Abstract
In the modern world, indiscriminate human activities impelled environmental toxicity through heavy metals such as cadmium (Cd) that poses significant health hazards to the flora and fauna. Multiple mechanisms such as oxidative stress, inflammation, apoptotic cell death, and chromosomal aberrations underlie the Cd-induced organ toxicity with the liver and kidneys bearing most of the brunt. Fumaric acid (FA) is an organic acid (C4H4O4) omnipresent in nature and attributed with such properties (e.g., antioxidant, anti-inflammatory, analgesic, chemopreventive, anti-psoriatic, immunomodulatory, and neuroprotective) that may bestow relief in Cd-induced liver damage. Hence, in the present study, the protective effects of FA were determined in Cd-induced hepatotoxicity in rats. Wistar rats were chronically exposed to Cd (5 mg/kg, p.o.) to induce liver dysfunction. The rats were subjected to FA (1.25, 2.5, 5 mg/kg; p.o.) pre-treatment for 28 days to observe effects on liver and serum biomarkers of oxidative stress, enzymatic activities, and hepatic damage (liver histopathology). Body weights, feed/water intake, body mass index (BMI), and non-invasive parameters (FIB-4 score; AST/ALT ratio) were quantified. Cd-triggered hepatic injury in rats through oxidative stress, derangement of hepatic serum biomarkers (ALT, AST, ALP, LDH, bilirubin, cholesterol, triglycerides, uric acid, and platelet count), and pathogenic alteration in non-invasive parameters. FA pre-treatment significantly protected rat livers against Cd toxicity by decreasing oxidative stress and improving the hepatic serum biomarkers and non-invasive parameters. In a histopathological analysis, FA prevented Cd-accrued hepatocellular damage. Fumaric acid showed potential to avert hepatic injury against cadmium in rats.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a well-known category-I (International Agency for Research on Cancer) carcinogen, having potential to induce prostate, renal, breast, and pulmonary cancers. In addition to environmental or occupational exposures, the other key sources of Cd intoxication are cigarette smoking, industrial emissions, farm products, sea food, and ingestion of contaminated food and water (Hartwig 2013). Chronic exposures to Cd pose toxic effects on the skeletal, reproductive, cardiovascular, renal, respiratory, and nervous systems (Rani et al. 2014). In human beings, Cd (t1/2 15–30 years) binds with sulfhydryl (–SH) group proteins mostly heavy metal binding metallothionein (MT) proteins, with maximum concentrations in the liver and kidneys. Higher levels of Cd depletes the –SH antioxidants (e.g., glutathione, MTs) and thereby, jeopardizes the cells to oxidative stress. Multiple mechanisms underlying Cd toxicity include free radicals (e.g., reactive oxygen species and nitrogen species), chromosomal aberrations, DNA strand break, and mutations (Bernhoft 2013). Cd enhances the intracellular calcium levels, inflammation (e.g., nuclear factor-kappaB) and activates apoptotic pathways through caspases, p53 triggered mitochondrial dysfunction, and apoptotic factors (e.g., Bcl-2, Bad) (Rani et al. 2014). Pre-existing metabolic disorders such as diabetes amplify the Cd-triggered toxicity manifolds (Bernhoft 2013).
The liver is the largest organ responsible for digestion, excretion, and detoxification, and storage of several nutrients and minerals. Liver-associated disorders are on the rise worldwide due to modified lifestyle, pollution, drug abuse, and infections that make liver diseases most fatal in the modern world. There are enough evidences for Cd-induced inflammation (Kupffer cell activation, neutrophil infiltration), necrotic hepatocellular death, non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH) in human adults (Hyder et al. 2013). Although hepatic cells and proximal tubule of nephrons are the two major sites of Cd toxicity, however, hepatocellular damage is far greater than renal at initial stages Cd exposure (Andjelkovic et al. 2019). Several previous studies indicate that restrain over free radical production may bestow benefits in curbing the Cd-induced liver toxicity (Andjelkovic et al. 2019; Rafati Rahimzadeh et al. 2017). In light of the above facts, prevention of Cd toxicity is need of the hour at present, whereas the pharmacological interventions available at present such as meso-2,3-dimercaptosuccinic acid, 2,3-dimercapto-1-propane sulfonic acid, and ethylenediaminetetraacetic acid (EDTA) are curative only (Rafati Rahimzadeh et al. 2017). Indiscriminate human activities made the Cd a ubiquitous environmental pollutant ranging from soil, food chain, water, and air. Hence, palliative therapy is the best alternative for eliminating the Cd-induced health hazards.
