Abstract
The present study aimed to investigate the potential protective effect of all-trans-retinoic acid (ATRA, a natural derivative of vitamin A) against doxorubicin (DOX)-induced in vivo cardiac toxicity and its underlying mechanisms. Forty male albino rats were allocated into control, ATRA (0.5 mg/kg bwt, intraperitoneally daily), DOX (2.5 mg/kg bwt, intraperitoneally twice weekly for 3 weeks), and DOX + ATRA groups. Serum lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase-cardiac type (CK-MB), troponin I, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) were measured. In addition, cardiac glutathione (GSH), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase (CAT), and malondialdehyde (MDA) were determined. Cardiac tissues were examined for histopathologic changes and immunoexpression of pro-apoptotic caspase 3 and tumor-suppressor p53 proteins. DOX caused severe myocardial damage; degenerative and necrotic changes and worsened cardiac function biomarkers; and elevated serum LDH, CK, CK-MB, and troponin I. In addition, DOX inhibited cardiac antioxidative enzymes (GSH, GSH-Px, SOD, CAT) activities and enhanced MDA level. DOX increased serum proinflammatory cytokines (TNF-α, IL-6) and area percent of caspase 3 and p53 immunoexpression in heart tissues. Pretreatment with ATRA maintained cardiac function biomarkers, and reduced proinflammatory cytokines, lipid peroxidation, and immunoexpression of caspase 3 and p53. Moreover, ATRA improved cardiac histoarchitecture, as well as the activities of antioxidative enzymes. Collectively, ATRA can counteract DOX-induced cardiomyopathy through antioxidative and anti-inflammatory properties, besides suppression of the activation of the mitochondrial apoptotic pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (DOX) is a broad-spectrum anti-neoplastic agent with a potent chemotherapeutic efficacy against various malignancies and tumorigenesis. It is widely used in the treatment of several tumors, such as liver carcinoma, breast cancer, and non-Hodgkin’s lymphoma (Jordan 2002; Weiss 1992). However, the cardiotoxicity is considered as the major adverse effect of DOX, which limits its clinical use (Barry et al. 2007; Gianni et al. 2008). Lipid peroxidation and subsequent production of free radicals are supposed to be integrated into DOX-induced cardiotoxicity (Simunek et al. 2009). In addition, the mitochondrial dysfunction, excessive production of proinflammatory cytokines (Pecoraro et al. 2016), and cardiac membrane injuries (Hiona et al. 2011) may be also implicated in the pathogenesis of cardiotoxicity. DOX cardiotoxicity has a poor prognosis for available treatment, and it is frequently fatal (Jordan 2002; Takemura and Fujiwara 2007). Accordingly, extensive researches on various cardioprotectant are being proposed to mitigate DOX-induced cardiotoxicity.
The dietary vitamin A is normally metabolized in the cytoplasm to several isomeric forms of retinoic acid, of which all-trans-retinoic acid (ATRA) is the major biologically active form (Lee and Lee 2006). Previous investigations indicated that ATRA has antioxidant, anti-inflammatory, and anti-neoplastic properties (Rao et al. 2010). Furthermore, it has been suggested that ATRA plays a role in the cellular proliferation and tissue hemostasis (Canete et al. 2017; Doldo et al. 2015), and in retrieving the adverse effect in various conditions, such as uterine toxicity (Chatterjee and Chatterji 2011), hepatic ischemia and hepatitis (Nagy 2012; Rao et al. 2010), neurotoxicity (Cheng et al. 2013; Kim et al. 2013), and cardiotoxicity (Choudhary et al. 2008; Lou et al. 2013; Nizamutdinova et al. 2013). A few studies have been proposed to investigate the role of combined chemotherapy of DOX and ATRA in a single nano-system in the enhancement of anti-neoplastic activity (Sun et al. 2015; Zhang et al. 2015). However, only one study was conducted to evaluate the in vitro cardioprotective potential of ATRA against DOX cardiotoxicity in H9C2 cells and primary cardiomyocytes (Yang et al. 2016), since the in vivo protective effect of ATRA against DOX-associated cardiac injury remains unclear. This study was thereby designed to assess the potential protective effects of ATRA against in vivo DOX cardiotoxicity, in the light of cytopathic effects, oxidative stress, and apoptotic signaling pathways.
Materials and methods
Animals and experimental design
Forty male Wistar albino rats, 6–8 weeks old and 175–200 g body weight, were used for the experimental procedures. They were obtained from the closed bred colony, Pharos University, Alexandria, Egypt. Prior to experimentation, rats were housed in metal cages under controlled environmental conditions (24–27 °C temperature, 55% RH, and 12 h light/dark cycle) for 2 weeks. All animals fed on a standard laboratory diet and received water ad libitum during the experimentation.
