Abstract
Cadmium (Cd), a heavy metal contaminant, seriously threatens human and animal health. Taurine (Tau) has been used against hepatotoxicity caused by different environmental toxins. However, it has not been elucidated whether Tau exerts its protective function against Cd-induced hepatotoxicity. The aim of this study was thus to evaluate the ameliorative function of Tau (500 mg/kg body weight intraperitoneally) on Cd-induced (2 mg/kg body weight intraperitoneally) liver toxicity in mice for 14 days. The histopathologic and ultrastructure changes as well as alterations in indexes related to liver function, antioxidant biomarkers, inflammatory, and apoptosis were evaluated. The results showed that Tau alleviated the vacuolar degeneration, nuclear condensation, mitochondria swelling, and cristae lysis of hepatocytes induced by Cd. In addition, Tau treatment significantly reduced the ALT, AST levels in serum, and inflammatory factor TNF-α and IL-1β in liver tissue. Furthermore, Tau treatment decreased the Bax/Bcl-2 ratio and cleaved caspase-3 protein expression levels. Taken together, these observations demonstrate that Tau has an important hepatic protective function against the inflammation and apoptosis induced by Cd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a ubiquitous environmental and industrial contaminant, which seriously threatens the health of animals and human beings due to its long metabolism rate [1, 2]. China is the largest producer of Cd worldwide, with an annual Cd production of approximately 8200 tons, accounting for one-third of the total global production (https://www.usgs.gov/centers/nmic/cadmium-statistics-and-information). Recently, the rapid increase in Cd contamination incidents has raised public concerns about its potential hazards. The general population is exposed to Cd primarily by inhalation of contaminated air and consumption of grains and vegetables produced from irrigated agricultural fields containing Cd wastewater [3]. As the primary organ for detoxification and metabolism, hence, liver is extremely susceptible to Cd exposure [1, 4]. Environmental Cd exposure was associated with, hepatic necroinflammation, non-alcoholic fatty liver disease, and liver fibrosis [5,6,7,8]. In addition, growing evidence reported potential relationships between Cd exposure and inflammation, oxidative stress, and apoptosis [9,10,11]. It is thus crucial to find the supplements or drugs to protect against hepatotoxicity resulted from Cd.
Antioxidants exist in many natural dietary sources, and daily consumption of antioxidants has a vital role in human and animal health [12, 13]. Taurine (Tau) is a semi-essential amino acid present in mammalian muscles, liver, brain, retina, adrenal gland, and other tissues. It is involved in several essential biological events, including the formation of bile salts, detoxification, biological membrane stabilization, osmoregulation, and regulation of intracellular Ca2+ concentration [14,15,16,17,18,19]. Tau has protective effects on various organ system diseases, such as cardiovascular [20, 21], integumentary [22], skeletal muscle [23], and endocrine systems [24]. Recent studies showed that Tau has functions against oxidative stress and inflammatory reactions, thus preventing apoptosis and necrotic cell death [25,26,27]. Oral administration of Tau prevented Cd-induced the decreases in antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), and glutathione reductase (GR) [28]. In addition, Tau significantly decreased inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in lipopolysaccharide-induced liver injury [29]. Most importantly, Tau has a strong potency in inhibiting apoptosis by increasing Bcl-2 levels and decreasing Bax and caspase-3 levels in hypoxic-ischemic brain injury in neonatal rats [30, 31].
Recently, Tau has been reported to ameliorate hepatotoxicity induced by various toxicants in the liver [26, 32]. However, the exact mechanism of Tau on Cd-induced liver disease has not been elucidated. Therefore, the purpose of this study was to explore the potential effects of Tau against Cd poisoning and elucidate the possible mechanisms in female mice. In addition, this study may provide a clue to the therapeutic application of Tau in Cd-induced hepatotoxicity.
