone. This finding shows that IR did not worsen the renal inflammatory response evoked by ALM exposure. The expression of TNF-α and other inflammatory mediators is triggered by the transcription factor NF-κB (Akira and Takeda 2004, Hemmati et al. 2014). In the present study, we observed significant elevations of NF-κB expression in the renal tissue of rats treated with ALM, FRCT, or AL/FR. However, the difference between AL/FR and ALM was not significant.
NF-κB is a downstream factor of TLRs, which play a significant role in the immune system and inflammatory responses. These receptors are widely distributed in different tissues and expressed in the kidney (Smith 2009). Our results showed that ALM, FRCT, and AL/FR caused significant elevations of the gene expression of TLR4 in the renal tissue compared with the CTRL group. This was also associated with increased expression of the TLR4 adaptor proteins, MyD88 and TRIF. These molecules are important for the activation of IκB kinase (IKK) responsible for the phosphorylation of IκB. This results in the phosphorylation of NF-κB, followed by its translocation to the nucleus. In this regard, the effects of the administration of AL/FR were comparable to ALM alone.
The renoprotective effects of GLYA might be attributed to its antioxidant and anti-inflammatory properties. It significantly decreased renal MDA and increased renal SOD activity, CAT activity, and GSH/GSSG ratio. Such antioxidant effects align with other studies using kidney injury models (Hou et al. 2014; Wu et al. 2015). Moreover, TNF-α was reduced significantly by GLYA, which might be mediated via the inhibition of the TLR4 pathway, including its adaptor proteins and the downstream transcription factor, NF-κB. The latter finding gives a new insight into the renoprotective mechanism of GLYA in this model.
In conclusion, the present study, as an extension to our previous work, broadens our understanding regarding the toxic effects of the combination of AL/FR in rats on different organs. In addition, we provide a piece of evidence for the pleiotropic protective effects of GLYA in different experimental models of organ injury. We assume that these findings support the clinical testing of this promising nutraceutical in future work.
References
Abdel Moneim AE, Othman MS, Mohmoud SM, El-Deib KM (2013) Pomegranate peel attenuates aluminum-induced hepatorenal toxicity. Toxicol Mech Methods 23:624–633
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Al Dera HS (2016) Protective effect of resveratrol against aluminum chloride induced nephrotoxicity in rats. Saudi Med J 37:369–378
Al Kahtani MA, Abdel-Moneim AM, El-Sayed WM (2014) The influence of taurine pretreatment on aluminum chloride induced nephrotoxicity in Swiss albino mice. Histol Histopathol 29:45–55
Ali NM, Mahmoud AAA, Mahmoud MF, El Fayoumi HM (2019) Glycyrrhizic acid and silymarin alleviate the neurotoxic effects of aluminum in rats challenged with fructose-induced insulin resistance: possible role of toll-like receptor 4 pathway. Drug Chem Toxicol 42:210–219
Aoyama M, Isshiki K, Kume S, Chin-Kanasaki M, Araki H, Araki S, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T (2012) Fructose induces tubulointerstitial injury in the kidney of mice. Biochem Biophys Res Commun 419:244–249
Belaid-Nouira Y, Bakhta H, Haouas Z, Flehi-Slim I, Ben Cheikh H (2013) Fenugreek seeds reduce aluminum toxicity associated with renal failure in rats. Nutr Res Pract 7:466–474
Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226
El-Kenawy Ael M, Hussein Osman HE, Daghestani MH (2014) Role of propolis (bee glue) in improving histopathological changes of the kidney of rat treated with aluminum chloride. Environ Toxicol 29:1000–1010
Feng C, Wang H, Yao C, Zhang J, Tian Z (2007) Diammonium glycyrrhizinate, a component of traditional Chinese medicine Gan-Cao, prevents murine T-cell-mediated fulminant hepatitis in IL-10- and IL-6-dependent manners. Int Immunopharmacol 7:1292–1298
Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, Bielenberg J (2008) Antiviral effects of Glycyrrhiza species. Phytother Res 22:141–148
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55:187–196
Fujisawa Y, Sakamoto M, Matsushita M, Fujita T, Nishioka K (2000) Glycyrrhizin inhibits the lytic pathway of complement--possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis. Microbiol Immunol 44:799–804
Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, Sautin YY, Johnson RJ, Nakagawa T (2007) Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Ren Physiol 293:F1256–F1261
Gumpricht E, Dahl R, Devereaux MW, Sokol RJ (2005) Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem 280:10556–10563
Guo Y, Wang S, Wang Y, Zhu T (2016) Silymarin improved diet-induced liver damage and insulin resistance by decreasing inflammation in mice. Pharm Biol 54:2995–3000