After the acclimatization period, rats were assigned into four groups (n = 10/each). Group I (control) was intraperitoneal (ip) injected daily with olive oil (2.5 ml/kg bwt), the vehicle used for DOX. Group II (ATRA) was administered daily with ATRA (Sigma-Aldrich Chemical Co., St. Louis, USA) 0.5 mg/kg bwt ip, for three consecutive weeks, which asserted as the optimum effective dosage (Chatterjee and Chatterji 2011). Group III (DOX) received DOX (BMC United Pharmaceuticals Co., Cairo, Egypt) 2.5 mg/kg bwt ip, twice weekly to a cumulative dose 15 mg/kg bwt, as described earlier (Kumar et al. 2001). Group IV (ATRA + DOX) was administered ATRA, an hour before DOX administration in the similar regime of Groups II and III, respectively. Twenty-four hours of the last injection, all animals were anesthetized and weighed. The experimental protocol and dosage regimen used in this study are summarized in Fig. 1. Blood samples were then collected from the inner eye canthus into heparinized capillary tubes. Next, rats were immediately euthanized and grossly examined, and hearts were rapidly harvested, washed, and weighed. The relative heart weight (RHW) was calculated according to the following equation: RHW = (heart weight/body weight) × 100. Instantly after weighting, hearts were cut longitudinally with one half, which was thoroughly washed with ice-cold phosphate buffer saline (PBS) and kept at − 80 °C. A 10% cardiac tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4) using a homogenizer with a Teflon pestle. The homogenate was centrifuged at 14,000×g for 15 min at 4 °C to remove cell debris. Aliquots of the supernatant were used for determination of lipid peroxidation and antioxidants biomarkers. The other split was collected in neutral buffered formalin 10% for cytopathological and immunocytochemical evaluations. The number of rats and their suffering were minimized.
Schematic summary of the study protocol. Allocated animals were intraperitoneally injected with olive oil (2.5 mg/kg bwt twice weekly), all-trans-retinoic acid (ATRA; 0.5 mg/kg bwt daily), doxorubicin (DOX; 2.5 mg/kg bwt twice weekly), or ATRA + DOX as in the previous dosage for 3 weeks. One day of the last injection, animals were anesthetized and weighed, and blood and heart samples were collected. *ATRA was given an hour before DOX administration
Measurement of cardiac function biomarkers
Collected blood samples were immediately centrifuged for 10 min at 3000 rpm for serum separation and further colorimetric estimation of lactate dehydrogenase (LDH, EC 1.1.1.27) (Buhl and Jackson 1978), creatine kinase (CK, EC 2.7.3.2) (Hughes 1962), and creatine kinase-cardiac type isoenzyme (CK-MB) (Wu and Bowers 1982) activities following the providers’ manual of commercial-supplied assay kits (BioAssay Systems, USA). Additionally, serum troponin I concentration was determined using enzyme-linked immunosorbent assay (ELISA) capture (Spectrum Diagnostics, Egypt) (Penttila et al. 1997).
Assessment of pro-inflammatory biomarkers
To test whether cardiac damage was associated with pro-inflammatory cytokine induction, we measured serum tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) using ELISA assay following the manufacturers’ instruction (Abcam, USA). All assays were performed in duplicates, and cytokine concentrations (pg/ml) were determined at 450 nm using a computerized, automated microplate ELISA reader (Sorin Biomedica SpA., Italy).
Oxidative damage and antioxidant capacity assay
Lipid peroxidation in cardiac tissues was estimated in heart homogenates as malondialdehyde (MDA). The principle of the colorimetric assay was based on the reaction between one molecule of MDA in homogenate with two molecules of thiobarbituric acid resulting in pink-colored complex, its absorbance measured at 532 nm (Mihara and Uchiyama 1978). The tissue content of reduced glutathione (GSH) was assayed by the colorimetric method using commercially supplied kits (Bio-Diagnostic, Egypt). The GSH assay was based on the reduction of 5,5′–dithiobis (2–nitrobenzoic acid) with GSH to a yellow-colored complex; its absorbance was read at 405 nm within 15 min (Adams et al. 1983). The superoxide dismutase (SOD, EC 1.15.1.1) activity was colorimetrically determined using commercially available kits (Bio-Diagnostic, Egypt). The assay depends on the ability of the SOD enzyme to inhibit the phenazine methosulphate-mediated reduction of the nitroblue tetrazolium dye (Sun et al. 1988). The activity of glutathione peroxidase (GSH-Px, EC 1.11.1.9) was evaluated according to the previously described methods (Paglia and Valentine 1967). Evaluation of catalase (CAT, EC 1.11.1.6) activity was performed on the basis of the decomposition rate of H2O2 at 240 nm (Aebi 1984). Protein was determined using Bradford’s reagent.
Histopathologic examination and semi-quantitative scoring
Collected cardiac tissue specimens of control and treated rats were immediately fixed in buffered formalin for 24 h then processed through the paraffin-embedding technique. Several sections of 5 μm thickness were deparaffinized with xylene, stained with hematoxylin and eosin (H&E), and finally examined under light microscopy. Additionally, the extent of cardiac tissue damage was then evaluated using a semiquantitative scoring assay, in which five random fields were examined from each section. The severity of lesions was scored and graded according to the percentage of tissue involvement, as follows:
-
None (−): representing no involvement of the examined field
-
Mild (+): representing involvement of 0–25% of the examined field
-
Moderate (++): representing involvement of 25–50% of the examined field
-
Severe (+++): represented involvements of 50–100% of the examined field
Immunohistochemical assessment
Several 4-μm-thick sections were prepared from paraffin blocks, deparaffinized in xylene, and rehydrated in descending grades of ethanol. The sections were pretreated with 0.01 mol/L citrate-buffered saline (pH 6.0) for antigen retrieval, quenched with 0.3% (v/v) H2O2 in phosphate-buffered saline for endogenous peroxidase activity, and incubated for an hour with 10% (v/v) normal goat serum to block nonspecific binding of the immunological reagents. After that, the sections were incubated overnight at 4 °C with mouse monoclonal antibodies against caspase 3 and p53 (Dako Corporation, Life Trade, Egypt). Streptavidin-biotin complex and horseradish peroxidase were then applied, and immunohistochemical staining kits were used to visualize the reaction products. Sections were incubated for a minute in diaminobenzidine tetra-hydrochloride to develop the peroxidase labeling. Counterstaining was finally conducted using Mayer’s hematoxylin solution. For quantitative analysis, area and area percentage of caspase 3 and p53 immunoexpression were measured in myocardial sections of control and treated groups using the ImageJ software (Wayne@codon. nih.gov, Rasband 1997–2016). Original immunohistochemical photographs were obtained from 10 random fields per section with total magnification 100 (magnification lens 10). Area of positive immunoreactivity was determined by a standard measuring frame and then masked by red binary color to be measured.
