Abstract
Spermatic cord torsion is a serious and common urologic emergency. It requires early diagnosis for prevention of subfertility and testicular necrosis. Vildagliptin and sitagliptin are anti-diabetic drugs of the dipeptidyl peptidase-4 (DPP-4) inhibitors that have a protective role against cerebral ischemic stroke and cardiac ischemia reperfusion. This study aimed to investigate the role and mechanism of action of vildagliptin and sitagliptin in a model of testicular ischemia/reperfusion injury by testicular torsion/detorsion (T/D). Testicular T/D was done and vildagliptin and sitagliptin were administered either alone or in combination with nitric oxide synthase (NOS) inhibitor. Serum total cholesterol and testosterone were measured, while in testicular tissue testosterone, malondialdehyde (MDA) level, total antioxidant capacity (TAC), nitric oxide level, caspase-3, superoxide dismutase (SOD), hypoxia-inducible factor-1α (HIF-1α), tumor necrosis factor-α (TNF-α) and endothelial NOS (eNOS), and inducible NOS (iNOS) and neuronal NOS (nNOS) were measured. Histopathology of testicular tissue was done. Vildagliptin and sitagliptin increased serum testosterone, expression, and activity of SOD and testicular TAC. It also reduced total serum cholesterol, testicular MDA, caspase-3, HIF-1α, TNF-α, and expression of eNOS, iNOS, and nNOS. Vildagliptin and sitagliptin also improved histopathological picture of testicular tissue. NOS inhibitor produced similar result to DDP-4 inhibitors; however, its co-administration augmented the effect of vildagliptin and sitagliptin on these parameters. DPP-4 inhibitors, vildagliptin, and sitagliptin were protective against testicular T/D-induced injury mostly by anti-oxidative stress, and anti-apoptotic and anti-inflammatory actions that was augmented by NOS inhibition with a possible role for HIF-1α expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A testicular torsion, which is a severe acute urological emergency, happens when the spermatic cord is twisted and it causes an interruption in the blood supply and testicular ischemia. This case must be diagnosed and treated to prevent subinfertility and infertility (Fehér and Bajory 2016).
The pathophysiology of testicular torsion/detorsion (T/D) is resulted from the overgeneration of reactive oxygen species (ROS) (Wei et al. 2011). It stimulates an intra-cellular signaling cascade in the testicular endothelial cells that leads to neutrophil recruitment, which is an increase in the intra-testicular ROS and germ cell–specific apoptosis (Asghari et al. 2016). It is accompanied by an increase of the hypoxia-inducible factor-1α (HIF-1α) which is increased in hypoxic conditions and is linked to the apoptotic cell death (Palladino et al. 2011; Guven et al. 2014).
The permanent cessation of spermatogenesis in rats is the result of germ cell–specific oxidative stress, apoptosis, and inflammation in spermatic cord torsion (Turner et al. 1997). The testicular injury due to spermatic cord T/D is the state of testicular ischemia/reperfusion (I/R) (Turner and Brown 1993).When ischemia happens, it causes a reduction in the oxygen supply, depletion of the cellular energy, and the accumulation of toxic metabolites which results in oxidative stress and germ cell death. Then the reperfusion causes an increase in the production of ROS and nitrogen species which in turn causes membrane lipid peroxidation that leads to tissue injury and the disorganization of the cell structure and function (Abdel-Gaber et al. 2018).
The treatment of testicular T/D is still a controversial area of urology as it seems to be unsalvageable because of the poor recognition, the absence of symptoms, and the limited options for treatment (Fehér and Bajory 2016). Several anti-inflammatory drugs, antioxidants, and free radical scavengers have been reported to prevent testicular injury (Yapanoglu et al. 2017; Vaos and Zavras 2017).
Vildagliptin is an inhibitor of dipeptidyl peptidase-4 (DPP-4) commonly used in treating type II diabetes mellitus with antioxidant, anti-inflammatory, and anti-apoptotic characters (Purnachander et al. 2016). In parallel with its antihyperglycemic actions, treatment with DPP-4 inhibitors has been reported to be protective in models of ischemia-reperfusion (I/R) injury in many tissues such as the brain (Purnachander et al. 2016), heart (Bayrami et al. 2018), kidney (Reichetzeder et al. 2017), and lung (Tang et al. 2017). Similarly, sitagliptin, another DDP-4 inhibitor, was reported to be protective against tissue injury induced by I/R in many tissues mediated by its antioxidant, anti-inflammatory, and anti-apoptotic mechanisms (Chang et al. 2015; El-Sahar et al. 2015).
The current study aimed to evaluate the protective effect of vildagliptin and sitagliptin in a model of testicular ischemia/reperfusion injury by testicular T/D method and clarify its mechanism of action.
Material and methods
Animals
All experiments were conducted in accordance with the guidelines of Animal Use and Care Committee of Minia University. Adult male Wistar rats aged 2–3 months weighing 180~200 g were obtained from the National Research Center, Cairo, Egypt. Rats were housed individually in cages at 23 ± 2 °C under standard environmental conditions and had free access to diet and tap water.
Chemicals
Vildagliptin was purchased from Eva Pharma, Cairo, Egypt. Omega N-nitro-L-arginine (LNNA) was purchased from Sigma Aldrich, Germany. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was purchased from spectrum diagnostic, Egypt. All other chemicals were of analytical grade and obtained from commercial sources.
