Abstract
Because of limitation of doxorubicin (DOX) clinical application in chemotherapy due to its cardiotoxicity, finding new strategies to reduce DOX challenge and improve patients’ outcomes is crucial. Due to positive cardiovascular impacts of troxerutin (TXR), here we have investigated the effect of TXR on DOX-induced cardiotoxicity by evaluating the myocardial oxidative stress and expression of genes regulating mitochondrial biogenesis. Male Wistar rats (250–300 g) were randomly allocated into four groups: control, TXR, DOX, and TXR + DOX. Troxerutin (150 mg/kg) was orally administrated once a day through a gavage tube for 4 weeks before DOX challenge. The TXR-treated and time-matched control rats received intraperitoneal injection of DOX (20 mg/kg). Three days after DOX challenge, the left ventricular samples were obtained to determine the expression of genes regulating mitochondrial biogenesis via real-time PCR. Myocardial creatine kinase (CK-mB), oxidative stress markers, and mitochondrial function (generation of reactive oxygen species or ROS and ATP levels) were also evaluated using commercial kits and spectrophotometric and fluorometric methods. DOX administration significantly increased the levels of CK-mB, malondialdehyde (MDA), and mitochondrial ROS levels, while reduced the cellular ATP production and expression levels of SIRT-1, PGC-1α, and NRF-2 as well as superoxide dismutase, glutathione peroxidase, and catalase activity in comparison to control group (P < 0.05 to P < 0.01). Pretreatment of DOX-received rats with TXR significantly upregulated the expression of all biogenesis genes and antioxidant enzymes with non-significant effect on catalase activity, and significantly reduced CK-mB and MDA levels toward control values (P < 0.05 to P < 0.01). Mitochondrial ROS and ATP levels were also restored significantly by pretreatment with TXR (P < 0.05). The data suggested that preconditioning of rats with TXR had protective effect on DOX-induced cardiotoxicity through inducing antioxidative properties and restoring the mitochondrial function and the expression profiles of myocardial SIRT-1/PGC-1α/NRF-2 network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anticancer drug doxorubicin (DOX) is an anthracycline antibiotic widely used in the treatment of a wide range of malignancies including solid and hematologic cancers and is one of the most effective drugs among the chemotherapeutic agents in this field (Meredith & Dass, 2016). The most important side effect of this drug is its cardiac toxicity, which limits its use in clinical practice. DOX-induced cardiomyopathy is a fatal disease that has a poor prognosis and can be acute, occurring within 2 to 3 days after its administration (Pugazhendhi et al., 2018). This condition has a major impact on the cancer-surviving patients’ life. Various cellular and molecular mechanisms have been proposed for the pathogenesis of DOX-induced cardiotoxicity, such as increased oxidative stress, sarcoplasmic-reticulum stress, disruption of ion homeostasis, altered gene expression patterns, and necrosis and apoptosis (Nebigil & Désaubry, 2018). Based on these mechanisms, various therapeutic strategies are being developed to protect the heart during treatment of cancer with DOX. However, the use of most of these therapeutic strategies has not been very satisfactory due to their side effects as well as less beneficial effects on cardiac tissue. So, finding new therapies in this major health challenge is one of the primary clinical needs.
Oxidative stress is one of the most common mechanisms of cardiac toxicity induced by DOX (Nebigil & Désaubry, 2018; Yousefi et al., 2017). Lipid peroxidation-induced cardiac injury occurs following an increase in the production of reactive oxygen species (ROS). The heart is highly susceptible to oxidative stress, due in part to its low levels of antioxidant enzymes, such as glutathione peroxidase, catalase, and superoxide dismutase. On the other hand, the main intracellular organelles damaged by DOX are the mitochondria (Nebigil & Désaubry, 2018; Gorini et al., 2018). DOX is a cationic drug that binds highly to cardiolipin and forms an irreversible complex in the inner membrane of the mitochondria. Cardiolipin is essential for the better function of electron transport chain proteins in mitochondria (Guo et al., 2015). DOX may disrupt the link between these proteins and cardiolipin, leading to the formation of superoxide radicals and mitochondrial damage.