Statistical analyses
All values in tables and figures were expressed as means ± standard error of means (SEM). Collected data were subjected to one-way analysis of variance (ANOVA) using the SAS® software followed by post hoc Duncan’s multiple range test to detect differences between groups. Differences were statistically considered significant at p < 0.05.
Results
Body weight and relative heart weight
DOX-treated rats showed a significant drop (p < 0.05) in their body weight (155.9 ± 4.21 g) as compared to control rats (211.9 ± 2.49 g). However, rats treated with ATRA + DOX showed significant (p < 0.05) improvement in their body weights (178.9 ± 3.21 g) compared to DOX-treated rats. Noticeably, Duncan’s multiple range post hoc test showed that there were no significant (p > 0.05) differences in rats’ body weights between ATRA-treated (204.9 ± 3.12 g) and control (211.9 ± 2.49 g) groups. Generally, the relative heart weights showed no significant (p > 0.05) changes between treated and control rats (Table 1).
Serum cardiac biomarkers and pro-inflammatory cytokines
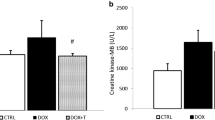
As shown in Table 1, DOX evoked cardiotoxicity as showed by the elevation of serum cardiac biomarkers. The LDH, CK, and CK-MP activities and troponin I level were significantly increased (p < 0.05) in DOX-treated rats (1673 ± 18.4, 793 ± 4.78, 547.0 ± 3.00, and 21.04 ± 1.12 U/L, respectively) compared to control values (590 ± 4.08, 206 ± 3.06, 182.4 ± 1.54, and 11.14 ± 0.17 U/L, respectively). Alternatively, these biomarkers were significantly reduced (p < 0.05) following pretreatment of DOX-treated rats with ATRA (1207 ± 5.33, 461 ± 3.32, 346.8 ± 3.38, and 17.04 ± 0.64 U/L, respectively) compared to DOX-treated rats, even though they were not identical to controls. Treatment with ATRA alone had no effect on the serum cardiac biomarkers compared to control.
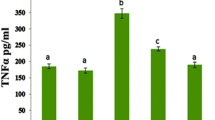
Furthermore, the data presented in Table 1 showed the levels of pro-inflammatory cytokines; IL-6 and TNF-α were significantly (p < 0.05) increased in DOX-treated rats (91.87 ± 3.25 and 84.78 ± 5.96 pg/mL, respectively) compared to controls (32.13 ± 2.63 and 31.83 ± 2.83 pg/mL, respectively). Meanwhile, their values were significantly (p < 0.05) diminished following pretreatment with ATRA (64.95 ± 6.66 and 62.87 ± 7.40 pg/mL, respectively) compared to DOX-treated rats. Moreover, administration of ATRA alone did not produce any alterations in the assessed proinflammatory cytokines.
Cardiac oxidative parameters
To estimate the protective effect of ATRA against DOX-induced cardiac oxidative damage in the rat, we examined the lipid peroxidation and antioxidant biomarkers in the myocardium. Lipid peroxidation was estimated as MDA, which was significantly increased (p < 0.05) in DOX-treated rats (86.11 ± 0.71 μmol/g protein) compared to controls (35.29 ± 0.43 μmol/g protein). However, cardiac antioxidant cellular molecules (GSH, GSH-Px, CAT, and SOD) were significantly (p < 0.05) lowered in DOX-treated animals (6.92 ± 0.20 nmol/g protein, 3.89 ± 0.15, 14.31 ± 0.37, and 17.28 ± 0.31 U/mg protein, respectively) than that of controls (16.19 ± 0.23 nmol/g protein, 7.43 ± 0.19, 27.26 ± 0.28 and 32.40 ± 0.19 U/mg protein, respectively). Administration of ATRA shortly before DOX resulted in an improvement of the cardiac oxidative state. It significantly (p < 0.05) decreased lipid peroxidation biomarker (53.55 ± 0.55 μmol/g protein) and increased antioxidant parameters (GSH 10.26 ± 0.25 nmol/g protein, GSH-Px 4.81 ± 0.15 U/mg protein, CAT 18.91 ± 0.29 U/mg protein, and SOD 22.82 ± 0.25 U/mg protein) compared to the DOX-treated group. Furthermore, ATRA injection alone did not produce any significant changes (p > 0.05) in oxidative parameters in relation to the control group (Table 2).
Histopathological observations and semiquantitative scoring
Myocardial tissues from the control and ATRA-treated rats showed normal histoarchitecture with a centrally located basophilic nucleus and normal striation of muscle fibers (Fig. 2a, b, Table 3). On the other hand, cardiomyocytes from the DOX-intoxicated rats revealed various degenerative changes: eosinophilia, fragmentation, hyalinization, and disorganization of myofibrillar arrays. Vacuolization of the sarcoplasm and formation of single large or multiple small vacuoles were evident in both muscle fibers and arteriolar wall (Fig. 2c, Table 3). Severe congestion and interfibrillar hemorrhage were also detected (Fig. 2d, Table 3). Additionally, numerous areas of interfibrillar edema (Fig. 2e, Table 3), coagulative necrosis, and myocytolysis were observed. Moreover, areas of myofibrillar loss were intensely infiltrated by various inflammatory cells including mononuclear cells and fibroblasts (Fig. 2f, Table 3). Co-treatment with ATRA resulted in an improvement of DOX-related myocardial damages; scattered patches of disorganized and fragmented cardiomyocytes with moderate vascular congestion were the commonly observed lesions (Fig. 2g, h, Table 3). The semiquantitative scoring of the reported lesions indicates a significant increase in myocardial injury in DOX-treated rats compared to control or ATRA-treated rats. Rats received ATRA shortly before DOX significantly prevented these myocardial damages as compared to DOX-intoxicated rats (Table 3).