Animal model and study design
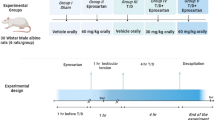
Rats randomly divided into 5 groups of 6 rats each: (1) sham group: rats received sham operations with no additional interventions; (2) T/D group: rats undergone torsion for 2 h followed by detorsion for 4 h; (3) vildagliptin-treated group: rats received vildagliptin (10 mg/kg; i.p.) (Glorie et al. 2012) 1 h before T/D; (4) sitagliptin-treated group: rats received sitagliptin (5 mg/kg; i.p.) (Youssef et al. 2015) 1 h before T/D; (5) LNNA-treated group: rats received LNNA (25 mg/kg; i.p.) (Veeresh et al. 2009) 1 h before T/D; (6) vildagliptin and LNNA-treated group; rats received LNNA and after 1 h received vildagliptin which administered 1 h before T/D; and (7) sitagliptin and LNNA-treated group; rats received LNNA and after 1 h received sitagliptin which administered 1 h before T/D.
The torsion and detorsion protocols lasted for 6 h. Rats were anesthetized with 50 mg/kg pentobarbital sodium injected intraperitoneally. The same surgeon under sterile conditions did all surgical procedures. To access the left testis, a left-sided ilioinguinal incision was performed. In the sham group, the left testis was taken out through the incision and an 11–0 atraumatic silk suture was placed through the tunica albuginea. The left testis was returned to the scrotum, and the incision was closed using a 4–0 silk suture. In the T/D group, a 720° rotation of the left testis in a counterclockwise direction was done to induce testicular ischemia and the left testis was fixed to the scrotal wall with an 11–0 atraumatic silk suture. After 2 h of ischemia, the fixing suture was removed, and the left testis was released in a clockwise direction to initiate reperfusion that was maintained for 4 h and bilateral orchiectomies performed at the end of protocol (Altunoluk et al. 2011; Wei et al. 2017).
At the end of the experiment (after 6 h from starting T/D protocol), blood samples were collected from tail of each rat for determination of blood glucose level (BGL) using the ACCU-CHEK active blood glucose meter (Roche, Mannheim, Germany) then rats were sacrificed.
Tissue samples were collected for biochemical analysis and morphological evaluation at the end of study protocol. Half of each fresh testis was washed with ice-cold phosphate-buffered saline (PBS) (pH = 7.4) and then kept at − 80 °C for measurement of testosterone, malondialdehyde (MDA) level, total antioxidant capacity (TAC), and expression of each of the following: superoxide dismutase (SOD), tumor necrosis factor-α (TNF-α), HIF-1α. The remaining half of the testis was divided into two parts and immersed in Bouin’s fluid for light microscopy. Sera were also collected and stored at − 80 °C for measurement of testosterone, total serum cholesterol, ALT, and AST.
Colorimetric measurements of parameters in testicular tissue
Testis MDA, an index of lipid peroxidation, was determined by using 1,1,3,3-tetramethoxypropane as standard (Buege and Aust 1978). TAC was assessed by the colorimetric technique using commercial kits (Biodiagnostic, Egypt). Testicular tissue total nitrites, the stable oxidation end products of nitric oxide, was served as an index of nitric oxide level and measured by reduction of nitrate into nitrite using activated cadmium granules, followed by color development with Griess reagent in acidic medium (Sastry et al. 2002). Activity of SOD activity was measured by method of Marklund and Marklund. This method is based on inhibition of the autoxidation of pyrogallol by SOD. The percentage of inhibition for the samples was calculated by the aid of running a control with no sample under the same conditions. SOD enzyme activity was expressed as units per milligrams of protein, where one unit was defined as the amount of the enzyme that inhibited the rate of pyrogallol autoxidation by 50% (Marklund and Marklund 1974). HIF-1α and TNF-α were measured by ELISA kit (Elabscience, USA). Caspase-3 was measured by ELISA kit (Cusabio, USA).
Evaluation of the serum parameters
Testosterone concentration in serum samples was determined by ELISA kit (“DRG,” Germany). Total serum cholesterol, ALT, and AST levels were measured using colorimetric kit according to the manufacturer’s instructions (Biomed, Egypt).
Sample preparation and RNA isolation
Gene expressions of eNOS, iNOS, nNOS, HIF-1α, TNF-α, and SOD in the testis were assessed using q RT-PCR technique. A total of 100 mg frozen tissue was weighed and homogenized in 1 ml Trizol reagent (Invitrogen, USA). After incubating for 5–10 min at 25 °C, 0.2 ml chloroform was added to the homogenate and the mixture was shaken vigorously for 15 s, followed by 3-min incubation. Then the mixture was centrifuged at 4 °C at 10000×g for 5 min. The upper layer of the mixture was obtained and 0.5 ml isopropanol was added. Next, the samples were centrifuged at 4 °C at 10000×g for 10 min. Supernatant was removed and the pellet was washed and suspended with 1 ml 75% ethanol.
Suspended pellet was centrifuged for 5 min at 4 °C at 7000×g. Majority of ethanol was removed and the remaining was air-dried. Completely dried pellet was dissolved collected in 50 μl RNase-free water. Quantity and quality of the extracted RNA were assessed by spectrometry. The concentrations and purity of RNA were determined by measuring the absorbance A260/A280. q RT-PCR for quantitative assessment of mRNA expression was performed on (Applied Biosyst 7500 fast, Techne (Cambridge) LTD., UK). RNA extract was reverse transcribed and q RT-PCR was performed according to the manufacturer’s instructions (Thermo Scientific one step kits plus ROX Vial, code no AB-4104/A).
All primers were obtained from Eurofins Genomics, Europe. The sequence of primers have been summarized in Table 1. Real-time polymerase chain reaction (q RT-PCR) was performed with 0.2 μg RNA per reaction using 20 μL of SYBER Green qPCR mix containing 10 pM of specific primer in the REAL TIME PCR DETECTION SYSTEM. The SYBER Green data were analyzed with a relative quantification to as reference gene. The relative expression level of the gene calculated using formula 2−∆∆Ct (VanGuilder et al. 2008). They were scaled relative to control. The results for all experimental samples were graphed as relative expression compared with the control. The reactions were as follows: reverse transcription step 42 °C for 15 min followed by an initial inactivation step at 95 °C for 10 min. The obtained cDNA was subjected to 40 cycles (15 s at 95 °C for denaturation followed by 60 s at 60 °C for annealing and 60 s at 72 °C for extension). The level of expression of each target gene was normalized relative to the expression of B-actin mRNA in that sample using ∆CT. Relative differences in gene expression among groups were determined using comparative ∆∆CT method and fold expression was calculated 2-∆∆CT, where ∆∆CT represents ∆CT values normalized relative to the mean ∆CT of control sample.