Decreased mitochondrial biogenesis results in decreased renewing of mitochondrial components, leading to the oxidation of lipid, protein, and DNA (Hosseini et al., 2019). It is suggested that maintaining the normal capacity of mitochondrial biogenesis in off-target organs during cancer treatment is a key factor in preventing the development of side effects caused by chemotherapy (Guo et al., 2015). One of the important factors involved in the regulation of mitochondrial biogenesis is the sirtuin family. Sirtoin-1 (SIRT1) is a member of this family that has been reported to affect the activity of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) through direct deacetylation (Mohamed et al., 2014). PGC-1α is a multifunctional transcription protein that acts as a switch molecule and regulates genes involved in energy metabolism. It is a major regulator of mitochondrial biogenesis, oxidative metabolism, and antioxidant defense (Fernandez-Marcos & Auwerx, 2011). The PGC-1α regulates mitochondrial biogenesis by activating transcription factors, such as nuclear respiratory factors-1 and 2 (NRF1/2). NRFs regulate the expression of many nuclear genes and DNA encoding mitochondrial proteins (Dinkova-Kostova & Abramov, 2015).
Known as vitamin P4, troxerutin (TXR) is a tri-hydroxylated derivative of natural bioflavonoids which is found in a variety of tea, coffee, bean sprouts, fruits, and vegetables (Najafi et al., 2018). Many reports have documented that TXR has diverse biological activities such as antioxidative, anti-inflammatory, anti-DNA damage, fibrinolytic, and neuroprotective activities (Najafi et al., 2018; Liu et al., 2010; Zhào et al., 2018). In addition, TXR has long been used to treat certain cardiovascular diseases including chronic venous insufficiency, varicose syndrome, and edema. Also, the effect of cardioprotective effects of TXR against myocardial ischemia-reperfusion injury has been reported (Najafi et al., 2018; Badalzadeh et al., 2016). Increasing the tissue tolerance to pathological stresses and decreasing the cellular injuries are the main ways of bioflavonoid efficiency, but the contribution of this process has not been demonstrated in the effectiveness of TXR in DOX-induced cardiotoxicity. Moreover, determining the mechanisms of action of this agent in DOX cardiotoxicity could help us to find the promising therapeutic targets for the prevention of related cardiac pathologies.
Considering the importance of DOX cardiotoxicity challenge and the potentials of TXR in generating a protective phenotype in the heart tissue, the aim of this study was to investigate the preventive effect of this bioflavonoid on DOX-induced cardiac toxicity and the levels of tissue oxidative stress, mitochondrial ROS generation, ATP levels, and expression of genes regulating mitochondrial biogenesis of the rat myocardium.
Materials and methods
Animals and materials
In this study, 24 male Wistar rats (in the weight of 250–300 g) were prepared and kept in the university’s animal room. The animals were housed under a controlled cycling of 12 h of light and 12 h of darkness at a constant room temperature of 23 ± 2 °C. They had free access to normal food and water. In order to acclimation of rats in animal room, they were kept in their cages for one week and then the interventions were started. DOX, anesthetizing drugs ketamine and xylazine, kits, and other reagents were obtained in their highest quality. All steps of this study were performed under the control of local animal research committee according to the standard guidelines with ethical approval number of 97-61754.
Study design
Twenty-four Wistar rats were randomly allocated in the following groups, each containing 6 rats: (1) non-treated healthy group (control); (2) troxerutin-receiving group (TXR); (3) DOX-receiving group (DOX); and (4) troxerutin plus DOX-receiving group (TXR + DOX).
In TXR-receiving groups, TXR (150 mg/kg) was orally administrated once a day through a gavage tube for four weeks before DOX challenge (Najafi et al., 2018). Doxorubicin hydrochloride at a dosage of 20 mg/kg of body weights of rats was intraperitoneally injected to the DOX-receiving rats. This dosage has been frequently used in the previous reports to induce cardiac injury (Wang et al., 2004). A similar volume of normal saline was injected to the rats of DOX-untreated groups. After 72 h of DOX challenge, all rats were sacrificed and the blood and tissue samples of all rats were immediately taken under general anesthesia.
Myocardial creatine kinase measurement
Measuring the myocardial creatine kinase (CK-mB) was used to evaluate the extent of myocardial cellular damage. CK-mB level from blood samples was measured by ELISA method using the commercially available assay kit (Roche Diagnostics, Germany).