Representative photomicrograph of myocardial tissue of rat following doxorubicin (DOX) and/or all-trans retinoic acid (ATRA) treatment (H&E, × 100). The light microscopic findings of myocardial tissue of control (a) and ATRA (0.5 mg/kg bw, ip daily)-administered (b) rats showed normal histological limits of myocardial tissues. The DOX (2.5 mg/kg bw, ip twice weekly)-administered rats showed severe cardiac injury with prominent vacuolization of sarcoplasm and arteriolar wall (arrows) (c), hyalinized myofibers with extensive inter-myofibrillar hemorrhage (asterisk) (d), interfibrillar edema (arrows) (e), and area of myocytolysis infiltrated with mononuclear inflammatory cells (yellow arrows) and fibroblasts (blue arrows) (f). ATRA + DOX-treated rats showed mild degeneration of myocardial tissues (arrows) (g) and least congestion of cardiac blood vessels (arrows) (h). CTR control, ATRA all-trans-retinoic acid, DOX doxorubicin
Immunohistochemistry and histomorphometric findings
Compared to control (Figs. 3a and 4a) and ATRA-treated rats (Figs. 3b and 4b), the caspase 3 and p53 immunoexpression showed strong immunopositivity in DOX-treated groups (Figs. 3c and 4c), while in pretreatment with ATRA there is a mild to moderate immunopositivity of caspase 3 and p53 levels compared to DOX-treated rats (Figs. 3d and 4d), respectively. In addition, comparing to control and ATRA-treated rats, DOX-treated groups showed significant (p < 0.001) increment of the mean area percent of caspase 3 and p53 immunoreactivity, but co-treatment of DOX with ATRA resulted in a significant decline (p < 0.001) in their immunostaining area percent compared to DOX-treated rats. Treatment with ATRA alone had no significant (p > 0.05) effect on area percent of both caspase 3 and p53 immunostaining compared to control rats (Figs. 3e and 4e).
Representative photomicrograph of caspase 3 immunoreactivity in rat’s myocardium following doxorubicin (DOX) and/or all-trans retinoic acid (ATRA) treatment. Cardiomyocytes from a control and b ATRA-treated rats showed negative immunoreactivity for caspase 3. c Cardiomyocytes from DOX-treated rats showed strong positive immunoreactivity for caspase 3 (arrows). d Cardiomyocytes from ATRA + DOX-treated rats showed mild to moderate immunoreactivity for caspase 3 (arrows). e Area percent of caspase 3 immunoexpression/field in control and treated groups; all values are expressed as mean ± SEM, n = 10. Mean value were significantly different from control (***p ≤ 0.001), ATRA (### p ≤ 0.001), DOX ($$$ p ≤ 0.001), one-way ANOVA with Duncan’s post hoc test for multiple comparisons
Representative photomicrograph of p53 immunoreactivity in rat’s myocardium following doxorubicin (DOX) and/or all-trans retinoic acid (ATRA) treatment. Cardiomyocytes from a control and b ATRA-treated rats showed negative immunoreactivity for p53. c Cardiomyocytes from DOX-treated rats showed strong positive immunoreactivity for caspase 3 (arrows). d Cardiomyocytes from ATRA-treated rats showed mild to moderate immunoreactivity for p53 (arrows). e Area percent of p53 immunoexpression/field in control and treated groups; all values are expressed as mean ± SEM, n = 10. Mean value were significantly different from control (***p ≤ 0.001), ATRA (### p ≤ 0.001), DOX ($$$ p ≤ 0.001), one-way ANOVA with Duncan’s post hoc test for multiple comparisons
Discussion
To the best of our knowledge, this is the first work that reveals the in vivo cardioprotective role of ATRA against DOX-induced cardiotoxicity. Serum cardiac enzymes, such as CK, CK-MB, and LDH, as well as troponin I act as highly sensitive and specific biomarkers that evaluate the severity of myocardial damages. Commonly, DOX-treated rats exhibited significant increment in the activities of these cardiac function biomarkers (Chen et al. 2015; Kelleni et al. 2015; Sun et al. 2013, 2016; Yu et al. 2013), reflecting cardiomyocyte damages and loss of their membrane permeability and integrity (Bertinchant et al. 2003; Rohilla et al. 2012). In contrast, co-treatment with ATRA significantly improved the DOX-disturbed cardiac function, supporting its promising role as a cardioprotectant, and hence preservation of cardiac function. Oxidative stress is one of the major cellular reactions against toxic insults (Winczura et al. 2012). Previous research demonstrated that DOX-induced cardiotoxicity is associated with the generation of free radicals in cardiac tissues (Hrdina et al. 2000; Naidu et al. 2002). Furthermore, DOX free radicals are non-enzymatically produced, and then react with iron; this DOX-iron complex is able to reduce oxygen to superoxide anion and hydrogen peroxide, which are involved in hydroxyl radicals formation (Malisza and Hasinoff 1995). In addition, MDA (the end product of lipid peroxidation process) is an indicator for free radicals-induced lipid breakdown (Abdel-Daim et al. 2017, 2016; El-Far et al. 2017; El-Sayed et al. 2015; Mohamed et al. 2016). In this work, a significant increment in MDA content and reduction of antioxidant enzymes activity involving GSH, GSH-Px, CAT, and SOD were recorded in DOX-intoxicated rats. Consistent with our findings, DOX-induced oxidative stress and depletion of antioxidant enzymes have been implicated in many tissues, such as the liver, kidneys (El-Moselhy and El-Sheikh 2014), brain (Pal et al. 2012), and in particular, heart tissues (Chen et al. 2015; Elberry et al. 2010; Sterba et al. 2013; Sun et al. 2013, 2015; Yu et al. 2013). Remarkably, pretreatment with ATRA decreased MDA content and improved antioxidant enzyme activity in cardiac tissue compared to DOX-treated rats, thereby indicating the antioxidative potential of ATRA against DOX-induced oxidative stress. The eventual mechanism by which ATRA acts as an antioxidant may be mediated through scavenging free radicals-induced oxidative stress and preventing the peroxyl radical-dependent peroxidation (Marnett and Ji 1994).