Fumaric acid (FA) is a naturally occurring organic acid (C4H4O4) omnipresent in flora and fauna. Chemically, it is an unsaturated dicarboxylic acid, first detected in fungus Boletus pseudoignarius. Winkler isolated FA from Fumaria officinalis L. in 1832 (Wollina 2011; Xu et al. 2012). FA is abundantly present in Papaveraceae family (poppy plants), lichens, and mushrooms in considerably higher concentrations. Fumaric acid esters (FAE) are used in various skin disorders such as psoriasis, sarcoidosis, granuloma annulare, necrobiosis lipoidica, and malignant melanoma since late 1950s. In the 1990s, Fumaderm® was a registered pharmaceutical product for treatment of psoriasis in Germany (Wollina 2011). In the search for novel pharmacologically active compounds, FA and its derivatives are important and promising compounds with high potential for development into therapeutically active pharmaceuticals. Fumaric acid and FAE are well-known antioxidants that bestow several health benefits due to their strong free radical scavenging properties, anti-inflammatory, immunomodulatory, and chemopreventive effects (Kronenberg et al. 2019; Ahuja et al. 2016; Shakya et al. 2014; Noura et al. 2017; McGuire et al. 2016). The FA induced protection of the brain through activation of nuclear factor-erythroid-2/antioxidant response element (Nrf-2/ARE) pathway, and expression of neuroprotective factors aptly shows its potential against oxidative stress (Kronenberg et al. 2019; Ahuja et al. 2016). In earlier studies, FA depicted promising results in hypercholesterolemic rabbits (Noura et al. 2017). These findings indicate that FA may provide benefits in Cd-accrued liver disorders. Hence, in the present study, we aimed to assess the hepatoprotective effects of fumaric acid FA against Cd-induced toxicity in rats.
Materials and methods
Experimental animals and protocol
Adult Wistar rats (either sex), weighing between 160–180 g, were procured from a CPCSEA registered breeder. The research protocol of this study was approved by the Institutional Animal Ethics Committee (IAEC) of ASBASJSM College of Pharmacy, Bela (Ropar) vide approval no. ASCB/IAEC/12/18/129. The animals were maintained under standard laboratory conditions controlled temperature (23 ± 2 °C), humidity (40 ± 10%), and light-dark cycle (12 h each) under the guidelines of the CPCSEA, New Delhi (India). The animals were kept on water and pellet diet (Ashirwad Industries, Mohali) ad libitum. The animals were acclimatized for a week before the initiation of experiments. Fumaric acid (FA doses 1.25, 2.5, 5 mg/kg) (Shakya et al. 2014) and cadmium chloride (Cd dose 5 mg/kg) (Sigma-Aldrich, India) (Adefegha et al. 2015) doses were prepared freshly using 3% carboxymethyl cellulose (CMC) and administered orally to rats for 28 days daily. Thirty rats were divided into 5 groups (n = 6) in a single-blind fashion using random allocation method. Disease control group (Cd) received cadmium chloride (5 mg/kg) to induce hepatotoxicity. Three separate groups (Cd + FA1.25, Cd + FA2.5, Cd + FA5) were given FA (doses 1.25 mg/kg, 2.5 mg/kg, and 5 mg/kg) 1 h prior to administration of Cd (5 mg/kg) daily for 28 days. Control group received same amount of vehicle only for 28 days daily (volume 5 ml/kg). The mean body weight, feed and water intake, and body mass index (BMI) of rats were analyzed at day 1 and thereafter each week. Afterwards, the blood samples were collected and the rats were euthanized by cervical dislocation on day 28. The liver tissues were harvested for biochemical analysis. Histopathological analysis of the liver tissue was accomplished using hematoxylin and eosin stain.
Analysis of blood biomarkers
After the treatment period, the blood samples (1.5–2 ml) were collected by puncturing retro-orbital plexus. Blood samples were centrifuged (REMI, Mumbai) at 1000×g for 10 min (room temperature) to separate the serum for further biochemical analysis. The liver enzyme markers such as alanine amino transferase (ALT/SGPT), aspartate aminotransferase (AST/SGOT), alkaline aminotransferase (ALP), and lactate dehydrogenase (LDH) were evaluated. Cholesterol, total bilirubin, triglyceride, uric acid, and platelet count were also analyzed in the blood samples. Standard procedures were employed for measuring the biochemical parameters as provided in the kits purchased from Arkray Healthcare Pvt., Ltd., Mumbai (India) (AutoSpan®) and Reckon Diagnostics P. Ltd., Vadodara (India). On the basis of the serum biomarkers, the non-invasive parameters such as FIB-4 score (Angulo et al. 2007) and AST/ALT ratios (Williams and Hoofnagle 1988) were assessed.
Assessment of biomarkers of oxidative stress
The rats were euthanized by cervical dislocation. The whole livers were harvested, minced to small pieces (1 cm3), and divided for homogenate preparation and histopathology. The liver tissues were homogenized (REMI, Mumbai) in 0.05 M ice-cold phosphate-buffered saline (PBS) and centrifuged (3000×g, 10 min, 4 °C) to collect the supernatant for evaluation of the antioxidant activities such as lipid peroxidation (Ohkawa et al. 1979), reduced glutathione (GSH) (Ellman 1959), superoxide dismutase (SOD) (Kono 1978), glutathione peroxidase (GPx) (Rotruck et al. 1973), and catalase activity (CAT) (Luck 1965) by the standard methods.
Histopathological evaluation
The liver tissue were rinsed in PBS buffer and fixed in 10% neutral buffered formalin (room temperature, 24 h) for histopathological analysis. Afterwards, the liver tissue was dehydrated using different concentrations of ethanol (70–100 %), cleared in xylene, and paraffin wax was used as embedding agent. The 5-μm sections were trimmed out using microtome and stained with hematoxylin and eosin (H&E). Permanent slides were prepared using cover slips and synthetic resin DPX. The pathologic structural changes were examined under light microscope under × 100 magnifications.