Tissue sampling
After DOX challenge, the animals were experienced deep anesthetization with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). Then, approximately 2 ml of blood was attained from portal vein and the heart was immediately isolated and removed from the animal body and the left ventricle was sampled and divided in two parts; one part was immersed in liquid nitrogen for biochemical study and the second part was placed in RNase Later solution (Qiagen, Germany) in order to preserve RNA from digestion. The latter part was used for assaying gene expression.
Measuring oxidative stress and endogenous antioxidative enzymes
Lipid peroxidation index: malondialdehyde
Measurement of malondialdehyde (MDA) level is often used as the indicator of lipid peroxidation, which is analyzed by measuring thiobarbituric acid reactive substances (TBARS) in sample homogenates. Briefly, 250 μl of samples was mixed with 1 ml of 10% trichloroacetic acid (TCA) and 1 ml of 0.67% thiobarbituric acid. Next, the samples were heated in a boiling water bath for 15 min and then n-butylalchohol (2:1 v:v) was added to the solution. After centrifugation at 3500g for 5 min in room temperature, TBARS was determined at 535 nm of absorbance, using a spectrophotometer (Pharmacia Biotech; England) and the obtained values were expressed as nmol per 100 mg of tissue protein. The maximal intra-assay and inter-assay coefficient of measurements for same samples was 5% and 7%, respectively.
Superoxide dismutase enzyme
The activity of antioxidant enzyme superoxide dismutase (SOD) was determined using a RANSOD kit (Randox Crumlin, UK). By using this kit, the xanthine and xanthine oxidase were used for generation of superoxide radicals capable to react with 2-[4-iodophenyl]-3-[4-nitrophenol]-5-phenyl tetrazolium chloride (ITN) to form a red formazan dye. Concentrations of substrates were 0.05 mmol/l for xanthine and 0.025 mmol/l for ITN. SOD activity (at 505 nm) was determined by the degree of inhibition of this reaction. After calculating the percent of inhibition using related formula, SOD activity was calculated by comparing with the standard curve and was expressed as U/mg protein. The inter-sample and intra-assay variations were less than 8%.
Glutathione peroxidase enzyme
Glutathione peroxidase (GPX) activity was determined using a RANSEL kit (Randox Crumlin, UK). In this method, GPX catalyzes the oxidation of glutathione (at a concentration of 4 mmol/l) by cumene hydroperoxide. In the presence of glutathione reductase (at a concentration ≥ 0.5 units/l) and 0.28 mmol/l of NADPH, oxidized glutathione is immediately convert into the reduced form with concomitant oxidation of NADPH to NAD+. The decrease in absorbance at 340 nm at 37 °C was measured using a spectrophotometer, then GPX concentration was calculated and expressed as U/mg protein. The coefficient of variations for intra- and inter-assay was 7% and 8%, respectively.
Catalase enzyme
Catalase activity was assayed by the method of Aebi, as previously described (Badalzadeh et al., 2015). In brief, 0.65 ml of the phosphate buffer (50 mmol/l; pH 7.0) and 50 μl sample were added to a cuvette tube, and the reaction was started by addition of 0.3 ml of 30 mM hydrogen peroxide (H2O2). The decomposition of H2O2 was monitored at 240 nm at 25 °C. Catalase activity was calculated as nmol of H2O2 consumed/min/mg of tissue protein and the value was expressed as U/mg protein.
Isolation of ventricular mitochondria
Mitochondria isolation was performed on the left ventricular samples obtained at the end of the experiment. Approximately 40 mg of samples was rapidly minced on ice at 4 °C and homogenized with a precooled 2-ml Dounce homogenizer having an isolation buffer containing 70 mmol/l sucrose, 210 mmol/l mannitol, and 1 mmol/l EDTA in 50 mmol/l Tris/HCl, pH 7.4. Then, the homogenates were centrifuged at 1300g for 3 min, and the resultant supernatants were centrifuged again at 10,000g for 10 min. The mitochondrial pellet was obtained and suspended in a 100-μl storage buffer containing sucrose 70 mmol/l, mannitol 210 mmol/l, and Tris/HCl 50 mmol/l, at pH 7.4. The final solution was used within 4 h for measurement of mitochondrial ROS generation. Protein content was assayed by the bicinchoninic acid method with bovine serum albumin as a standard.