Another theory of DOX-induced cardiotoxicity is an initiation of pro-inflammatory cytokines production (Abd El-Aziz et al. 2012; Pecoraro et al. 2016), which is associated with chronic heart failure (Hedayat et al. 2010), a leading cause of death worldwide. It is well documented that overexpression of IL-6 (Kanda and Takahashi 2004) and TNF-α (Feldman et al. 2000) is involved in the pathogenesis of cardiac failure. Similarly, DOX induced in vivo overexpression of pro-inflammatory cytokines, IL-6 and TNF-α; therefore, it elicits an inflammatory response in cardiac tissues, and further cardiotoxicity. Several reports have also demonstrated overexpression and release of inflammatory mediators including TNF-α and IL-6 in the serum of DOX-intoxicated rats (Abd El-Aziz et al. 2012; Sun et al. 2016) and plasma of heart failure patients (Gullestad et al. 2012). Accumulating evidence suggests a primary role for IL-6 and TNF-α in cardiovascular toxicity as reliable predictors of morbidity and mortality (Hedayat et al. 2010). Conversely, ATRA pretreatment inhibited pro-inflammatory mediators production, which might be mediated through reduction of ERK1/2 phosphorylation (Kirchmeyer et al. 2008), thereby improving its anti-inflammatory activity against DOX-induced cytokines release.
It has been reported that DOX-induced cardiotoxicity depends mainly upon the apoptotic death of cardiomyocytes (Kalay et al. 2006). The most direct cause of apoptotic death is the activation of caspases that have a major role in controlling apoptosis-associated chemical and cellular alterations (Lebda et al. 2017). DOX has the ability to activate caspase 3, the hallmark of the apoptotic signaling pathway (Kalyanaraman et al. 2002). The strong immunopositivity and significant increment of immunostaining area of caspase 3 were apparent following DOX administration (Sun et al. 2013), indicating cardiomyocyte apoptosis. However, the reduced immunoexpression of caspase 3 follwoing co-treatment with ATRA was reflecting its anti-apoptotic effect against DOX-induced apoptotic cell death. Caspases were activated through two distinct pathways: the intrinsic (mitochondrial-dependent) apoptotic pathway and the extrinsic (death receptor-dependent) pathway (Pryor et al. 2006). The mitochondrial-dependent apoptotic pathway is the major pathway for DOX-induced myocardial apoptosis (Xiao et al. 2012). Furthermore, initiation of this intrinsic pathway has been directly dependent on activation of the p53 tumor-suppressor protein. p53 protein has a critical role in the regulation of cellular response to DNA damage and has the ability to interfere with the release of cytochrome c leading to subsequent initiation of cardiomyocytes apoptosis (Sun et al. 2013; Yoshida et al. 2009). Our findings revealed that DOX also induced overexpression with marked elevation in the immunostaining area of p53 tumor-suppressor protein. It has been reported that DOX evoked p53 overexpression in H9C2 cardiomyocytes (Sun et al. 2013), which contributes to the development of apoptosis (Liu et al. 2008). Interestingly, the protective intervention with ATRA shortly prior to DOX injection effectively reduced the area of p53 immunoexpression, signifying in vivo anti-apoptotic efficacy of ATRA. Recently, it has been demonstrated that ATRA reduced caspase 3 immunopositivity in both H9C2 cells and primary cardiomyocytes compared to DOX-treated cells (Yang et al. 2016). Others summarize the role of ATRA in activation of caspase 3 and p53 in tumor cell lines as a possible explanation for its tumor preventive activity (Gumireddy et al. 2003; Hormi-Carver et al. 2007; Mrass et al. 2004). As well, the normal cells were found more sensitive to DOX-induced apoptosis than the cancer cell lines (Bruynzeel et al. 2007). In this context, more than one mechanism can mediate apoptosis, which depends on the provided stimulus of cell death and the studied cell type (Bruynzeel et al. 2007). Moreover, the difference may be attributed to the diverse dose schedule of ATRA, and because we held our comparison with DOX, which is a potent activator for caspase 3 and p53 proteins. Altogether, ATRA pretreatment appears to protect against the diverse DOX-induced cell death pathways, because of its ability to scavenge ROS and then reducing the apoptotic myocardial cell death.