Materials and Methods
Materials
Cadmium chloride was purchased from Sigma-Aldrich (St. Louis, MO, USA). Assay kits including total protein (TP), lactate dehydrogenase (LDH), alanine transaminase (ALT), and aspartate aminotransferase (AST) were purchased from Beijing Xinchuangyuan Bioengineering Institute (Beijing, China). Glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The bicinchoninic acid (BCA) protein assay kit was purchased from the Beyotime Institute of Biotechnology (Shanghai, China). Antibody against Bcl-2 (ab32124) was procured from Abcam (Cambridge, MA, USA). Antibodies against cleaved caspase-3 (9664), Bax (14796), and Horseradish Peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) were purchased from Cell Signaling Technology (Boston, MA, USA). Other chemicals used in this work were purchased from Shenggong Bioengineering Ltd. Company (Shanghai, China).
Experimental Animals
Twenty-four five-week-old ICR female mice were purchased from the Laboratory Animal Center of Yangzhou University. All animals were housed in an animal facility with a 12-/12-h light/dark cycle temperature of 20–22 °C, and humidity of 55 ± 5%. The mice had free access to water and food. All procedures were approved by the Animal Care and Use Committee at Yangzhou University (approval ID: SYXK (Su) 2017–0044). The animals were acclimatized for 1 week before starting the experiments.
Experimental Design
Both Cd and Tau solutions were prepared by dissolving in sterile saline. The concentrations of Cd solution and Tau solution were 0.4 mg/ml and 100 mg/ml, respectively. The doses of Cd and Tau were 2 and 500 mg/kg, respectively, according to the earlier literature [9, 33]. The animals were divided into 4 groups as (1) control group: received an equal volume of normal saline; (2) Tau group: received Tau intraperitoneally at a dose of 500 mg/kg body weight for 14 days, once daily; (3) Cd group: received Cd intraperitoneally at a dose of 2 mg/kg body weight for 14 days, once daily; and (4) Cd + Tau group: received Tau one hour prior to Cd injection for 14 days, once daily. The flow diagram of animal grouping and treatment is shown in Fig. S2.
Collection of Samples
The body weights of the mice were weighed from the beginning of the experiment and then every 2 days until day 14. After the end of the experiment, the mice were anesthetized with sodium pentobarbital (50 mg/kg). Blood samples were collected by excise the eyeballs and centrifuged at 3000 rpm for 10 min at 4 °C to obtain the serum. The livers were quickly isolated and weighed. Part of the liver was taken then fixed in 4% paraformaldehyde solution for histological analysis. The remained part of the liver was immediately frozen in liquid nitrogen and stored at − 80 °C. The relative weight liver was calculated as: Relative liver weight = (liver weight/body weight) × 100.
Histopathological Analysis
Liver samples were embedded in paraffin after gradient dehydration and xylene transparency. Sections were cut into 5 μm thickness, deparaffinized with xylene, and stained with hematoxylin and eosin (H&E) according to previous literature [9]. The sections were analyzed by light microscope (IX71; Olympus, Tokyo, Japan).
Electron Microscopic Investigations
Electron microscopic investigations protocol was according to previous literature [32]. In brief, the fresh liver tissues were cut into 1 mm3 sections and being fixed with 2.5% glutaraldehyde at 4 °C overnight. After fixed with osmium tetroxide, the samples were washed with 0.1M PBS and dehydrated by ethanol gradient and embedded in the resin to prepare ultrathin sections. After being stained in uranyl acetate and lead citrate, sections were observed under a transmission electron microscope (TEM) (H7800, Hitachi, Japan).
Measurement of Liver Function Biomarkers
Serum alanine transaminase (ALT), aspartate aminotransferase (AST), serum lactic dehydrogenase (LDH), and total protein (TP) levels were measured by commercial kits according to the manufacturer’s instructions.
Determination of GSH, MDA, and SOD Levels in the Liver
Liver tissues were homogenized in normal saline and centrifuged to obtain the supernatant. The contents of GSH, MDA, and the activities of SOD were detected by using commercial kits following the manufacturer’s instructions. The protein concentrations were determined using the BCA protein assay reagent.