Mitochondrial ROS generation
Cardiac mitochondrial ROS generation was assessed by dichlorohydro-fluorescein diacetate (DCFDA) dyes using a fluorometric method. The mitochondrial pellets in storage buffer were incubated with 2 μM DCFDA dye at room temperature for 30 min. DCFDA is a fluorogenic dye which oxidized by mitochondrial ROS including hydroxyl and peroxyl to produce a highly fluorescent compound. The fluorescence was detected by a fluorescent microplate reader with the excitation and emission wavelengths of 480 nm and 530 nm, respectively. Mitochondrial ROS levels were reported as the fluorescence intensity per mg protein of samples.
ATP production levels
Cellular ATP content was determined using a related bioluminescent assay kit (MAK190, Sigma, USA), based on the manufacturer’s instructions. In brief, left ventricular samples (10 mg) were lysed in 100 μl of ATP assay buffer. After adding the ATP probe in the presence of developer (provided in the kit), the absorbance of the solution was read at 570 nm. The ATP content of the samples was expressed as nmol/mg protein.
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Firstly, the RNA extraction process was employed on approximately 100 mg of fresh samples obtained from left ventricles which were immersed in RNase Later solution, using Trizol method and according to the manufacturer’s instructions (Roche, Germany). A NanoDrop spectrophotometer at wavelength of 260/280 nm (NanoDrop ND-2000C, Thermo Fisher Scientific, USA) was used to detect the RNA yield and purity. Thereafter, first-strand complementary DNA (cDNA) was synthesized from the obtained samples’ RNAs using an Exiqon cDNA Synthesis Kit, according to the manufacturer’s instructions. In brief, 1 μl of extracted RNA (30 μg) was firstly mixed with 1 μl of random-hexamer primer and 6 μl RNase free H2O. Then, the solution was incubated at 65 °C for 5 min. Subsequently, micro-tubes were chilled on ice, and a mixture of 4 μl reaction buffer, 1 μl RNase inhibitor, 2 μl dNTP mix, and 1 μl reverse transcriptase was added to each sample. Then, the resultant solutions were immediately incubated at 25 °C for 5 min followed by 42 °C for 60 min; the reaction was finally ended by heating at 70 °C for 5 min. The reverse transcription was performed with the final volume of 20 μl pre tube. To assay the gene expressions of SIRT-1, PGC-1α, and NRF-2, a Light Cycler-96 Roche device was used. Forward and reverse primers of genes were produced by using the custom oligonucleotide synthesis service Metabion (Martinsried, Germany). Primers (listed in Table 1) were designed using Primer-3 software and blasted through Basic Local Alignment Search Tool on the NCBI website (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) in order to check out their specificity. The purity of amplified products was confirmed by using of melting curve and analyzed to ensure the identity of the specific PCR product. Relative quantification of target mRNAs was calculated using Livak method and the relative amounts of the mRNAs were normalized to the housekeeping GAPDH transcript levels.
Statistical analysis
Quantitative data was demonstrated as mean ± standard errors of mean. Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, La Jolla California USA). One-way ANOVA and Tukey post hoc test were performed to compare the difference between different groups. The significant deference was considered when P < 0.05.
Results
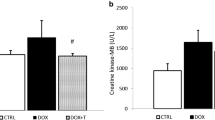
CK-mB release
The levels of CK-mB in blood of animals were measured to compare the cardiac damage between experimental groups (Fig. 1). TXR had a negligible effect on CK-mB level as compared to the control group. However, CK-mB level was significantly increased in DOX-receiving group in compression to the controls (P < 0.01). Additionally, TXR pretreatment significantly reduced the level of CK-mB as compared to those of DOX group (P < 0.01, Fig. 1).
MDA levels
As shown in Fig. 2, the level of lipid peroxidation indicated by MDA levels was increased significantly in DOX group in comparison with control and TXR groups (P < 0.01). Four weeks of pretreatment of rats with TXR significantly decreased the DOX-induced elevated level of MDA as compared with DOX group (P < 0.05).
SOD levels
Results demonstrated that induction of DOX challenge significantly reduced the SOD activity as compared with controls (P < 0.01). However, TXR administration significantly increased the activity of this enzyme in both DOX-receiving rats (P < 0.01) and non-receiving intact rats (P < 0.05) as compared to corresponding controls, respectively (Fig. 3).