Hayashi H, Sudo H (2009) Economic importance of licorice. Plant Biotechnol 26:101–104
Hemmati F, Ghasemi R, Mohamed Ibrahim N, Dargahi L, Mohamed Z, Raymond AA, Ahmadiani A (2014) Crosstalk between insulin and Toll-like receptor signaling pathways in the central nervous system. Mol Neurobiol 50:797–810
Hibasami H, Iwase H, Yoshioka K, Takahashi H (2006) Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int J Mol Med 17:215–219
Hoever G, Baltina L, Michaelis M, Kondratenko R, Baltina L, Tolstikov GA, Doerr HW, Cinatl J Jr (2005) Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem 48:1256–1259
Hou S, Zheng F, Li Y, Gao L, Zhang J (2014) The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose. Int J Mol Sci 15:15026–15043
Kizhner T, Werman MJ (2002) Long-term fructose intake: biochemical consequences and altered renal histology in the male rat. Metabolism 51:1538–1547
Kutlubay R, Oguz EO, Guven C, Can B, Sinik Z, Tuncay OL (2007) Histological and ultrastructural evidence for protective effects on aluminium-induced kidney damage by intraperitoneal administration of alpha-tocopherol. Int J Toxicol 26:95–101
Lin JC (2003) Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antivir Res 59:41–47
Lin JL, Yang YJ, Yang SS, Leu ML (1997) Aluminum utensils contribute to aluminum accumulation in patients with renal disease. Am J Kidney Dis 30:653–658
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Mahieu ST, Gionotti M, Millen N, Elias MM (2003) Effect of chronic accumulation of aluminum on renal function, cortical renal oxidative stress and cortical renal organic anion transport in rats. Arch Toxicol 77:605–612
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Mudge DW, Johnson DW, Hawley CM, Campbell SB, Isbel NM, van Eps CL, Petrie JJ (2011) Do aluminium-based phosphate binders continue to have a role in contemporary nephrology practice? BMC Nephrol 12:20
Nakayama T, Kosugi T, Gersch M, Connor T, Sanchez-Lozada LG, Lanaspa MA, Roncal C, Perez-Pozo SE, Johnson RJ, Nakagawa T (2010) Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Ren Physiol 298:F712–F720
Oudot C, Lajoix AD, Jover B, Rugale C (2013) Dietary sodium restriction prevents kidney damage in high fructose-fed rats. Kidney Int 83:674–683
Qiao Y, Xu L, Tao X, Yin L, Qi Y, Xu Y, Han X, Tang Z, Ma X, Liu K, Peng J (2018) Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol Lett 284:37–45
Sargazi M, Shenkin A, Roberts NB (2006) Aluminium-induced injury to kidney proximal tubular cells: effects on markers of oxidative damage. J Trace Elem Med Biol 19:267–273
Sen S, Roy M, Chakraborti AS (2011) Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J Pharm Pharmacol 63:287–296
Sil R, Ray D, Chakraborti AS (2013) Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Indian J Exp Biol 51:129–138
Smith KD (2009) Toll-like receptors in kidney disease. Curr Opin Nephrol Hypertens 18:189–196
Takii H, Kometani T, Nishimura T, Nakae T, Okada S, Fushiki T (2001) Antidiabetic effect of glycyrrhizin in genetically diabetic KK-ay mice. Biol Pharm Bull 24:484–487
Wu CH, Chen AZ, Yen GC (2015) Protective effects of glycyrrhizic acid and 18beta-glycyrrhetinic acid against cisplatin-induced nephrotoxicity in BALB/c mice. J Agric Food Chem 63:1200–1209
Zakaria S, Hasan RA, Mahmoud MF, El Fayoumi HM, Mahmoud AAA (2020) The concurrent exposure to aluminium and fructose induces liver injury in rats: protection by monoammonium glycyrrhizinate. Clin Exp Pharmacol Physiol 47:809–820
Zheng QZ, Lou YJ (2003) Pathologic characteristics of immunologic injury in primary cultured rat hepatocytes and protective effect of glycyrrhizin in vitro. Acta Pharmacol Sin 24:771–777
Acknowledgments
The authors acknowledge Dr. Rehab Hasan, Department of Histology, Faculty of Medicine, Al-Azhar University, Egypt, for her great effort in the histopathological examination.
Author information
Authors and Affiliations
Contributions
MFM, HME, and AAAM conceived and designed research. NAE conducted the experiments. NAE and AAAM analyzed the data. NAE and AAAM wrote the first draft of manuscript. MFM, HME, and AAAM revised and edited the manuscript. All the authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
The study protocol and animal handling procedures were approved by the Ethical Committee for Animal Handling at Zagazig University (ECAH ZU), Faculty of Pharmacy, Zagazig University, Egypt based on the recommendations of the Weatherall report.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Emara, N.A., Mahmoud, M.F., El Fayoumi, H.M. et al. The renoprotective effect of glycyrrhizic acid in insulin-resistant rats exposed to aluminum involves the inhibition of TLR4/NF-κB signaling pathway. Naunyn-Schmiedeberg's Arch Pharmacol 394, 863–872 (2021). https://doi.org/10.1007/s00210-020-02012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-02012-y