Histopathological examination
The testes were immersed in Bouin’s fluid then embedded in paraffin. Cross-sections 5 μm thick were cut using a microtome. The sections were subjected to hematoxylin-eosin staining and observed under an optical microscope and histopathologically evaluated (Cosentino et al. 1986).
Statistical analysis
Results were expressed as mean ± standard error of mean (SEM). Differences among groups were statistically analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s HSD post-hoc test for multiple comparisons. Some data of the study groups were compared using Kruskal–Wallis test. For all tests, probability (p) was considered: Non-significant if P value ≥ 0.05 and significant if P value < 0.05. All data analyses were performed using Graph Pad Prism7 program (Graph pad Software, San Diego California, USA, and www.graphpad.com).
Results
Effect of vildagliptin and sitagliptin on testicular MDA and TAC
In T/D model, there was a significant increase in MDA with reduction of TAC in testis as compared with sham group. On the other hand, treatment with vildagliptin, sitagliptin, LNNA, and their combination reversed the condition shown by an increase in TAC and reduction of MDA, as compared with T/D model. Combination of vildagliptin or sitagliptin, and LNNA caused a significant increase in TAC and reduction of MDA, as compared with vildagliptin- and sitagliptin-treated groups, respectively (Table 2).
Effect of vildagliptin and sitagliptin on total serum cholesterol, serum, and testicular testosterone
Table 3 showed that both serum and testicular testosterone significantly reduced and a significant increase in total serum cholesterol was noticed in T/D model, as compared with sham group. Meanwhile, groups treated with vildagliptin, sitagliptin, LNNA either solely or in combination significantly increased serum and testicular testosterone with a significant reduction in total serum cholesterol in comparison with T/D model. Furthermore, such changes occurred in the group treated with combination of either vildagliptin or sitagliptin with LNNA was significant, as compared with vildagliptin- and sitagliptin-treated groups, respectively.
Effect of vildagliptin and sitagliptin on BGL, ALT, and AST levels
No significant changes in blood glucose, serum ALT, and AST levels between different rat groups were observed (Table 4).
Effect of vildagliptin and sitagliptin on testicular nitric oxide
In T/D model, there was significant increase total nitrites in the testis, as compared with sham group. On the other hand, treatment with vildagliptin, sitagliptin, LNNA, and their combination reduced total nitrites, as compared with T/D model. Combination of vildagliptin or sitagliptin, and LNNA caused a significant reduction of total nitrites, as compared with vildagliptin- and sitagliptin-treated groups, respectively (Fig. 1).
Effect of vildagliptin and sitagliptin on testicular total nitrites (A) and testicular SOD activity (B). Results represent the mean ± SEM (n = 6): (a) Significance from sham operated group, (b) significance from T/D model group, (c) significance from V-treated group, (d) significance from S-treated group. Significance is at P ˂ 0.05 [T/D, torsion/detorsion; V, vildagliptin; S, sitagliptin; L, omega N-nitro-L-arginine]
Effect of vildagliptin and sitagliptin on gene expression and activity of SOD
Gene expression and activity of SOD showed a significant reduction in T/D model when compared with sham group. Meanwhile, its gene expression and activity was increased significantly in vildagliptin-, sitagliptin-, and LNNA-treated groups, as compared with T/D model. At the same time, in the group treated with LNNA combined with vildagliptin or sitagliptin, SOD gene expression and activity was increased significantly as compared with vildagliptin-and sitagliptin-treated groups, respectively (Figs. 1 and 3c).
Effect of vildagliptin and sitagliptin on gene expression of eNOS, iNOS, and nNOS
In T/D model, gene expression of eNOS, iNOS, and nNOS was increased significantly when compared with sham group. In groups treated with vildagliptin, sitagliptin and LNNA, gene expression of eNOS, iNOS, and nNOS was significantly reduced, as compared with T/D model. At the same time, in groups treated with LNNA combined with vildagliptin or sitagliptin, eNOS, iNOS, and nNOS gene expressions showed a significant reduction, as compared with vildagliptin- and sitagliptin-treated groups, respectively (Fig. 2).
Effect of vildagliptin and sitagliptin on expression of testicular nitric oxide synthases. Results represent the mean ± SEM (n = 6): (a) Significance from sham operated group, (b) significance from T/D model group, (c) significance from V-treated group, (d) significance from S-treated group. Significance is at P ˂ 0.05 [T/D, torsion/detorsion; V, vildagliptin; S, sitagliptin; L, omega N-nitro-L-arginine]
Effect of vildagliptin and sitagliptin on gene expression and concentration of TNF-α in the testis
In T/D model, gene expression and concentration of TNF-α was increased significantly when compared with sham group. Treatment with either vildagliptin, sitagliptin, or LNNA causes a significant reduction in TNF-α gene expression and concentration, as compared with T/D model. Further significant reduction in TNF-α expression and concentration was noticed in the group treated with LNNA combined with vildagliptin or sitagliptin, as compared with vildagliptin- and sitagliptin-treated groups, respectively (Table 5 and Fig. 3a).