GPX levels
One-way ANOVA showed that the GPX levels were significantly lower in DOX treatment rats than in control ones (P < 0.05) (Fig. 4). TXR pretreatment in control rats showed no effect on GPX activity. However, the comparison of data between DOX-receiving groups showed that TXR could significantly improve GPX activity in DOX-receiving rats (P < 0.05).
CAT levels
Figure 5 shows that induction of DOX challenge resulted in a significant decrease in catalase (CAT) activity in the myocardium of rats compared with control group (P < 0.05). Nevertheless, TXR administration had no effect on the activity of this enzyme in DOX-receiving rats or control ones.
Mitochondrial ROS levels
Mitochondrial ROS levels were determined by measuring the fluorescent intensity of DCFDA in cardiac samples. An increase in the fluorescent intensity indicated an increased mitochondrial ROS production. Intergroup analysis showed that TXR had no significant effect on mitochondrial ROS generation in untreated control rats (Fig. 6). However, DOX challenge significantly increased ROS level as compared to control group (P < 0.01). Administration of TXR prior to DOX challenge significantly lowered mitochondrial ROS level toward its normal values in comparison to DOX group (P < 0.05).
ATP levels
As shown in Fig. 7, the hearts of rats with DOX toxicity had significant decreased levels of ATP compared to control group (P < 0.05). TXR pretreatment significantly improved ATP levels in DOX-treated rats (P < 0.05), with no significant effect in control healthy rats (Fig. 7).
SIRT-1 expression
Our experiments demonstrated that TXR treatment in control rats significantly upregulated the mRNA of SIRT-1 gene in comparison to the control group (P < 0.05). Nonetheless, in DOX-receiving group, SIRT-1 mRNA was significantly downregulated when compared with control group (P < 0.05). The mRNA of SIRT-1 gene in DOX rats receiving TXR showed a significant increase as compared with that of DOX group (P < 0.05) (Fig. 8).
PGC-1α expression
Following 4 weeks of troxerutin treatment, the expression level of PGC-1α gene was not statistically different in control rats (Fig. 9). However, DOX treatment significantly decreased the level of PGC-1α expression as compared with control group (P < 0.01). On the other hand, pretreatment of DOX-receiving rats with TXR significantly restored the PGC-1α expression level in comparison to DOX group (P < 0.05; Fig. 9).
NRF-2 expression
Besides PGC-1α and SIRT1 genes, the expression of NRF-2 mRNA was significantly upregulated in TXR group versus control rats (P < 0.05). As seen in Fig. 10, DOX challenge significantly downregulated the expression of NRF-2 mRNA as compared with those of control group (P < 0.05). In addition, the expression of this gene was significantly increased in TXR + DOX group versus DOX group (P < 0.05).
Discussion
In the present study, we found that treatment of rats with TXR regimen had positive influences on the oxidative stress and mRNA levels of genes regulating mitochondrial biogenesis in the myocardium of DOX-treated rats as well as healthy ones. Four-week treatment of healthy rats with TXR could significantly increase the expression of NRF-2 and SIRT-1. Importantly, TXR significantly reduced the DOX-induced cardiotoxicity and prevented the downregulation of SIRT-1, PGC-1α, and NRF-2 mRNAs. In addition, DOX treatment overproduced lipid peroxidation level and reduced antioxidant enzymes, while preconditioning of DOX-treated rats with TXR significantly increased the levels of SOD and GPX and reduced the levels of MDA toward normal values. Moreover, TXR significantly restored the DOX-induced elevation of mitochondrial ROS levels and reduction of ATP levels. As a result, TXR recovered the DOX-induced alterations of oxidative stress markers and mitochondrial parameters in myocardial tissue. These findings reveal the beneficial effects of TXR against DOX-induced cardiotoxicity.