In the present study, the biochemical, oxidative, inflammatory, and immunohistochemical alterations in cardiac tissues of DOX-treated rats were also confirmed by histopathological examination. Toxopathologic analysis of DOX-treated rats revealed severe myocardial damages as reported in various DOX-related cardiotoxicity models (Abdel-Raheem and Abdel-Ghany 2009; Ashour et al. 2011; Sun et al. 2013). DOX caused severe cardiac lesions ranging from degenerative changes (hyalinization, vacuolization, and sarcoplasmic fragmentation) to myocardial necrosis, myocytolysis, intense inflammatory infiltration, interfibrillar edema, and circulatory disorders. Consequently, it appears that oxidative stress evoked by DOX plays an important role in promoting cardiomyocyte apoptosis and necrosis with subsequent cardiotoxicity (Minotti et al. 2004; Zhang et al. 2009) through various signaling pathways including activation of caspase 3 and p53. Interestingly, pretreatment with ATRA extensively ameliorated DOX-induced cardiomyopathy, suggesting antioxidative, anti-inflammatory, and anti-apoptotic potentials against DOX cardiotoxicity. Noteworthy, administration of ATRA shortly before DOX is a regime of choice that is successfully used in the treatment of acute promyelocytic leukemia. It has been shown that combination of both drugs improved the treatment outcome, and could increase the disease-free survival and overall survival compared to the DOX alone (Tallman et al. 1997). Moreover, recent studies have been concluded that the combined chemotherapy of ATRA and DOX in a single nano-system enhanced the antitumor effect of DOX (Sun et al. 2015; Zhang et al. 2015).
Conclusion
In summary, these results demonstrated that ATRA can be a novel candidate that could be used in combination with DOX as a cardioprotective agent against in vivo DOX-induced cardiomyopathy. The proposed mechanisms may be mediated via diminishing excess release of proinflammatory cytokines, amelioration of cardiac oxidative stress, and suppression of caspase 3 and p53 activation. Further research will be carried out to clarify whether ATRA modulates the in vivo cancer-killing ability of DOX.
References
Abd El-Aziz TA, Mohamed RH, Pasha HF, Abdel-Aziz HR (2012) Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med 12:233–240. https://doi.org/10.1007/s10238-011-0165-2
Abdel-Daim MM, Taha R, Ghazy EW, El-Sayed YS (2016) Synergistic ameliorative effects of sesame oil and alpha-lipoic acid against subacute diazinon toxicity in rats: hematological, biochemical, and antioxidant studies. Can J Physiol Pharmacol 94:81–88. https://doi.org/10.1139/cjpp-2015-0131
Abdel-Daim MM, El-Sayed YS, Eldaim MA, Ibrahim A (2017) Nephroprotective efficacy of ceftriaxone against cisplatin-induced subchronic renal fibrosis in rats. Naunyn Schmiedeberg's Arch Pharmacol 390:301–309. https://doi.org/10.1007/s00210-016-1332-5
Abdel-Raheem IT, Abdel-Ghany AA (2009) Hesperidin alleviates doxorubicin-induced cardiotoxicity in rats. J Egypt Natl Canc Inst 21:175–184
Adams JD Jr, Lauterburg BH, Mitchell JR (1983) Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther 227:749–754
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ashour OM et al (2011) Protective effect of bilberry (Vaccinium myrtillus) against doxorubicin-induced oxidative cardiotoxicity in rats. Med Sci Monit 17:BR110–BR115
Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE (2007) Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother 8:1039–1058. https://doi.org/10.1517/14656566.8.8.1039
Bertinchant JP et al (2003) Evaluation of cardiac troponin I and T levels as markers of myocardial damage in doxorubicin-induced cardiomyopathy rats, and their relationship with echocardiographic and histological findings. Clin Chim Acta 329:39–51
Bruynzeel AM, Abou El Hassan MA, Torun E, Bast A, van der Vijgh WJ, Kruyt FA (2007) Caspase-dependent and -independent suppression of apoptosis by monoHER in doxorubicin treated cells. Br J Cancer 96:450–456. https://doi.org/10.1038/sj.bjc.6603598
Buhl SN, Jackson KY (1978) Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate-to-pyruvate and pyruvate-to-lactate reactions in human serum at 25, 30, and 37 degrees C. Clin Chem 24:828–831
Canete A, Cano E, Munoz-Chapuli R, Carmona R (2017) Role of vitamin A/retinoic acid in regulation of embryonic and adult hematopoiesis. Nutrients 9. https://doi.org/10.3390/nu9020159
Chatterjee A, Chatterji U (2011) All-trans retinoic acid protects against arsenic-induced uterine toxicity in female Sprague-Dawley rats. Toxicol Appl Pharmacol 257:250–263. https://doi.org/10.1016/j.taap.2011.09.011
Chen CT, Wang ZH, Hsu CC, Lin HH, Chen JH (2015) In vivo protective effects of diosgenin against doxorubicin-induced cardiotoxicity. Nutrients 7:4938–4954. https://doi.org/10.3390/nu7064938
Cheng B, Martinez AA, Morado J, Scofield V, Roberts JL, Maffi SK (2013) Retinoic acid protects against proteasome inhibition associated cell death in SH-SY5Y cells via the AKT pathway. Neurochem Int 62:31–42. https://doi.org/10.1016/j.neuint.2012.10.014
Choudhary R, Baker KM, Pan J (2008) All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol 215:172–181. https://doi.org/10.1002/jcp.21297
Doldo E et al (2015) Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins. Biomed Res Int 2015:624627. https://doi.org/10.1155/2015/624627
Elberry AA, Abdel-Naim AB, Abdel-Sattar EA, Nagy AA, Mosli HA, Mohamadin AM, Ashour OM (2010) Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol 48:1178–1184. https://doi.org/10.1016/j.fct.2010.02.008