Statistical analysis
The data were expressed as mean ± standard error of mean (SEM). The data was analyzed using a one-way ANOVA followed by Tukey’s multiple comparison tests or a two-way ANOVA followed by Bonferroni’s post hoc test using Graph Pad Prism 5.0 software package. A value of p < 0.05 was considered to be significant.
Results
Fumaric acid prevents cadmium-triggered decrease in feed and water intake, and mean body weights
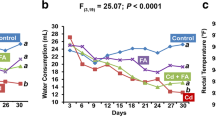
The Cd group showed marked decrease in the feed intake (Fig. 1b) on 1st (p < 0.05), 2nd (p < 0.01), 3rd (p < 0.001), and 4th week (p < 0.001); and water intake on 2nd (p < 0.01), 3rd (p < 0.01), and 4th week (p < 0.001) (Fig. 1c) when compared with vehicle control group. Consistently, administration of Cd (5 mg/kg) in rats significantly (3rd week, p < 0.05; 4th week, p < 0.001) decreased the mean body weight when compared with the rats given vehicle only (Fig. 1a). FA pre-treatment gradually increased the feed/water consumption against Cd over the duration of 4 weeks. Cd + FA1.25 group showed significant enhancement in feed (1st week, p < 0.01; 2nd, 3rd, and 4th week, p < 0.001) and water consumption (2nd, 3rd, and 4th week, p < 0.001) when compared with Cd control group. Cd + FA2.5 group depicted significant improvement in feed (2nd week, p < 0.05; 3rd and 4th week, p < 0.001), and water consumption (3rd week, p < 0.05) when compared with Cd control group. Pre-treatment with a high dose of FA (5 mg/kg) significantly prevented the Cd-triggered decline of feed (1st week, p < 0.05; 3rd week, p < 0.05; 4th week, p < 0.001) and water intake (3rd week, p < 0.05; 4th week, p < 0.01) in relation with rats that were subjected to Cd alone. In harmony with these results, pre-treatment with FA at a low dose (1.25 mg/kg) significantly prevented the Cd-induced reduction in body weight of rats on 2nd (p < 0.05), 3rd (p < 0.001), and 4th week (p < 0.001) when compared with rats which received Cd only. FA (2.5 mg/kg) averted the body weight reduction at 3rd (p < 0.05) and 4th week (p < 0.01) in Cd-treated rats when compared with rats administered with Cd only. Pre-treatment with a high dose of FA (5 mg/kg) significantly (p < 0.001) prevented the mean body weight reduction against Cd in 3rd and 4th week when compared with Cd control group.
Effect of fumaric acid (FA; 1.25, 2.5, and 5 mg/kg; p.o.) pre-treatment for 28 days on (a) mean body weight, (b) feed intake, (c) water intake, and (d) body mass index (BMI) of cadmium (Cd; 5 mg/kg; p.o.)-administered rats. Body weight (g) of each rat was quantified daily. Each rat was placed on digital animal weighing machine and weight (g) was recorded after stabilization of the rat. A predetermined quantity of food/water was placed in cages each morning (0900 h) and the left-over was measured after 24 h each day. The difference was noted as food/water consumed (g or ml) for each animal. For BMI [BMI (g/cm2) = weight (g)/length (cm2)], the length of the rats was measured between nasal and anal region. Mean body weights, food/water intake, and BMI of each group were analyzed on day 1 (before treatments) and weekly thereafter (days 7, 14, 21, 28). A gradual increase in mean body weight, food/water intake, and BMI was noted in control (vehicle) group. Cd administration significantly decreased these parameters in comparison with vehicle treatment. Pre-treatment with FA significantly prevented the Cd-induced decrease in body weights, feed/water intake, and BMI rats. Values are expressed as mean ± SEM (n = 6) and analyzed using two-way ANOVA followed by Bonferroni’s post hoc test: ap < 0.05, bp < 0.01, cp < 0.001 vs control group; xp < 0.05, yp < 0.01, zp < 0.001 vs Cd group
Fumaric acid averts cadmium-induced reduction in BMI
Exposure of rats to Cd decreased the BMI significantly on days 14 (p < 0.05), 21 (p < 0.001), and 28 (p < 0.001) when compared with rats that received vehicle only. Pre-treatment of Cd-administered rats with FA showed gradual increase in the BMI. Cd + FA1.25 group showed significant increase in BMI on days 7 (p < 0.01), 14 (p < 0.001), 21 (p < 0.001), and 28 (p < 0.001) in relation with Cd control group. Cd + FA2.5 group depicted marked rise in BMI on days 21 (p < 0.01) and 28 (p < 0.001) with respect to Cd group. Pre-treatment with high dose of FA (5 mg/kg) significantly increased the BMI on days 14 (p < 0.05), 21 (p < 0.001), and 28 (p < 0.001) against Cd when compared with rats subjected to Cd treatment only (Fig. 1d).
Mean liver weight remain unaltered
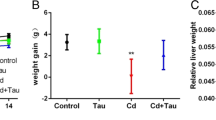
There was no significant inter-group variation in relation with mean liver weights between different groups. The Cd group showed no significant change in liver weights of rats in relation to control group. FA pre-treatment in rats exposed to Cd had no significant effect on mean liver weights with respect to rats subjected to Cd alone treatment (Fig. 2).