The previous findings have demonstrated that the development of oxidative stress and increased lipid peroxidation serve as a central pathophysiology of cardiotoxicity in DOX-treated hearts (Nebigil & Désaubry, 2018). In the present study, the elevated levels of MDA represented the significant increase in peroxidation and ROS levels by DOX challenge. Although, we had some limitations to measure the levels of oxidant anions and free radicals themselves in this study due to their highly active and easily metabolized features, instead, we quantified cardiac contents of endogenous antioxidant enzymes SOD, GPX, and CAT as well as mitochondrial content of ROS. The ROS- and MDA-increasing effect of DOX was associated with its lowering effects on antioxidant enzymes, supporting its peroxidative mechanism to induce imbalance between ROS production and antioxidant defensive system. This oxidative condition can damage several macromolecular and cellular components including cell membranes, proteins, DNAs, and mitochondria itself (Pugazhendhi et al., 2018; Yousefi et al., 2017). However, preadministration of TXR significantly reversed the DOX-induced oxidative and antioxidative alterations, as indicated by reduced MDA and mitochondrial ROS levels and increased SOD and GPX levels in the myocardium. In addition, TXR significantly increased the cellular ATP levels in DOX-treated rats. These findings reveal the specific antioxidative potentials of TXR and its protective effect on mitochondrial activity. TXR has been increasingly shown to provide powerful protection against many abnormalities such as cardiovascular diseases including diabetes-induced micro- and macrovascular dysfunctions (Badalzadeh et al., 2015), myocardial lipid abnormalities (Geetha et al., 2014), myocardial ischemia-reperfusion injuries, and heart failure (Najafi et al., 2018; Yu & Zheng, 2017). In addition, TXR significantly protected the renal (Fan et al., 2009), hepatic (Zhang et al., 2009), and cerebral tissues (Lu et al., 2010) from several oxidative, apoptotic, and inflammatory injuries.
Although the causes and specific roles of oxidative stress in the DOX-treated hearts have not been fully determined, the involvement of mitochondrial dysfunction may has crucial importance in this situation. Cardiomyocytes contain many mitochondria which their normal function and biogenesis guarantee the survival of cardiomyocytes during pathological circumstances (Guo et al., 2015). Various stimuli such as oxidative stress and inflammation can target the mitochondria and lead to dysregulation of its biogenesis and function. DOX binds highly to cardiolipin in the inner membrane of the mitochondria and thereby accumulates in cardiac mitochondria. DOX may disrupt the functional link between cardiolipin and electron transport chain proteins, leading to the overproduction of mitochondrial free radicals and disturbances in bioenergetics (Yousefi et al., 2017; Gorini et al., 2018). Dysfunctional mitochondria can now become a source of oxidative damages. Thus, targeting the mitochondria to restore its abnormalities can minimize the DOX toxicities. In our study, reduction of mitochondrial ROS and elevation of ATP levels after TXR administration indicate that this bioflavonoid can improve mitochondrial function and thereby reverse the DOX-induced cardiac oxidative stress.
Moreover, DOX challenge significantly reduced the expression of master regulator of mitochondrial biogenesis PGC-1α and two related genes namely SIRT-1 and NRF-2. Excessive oxidative stress induced by DOX could attack these components of mitochondria, to induce dysregulation of mitochondrial biogenesis. However, our results showed that TXR pretreatment significantly improved DOX-induced alteration in the expression of mitochondrial biogenesis genes. These observations are in agreement with previous reports showing that restoring mitochondrial gene expression and biogenesis through strategies like high-intensity interval training or pharmacological modalities can ameliorate DOX-induced cardiotoxicity in rats (Zare et al., 2019; Huang et al., 2017a; Abbas & Kabil, 2017; Khafaga & El-Sayed, 2018).
TXR upregulated the expression of SIRT-1 in the hearts of rats treated with DOX, which in turn, it could subsequently enhance the transcriptional activity of PGC-1α and thereby promotes the mitochondrial gene transcription, biogenesis, and function. In addition, increased expression of SIRT-1 was associated with the upregulation of transcription factor NRF-2. Over-activation of NRF-2 following TXR administration can positively influence the activity of antioxidant genes and proteins (Dinkova-Kostova & Abramov, 2015; Holmström et al., 2016). In this line, pretreated rats with TXR prior to DOX toxicity increased myocardial antioxidant SOD and GPX levels in comparison to untreated rats. This finding indicates the cardioprotective effect of TXR with preservation of mitochondrial biogenesis/function and suggests the functional interplay between SIRT-1 and NRF-2 to counterbalance the DOX-induced oxidative stress, which is considered as one of the underlying mechanisms of DOX cardiotoxicity (Huang et al., 2017b). Moreover, modulating effect of NRF-2 and SIRT-1 on inflammatory responses can also contribute to this cardioprotection by TXR (Holmström et al., 2016), which warrants further investigation.