El-Far AH, Korshom MA, Mandour AA, El-Bessoumy AA, El-Sayed YS (2017) Hepatoprotective efficacy of Nigella sativa seeds dietary supplementation against lead acetate-induced oxidative damage in rabbit—purification and characterization of glutathione peroxidase. Biomed Pharmacother 89:711–718. https://doi.org/10.1016/j.biopha.2017.02.044
El-Moselhy MA, El-Sheikh AA (2014) Protective mechanisms of atorvastatin against doxorubicin-induced hepato-renal toxicity. Biomed Pharmacother 68:101–110. https://doi.org/10.1016/j.biopha.2013.09.001
El-Sayed YS, Lebda MA, Hassinin M, Neoman SA (2015) Chicory (Cichorium intybus L.) root extract regulates the oxidative status and antioxidant gene transcripts in CCl4-induced hepatotoxicity. PLoS One 10:e0121549. https://doi.org/10.1371/journal.pone.0121549
Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C (2000) The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol 35:537–544
Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB (2008) Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 26:3777–3784. https://doi.org/10.1200/JCO.2007.14.9401
Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P (2012) Inflammatory cytokines in heart failure: mediators and markers. Cardiology 122:23–35. https://doi.org/10.1159/000338166
Gumireddy K, Sutton LN, Phillips PC, Reddy CD (2003) All-trans-retinoic acid-induced apoptosis in human medulloblastoma: activation of caspase-3/poly(ADP-ribose) polymerase 1 pathway. Clin Cancer Res 9:4052–4059
Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N (2010) Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev 15:543–562. https://doi.org/10.1007/s10741-010-9168-4
Hiona A, Lee AS, Nagendran J, Xie X, Connolly AJ, Robbins RC, Wu JC (2011) Pretreatment with angiotensin-converting enzyme inhibitor improves doxorubicin-induced cardiomyopathy via preservation of mitochondrial function. J Thorac Cardiovasc Surg 142:396–403.e393. https://doi.org/10.1016/j.jtcvs.2010.07.097
Hormi-Carver K, Feagins LA, Spechler SJ, Souza RF (2007) All trans-retinoic acid induces apoptosis via p38 and caspase pathways in metaplastic Barrett’s cells. Am J Physiol Gastrointest Liver Physiol 292:G18–G27. https://doi.org/10.1152/ajpgi.00237.2006
Hrdina R, Gersl V, Klimtova I, Simunek T, Machackova J, Adamcova M (2000) Anthracycline-induced cardiotoxicity. Acta Med (Hradec Kralove) 43:75–82
Hughes BP (1962) A method for the estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 7:597–603
Jordan MA (2002) Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents 2:1–17
Kalay N et al (2006) Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 48:2258–2262. https://doi.org/10.1016/j.jacc.2006.07.052
Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S (2002) Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem 234-235:119–124
Kanda T, Takahashi T (2004) Interleukin-6 and cardiovascular diseases. Jpn Heart J 45:183–193
Kelleni MT, Amin EF, Abdelrahman AM (2015) Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: impact of oxidative stress, inflammation, and apoptosis. J Toxicol 2015:424813. https://doi.org/10.1155/2015/424813
Kim JH et al (2013) All-trans-retinoic acid rescues neurons after global ischemia by attenuating neuroinflammatory reactions. Neurochem Res 38:2604–2615. https://doi.org/10.1007/s11064-013-1178-x
Kirchmeyer M, Koufany M, Sebillaud S, Netter P, Jouzeau JY, Bianchi A (2008) All-trans retinoic acid suppresses interleukin-6 expression in interleukin-1-stimulated synovial fibroblasts by inhibition of ERK1/2 pathway independently of RAR activation. Arthritis Res Ther 10:R141. https://doi.org/10.1186/ar2569
Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK (2001) Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal 3:135–145. https://doi.org/10.1089/152308601750100641
Lebda MA, Sadek KM, El-Sayed YS (2017) Aspartame and soft drink-mediated neurotoxicity in rats: implication of oxidative stress, apoptotic signaling pathways, electrolytes and hormonal levels. Metab Brain Dis Accepted (June 1, 2017) doi:https://doi.org/10.1007/s11011-017-0052-y
Lee KW, Lee HJ (2006) Biphasic effects of dietary antioxidants on oxidative stress-mediated carcinogenesis. Mech Ageing Dev 127:424–431. https://doi.org/10.1016/j.mad.2006.01.021
Liu J, Mao W, Ding B, Liang CS (2008) ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol 295:H1956–H1965. https://doi.org/10.1152/ajpheart.00407.2008
Lou S et al (2013) Efficacy of all-trans retinoid acid in preventing nickel induced cardiotoxicity in myocardial cells of rats. Food Chem Toxicol 51:251–258. https://doi.org/10.1016/j.fct.2012.09.007
Malisza KL, Hasinoff BB (1995) Production of hydroxyl radical by iron(III)-anthraquinone complexes through self-reduction and through reductive activation by the xanthine oxidase/hypoxanthine system. Arch Biochem Biophys 321:51–60. https://doi.org/10.1006/abbi.1995.1367
Marnett LJ, Ji C (1994) Modulation of oxidant formation in mouse skin in vivo by tumor-promoting phorbol esters. Cancer Res 54:1886s–1889s
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229. https://doi.org/10.1124/pr.56.2.6
Mohamed OI, El-Nahas AF, El-Sayed YS, Ashry KM (2016) Ginger extract modulates Pb-induced hepatic oxidative stress and expression of antioxidant gene transcripts in rat liver. Pharm Biol 54:1164–1172. https://doi.org/10.3109/13880209.2015.1057651
Mrass P et al (2004) Retinoic acid increases the expression of p53 and proapoptotic caspases and sensitizes keratinocytes to apoptosis: a possible explanation for tumor preventive action of retinoids. Cancer Res 64:6542–6548. https://doi.org/10.1158/0008-5472.CAN-04-1129