Effect of fumaric acid (1.25, 2.5, and 5 mg/kg; p.o.) and/or cadmium (5 mg/kg; p.o.) treatments on mean liver weight in rats. The whole liver collected 3 h after the last dose of fumaric acid (FA), and cadmium (Cd) on day 28 were rinsed in isotonic PBS and weighed. Mean liver weight (g) of each group was calculated and analyzed using one-way ANOVA. There was no significant inter-group variation with respect to liver weights. Treatment with FA (doses 1.25, 2.5, and 5 mg/kg; p.o.) and/or Cd (5 mg/kg; p.o.) caused no significant variation in the mean liver weights in comparison with vehicle treatment (control rats). Values are expressed as mean ± SEM (n = 6)
Fumaric acid prevents the cadmium-induced derangement of serum biomarkers
Chronic exposure to Cd significantly (p < 0.001) increased the serum levels of ALT, AST, ALP, LDH, cholesterol, total bilirubin, triglycerides, and uric acid levels, and decreased (p < 0.001) the platelet count when compared with vehicle alone treatment. However, pre-treatment of rats with FA (1.25 mg/kg) significantly prevented the Cd-triggered rise in levels of ALT (p < 0.05), AST (p < 0.05), ALP (p < 0.05), LDH (p < 0.05), cholesterol (p < 0.01), triglycerides (p < 0.001), and uric acid (p < 0.05). Cd + FA2.5 group showed decrease in the ALT (p < 0.001), AST (p < 0.01), ALP (p < 0.001), LDH (p < 0.01), cholesterol (p < 0.001), total bilirubin (p < 0.05), triglycerides (p < 0.001), and uric acid (p < 0.05) content in comparison with Cd group. Cd + FA5 group showed decrease in the ALT (p < 0.001), AST (p < 0.001), ALP (p < 0.001), LDH (p < 0.01), cholesterol (p < 0.001), total bilirubin (p < 0.01), triglycerides (p < 0.001), and uric acid (p < 0.01) content in comparison with Cd group. FA also averted the decline (2.5 mg/kg, p < 0.05; 5 mg/kg, p < 0.01) in platelet count with respected to rats that received Cd alone (Table 1).
Fumaric acid prevents the cadmium-induced increase in FIB-4 score
Chronic treatment with Cd caused a significant (p < 0.001) increase in FIB-4 score of rats as compared with vehicle only treatment. However, pre-treatment with FA (1.25, 2.5, and 5 mg/kg) for 28 days significantly (p < 0.001) prevented the Cd-triggered increase in FIB-4 score of rats in comparison with rats that received Cd alone (Fig. 3a).
Effect of fumaric acid (FA; 1.25, 2.5, and 5 mg/kg; p.o.) pre-treatment for 28 days on FIB-4 score and AST/ALT ratio in rats administered with cadmium (Cd; 5 mg/kg; p.o.). Blood samples from retro-orbital plexus taken on day 28 and serum separated for enzymatic measurements. (a) Fibrosis-4 index for liver fibrosis [FIB-4 = {Age (years) × AST(IU/l)}/{platelet count(103/mm3) × (ALT(IU/l))½}] and (b) ratio of aspartate aminotransferase/alanine aminotransferase (AST/ALT ratio) were calculated for different groups. Vehicle-treated control group showed normal FIB-4 score and AST/ALT ratio. Cadmium (Cd) enhanced FIB-4~4.5 and AST/ALT ratio > 1 that signified liver dysfunction. Cadmium (5 mg/kg; p.o.)-induced increase in FIB-4 score was significantly prevented by FA (doses 1.25, 2.5, and 5 mg/kg; p.o.) and AST/ALT ratio was lowered (< 1). Values are expressed as mean ± SEM (n = 6) and analyzed using one-way ANOVA followed by Tukey’s multiple comparison test: cp < 0.001 vs control group; zp < 0.001 vs Cd group
Fumaric acid averts the cadmium-triggered increase in AST/ALT ratio
Chronic exposure of rats to Cd caused a significant (p < 0.001) increase in AST/ALT ratio as compared with rats that were subjected to vehicle alone treatment. The AST/ALT ratio value was more than 1 that indicated advanced liver fibrosis. Pre-treatment of Cd-administered rats with FA (1.25, 2.5, and 5 mg/kg) showed moderate (p > 0.05) decrease in AST/ALT ratio (Fig. 3b). However, the AST/ALT ratio of FA pre-treated rats was less than 1 that indicated prevention of liver fibrosis against Cd toxicity.