Effect of vildagliptin and sitagliptin on expression of testicular tumor necrosis factor-α (A), testicular hypoxia inducible-factor-1α (B), and testicular superoxide dismutase (C). Results represent the mean ± SEM (n = 6): (a) Significance from sham operated group, (b) significance from T/D model group, (c) significance from V-treated group, (d) significance from S-treated group. Significance is at P ˂ 0.05 [T/D, torsion/detorsion; V, vildagliptin; S, sitagliptin; L, omega N-nitro-L-arginine]
Effect of vildagliptin and sitagliptin on gene expression and tissue level of HIF-1a
Gene expression and concentration of HIF-1α increased significantly in T/D model when compared with sham group. Meanwhile, in groups treated with vildagliptin, sitagliptin, and LNNA, HIF-1α gene expression and concentration reduced significantly, as compared with T/D model. At the same time, group treated with LNNA combined with vildagliptin or sitagliptin, HIF-1α gene expression and concentration was significantly reduced, as compared with vildagliptin- and sitagliptin-treated groups, respectively (Table 5 and Fig. 3b).
Effect of vildagliptin and sitagliptin on tissue level of caspase-3
In T/D model, caspase-3 was increased significantly when compared with sham group. Treatment with either vildagliptin, sitagliptin, or LNNA causes a significant reduction in caspase-3, as compared with T/D model. Further significant reduction was noticed in the groups treated with LNNA combined with vildagliptin or sitagliptin, as compared with vildagliptin- and sitagliptin-treated groups, respectively (Table 5).
Effect of vildagliptin and sitagliptin on histopathological picture of testicular tissue
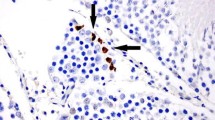
In sham group, germinal epithelium cells were well organized and intact seminiferous tubules with normal interstitial cells of Leydig. In T/D model, necrosis in superficial and deep cells, with structural damage, distal necrosis in germ cells occurred. Necrosis of interstitial cells of Leydig was also seen. Furthermore, seminiferous tubule lumens were filled with amorphous material containing cellular debris. Also, some testes revealed disappearance of tubular structures. In contrast, groups treated with vildagliptin, or LNNA, germinal epithelium cells were well organized, while some testes revealed necrosis limited only to superficial cells with mild degeneration of interstitial cells of Leydig. In sitagliptin-treated group, sections revealed well-organized seminiferous tubules with degeneration of superficial cells; only few tubules revealed degeneration of both superficial and deep layers of cells. In groups, received vildagliptin or sitagliptin in combination with LNNA, normal germ cells with preserved tubular structures and interstitial cells of Leydig were noticed (Fig. 4).
Effect of vildagliptin and sitagliptin on testis histopathological picture. A representative photomicrograph of a section in testis tissue (× 40). a Sham group; b T/D group, arrows refer to necrosis in superficial and deep cells, with structural damage and necrosis in germ cells; c Vildagliptin-treated group, well-organized germinal epithelium, while other ones reveals necrosis in superficial cells; d sitagliptin-treated group, arrows refer to well-organized germinal epithelium, while other ones reveals necrosis in superficial and deep cells; e LNNA-treated group; arrows refer to well-organized germinal epithelium with necrosis in superficial cells; e Vildagliptin + LNNA-treated group; and f Sitagliptin + LNNA-treated group, a well-preserved tubular structures and normal germ cells
Discussion
Twisting of the spermatic cord causes a severe acute urological emergency, which is the testicular torsion, resulting in an interrupted blood supply and testicular ischemia (Fehér and Bajory 2016). In this study, a rat T/D model is used to investigate the protective effects of vildagliptin and sitagliptin on testicular I/R injury.
It has been established before that the oxidative stress is usually associated with an imbalance between ROS and cellular anti-oxidative defensive mechanisms. On the reperfusion of the ischemic tissue, superoxide anions, hydrogen peroxide, and hydroxyl radicals are released. Lipid peroxidation can cause biochemical and morphological changes; in this case, protein denaturation, DNA damage, and apoptosis may occur with repeated succession of I/R injury in testicular cells (Akgur et al. 1993; Kanter 2010). ROS production seems to possess two phases in I/R tissues. First phase is reversible in cellular injury and typically oxidative stress situation. It immediately occurs after reperfusion and lasts for few hours. The second phase causes irreversible tissue damage and inflammation depending on the maintenance of the oxidative stress insult. It extends for hours and sometimes for days (Cutrìn et al. 2000).
Grievously, the overproduction of free radicals is prominent in testicular I/R injury and mammalian testis are highly susceptible to oxidative stress (Wei et al. 2007). Many studies proved that MDA level in testicular tissue increased after testicular T/D. In contrast, SOD was reported to protect the testis against IR injury following testicular torsion (Ghasemnejad-Berenji et al. 2017). The TAC level decreases due to the inactivation of one or more sulfhydryl group residues in the antioxidant enzymes, as these are responsible for their catalytic activities (Amien et al. 2015).
Fortunately, this study revealed that the treatment with vildagliptin and sitagliptin attenuated the oxidative stress in the damaged testes through reducing testicular MDA accompanied by the elevation of TAC and the increased expression of SOD. Those results showed the anti-oxidative effect of both drugs in testicular T/D, that concurs with the previous reports stated the antioxidant actions of vildagliptin and sitagliptin in different tissues (Shah et al. 2011; Apaijai et al. 2013; Chen et al. 2013; Abdelsalam and Safar 2015; Nuransoy et al. 2015; Tsai et al. 2015).
The results showed that testicular injury was associated with the decrease of both the serum and the testicular testosterone which indicates a functional injury in response to testicular I/R. These results are in line with the previous studies (Kanter 2010; Refaie et al. 2017; Abdel-Gaber et al. 2018). A metabolic disturbance also was observed in the form of high serum cholesterol levels in T/D model. Hypercholesterolemia was previously reported in response to I/R which can be explained by the decline in testosterone production due to Leydig cell dysfunction (Naito et al. 2009; Elshaari et al. 2012). Propitiously, in the current study, both studied DPP-4 inhibitors were able to ameliorate the functional and metabolic disturbances associated with testicular I/R that is indicative to its protective effect.