Quantitative Real-Time PCR Analysis
Total RNA from livers was isolated using TRIzol reagent (Vazyme Biotech, Nanjing, China). Then, the cDNA was synthesized by a commercial reverse transcriptase kit (Vazyme Biotech, Nanjing, China) according to the manufacturer’s instructions. Real-time PCR was performed using the SYBR Green master mix (Vazyme Biotech, Nanjing, China). The primer sequence of GAPDH (XM_036165840.1) is, forward: 5′-GGTTGTCTCCTGCGACTTCA-3′, reverse: 5′-GGGTGGTCCAGGGTTTCTTA-3′. The primer sequence of TNF-α (NM_013693.3) is, forward: 5′-ACTGAACTTCGGGGTGATCG-3′, reverse: 5′-TGATCTGAGTGTGAGGGTCTGG-3′. The primer sequence of IL-1β (XM_006498795.5) is, forward: 5′-ATGAAAGACGGCACACCCAC-3′, reverse: 5′-GCTTGTGCTCTGCTTGTGAG-3′. The primer sequence of IL-6 (NM_001314054.1) is, forward: 5′-TGCAAGAGACTTCCATCCAGT-3′, reverse: 5′-GTGAAGTAGGGAAGGCCG-3′. The PCR protocol was as following: denaturation for 30 s at 95 °C, followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. The mRNA relative expression was calculated by the 2−△△Ct method and normalized to the mean of the values for GAPDH.
Western Blot Analysis
Western blot was performed as described previously [34]. Protein concentrations were quantified with a BCA protein assay kit. Equal amounts of protein were separated by 12% SDS–polyacrylamide gels and transferred onto PVDF membranes. Membranes were blocked with 5% (w/v) skim milk at room temperature for 1 h, followed by incubation with primary antibodies at 4 °C overnight. After incubated in horseradish peroxidase labeled-secondary antibodies for 1 h at room temperature, the protein bands were detected by enhanced chemiluminescence reagents (Vazyme Biotech, Nanjing, China).
Statistical Analyses
The data were analyzed using SPSS V22.0 Software (Armonk, NY, USA). Experimental groups were evaluated using one-way analysis of variance (ANOVA) and the group means were compared by Duncan’s multiple range test (DMRT). Data were expressed as mean ± SD from at least three independent experiments. P < 0.05 was regarded as statistically significant.
Results
Tau Alleviates Body Weight Loss Induced by Cd
The results showed that Cd treatment significantly reduced the body weight (P < 0.01). However, there were no significant differences in body weight increase between control groups, Tau-treated groups, and Cd + Tau -treated groups (Fig. 1A, B). In addition, Cd treatment significantly increased the liver weight (P < 0.01), but which was not restored by Tau supplement (Fig. 1C).
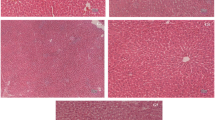
Tau Alleviates Cd-Induced Histological and Ultrastructural Changes
It was observed that the Cd treatment resulted in notable lesions including irregular hepatic cord arrangement, intracellular vacuolization, nuclear condensation, and inflammatory cell infiltration (Fig. 2). However, the Cd + Tau group demonstrated a great degree of alleviation in destruction of hepatocytes compared with Cd, although the inflammatory cells were still visible.
Tau treatment prevented liver damage induced by Cd (H&E staining × 640). After treatment, mice were sacrificed and the livers from each experimental group were processed for histological evaluation. Representative histological changes of liver tissues obtained from mice in different groups. Black arrows refer to central vein, blue arrow refers to area of inflammatory cell infiltration, and red arrow refers to intracellular vacuolization
Furthermore, TEM results showed that the hepatocytes appeared the apoptosis characteristics, such as nuclear condense, disintegration, and even necrosis, and the mitochondria were swollen and appeared with obvious cristolysis in the Cd group. Tau treatment partially reduced the damage in hepatocytes; the nuclear morphology and mitochondria cristae were preserved (Fig. 3). These results demonstrate that Tau protects the liver from Cd-induced damage.
Tau Attenuates Liver Dysfunction Induced by Cd
We next determined serum ALT, AST, LDH, and TP levels, the indicators of liver function. As shown in Fig. 4, compared to control group, the levels of serum ALT and AST in Cd treatment group were significantly elevated by 160.7% and 109.3% respectively (P < 0.01) (Fig. 4A, B). However, treatment with Tau reduced the levels of ALT and AST by 44.8% and 25.1%, respectively, compared to the Cd exposed group (P < 0.05). In addition, there was no significant difference in LDH and TP levels among the treatment groups (Fig. 4C, D).