Fumaric acid prevents cadmium-induced increase in hepatic oxidative stress
Chronic exposure of rats to Cd caused a significant (p < 0.001) increase in the levels of thiobarbituric acid reactive substances (TBARS) and decrease (p < 0.001) in the levels of GSH, SOD, GPx, and CAT activity in the liver when compared with vehicle-treated rats. FA (1.25, 2.5, 5 mg/kg) pre-treatment significantly (p < 0.001) decreased the hepatic TBARS level against Cd in comparison with Cd alone–administered rats (Fig. 4a). The Cd-induced decrease in GSH levels was significantly prevented by FA pre-treatment (1.25 mg/kg, p < 0.05; 2.5 mg/kg, p < 0.01; 5 mg/kg, p < 0.01) with respect to rats treated with Cd only (Fig. 4b). FA prevented (1.25 mg/kg, p < 0.05; 2.5 mg/kg, p < 0.01; 5 mg/kg, p < 0.001) the Cd-triggered decline of SOD and GPx activity in the liver of rats when compared with rats treated with Cd alone (Fig. 4 c and d). Furthermore, the CAT activity was considerably increased in the liver of rats that were exposed to FA (1.25 mg/kg, p < 0.05; 2.5 mg/kg, p < 0.001; 5 mg/kg, p < 0.001) and Cd in comparison with rats that were subjected to Cd alone (Fig. 4e).
Effect of fumaric acid (FA; 1.25, 2.5, and 5 mg/kg; p.o.) pre-treatment for 28 days on biomarkers of hepatic oxidative stress in cadmium (Cd; 5 mg/kg; p.o.)-administered rats. (a) Thiobarbituric acid reactive substances (TBARS), (b) reduced glutathione (GSH) content, (c) superoxide dismutase (SOD), (d) glutathione peroxidase (GPx), and (e) catalase (CAT) activity were measured on day 28 using standard methods. Absorbance (Abs) of chromophores MDA-TBA2 adduct (λmax = 532 nm, TBARS) and 2-nitro-5-thiobenzoic acid (λmax = 412 nm, GSH) was noted using a double-beam UV-spectrophotometer (Shimadzu UV-1700, PharmaSpec) and molar extinction coefficients (ε) used to calculate TBARS (ε = 1.56 × 105 M−1 cm−1) and GSH (ε = 1.36 × 104 M−1 cm−1). Change in absorbance (∆Abs) of NBT-diformazan blue (λmax = 560 nm, SOD), 2-nitro-5-thiobenzoic acid (λmax = 412 nm, GPx), and hydrogen peroxide (λmax = 240 nm, CAT) was noted for 3–5 min at 30-s interval and molar extinction coefficients (ε) were used to calculate rate SOD (ε = 15,000 M−1 cm−1), GPx (ε = 1.36 × 104 M−1 cm−1), and CAT (ε = 43.6 M−1 cm−1) activity on basis of slope obtained during first 90 s. For SOD, 1 unit = 50% inhibition of rate of NBT-formazan formation; CAT, 1 unit = decomposition of 1 μM H2O2/min under standard assay conditions (pH 7, 25 °C); GPx, 1 unit = 1 μM GSH reduced/min. Cd significantly enhanced the oxidative stress in comparison with vehicle (3% CMC) treatment (control). Pre-treatment with FA (1.25, 2.5, and 5 mg/kg) significantly prevented Cd-induced increase in TBARS content and decrease in GSH, SOD, CAT, and GPx activity in hepatic tissues homogenate. Values are expressed as mean ± SEM (n = 6) and analyzed using one-way ANOVA followed by Tukey’s multiple comparison test: a(p < 0.05), b(p < 0.01), c(p < 0.001) vs control group; x(p < 0.05), y(p < 0.01), z(p < 0.001) vs Cd group
Liver histopathological analysis
Vehicle control group depicted no vacuolar and granular degeneration, sinusoidal dilatations, and portal triad widening. The Cd-treated group clearly showed signs of vacuolar and granular degeneration sites (V), sinusoidal dilatations (S), and portal triad widening (PT). Cd + FA1.25 group showed moderate signs of V, S, and PT. In Cd + FA2.5 group, no V, minor S, and PT were observed. In Cd + FA5 group, only minor PT was noted, however, an improvement in hepatocyte damage was also observed (Fig. 5).
Effect of fumaric acid (FA; 1.25, 2.5, and 5 mg/kg; p.o.) pre-treatment on histopathology of liver tissue of cadmium (Cd; 5 mg/kg; p.o.)-administered rats. The liver sections (5 μm) were stained with hematoxylin and eosin on day 28 and analyzed under magnification × 100 using a light microscope. Control (3% CMC-vehicle) group showed normal hepatic structure. Chronic exposure of rats to Cd (28 days) caused vacuolar and granular degeneration (V), sinusoidal dilatations (S), and portal triad widening (PT) in the liver. FA (1.25, 2.5 and 5 mg/kg) pre-treatment for 28 days prevented the Cd-induced hepatic architectural destruction in rats. Study groups: (a) control, (b) Cd, (c) Cd + FA1.25, (d) Cd + FA2.5, and (e) Cd + FA5
Discussion
Cd is an environmental pollutant which enters into the food chain through diverse means that necessitates the prevention of Cd-triggered organ toxicity in humans (Bernhoft 2013). World Health Organization (WHO) and US Agency for Toxic Substances and Disease Registry (ATSDR) have grouped Cd in most hazardous chemicals list (Andjelkovic et al. 2019). Many of the literature reports indicate adverse effects of Cd on the liver (Rani et al. 2014; Andjelkovic et al. 2019). In the present study, we observed considerable hepatotoxic effects of long-term exposure of Cd in rats, which were prevented by FA pre-treatment.