Significantly, the current study showed no considerable changes in the BGL as vildagliptin and sitagliptin did not cause hypoglycemic effect in non-diabetic rats. Also, it showed that no momentous changes occurred in ALT and AST levels. Furthermore, it was reported that HIF-1α is increased within the periods of testicular ischemia, even though the cell type expressing HIF-1α was not determined. Interestingly, the von Hippel-Lindau protein, which is necessary for the process of HIF-1α degradation, was found to be inactive in the mice having oligospermia and are infertile (Lysiak et al. 2009). In the current work, HIF-1α expression was increased in the T/D non-treated rats indicating the harmful role of HIF-1α in various tissues. Under normoxic conditions, HIF-1α is rapidly degraded via the ubiquitin dependent proteasome 26S pathway after hydroxylation and ubiquitination. Under hypoxic conditions, it is generally regarded that HIF-1α is accumulated due to hydroxylation inhibition (Jiang et al. 1996).
It was reported that there is a directly proportional relationship between NO and HIF-1α; when NO is generated in ample quantities, it upregulates HIF-1α expression in hypoxic conditions (Huang et al. 2014). On the other hand, HIF-1 has also been known to enhance iNOS gene expression in a variety of cell types (Melillo 2004; Jung et al. 2000; Matrone et al. 2004). Therefore, during hypoxic conditions, activated iNOS and HIF-1 can form an amplification loop. Indeed, we can claim that our data underscores new opportunities of drug development afforded by the relationship between NO and HIF-1α for various ischemic conditions.
In the current study, such increase in expression of HIF-1α was associated with the induction in the oxidative stress, apoptosis, and inflammation. That complies with the numerous reports which stated that an increase in oxidative stress, apoptosis, and inflammatory parameters is strongly linked to the increase of HIF-1α in various tissues (Ghosh et al. 2013; Wang et al. 2016; Kowalski et al. 2018; Ma et al. 2018; Guan et al. 2018). Increased expression of eNOS, iNOS, and nNOS in I/R injury of the testis, which has been reported previously (Lue et al. 2003; Moon et al. 2005; Ustün et al., 2008; Erol et al., 2009), produces toxic levels of nitric oxide in apoptotic germ cells of mice testis (Shiraishi et al. 2003; Rossoni et al. 2007). The studies showed that the detection of strong immunostaining in apoptotic germ cells supports a role of eNOS, iNOS, and nNOS in germ cell degeneration after testicular I/R, and suggested that nitric oxide is associated with germ cell apoptosis (Zini et al., 1998; Moon et al. 2005). There are multiple modes of regulation that control eNOS expression together with the low O2 dependency of the eNOS enzyme that allows a dynamic manner of the response of endothelial cells to changes in O2 levels. The biological adaptations give eNOS the ability to continue, at least on the short term to produce NO which is important for the transduction of hypoxia signals in tissues, especially at lower O2 levels (Ho et al. 2012). The current study procured similar results in the form of an elevation of total nitrite level and caspase-3 level together with increase in iNOS, eNOS, and nNOS expressions in testicular tissues after an I/R injury. Vildagliptin and sitagliptin momentously reduced total nitrites level, caspase-3 and expression of eNOS, iNOS, and nNOS compared with the T/D group.
It is well established that following a testicular torsion, inflammation contributes substantially to the pathogenesis of I/R injury. That is evident by the increase in the pro-inflammatory cytokines such as TNF-α (Yapanoglu et al. 2017; Tamer et al. 2018). In this study, vildagliptin- and sitagliptin-treated groups showed lower TNF-α level compared with the T/D group. This finding concurs with other previous researches, which reported that DPP-4 inhibition reduces inflammation via inhibition of monocyte activation/chemotaxis (Shah et al. 2011). In addition, vildagliptin and sitagliptin reported to reduce the expression of TNF-α and NFκB and caused reduction of the pro-inflammatory cytokines in various tissues (Chen et al. 2013; Miyoshi et al. 2014; Tsai et al. 2015; Atkin et al. 2017). This study proposes that the protective effect of vildagliptin and sitagliptin in testicular I/R injury was partially due to its anti-inflammatory properties.
Running in the same stream, vildagliptin and sitagliptin repressed the expression of HIF-1α, which was associated with the reduction in oxidative stress, apoptosis, and inflammatory parameters. That action highlighted the role of HIF-1α repression in protecting the testis against ischemic damage induced by T/D model. This effect on HIF-1α is supported by the fact that there is an inversely proportional relationship between SOD (Gao et al. 2013) and the HIF-α, which were increased with the vildagliptin treatment. At the same time, there is a direct correlation between TNF-α (Ghosh et al. 2013), caspase-3, eNOS, iNOS, nNOS (Tsui et al. 2011; Mashmoushi and Oates 2015; Wang et al. 2016; Yoon et al. 2016), and the HIF-α, which were reduced with both vildagliptin and sitagliptin treatment and HIF-α.
Treatment with LNNA showed reduction in serum cholesterol, MDA, total nitrites, caspase-3, NOS, HIF-1α, and TNF-α together with increase in serum and testicular testosterone, and TAC and SOD expressions which explore the antioxidant, anti-apoptotic, and anti-inflammatory actions of LNNA in testicular ischemia. Moreover, LNNA when combined with either vildagliptin or sitagliptin further reduced nitric oxide level, caspase-3, and the expression of eNOS, iNOS and nNOS that lead to an increase in the anti-oxidative stress, anti-apoptotic, and the anti-inflammatory actions of both DPP-4 inhibitors which augmented their protective effect against testicular I/R. This finding was supported by the notable improvement in the histopathological findings, which showed the well-organized germinal epithelium cells and the intact seminiferous tubules and interstitial cells of Leydig.