Tau Has No Significant Effect on Oxidative Markers
Numerous studies have shown that oxidative stress plays a critical role in Cd induced hepatotoxicity. As shown in Fig. 5, Cd increased GSH contents by 41.4% compared with control, but Tau failed to restore the increase of GSH (P < 0.01). Furthermore, SOD activities and MDA contents were not significantly different between the control and Cd groups.
Tau Reduced Cd-Induced Inflammation
The mRNA expressions of pro-inflammatory cytokines in the liver were detected. As shown in Fig. 6, Cd caused a significant increase of TNF-α, IL-1β, and IL-6 levels by 260.2%, 239.0%, and 139.2%, respectively, compared with control group (P < 0.01). Tau supplement significantly reduced TNF-α and IL-1β expressions by 38.8% and 57.1%, respectively, compared to the Cd group (P < 0.05 or P < 0.01) (Fig. 6A, B), and partly restore IL-6 levels in our study (Fig. 6C).
Tau Alleviates Cd-Induced Apoptosis
Western blotting showed that Cd significantly increased the Bax/Bcl-2 ratio (Fig. 7B), and levels of active caspase-3 by 887.0% and 43.9%, respectively, compared with control (Fig. 7C), but all of which were decreased by Tau supplementation by 50.3% and 33.0%, respectively, compared to the Cd group. These results suggest Tau has the function to inhibit the apoptosis induced by Cd.
Effects of Tau on Cd-induced the protein expression levels of Bax, Bcl-2 and cleaved caspase-3. GAPDH served as the loading control. Data were presented as the means ± SD. **P < 0.01 compared with control group, #P < 0.05, ##P < 0.01 compared with Cd group. (A) Representative immunoblot analysis. (B) The ratio of Bax and Bcl-2. (C) The expressions of cleaved caspase-3
Discussion
Previous studies have shown that Tau supplementation provides beneficial effects against various hepatotoxic substances such as arsenic [35], lead [36], and mercury [37]. In this study, Tau has been observed to reverse the alterations of Cd induced hepatic damages. Moreover, Tau attenuated Cd-induced hepatocyte inflammation and apoptosis, supporting the beneficial role of Tau against Cd-induced hepatotoxicity. A graphical abstract of the protective effect of Tau against Cd-induced hepatic damage is shown in Fig. S1.
Body and organ weights are important indexes to reflect animal health status and organ toxicity [38,39,40]. Studies have shown that Cd exposure can significantly decrease the weight of mice [41, 42]. In this research, we observed that Tau reversed the inhibitory effect of Cd on weight gain. In addition, Tau treatment reversed the cellular abnormalities and kept the liver histologically almost normal. These findings are also supported by studies that Tau supplementation reduced bisphenol A-induced liver injury [43]. Hepatic injury is directly reflected by the elevated serum hepatic enzymes, such as AST, ALT, and LDH [44, 45]. In the present study, the serum ALT and AST were remarkably ameliorated by Tau, further supporting the histopathologic analysis. This result is also reported by other papers that Tau significantly reduced the levels of AST and ALT in serum of rats induced by Cd [44] and fipronil [26].
Studies have demonstrated Cd can induce oxidative damage in different tissues by enhancing membrane lipid peroxidation and changing the cellular antioxidant system [46,47,48]. Tau can affect a series of enzymes involved in oxidative insult in Cd exposed tissues [49]. Moreover, Tau administration can increases GSH levels by inducing cysteine to synthesize GSH as cysteine is a precursor of Tau and GSH [50]. In this study, the activities of GSH were significantly increased, but the contents of SOD and MDA were not affected by Cd treatment. These results are in line with previous studies [51], which reported that the hepatic GSH contents increased significantly and the MDA content was not affected after Cd exposure. On the contrary, other studies have shown that Cd-induced liver injury was accompanied by a significant increase in MDA levels, while GSH levels and SOD activities were significantly decreased [52]. These different results may be attributed to the difference of dosage, time, and method of Cd treatment. In addition, the toxic effect of Cd varies with sex in experimental animals, which might be a factor that affected the results [53]. More importantly, Tau administration failed to restore the increase in GSH, which indicated, to a certain extent, that the endogenous antioxidant capacity of the liver was in a compensatory state.