Nagy L (2012) Would eating carrots protect your liver? A new role involving NKT cells for retinoic acid in hepatitis. Eur J Immunol 42:1677–1680. https://doi.org/10.1002/eji.201242705
Naidu MU, Kumar KV, Mohan IK, Sundaram C, Singh S (2002) Protective effect of Gingko biloba extract against doxorubicin-induced cardiotoxicity in mice. Indian J Exp Biol 40:894–900
Nizamutdinova IT, Guleria RS, Singh AB, Kendall JA, Jr., Baker KM, Pan J (2013) Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-kappaB signaling pathway J Cell Physiol 228:380–392 doi:https://doi.org/10.1002/jcp.24142
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pal S, Ahir M, Sil PC (2012) Doxorubicin-induced neurotoxicity is attenuated by a 43-kD protein from the leaves of Cajanus indicus L. via NF-kappaB and mitochondria dependent pathways. Free Radic Res 46:785–798. https://doi.org/10.3109/10715762.2012.678841
Pecoraro M et al (2016) Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol 293:44–52. https://doi.org/10.1016/j.taap.2016.01.006
Penttila K, Penttila I, Bonnell R, Kerth P, Koukkunen H, Rantanen T, Svanas G (1997) Comparison of the troponin T and troponin I ELISA tests, as measured by microplate immunoassay techniques, in diagnosing acute myocardial infarction. Eur J Clin Chem Clin Biochem 35:767–774
Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ (2006) Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291:R491–R511. https://doi.org/10.1152/ajpregu.00614.2005
Rao J, Zhang C, Wang P, Lu L, Zhang F (2010) All-trans retinoic acid alleviates hepatic ischemia/reperfusion injury by enhancing manganese superoxide dismutase in rats. Biol Pharm Bull 33:869–875
Rohilla A, Khan MU, Khanam R (2012) Cardioprotective potential of simvastatin in the hyperhomocysteinemic rat heart. J Adv Pharm Technol Res 3:193–198. https://doi.org/10.4103/2231-4040.101018
Simunek T, Sterba M, Popelova O, Adamcova M, Hrdina R, Gersl V (2009) Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 61:154–171
Sterba M, Popelova O, Vavrova A, Jirkovsky E, Kovarikova P, Gersl V, Simunek T (2013) Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid Redox Signal 18:899–929. https://doi.org/10.1089/ars.2012.4795
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Sun J et al (2013) Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One 8:e64526. https://doi.org/10.1371/journal.pone.0064526
Sun R et al (2015) Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials 37:405–414. https://doi.org/10.1016/j.biomaterials.2014.10.018
Sun Z, Yan B, WY Y, Yao X, Ma X, Sheng G, Ma Q (2016) Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of FOXO3a. Exp Ther Med 12:1879–1884. https://doi.org/10.3892/etm.2016.3518
Takemura G, Fujiwara H (2007) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49:330–352. https://doi.org/10.1016/j.pcad.2006.10.002
Tallman MS et al (1997) All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 337:1021–1028. https://doi.org/10.1056/NEJM199710093371501
Weiss RB (1992) The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 19:670–686
Winczura A, Zdzalik D, Tudek B (2012) Damage of DNA and proteins by major lipid peroxidation products in genome stability. Free Radic Res 46:442–459. https://doi.org/10.3109/10715762.2012.658516
Wu AH, Bowers GN Jr (1982) Evaluation and comparison of immunoinhibition and immunoprecipitation methods for differentiating MB and BB from macro forms of creatine kinase isoenzymes in patients and healthy individuals. Clin Chem 28:2017–2021
Xiao J et al (2012) Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology 292:53–62. https://doi.org/10.1016/j.tox.2011.11.018
Yang L, Luo C, Chen C, Wang X, Shi W, Liu J (2016) All-trans retinoic acid protects against doxorubicin-induced cardiotoxicity by activating the ERK2 signalling pathway. Br J Pharmacol 173:357–371. https://doi.org/10.1111/bph.13377
Yoshida M, Shiojima I, Ikeda H, Komuro I (2009) Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J Mol Cell Cardiol 47:698–705. https://doi.org/10.1016/j.yjmcc.2009.07.024
Yu X, Cui L, Zhang Z, Zhao Q, Li S (2013) Alpha-linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim Biophys Sin Shanghai 45:817–826. https://doi.org/10.1093/abbs/gmt082
Zhang YW, Shi J, Li YJ, Wei L (2009) Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp 57:435–445. https://doi.org/10.1007/s00005-009-0051-8
Zhang T et al (2015) Combination chemotherapy of doxorubicin, all-trans retinoic acid and low molecular weight heparin based on self-assembled multi-functional polymeric nanoparticles. Nanotechnology 26:145101. https://doi.org/10.1088/0957-4484/26/14/145101
Acknowledgements
The author greatly thanks Dr. Ayman Taha (Assist. Prof. of Poultry Breeding and Production, Faculty of Veterinary Medicine, Alexandria University) for the statistical handling of raw data.
Funding sources
This research work received no funding from any organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental approach was done in accordance with National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals and certified by the Committee of the Faculty of Veterinary Medicine, Alexandria University.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khafaga, A.F., El-Sayed, Y.S. All-trans-retinoic acid ameliorates doxorubicin-induced cardiotoxicity: in vivo potential involvement of oxidative stress, inflammation, and apoptosis via caspase-3 and p53 down-expression. Naunyn-Schmiedeberg's Arch Pharmacol 391, 59–70 (2018). https://doi.org/10.1007/s00210-017-1437-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1437-5