It is worth noting that LNNA administration with either vildagliptin or sitagliptin reduced significantly the HIF-1α expression. This reduction was accompanied by an improvement in biochemical findings and there was a positive correlation between NOS and HIF-1α in different tissues, and this was asserted by previously published studies (Rodriguez-Miguelez et al. 1985; Zhang et al. 2015). Our findings show that NOS inhibition further reduced HIF-1α expression, which shared in the augmentation of vildagliptin and sitagliptin’s protective effect.
Conclusion
Vildagliptin and sitagliptin can protect the testis from T/D-induced injury mostly; and we hypothesize that this occurred by anti-oxidative stress, anti-apoptotic, and anti-inflammatory actions, which was augmented by NOS inhibition. We recommend HIF-1α as a transcription factor that was activated by NO and might be linked to ischemic damage of testes.
References
Abdel-Gaber SA, Mohammed RK, Refaie MMM (2018) Mechanism mediating the protective effect of diacerein in ischemia reperfusion-induced testicular injury in rats. Life Sci 209:57–62
Abdelsalam RM, Safar MM (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J Neurochem 133(5):700–707
Akgur FM, Kilinc K, Aktug T (1993) Reperfusion injury after detorsion of unilateral testicular torsion. Urol Res 21:395–399
Altunoluk B, Soylemez H, Bakan V, Ciralik H, Tolun FI (2011) Protective effects of zofenopril on testicular torsion and detorsion injury in rats. Urol J 8(4):313–319
Amien AI, Fahmy SR, Abd-Elgleel FM, Elaskalany SM (2015) Renoprotective effect of Mangifera indica polysaccharides and silymarin against cyclophosphamide toxicity in rats. J. Basic Appl Zool 72:154–162
Apaijai N, Pintana H, Chattipakorn SC, Chattipakorn N (2013) Effects of vildagliptin versus sitagliptin, on cardiac function, heart rate variability and mitochondrial function in obese insulin-resistant rats. Br J Pharmacol 169(5):1048–1057
Asghari A, Akbari G, Meghdadi A, Mortazavi P (2016) Protective effect of metformin on testicular ischemia/reperfusion injury in rats. Acta Cir Bras 31(6):411–416
Atkin SL, Katsiki N, Banach M, Mikhailidis DP, Pirro M, Sahebkar A (2017) Effect of dipeptidyl peptidase-4 inhibitors on circulating tumor necrosis factor-α concentrations: a systematic review and meta-analysis of controlled trials. J Diabetes Complicat 31(9):1458–1464
Bayrami G, Karimi P, Agha-Hosseini F, Feyzizadeh S, Badalzadeh R (2018) Effect of ischemic postconditioning on myocardial function and infarct size following reperfusion injury in diabetic rats pretreated with vildagliptin. J Cardiovasc Pharmacol Ther 23(2):174–183
Buege J, Aust S (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Chang MW, Chen CH, Chen YC, Wu YC, Zhen YY, Leu S, Tsai TH, Ko SF, Sung PH, Yang CC, Chiang HJ, Chang HW, Chen YT, Yip HK (2015) Sitagliptin protects rat kidneys from acute ischemia-reperfusion injury via upregulation of GLP-1 and GLP-1 receptors. Acta Pharmacol Sin 36(1):119–130
Chen YT, Tsai TH, Yang CC, Sun CK, Chang LT, Chen HH, Chang CL, Sung PH, Zhen YY, Leu S, Chang HW, Chen YL, Yip HK (2013) Exendin-4 and sitagliptin protect kidney from ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med 11:270
Cosentino MJ, Nishida M, Rabinowitz R, Abraham TK, Cockett ATK (1986) Histopathology of prepubertal rat testes subjected to various durations of spermatic cord torsion. J Androl 7:23–31
Cutrìn JC, Boveris A, Zingaro B, Corvetti G, Poli G (2000) In situ determination by surface chemiluminescence of temporal relationships between evolving warm ischemia-reperfusion injury in rat liver and phagocyte activation and recruitment. Hepatology 31(3):622–632
El-Sahar AE, Safar MM, Zaki HF, Attia AS, Ain-Shoka AA (2015) Sitagliptin attenuates transient cerebral ischemia/reperfusion injury in diabetic rats: implication of the oxidative-inflammatory-apoptotic pathway. Life Sci 126:81–86
Elshaari FA, Elfagih RI, Sheriff DS, Barassi IF (2012) Testicular torsion-Detorsion histological and biochemical changes in rat testis. J Cytol Histol 3:136
Erol B, Tokgoz H, Hanci V, Bektas S, Akduman B, Yencilek F, Mungan G, Mungan A (2009) Vardenafil reduces testicular damage following ischemia/reperfusion injury in rats.Kaohsiung. J Med Sci 25(7):374–380
Fehér AM, Bajory Z (2016) A review of main controversial aspects of acute testicular torsion. J Acute Dis 5(1):1–8
Gao YH, Li CX, Shen SM, Li H, Chen GQ, Wei Q, Wang LS (2013) Hypoxia-inducible factor 1α mediates the down-regulation of superoxide dismutase 2 in von Hippel-Lindau deficient renal clear cell carcinoma. Biochem Biophys Res Commun 435(1):46–51
Ghasemnejad-Berenji M, Ghazi-Khansari M, Yazdani I, Saravi SSS, Nobakht M, Abdollahi A, Ansari JM, Ghasemnejad-Berenji H, Pashapour S, Dehpour AR (2017) Rapamycin protects testes against germ cell apoptosis and oxidative stress induced by testicular ischemia-reperfusion. Iran J Basic Med Sci 20(8):905–911