Cd induces different inflammatory markers such as TNF-α and IL-6, -8, and -1β, which are involved in Cd induced inflammation, apoptosis, and cancer development [54]. It was also observed in our study that these cytokine levels were significantly increased after Cd administered. Similar results were also demonstrated by [51]. Nuclear factor-κB (NF-κB) is a key regulator of inflammatory genes. Tau could react with hypochlorous acid to generate taurine chloramines, which could inhibit the activation of NF-κB by stabilizing the I-κB and prevents inflammatory reactions [23, 55]. Tau has been shown to inhibit TNF-α, IL-6, and TGF-β1 secretion in CCl4 induced rat liver damage [32]. In addition, Liu et al. [29] also reported that Tau protects rat liver by relieving the inflammatory response and oxidative stress induced by lipopolysaccharide. In our study, Tau significantly restored the transcription level of TNF-α, IL-1β, and IL-6, which partially contributing to reduction of hepatic inflammation.
Tau serves as a sulfur-containing β-amino acid synthesized in the liver from cysteine. It may enhance the elimination of Cd through the chelation of its two functional groups (–NH2 and –SO3H) [28]. The cytoprotective action of Tau is also evidenced by its potential to regulate mitochondrial protein synthesis, enhance electron transport chain activity, and prevent mitochondrial oxidant production [56]. Previous studies suggested that Tau alleviates the liver damage induced by γ-irradiation in rats through anti-inflammatory and anti-apoptotic pathways [57]. Furthermore, Tau has been shown to downregulate Bax and caspase 3 expression and upregulate Bcl-2 expression in ethanol induced hepatocytes [58]. In this study, Tau treatment could effectively inhibit Bax/Bcl-2 ratio and prevent cleaved caspase-3 activation induced by Cd. These results suggested that Tau could exert a positive role in maintaining mitochondrial homeostasis, and thereby significantly inhibited the activation of caspase-3, which is in line with previous studies that oral administration of Tau protects mouse liver against arsenic-induced apoptosis [35] and Cd-induced oxidative stress [28].
In conclusion, the present study demonstrated that Tau has a protective function against Cd-induced liver injury. The underlying mechanisms are mainly through anti-inflammatory and anti-apoptotic effects. However, the findings of this study have to be seen in light of some limitations. The precise signaling pathway of Tau hepatoprotective toxicity and the appropriate therapeutic dose of Tau on long-term Cd poisoning under environmental conditions still require further in-depth studies.
Data Availability
All data used to support the findings of this study are included within the article.
References
Zhang C, Ge J, Lv M, Zhang Q, Talukder M, Li J (2020) Selenium prevent cadmium-induced hepatotoxicity through modulation of endoplasmic reticulum-resident selenoproteins and attenuation of endoplasmic reticulum stress. Environ Pollut 260:113873
Ansari M, Ganaie M, Rehman N, Alharthy K, Khan T, Imam F, Ansari M, Al-Harbi N, Jan B, Sheikh I, Hamad A (2019) Protective role of Roflumilast against cadmium-induced cardiotoxicity through inhibition of oxidative stress and NF-κB signaling in rats. Saudi Pharm J 27(5):673–681
Liu X, Tian G, Jiang D, Zhang C, Kong L (2016) Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ Sci Pollut Res Int 23(18):17941–17952
Almeer R, Alarifi S, Alkahtani S, Ibrahim S, Ali D, Moneim A (2018) The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed Pharmacother 106:1490–1498
Hyder O, Chung M, Cosgrove D, Herman J, Li Z, Firoozmand A, Gurakar A, Koteish A, Pawlik T (2013) Cadmium exposure and liver disease among US adults. J Gastrointest Surg 17(7):1265–1273