In conclusion, the findings of the present investigation depict a novel property of TXR in protection against DOX-induced cardiotoxicity and suggest that this cardioprotective effect of TXR is partly achieved by restoring the DOX-induced elevation in cellular and mitochondrial oxidative stress and impairment in SIRT-1/PGC-1α/NRF-2 pathway regulating mitochondrial biogenesis/function and antioxidative responses. In future studies, it is important to investigate whether TXR has antitumor activity. Obtaining more information about the pharmacokinetics and effective dose of TXR may reduce the gap between its preclinical promising effects and clinical application in protecting patients from myocardial side effects during DOX chemotherapy.
References
Abbas NAT, Kabil SL (2017) Liraglutide ameliorates cardiotoxicity induced by doxorubicin in rats through the Akt/GSK-3β signaling pathway. Naunyn Schmiedeberg's Arch Pharmacol 390(11):1145–1153. https://doi.org/10.1007/s00210-017-1414-z
Badalzadeh R, Layeghzadeh N, Alihemmati A, Mohammadi M (2015) Beneficial effect of troxerutin on diabetes-induced vascular damages in rat aorta: histopathological alterations and antioxidation mechanism. Int J Endocrinol Metab 13(2):e25969. https://doi.org/10.5812/ijem.25969
Badalzadeh R, Baradaran B, Alihemmati A, Yousefi B, Abbaszadeh A (2016) Troxerutin preconditioning and ischemic postconditioning modulate inflammatory response after myocardial ischemia/reperfusion injury in rat model. Inflammation 40(1):136–143. https://doi.org/10.1007/s10753-016-0462-8
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88:179–188. https://doi.org/10.1016/j.freeradbiomed.2015.04.036
Fan SH, Zhang ZF, Zheng YL, Lu J, Wu DM, Shan Q, Hu B, Wang YY (2009) Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int Immunopharmacol 9(1):91–96. https://doi.org/10.1016/j.intimp.2008.10.008
Fernandez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93(4):884S–890S. https://doi.org/10.3945/ajcn.110.001917
Geetha R, Yogalakshmi B, Sreeja S, Bhavani K, Anuradha CV (2014) Troxerutin suppresses lipid abnormalities in the heart of high-fat-high-fructose diet-fed mice. Mol Cell Biochem 387(1–2):123–134. https://doi.org/10.1007/s11010-013-1877-2
Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E (2018) Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxidative Med Cell Longev 2018:7582730. https://doi.org/10.1155/2018/75827303
Guo Q, Guo J, Yang R, Peng H, Zhao J, Li L, Peng S (2015) Cyclovirobuxine D attenuates doxorubicin-induced cardiomyopathy by suppression of oxidative damage and mitochondrial biogenesis impairment. Oxidative Med Cell Longev 2015:151972. https://doi.org/10.1155/2015/151972
Holmström KM, Kostov RV, Dinkova-Kostova AT (2016) The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol 1:80–91. https://doi.org/10.1016/j.cotox.2016.10.002
Hosseini L, Vafaee MS, Mahmoudi J, Badalzadeh R (2019) Nicotinamide adenine dinucleotide emerges as a therapeutic target in aging and ischemic conditions. Biogerontology 20(4):381–395. https://doi.org/10.1007/s10522-019-09805-6
Huang L, Zhang K, Guo Y, Huang F, Yang K, Chen L, Huang K, Zhang F, Long Q, Yang Q (2017a) Honokiol protects against doxorubicin cardiotoxicity via improving mitochondrial function in mouse hearts. Sci Rep 7:11989. https://doi.org/10.1038/s41598-017-12095-y
Huang K, Gao X, Wei W (2017b) The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp Cell Res 361(1):63–72. https://doi.org/10.1016/j.yexcr.2017.09.042
Khafaga AF, El-Sayed YS (2018) All-trans-retinoic acid ameliorates doxorubicin-induced cardiotoxicity: in vivo potential involvement of oxidative stress, inflammation, and apoptosis via caspase-3 and p53 down-expression. Naunyn Schmiedeberg's Arch Pharmacol 391(1):59–70. https://doi.org/10.1007/s00210-017-1437-5
Liu CM, Ma JQ, Lou Y (2010) Chronic administration of troxerutin protects mouse kidney against D-galactose-induced oxidative DNA damage. Food Chem Toxicol 48(10):2809–2817. https://doi.org/10.1016/j.fct.2010.07.011