In this study, exposure to Cd decreased the feed and water intake of rats. The mean body weight and BMI of rats were also decreased significantly by Cd. These results are in harmony with the previous findings that indicated reduction in weights and BMI by Cd, owing largely to detrimental effects on liver functions (Padilla et al. 2010). Non-localized pain, distress, inflammation, anorexia, and hampering of animal locomotion are the other major causes of Cd-induced decline in mean body weight and BMI. However, administration of FA before Cd significantly prevented the decline in body weight, feed intake, water intake, and BMI of rats over the duration of 4 weeks. These observations are well supported by histopathological assessments of the liver tissue that corroborated reduction in key signs of hepatocellular damage by FA pre-treatment in Cd-administered animals. The biochemical findings also revealed that FA prevented the Cd-triggered derangements of hepatic transaminase enzymes and other parameters (e.g., LDH activity, cholesterol, bilirubin, uric acid, triglyceride, oxidative stress, and redox imbalance). Hence, the improvement in liver functions by FA against Cd toxicity may be attributed to the observed increase in body weights and feed and water intake of animals.
AST, ALT, and ALP are involved in amino acid catabolism and bile production, and serve as important diagnostic biomarkers of liver function. Sever damage to the plasma membrane structural integrity causes the leakage of liver enzymes in the blood stream that leads to increase in serum levels of hepatic transaminases (Hall and Cash 2012). The hepatic content of AST and ALT enzymes is high; hence, the occurrence of these enzymes in serum depicts the amount of hepatic tissue damage. Pathogenic rise in serum ALP content indicates the excretory disturbances that also correlate with impaired membrane and hepatobiliary duct integrity. A higher value of AST, ALT, and ALP activity than the standard correlates with liver disorders and damage (Hall and Cash 2012). The pathogenic increase of these enzymes in the blood owing to compromised hepatocellular integrity is associated with Cd levels in previous reports (Kang et al. 2013). The above findings correlate with the present study, which revealed that exposure of rats to Cd elevated the enzyme (AST, ALT, and ALP) levels in serum considerably. FA pre-treatment caused a significant reduction in AST, ALT, and ALP levels in Cd-treated rats. In support with histopathological assessment, the prevention of Cd-induced liver damage by FA may be responsible for the observed decrease in serum content of these enzymes. LDH is released in response to damage to plasma membrane, and cells undergoing apoptosis and necrosis. Basically a measure of cytotoxicity, LDH serve as cell death marker (Li et al. 2015). In this study, Cd enhanced the serum LDH content considerably that showed hepatocellular damage. Pre-treatment with FA prevented the Cd-triggered hepatic cell death significantly and thus, showed hepatoprotective potential. Furthermore, the serum levels of cholesterol, total bilirubin, triglycerides, and uric acid were markedly increased in rats treated with Cd alone. These pathogenic changes are possibly accrued through Cd-induced alteration in enzymatic activities, oxidative stress, and inflammation that aptly shows liver dysfunction. However, FA administration significantly prevented the increase of these parameters in serum of Cd-treated rats, which showed that FA prevents liver malfunctioning.
The non-invasive approach was used to quantify the liver function or fibrosis (Angulo et al. 2007; Williams and Hoofnagle 1988). Chronic exposure of rats to Cd caused significant alteration in non-invasive parameters viz. FIB-4 score (4.5) and AST/ALT ratio (> 1), which indicated the advanced fibrosis in Cd control rats. Pre-treatment of Cd-administered rats with fumaric acid (1.25, 2.5, and 5 mg/kg) significantly averted the rise in FIB-4 score (p < 0.001) and modestly decreased the AST/ALT ratio (p > 0.05). However, the AST/ALT ratio of FA pre-treated rats was found to be less than 1. These findings indicated prevention of Cd-triggered fibrosis in FA-treated groups.
The hepatotoxic activity of Cd has been attributed to reactive oxygen species (ROS), lipid and protein peroxidation, and inflammation. Cd lowers the antioxidant defense (e.g., GSH, SOD, and catalase) that jeopardizes the cell to oxidative damage (Hartwig 2013; Rani et al. 2014; Bernhoft 2013; Hyder et al. 2013; Andjelkovic et al. 2019; Rafati Rahimzadeh et al. 2017). ROS such as hydrogen peroxide (H2O2), hydroxyl, and superoxide radicals modify the cell components such as lipids, carbohydrates, and proteins leading to disruption of cell integrity and metabolic dysfunction. ROS trigger biogenesis of lipid peroxidation products such as malondialdehyde (MDA), 4-hydroxy 2-nonenal (4-HNE), and isoprostanes that further potentiate formation of highly stable toxic adducts by reacting with diverse biological substrates. Measurement of TBARS directly