Ghosh S, Paul A, Sen E (2013) Tumor necrosis factor α-induced hypoxia inducible factor 1α-β-catenin axis regulates major histocompatibility complex class I gene activation through chromatin remodeling. Mol Cell Biol 33(14):2718–2731
Glorie LL, Verhulst A, Matheeussen V, Baerts L, Magielse J, Hermans N, D’Haese PC, De Meester I, De Beuf A (2012) DPP4 inhibition improves functional outcome after renal ischemia-reperfusion injury. Am J Physiol Ren Physiol 303(5):F681–F688
Guan R, Wang J, Li Z, Ding M, Li D, Xu G, Wang T, Chen Y, Yang Q, Long Z, Cai Z, Zhang C, Liang X, Dong L, Zhao L, Zhang H, Sun D, Lu W (2018) Sodium tanshinone IIA sulfonate decreases cigarette smoke-induced inflammation and oxidative stress via blocking the activation of MAPK/HIF-1α signaling pathway. Front Pharmacol 9:263
Guven A, Ickin M, Uzun O, Bakar C, Balbay EG, Balbay O (2014) Erdosteine protects rat testis tissue from hypoxic injury by reducing apoptotic cell death. Andrologia 46(1):50–58
Ho JJ, Man HS, Marsden PA (2012) Nitric oxide signaling in hypoxia. J Mol Med (Berl) 90(3):217–231
Huang T, Huang W, Zhang Z, Yu L, Xie C, Zhu D, Peng Z, Chen J (2014) Hypoxia-inducible factor-1α upregulation in microglia following hypoxia protects against ischemia-induced cerebral infarction. Neuroreport 25(14):1122–1128
Jiang BH, Semenza GL, Bauer C, Marti HH (1996) Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Phys 271(4 Pt 1):C1172–C1180
Jung F, Palmer LA, Zhou N, Johns RA (2000) Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res 86:319–325
Kanter M (2010) Protective effects of melatonin on testicular torsion/detorsion-induced ischemia-reperfusion injury in rats. Exp Mol Pathol 89(3):314–320
Kowalski E, Geng S, Rahtes A, Lu R, Li L (2018) Toll-interacting protein differentially modulates HIF1α and STAT5-mediated genes in fibroblasts. J Biol Chem 293(31):12239–12247
Lue Y, Sinha Hikim AP, Wang C, Leung A, Swerdloff RS (2003) Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: evidence from null mutant mice. Endocrinology 144:3092–3100
Lysiak JJ, Kirby JL, Tremblay JJ, Woodson RI, Reardon MA, Palmer LA, Turner TT (2009) Hypoxia-inducible factor-1alpha is constitutively expressed in murine Leydig cells and regulates 3beta-hydroxysteroid dehydrogenase type 1 promoter activity. J Androl 30(2):146–156
Ma J, Zhen X, Huang X, Jiang X (2018) Folic acid supplementation repressed hypoxia induced inflammatory response via ROS and JAK2/STAT3 pathway in human promyelomonocytic cells. Nutr Res 53:40–50
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Mashmoushi AK, Oates JC (2015) Lipopolysaccharide induces inducible nitric oxide synthase-dependent podocyte dysfunction via a hypoxia-inducible factor 1α and cell division control protein 42 and Ras-related C3 botulinum toxin substrate 1 pathway. Free Radic Biol Med 84:185–195
Matrone C, Pignataro G, Molinaro P, Irace C, Scorziello A, Di Renzo GF, Annunziato L (2004) HIF-1alpha reveals a binding activity to the promoter of iNOS gene after permanent middle cerebral artery occlusion. J Neurochem 90:368–378
Melillo G (2004) HIF-1: a target for cancer, ischemia and inflammation too good to be true? Cell Cycle 3:154–155
Miyoshi T, Nakamura K, Yoshida M, Miura D, Oe H, Akagi S, Sugiyama H, Akazawa K, Yonezawa T, Wada J, Ito H (2014) Effect of vildagliptin, a dipeptidyl peptidase 4 inhibitor, on cardiac hypertrophy induced by chronic beta-adrenergic stimulation in rats. Cardiovasc Diabetol 13:43
Moon C, Ahn M, Kim S, Yasuzumi F, Shin T (2005) Increased expression of both constitutive and inducible forms of nitric oxide synthase in the delayed phase of acute experimental testicular torsion. J Vet Med Sci 67(4):453–456
Naito M, Bomsztyk K, Zager RA (2009) Renal ischemia-induced ol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol 174(1):54–62
Nuransoy A, Beytur A, Polat A, Samdanci E, Sagir M, Parlakpinar H (2015) Protective effect of sitagliptin against renal ischemia reperfusion injury in rats. Ren Fail 37(4):687–693
Palladino MA, Pirlamarla PR, McNamara J, Sottas CM, Korah N, Hardy MP (2011) Normoxic expression of hypoxia-inducible factor 1 in rat Leydig cells in vivo and in vitro. J Androl 32(3):307–323
Purnachander K, Srinivasa Rao D, Kannappan N (2016) Anti neuroinflammatory effect of Vildagliptin in ischaemia-reperfusion induced cerebral infarction in normal and STZ induced type-II diabetic rats. IJPR 6(3):97–103
Refaie MMM, Abdel-Gaber SA, Mohammed RK (2017) Effect of telmisartan on ischemia reperfusion induced testicular injury in rats. EJBCP 7:2
Reichetzeder C, von Websky K, Tsuprykov O, Mohagheghi Samarin A, Falke LG, Dwi Putra SE (2017) Head-to-head comparison of structurally unrelated dipeptidyl peptidase 4 inhibitors in the setting of renal ischemia reperfusion injury. Br J Pharmacol 174(14):2273–2286
Rodriguez-Miguelez P, Lima-Cabello E, Martínez-Flórez S, Almar M, Cuevas MJ, González-Gallego J (2015) Hypoxia-inducible factor-1 modulates the expression of vascular endothelial growth factor and endothelial nitric oxide synthase induced by eccentric exercise. J Appl Physiol (1995) 118(8):1075–1083