Lu J, Wu DM, Hu B, Cheng W, Zheng YL, Zhang ZF, Ye Q, Fan SH, Shan Q, Wang YJ (2010) Chronic administration of troxerutin protects mouse brain against D-galactose-induced impairment of cholinergic system. Neurobiol Learn Mem 93(2):157–164. https://doi.org/10.1016/j.nlm.2009.09.006
Meredith AM, Dass CR (2016) Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol 68(6):729–741. https://doi.org/10.1111/jphp.12539
Mohamed JS, Hajira A, Pardo PS, Boriek AM (2014) MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1α network in skeletal muscle. Diabetes 63(5):1546–1559. https://doi.org/10.2337/db13-1364
Najafi M, Noroozi E, Javadi A, Badalzadeh R (2018) Anti-arrhythmogenic and anti-inflammatory effects of troxerutin in ischemia/reperfusion injury of diabetic myocardium. Biomed Pharmacother 102:385–391. https://doi.org/10.1016/j.biopha.2018.03.047
Nebigil CG, Désaubry L (2018) Updates in anthracycline-mediated cardiotoxicity. Front Pharmacol 9:1262. https://doi.org/10.3389/fphar.2018.01262
Pugazhendhi A, Edison TNJI, Velmurugan BK, Jacob JA, Karuppusamy I (2018) Toxicity of doxorubicin (Dox) to different experimental organ systems. Life Sci 200:26–30. https://doi.org/10.1016/j.lfs.2018.03.023
Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B (2004) Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. Intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem 11 279(24):25535–25543
Yousefi B, Azimi A, Majidinia M, Shafiei-Irannejad V, Badalzadeh R, Baradaran B, Zarghami N, Samadi N (2017) Balaglitazone reverses P-glycoprotein-mediated multidrug resistance via upregulation of PTEN in a PPARγ-dependent manner in leukemia cells. Tumour Biol 39(10):1010428317716501. https://doi.org/10.1177/1010428317716501
Yu Y, Zheng G (2017) Troxerutin protects against diabetic cardiomyopathy through NF-κB/AKT/IRS1 in a rat model of type 2 diabetes. Mol Med Rep 15(6):3473–3478. https://doi.org/10.3892/mmr.2017.6456
Zare P, Moghadaszadeh M, Asadi M, Ebadi F, Badalzadeh R (2019) Effect of high-intensity interval training on expression of microRNA-149 and genes regulating mitochondrial biogenesis in doxorubicin-cardiotoxicity in rats. Comp Clin Pathol 2019:1–7. https://doi.org/10.1007/s00580-019-03077-9
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B (2009) Troxerutin protects the mouse liver against oxidative stress-mediated injury induced by D-galactose. J Agric Food Chem 57(17):7731–7736. https://doi.org/10.1021/jf9012357
Zhào H, Liu Y, Zeng J, Li D, Zhang W, Huang Y (2018) Troxerutin and cerebroprotein hydrolysate injection protects neurovascular units from oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Evid Based Complement Alternat Med 2018:9859672. https://doi.org/10.1155/2018/9859672
Acknowledgments
The authors thank the staff of the Biotechnology and Drug Applied Research Center, Tabriz University of Medical Sciences. This study was the PhD thesis of Sara Babaei Kouchaki and granted by Sciences and Research Branch, Islamic Azad University, Tehran-Iran, and Clinical Development Research Unit of Shohada Hospital, Tabriz University of Medical Sciences, Tabriz-Iran.
Author contribution statement
SB, VB, and RB conceived and designed research. SB and RB conducted experiments. NP contributed new reagents. VB, NP, and RB analyzed data. SB and RB wrote the manuscript. All authors read, edited, and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All steps of this study were performed under the control of local animal research committee according to the standard guidelines with ethical approval number of 97-61754.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babaei-Kouchaki, S., Babapour, V., Panahi, N. et al. Effect of troxerutin on oxidative stress and expression of genes regulating mitochondrial biogenesis in doxorubicin-induced myocardial injury in rats. Naunyn-Schmiedeberg's Arch Pharmacol 393, 1187–1195 (2020). https://doi.org/10.1007/s00210-020-01818-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01818-0