correlates with the MDA content and depicts the level of lipid peroxidation. In the present study, exposure to Cd conspicuously elevated the lipid peroxidation (TBARS) in hepatic cells and lowered the antioxidant levels (GSH, GPx, SOD, and CAT). MDA is the most notorious player of lipid peroxidation whose maliferous activities result in parenchymal cell injury (Andjelkovic et al. 2019). MDA forms adducts with many biomolecules such as DNA, advanced glycation end products (AGEs), and acetaldehyde that compromise the cell integrity (Li et al. 2015). FA is a food additive having diverse pharmacological actions (e.g., anti-carcinogenic, anti-psoriasis, immunosuppressive, neuroprotective), based chiefly on restoration of cellular redox balance through an increase in expression of thiol antioxidants (GSH), NAD(P)H:quinine reductase (NQO-1), and Nrf2-dependent pathways. The antioxidant activity of FA (or FAE) causes a decrease in nuclear translocation of nuclear factor-kappaB (NFκB), lymphocytes (T-cells, B-cells), peripheral blood mononuclear cells, adhesion molecules (ICAM-1, VCAM-1, selectins), pro-inflammatory cytokines (e.g., inducible nitric oxide synthase, interferon-γ, tumor necrosis factor-α, interleukins), and induction of hemeoxygenase-1 (anti-inflammatory stress protein) that are responsible for anti-inflammatory effects (Moharregh-Khiabani et al. 2009; Balak 2015). Consistent with the findings from previous studies, in this study, FA pre-treated rats showed significant decrease in the lipid peroxidation, and improvement in the GSH levels and GPx, SOD, and CAT activities against Cd-accrued oxidative stress. These findings indicate that FA can maintain cellular membrane integrity against free radicals and lipid peroxidation products through fortification of endogenous antioxidants. Furthermore, the reduction in oxidative stress by FA was dose-dependent in Cd-treated rats. The histopathological analysis of liver tissue showed considerable vacuolar and granular degeneration sites, sinusoidal dilatations, and portal triad widening in Cd-treated rats. These findings indicated hepatocellular damage by Cd. However, FA showed protective effect, and a marked reduction in liver tissue damage was noted in a dose-dependent manner. Hence, FA pre-treatment restricted the Cd-accrued hepatic cytotoxicity in the rats.
Data from human studies indicates very mild acute/chronic toxicities such as gastrointestinal symptoms (diarrhea, flatulence, stomach cramps), flushing, headache, mild lymphopenia, leukopenia, and eosinophilia with no drug interaction and teratogenic or mutagenic effects associated with FA or FAEs (Wollina 2011; Balak 2015). Some of these side effects (flushing, headache, and eosinophilia) occur at the beginning of therapy and later diminish with further exposure. The clinical efficacy and favorable safety profile permitted long-term use of FA esters (Fumaderm) against psoriasis in Germany and other European countries (Wollina 2011; Moharregh-Khiabani et al. 2009). In clinical trials, inhibition of NFκB activity, strengthening of –SH antioxidants (GSH), and induction of Nrf2/hemeoxygenase-1 pathway and type II dendritic cells by orally administered FAEs (Fumaderm) showed good therapeutic potential in patients suffering from inflammatory autoimmune diseases such as multiple sclerosis (MS). Suppression of immune-mediated inflammatory cascades by regulating redox homeostasis is responsible for therapeutic efficacy of FAE against psoriasis and MS (Moharregh-Khiabani et al. 2009; Balak 2015). Cd is an environmental toxicant that depletes –SH antioxidants (GSH, GPx) and triggers free radical biogenesis, inflammatory apoptosis, and NFκB activity in the liver (Rani et al. 2014; Bernhoft 2013; Hyder et al. 2013). In the current protocol, administration of FA for 28 days daily prevented the deleterious effects of Cd-induced oxidative stress, derangement of liver and serum biomarkers, and associated degenerative changes in the liver tissues of rats. The protection of liver functions by FA caused improvement in feed and water intake, body weights, and BMI of rats exposed to Cd. From the above discussion, it can be inferred that long-term usage of FA can prevent Cd hepatotoxicity in humans, however, monitoring of blood count (leucocytes/lymphocytes) at regular intervals (every 2 months) is recommended when taken chronically, as the anti-inflammatory effect of FA is associated with decrease in lymphocytes and leucocytes. It is suggested that the side effects can be reduced by taking FA with aspirin, antihistamine, proton-pump inhibitor drugs, and food or milk (Balak 2015). However, further investigations by using different agonist and antagonists are pivotal in order to establish the mechanistic pathways contributing to the therapeutic significance of FA.
Conclusion
Fumaric acid has the potential to prevent the cadmium-induced hepatotoxic damage. The hepatoprotective activity of fumaric acid is attributed to protection from oxidative stress, hepatocellular injury and improvement of various enzymatic activities.