Alshammari G, Al-Qahtani W, AlFaris N, Alzahrani N, Alkhateeb M, Yahya M (2021) Quercetin prevents cadmium chloride-induced hepatic steatosis and fibrosis by downregulating the transcription of miR-21. BioFactors 47(3). https://doi.org/10.1002/biof.1724
He X, Gao J, Hou H, Qi Z, Chen H, Zhang X (2019) Inhibition of Mitochondrial Fatty Acid Oxidation Contributes to Development of Nonalcoholic Fatty Liver Disease Induced by Environmental Cadmium Exposure. Environ Sci Technol 53(23):13992–14000
Kaur G, Shivanandappa T, Kumar M, Kushwah A (2020) Fumaric acid protect the cadmium-induced hepatotoxicity in rats: owing to its antioxidant, anti-inflammatory action and aid in recast the liver function. Naunyn Schmiedebergs Arch Pharmacol 393(10):1911–1920
Cao Z, Fang Y, Lu Y, Tan D, Du C, Li Y, Ma Q, Yu J, Chen M, Zhou C, Pei L, Zhang L, Ran H, He M, Yu Z, Zhou Z (2017) Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J Pineal Res 62(3). https://doi.org/10.1111/jpi.12389
Li X, Li H, Cai D, Li P, Jin J, Jiang X, Li Z, Tian L, Chen G, Sun J, Bai W (2021) Chronic oral exposure to cadmium causes liver inflammation by NLRP3 inflammasome activation in pubertal mice. Food Chem Toxicol 148:111944
Arab-Nozari M, Mohammadi E, Shokrzadeh M, Ahangar N, Amiri F, Shaki F (2020) Co-exposure to non-toxic levels of cadmium and fluoride induces hepatotoxicity in rats via triggering mitochondrial oxidative damage, apoptosis, and NF-kB pathways. Environ Sci Pollut Res Int 27(19):24048–24058
Abdel-Daim M, El-Tawil O, Bungau S, Atanasov A (2019) Applications of antioxidants in metabolic disorders and degenerative diseases: mechanistic approach. Oxid Med Cell Longev 2019:4179676
Yeung A, Tzvetkov N, El-Tawil O, Bungǎu S, Abdel-Daim M, Atanasov A (2019) Antioxidants: scientific literature landscape analysis. Oxid Med Cell Longev 2019:8278454
Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, Zhou X, Li Q, Wang X, Chen J, Zeng X, Zhao Z, He H, Liu D, Zhu Z (2016) Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: randomized, double-blind, placebo-controlled study. Hypertension 67(3):541–9
Marcinkiewicz J, Kontny E (2014) Taurine and inflammatory diseases. Amino acids 46(1):7–20
Wen C, Li F, Zhang L, Duan Y, Guo Q, Wang W, He S, Li J, Yin Y (2019) Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol Nutr Food Res 63(2):e1800536
Xu H, Zhang Q, Kim S, Liao Z, Wei Y, Sun B, Jia L, Chi S, Liang M (2020) Takifugu rubripesDietary taurine stimulates the hepatic biosynthesis of both bile acids and cholesterol in the marine teleost, tiger puffer (). Br J Nutr 123(12):1345–1356
Qvartskhava N, Jin C, Buschmann T, Albrecht U, Bode J, Monhasery N, Oenarto J, Bidmon H, Görg B, Häussinger D (2019) Taurine transporter (TauT) deficiency impairs ammonia detoxification in mouse liver. Proc Natl Acad Sci U S A 116(13):6313–6318
El Idrissi A (2019) Taurine regulation of neuroendocrine function. Adv Exp Med Biol 1155:977–985
Ahmadian M, Roshan V, Aslani E, Stannard S (2017) Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther Adv Cardiovasc Dis 11(7):185–194
Ghosh J, Das J, Manna P, Sil P (2009) Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol Appl Pharmacol 240(1):73–87
Ashkani-Esfahani S, Zarifi F, Asgari Q, Samadnejad A, Rafiee S, Noorafshan A (2014) Taurine improves the wound healing process in cutaneous leishmaniasis in mice model, based on stereological parameters. Adv Biomed Res 3:204
Terrill J, Pinniger G, Graves J, Grounds M, Arthur P (2016) Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J Physiol 594(11):3095–3110