Rossoni G, Manfredi B, De Gennaro CV, Berti M, Guazzi M, Berti F (2007) Sildenafil reduces L-NAME induced severe hypertension and worsening of myocardial ischaemia-reperfusion damage in the rat. Br J Pharmacol 150:567–576
Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS (2002) Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem 306:79–82
Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S (2011) Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124:2338–2349
Shiraishi K, Yoshida K, Naito K (2003) Activation of endothelial nitric oxide synthase in contralateral testis during unilateral testicular torsion in rats. Arch Androl 49:179–190
Tamer SA, Yildirim A, Kutay Köroğlu M, Çevik Ö, Ercan F, Yeğen BÇ (2018) Nesfatin-1 ameliorates testicular injury and supports gonadal function in rats induced with testis torsion. Peptides 107:1–9
Tang Z, Zhai W, Wang Z, Hu Z, Zhang M (2017) Profiling Proteinic changes induced by vildagliptin treatment in a mouse lung transplantation model: the role of kininogen-1. Ann Transplant 22:128–137
Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YY, Leu S, Chang HW, Yang JL, Yip HK (2015) Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypo-perfusion through suppressing oxidative stress and inflammatory reaction. J Hypertens 33(5):1001–1013
Tsui AK, Marsden PA, Mazer CD, Adamson SL, Henkelman RM, Ho JJ, Wilson DF, Heximer SP, Connelly KA, Bolz SS, Lidington D, El-Beheiry MH, Dattani ND, Chen KM, Hare GM (2011) Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia. Proc Natl Acad Sci U S A 108(42):17544–17549
Turner TT, Brown KJ (1993) Spermatic cord torsion: loss of spermatogenesis despite return of blood flow. Biol Reprod 49:401–407
Turner TT, Tung KS, Tomomasa H, Wilson LW (1997) Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod 57:1267–1274
Ustün H, Akgül KT, Ayyildiz A, Cicek T, Basal S, Ozgok A, Oğüş E, Germiyanoğlu C (2008) Effect of phosphodiesterase 5 inhibitors on apoptosis and nitric oxide synthases in testis torsion: an experimental study. Pediatr Surg Int 24:205–211
VanGuilder HD, Vrana KE, Freeman WM (2008) Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques 44:619–626
Vaos G, Zavras N (2017) Antioxidants in experimental ischemia-reperfusion injury of the testis: where are we heading towards? World J Methodol 7(2):37–45
Veeresh B, Patil BM, Veeresh Babu SV, Jeedi NM, Unger BS (2009) Involvement of nitric oxide in 5-HT(3) receptor agonist-induced fluid accumulation in jejunum and colon of anesthetized rats. Indian J Pharm 41(5):221–223
Wang X, Li J, Wu D, Bu X, Qiao Y (2016) Hypoxia promotes apoptosis of neuronal cells through hypoxia-inducible factor-1α-microRNA-204-B-cell lymphoma-2 pathway. Exp Biol Med (Maywood) 241(2):177–183
Wei SM, Yan ZZ, Zhou J (2007) Beneficial effect of taurine on testicular ischemia reperfusion injury in rats. Urology 70(6):1237–1242
Wei SM, Yan ZZ, Zhou J (2011) Protective effect of rutin on testicular ischemia-reperfusion injury. J Pediatr Surg 46(7):1419–1424
Wei SM, Huang YM, Zhou J (2017) Probucol reduces testicular torsion/detorsion-induced ischemia/reperfusion injury in rats. Oxidative Med Cell Longev 2017:5424097
Yapanoglu T, Ozkaya F, Yilmaz AH, Mammadov R, Cimen FK, Hirik E, Altuner D (2017) Effect of etoricoxib on experimental oxidative testicular ischemia-reperfusion damage in rats induced with torsion-detorsion. Korean J Physiol Pharmacol 21(5):457–464
Yoon G, Oh CS, Kim HS (2016) Distinctive expression patterns of hypoxia-inducible factor-1α and endothelial nitric oxide synthase following hypergravity exposure. Oncotarget 7(23):33675–33688
Youssef MI, Mahmoud AA, Abdelghany RH (2015) A new combination of sitagliptin and furosemide protects against remote myocardial injury induced by renal ischemia/reperfusion in rats. Biochem Pharmacol 96(1):20–29
Zhang F, Wu W, Deng Z, Zheng X, Zhang J, Deng S, Chen J, Ma Q, Wang Y, Yu X, Kang S, Wang X (2015) High altitude increases the expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase with intestinal mucosal barrier failure in rats. Int J Clin Exp Pathol 8(5):5189–5195
Zini A, Abitbol J, Girardi SK, Schulsinger D, Goldstein M, Schlegel PN (1998) Germ cell apoptosis and endothelial nitric oxide synthase (eNOS) expression following ischemia-reperfusion injury to testis. Arch Androl 41:57–65
Author information
Authors and Affiliations
Contributions
Walaa Y. Abdelzaher and Remon R. Rofaeil equally carried out study design, performing experiment, biochemical measurements, analysis, interpretation of data, and writing the manuscript. Dr. Doaa performed and wrote PCR part. Mina E. Attya performed and wrote the histopathology part. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelzaher, W.Y., Rofaeil, R.R., Ali, D.M.E. et al. Protective effect of dipeptidyl peptidase-4 inhibitors in testicular torsion/detorsion in rats: a possible role of HIF-1α and nitric oxide. Naunyn-Schmiedeberg's Arch Pharmacol 393, 603–614 (2020). https://doi.org/10.1007/s00210-019-01765-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01765-5