References
Adefegha SA, Omojokun OS, Oboh G (2015) Modulatory effect of protocatechuic acid on cadmium induced nephrotoxicity and hepatoxicity in rats in vivo. Springerplus. 4:619. https://doi.org/10.1186/s40064-015-1408-6
Ahuja M, Ammal Kaidery N, Yang L, Calingasan N, Smirnova N, Gaisin A, Gaisina IN, Gazaryan I, Hushpulian DM, Kaddour-Djebbar I, Bollag WB, Morgan JC, Ratan RR, Starkov AA, Beal MF, Thomas B (2016) Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson’s-like disease. J Neurosci. 36(23):6332–6351. https://doi.org/10.1523/JNEUROSCI.0426-16.2016
Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health. 16(2):274. https://doi.org/10.3390/ijerph16020274
Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 45(4):846–854. https://doi.org/10.1002/hep.21496
Balak DM (2015) Fumaric acid esters in the management of psoriasis. Psoriasis (Auckl). 5:9–23. https://doi.org/10.2147/PTT.S51490
Bernhoft RA (2013) Cadmium toxicity and treatment. Scientific World Journal. 394652:1–7. https://doi.org/10.1155/2013/394652
Ellman G (1959) Tissue sulfhydryl groups. Arch Biochem Biophys. 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Hall P, Cash J (2012) What is the real function of the liver ‘function’ tests? Ulster Med J. 81(1):30–36
Hartwig A (2013) Cadmium and cancer. Met Ions Life Sci. 11:491–507. https://doi.org/10.1007/978-94-007-5179-8_15
Hyder O, Chung M, Cosgrove D, Herman JM, Li Z, Firoozmand A, Gurakar A, Koteish A, Pawlik TM (2013) Cadmium exposure and liver disease among US adults. J Gastrointest Surg. 17(7):1265–1273. https://doi.org/10.1007/s11605-013-2210-9
Kang MY, Cho SH, Lim YH, Seo JC, Hong YC (2013) Effects of environmental cadmium exposure on liver function in adults. Occup Environ Med. 70(4):268–273. https://doi.org/10.1136/oemed-2012-101063
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 186(1):189–195. https://doi.org/10.1016/0003-9861(78)90479-4
Kronenberg J, Pars K, Brieskorn M, Prajeeth CK, Heckers S, Schwenkenbecher P, Skripuletz T, Pul R, Pavlou A, Stangel M (2019) Fumaric acids directly influence gene expression of neuroprotective factors in rodent microglia. Int J Mol Sci. 20(2):325. https://doi.org/10.3390/ijms20020325
Li S, Tan HY, Wang N, Zhang Z-J, Lao L, Wong C-W, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 16(11):26087–26124. https://doi.org/10.3390/ijms161125942
Luck H (1965) Catalase. In: Bergmeyer HU (ed) Method of enzymatic analysis. Academic Press, New York, USA, pp 885–894. https://doi.org/10.1016/B978-0-12-395630-9.50158-4
McGuire VA, Ruiz-Zorrilla Diez T, Emmerich CH, Strickson S, Ritorto MS, Sutavani RV et al (2016) Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci Rep. 6:31159. https://doi.org/10.1038/srep31159
Moharregh-Khiabani D, Linker RA, Gold R, Stangel M (2009) Fumaric acid and its esters: an emerging treatment for multiple sclerosis. Curr Neuropharmacol. 7(1):60–64. https://doi.org/10.2174/157015909787602788
Noura OA, Shehatou GSG, Rahim MA, El-Awady MS, Suddek GM (2017) Antioxidant and anti-inflammatory effects of dimethyl fumarate in hypercholesterolemic rabbits. Egypt J Basic Appl Sci. 4(3):153–159. https://doi.org/10.1016/j.ejbas.2017.07.003
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Padilla MA, Elobeid M, Ruden DM, Allison DB (2010) An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int J Environ Res Public Health. 7(9):3332–3347. https://doi.org/10.3390/ijerph7093332
Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA (2017) Cadmium toxicity and treatment: an update. Caspian J Intern Med. 8(3):135–145. https://doi.org/10.22088/cjim.8.3.135
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res. 24(4):378–399. https://doi.org/10.1080/09603123.2013.835032
Rotruck J, Pope A, Ganther H, Swanson A, Hafeman D, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science. 179(4073):588–590. https://doi.org/10.1126/science.179.4073.588
Shakya A, Singh GK, Chatterjee SS, Kumar V (2014) Role of fumaric acid in anti-inflammatory and analgesic activities of a Fumaria indica extracts. J Intercult Ethnopharmacol. 3(4):173–178. https://doi.org/10.5455/jice.20140912021115
Williams AL, Hoofnagle JH (1988) Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 95(3):734–739. https://doi.org/10.1016/s0016-5085(88)80022-2
Wollina U (2011) Fumaric acid esters in dermatology. Indian Dermatol Online J. 2(2):111–119. https://doi.org/10.4103/2229-5178.86007
Xu G, Zou W, Chen X, Xu N, Liu L, Chen J (2012) Fumaric acid production in Saccharomyces cerevisiae by in silico aided metabolic engineering. Plos one. 7(12):e52086. https://doi.org/10.1371/journal.pone.0052086
Acknowledgments
The authors are thankful to the all the management committee member of the Amar Shaheed Baba Ajit Singh Jujhar Singh Memorial College of Pharmacy, BELA (Ropar), for providing the necessary research facilities.
Author information
Authors and Affiliations
Contributions
ASK conceptualized and designed the study. GK performed the experiments and collected the data. TBS and MK analyzed the data. ASK and MK drafted and revised the manuscript. All authors read and approved the manuscript. It is stated that all data were generated in-house and no paper mill was used.
Corresponding author
Ethics declarations
All the experiments were performed as per the ethical guidelines on animal experimentations provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), GOI, New Delhi.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 82 kb)
Rights and permissions
About this article
Cite this article
Kaur, G., Shivanandappa, T.B., Kumar, M. et al. Fumaric acid protect the cadmium-induced hepatotoxicity in rats: owing to its antioxidant, anti-inflammatory action and aid in recast the liver function. Naunyn-Schmiedeberg's Arch Pharmacol 393, 1911–1920 (2020). https://doi.org/10.1007/s00210-020-01900-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01900-7