Sirdah M (2015) Protective and therapeutic effectiveness of taurine in diabetes mellitus: a rationale for antioxidant supplementation. Diabetes Metab Syndr 9(1):55–64
Hsiao E, McBride S, Hsien S, Sharon G, Hyde E, McCue T, Codelli J, Chow J, Reisman S, Petrosino J, Patterson P, Mazmanian S (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155(7):1451–1463
Abdel-Daim M, Dessouki A, Abdel-Rahman H, Eltaysh R, Alkahtani S (2019) Hepatorenal protective effects of taurine and N-acetylcysteine against fipronil-induced injuries: the antioxidant status and apoptotic markers expression in rats. Sci Total Environ 650:2063–2073
Han H, Zhang J, Chen Y, Shen M, Yan E, Wei C, Yu C, Zhang L, Wang T (2020) Dietary taurine supplementation attenuates lipopolysaccharide-induced inflammatory responses and oxidative stress of broiler chickens at an early age. J Anim Sci 98(10). https://doi.org/10.1093/jas/skaa311
Sinha M, Manna P, Sil PC (2009) Induction of necrosis in cadmium-induced hepatic oxidative stress and its prevention by the prophylactic properties of taurine. J Trace Elem Med Biol 23(4):300–313
Liu Y, Li F, Zhang L, Wu J, Wang Y, Yu H (2017) Taurine alleviates lipopolysaccharide-induced liver injury by anti-inflammation and antioxidants in rats. Mol Med Rep 16(5):6512–6517
Jeong JE, Kim TY, Park HJ, Lee KH, Kim WT (2009) Taurine exerts neuroprotective effects via anti-apoptosis in hypoxic-ischemic brain injury in neonatal rats. Korean J Pediatr 52(12):1337–1347
Zhu X, Ma P, Wu W, Zhou R, Hao Y, Niu Y, Sun T, Li Y, Yu J (2016) Neuroprotective actions of taurine on hypoxic-ischemic brain damage in neonatal rats. Brain Res Bull 124:295–305
Abdel-Moneim A, Al-Kahtani M, El-Kersh M, Al-Omair M (2015) Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS ONE 10(12):e0144509
Wu G, Ren S, Tang R, Xu C, Zhou J, Lin S, Feng Y, Yang Q, Hu J, Yang J (2017) Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci Rep 7(1):4989
Zheng J, Zhuo L, Ran D, Ma Y, Luo T, Zhao H, Song R, Zou H, Zhu J, Gu J, Bian J, Yuan Y, Liu Z (2020) Cadmium induces apoptosis via generating reactive oxygen species to activate mitochondrial p53 pathway in primary rat osteoblasts. Toxicology 446:152611
Li S, Yang L, Dong G, Wang X (2017) Taurine protects mouse liver against arsenic-induced apoptosis through JNK pathway. Adv Exp Med Biol :855–862. https://doi.org/10.1007/978-94-024-1079-2_67
Aglan H, Safar M, Ain-Shoka A, Kandil A, Gebremedhn S, Salilew-Wondim D, Schellander K, Tesfaye D (2021) Developmental toxicity of lead in rats after gestational exposure and the protective role of taurine. J Biochem Mol Toxicol 35(8):e22816
Jagadeesan G, Sankarsami Pillai S (2007) Hepatoprotective effects of taurine against mercury induced toxicity in rats. J Environ Biol 28(4):753–756
Yuet Ping K, Darah I, Chen Y, Sreeramanan S, Sasidharan S (2013) Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Res Int 2013:182064
Abdel-Wahab W (2014) Thymoquinone attenuates toxicity and oxidative stress induced by bisphenol A in liver of male rats. Pak J Biol Sci 17(11):1152–1160
Sanjeev S, Bidanchi R, Murthy M, Gurusubramanian G, Roy V (2019) Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res Int 26(20):20631–20653
Yang Q, Zhu J, Luo X, Li F, Cong L, Wang Y, Sun Y (2019) Melatonin attenuates cadmium-induced ovulatory dysfunction by suppressing endoplasmic reticulum stress and cell apoptosis. Reprod Biol Endocrinol 17(1):61
Nna V, Usman U, Ofutet E, Owu D (2017) Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride-induced oxidative stress in the uterus and ovaries of female Wistar rats. Food Chem Toxicol 102:143–155
Uzunhisarcikli M, Aslanturk A (2019) Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ Sci Pollut Res Int 26(36):37242–37253
Hwang D, Wang L (2001) Effect of taurine on toxicity of cadmium in rats. Toxicology 167(3):173–180
Gong Z, Wang X, Wang J, Fan R, Wang L (2019) Trehalose prevents cadmium-induced hepatotoxicity by blocking Nrf2 pathway, restoring autophagy and inhibiting apoptosis. J Inorg Biochem 192:62–71
Dai Z, Cheng J, Bao L, Zhu X, Li H, Chen X, Zhang Y, Zhang J, Chu W, Pan Y, Huang H (2020) Exposure to waterborne cadmium induce oxidative stress, autophagy and mitochondrial dysfunction in the liver of Procypris merus. Ecotoxicol Environ Saf 204:111051
Ren L, Qi K, Zhang L, Bai Z, Ren C, Xu X, Zhang Z, Li X (2019) Glutathione might attenuate cadmium-induced liver oxidative stress and hepatic stellate cell activation. Biol Trace Elem Res 191(2):443–452
Amamou F, Nemmiche S, Meziane R, Didi A, Yazit S, Chabane-Sari D (2015) Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem Toxicol 78:177–184
Zhang Z, Liu D, Yi B, Liao Z, Tang L, Yin D, He M (2014) Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol Med Rep 10(5):2255–2262
Parildar-Karpuzoğlu H, Mehmetçik G, Ozdemirler-Erata G, Doğru-Abbasoğlu S, Koçak-Toker N, Uysal M (2008) Effect of taurine treatment on pro-oxidant-antioxidant balance in livers and brains of old rats. Pharmacol Rep 60(5):673–678
Liu L, Tao R, Huang J, He X, Qu L, Jin Y, Zhang S, Fu Z (2015) Hepatic oxidative stress and inflammatory responses with cadmium exposure in male mice. Environ Toxicol Pharmacol 39(1):229–236
Eşrefoglu M, Gül M, Dogru M, Dogru A, Yürekli M (2007) Adrenomedullin fails to reduce cadmium-induced oxidative damage in rat liver. Exp Toxicol Pathol 58(5):367–374
Shimada H, Hashiguchi T, Yasutake A, Waalkes M, Imamura Y (2012) Sexual dimorphism of cadmium-induced toxicity in rats: involvement of sex hormones. Arch Toxicol 86(9):1475–1480
Lee J, Lim K (2011) Preventive effect of phytoglycoprotein (27 kDa) on inflammatory factors at liver injury in cadmium chloride-exposed ICR mice. J Cell Biochem 112(2):694–703
Barua M, Liu Y, Quinn M (2001) Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: decreased NF-kappaB activation and IkappaB kinase activity. J immunol 167(4):2275–81
Jong C, Azuma J, Schaffer S (2012) Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42(6):2223–2232
El-Maraghi E, Abdel-Fattah K, Soliman S, El-Sayed W (2020) Taurine abates the liver damage induced by γ-irradiation in rats through anti-inflammatory and anti-apoptotic pathways. Int J Radiat Biol 96(12):1550–1559
Wu G, Yang J, Lv H, Jing W, Zhou J, Feng Y, Lin S, Yang Q, Hu J (2018) Taurine prevents ethanol-induced apoptosis mediated by mitochondrial or death receptor pathways in liver cells. Amino Acids 50(7):863–875
Funding
This work is supported by the Natural Science Foundation of China (31772692) and the Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Sheng Cui contributed to the conception of the study. Jiaming Zheng and Guobin Qiu performed experiment, collected and analyzed data, and wrote the paper. Yewen Zhou and Kezhe Ma contributed significantly to analysis and manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All procedures were approved by the Animal Care and Use Committee at Yangzhou University (approval ID: SYXK (Su) 2017–0044).
Consent to Participate
Not applicable.
Consent for Publication
The authors confirm that the work described has not been published before.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, J., Qiu, G., Zhou, Y. et al. Hepatoprotective Effects of Taurine Against Cadmium-Induced Liver Injury in Female Mice. Biol Trace Elem Res 201, 1368–1376 (2023). https://doi.org/10.1007/s12011-022-03252-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03252-0